Abstract
AIM: To present evidence and formulate recommendations for sedation in pediatric gastrointestinal (GI) endoscopy by non-anesthesiologists.
METHODS: The databases MEDLINE, Cochrane and EMBASE were searched for the following keywords “endoscopy, GI”, “endoscopy, digestive system” AND “sedation”, “conscious sedation”, “moderate sedation”, “deep sedation” and “hypnotics and sedatives” for publications in English restricted to the pediatric age. We searched additional information published between January 2011 and January 2014. Searches for (upper) GI endoscopy sedation in pediatrics and sedation guidelines by non-anesthesiologists for the adult population were performed.
RESULTS: From the available studies three sedation protocols are highlighted. Propofol, which seems to offer the best balance between efficacy and safety is rarely used by non-anesthesiologists mainly because of legal restrictions. Ketamine and a combination of a benzodiazepine and an opioid are more frequently used. Data regarding other sedatives, anesthetics and adjuvant medications used for pediatric GI endoscopy are also presented.
CONCLUSION: General anesthesia by a multidisciplinary team led by an anesthesiologist is preferred. The creation of sedation teams led by non-anesthesiologists and a careful selection of anesthetic drugs may offer an alternative, but should be in line with national legislation and institutional regulations.
Keywords: Gastro-intestinal endoscopy, Gastroscopy, Colonoscopy, Sedatives, Pediatric ages, Anesthetics, Analgesics
Core tip: Sedation for pediatric gastro-intestinal endoscopy is preferably performed by pediatric anesthesiologists, as part of a multidisciplinary team. However, in many hospitals pediatric anesthesiology is insufficiently developed. The creation of sedation teams led by non-anesthesiologists and a careful selection of anesthetic drugs may offer an effective and safe alternative. These teams should be in line with national legislation and institutional regulations. This paper will help non-anesthesiologists to provide as good-as-possible sedation for children undergoing endoscopy. Practical protocols were developed providing up-to-date information on the most effective and most safe options.
INTRODUCTION
Esophago-gastro-duodenoscopy in children needs almost always to be performed under anesthesia or deep sedation. Procedural analgesia and sedation for procedures performed in ambulatory care are changing. The authors reviewed the literature on sedation and for endoscopy by non-anesthesiologists and to propose practical algorithms.
In order to obtain the greatest yield from a pediatric gastrointestinal (GI) endoscopic procedure and to perform these with the highest quality and with the maximum level of safety, some prerequisites must be fulfilled. A pediatric gastroenterologist or dedicated pediatrician must have judged the necessity of the procedure to optimize patient management. The procedure must be performed by a skilled endoscopic team with appropriate equipment in a suitable environment. The patient and parents or guardians must be informed as much and good as possible.
General anesthesia is only possible in a limited number of centers because of shortness of anesthesiologists. The aim of this review is to present and discuss different sedation protocols for non-anesthesiologists for pediatric GI endoscopies. Several protocols for procedural sedation by non-anesthesiologists have been produced by different professional bodies and organizations. However, practical algorithms for these procedures have not been published[1].
MATERIALS AND METHODS
The search for studies on pediatric sedation for GI endoscopy was an update of van Beek and Leroy[2]’s search strategy for the period between January 2011 (when their search was finished) and January 2014 and utilized the following databases: MEDLINE, Cochrane, and EMBASE[2]. These were searched for the keywords “endoscopy, GI”, “endoscopy, digestive system” and “sedation”, “conscious sedation”, “moderate sedation”, “deep sedation”, and “hypnotics and sedatives” for publications in English restricted to the pediatric age group, which was defined as 0 to 18 years. Subsequently a search for pediatric GI endoscopy sedation guidelines for the same keywords as above for the last 20 years with the same limits (publications in English, pediatric population) was undertaken. The search was expanded to include guidelines for GI endoscopy sedation by non-anesthesiologists for the adult population for the last 10 years. Furthermore a search for guidelines for pediatric procedural sedation published in the last 10 years was made.
RESULTS
The first search revealed 12 studies of which 8 are listed in Table 1[3-10]. Four of them were not relevant: Liu et al[11] analyzed anesthesia for outpatient gastroscopies and colonoscopies in adults only, Yen et al[12] studied sex differences in sedation with midazolam and alfentanil for gastroscopy only in adults, too[3,4]. The aim of the study of Vadlamudi et al[13] was evaluation of ileoscopy via stoma and not a sedation[13]. And finally, Siwiec et al[14] tested transnasal gastroscopy with ultrathin endoscope in non-sedated healthy volunteers or patients with the signs or symptoms of gastro-esophageal reflux disease.
Table 1.
Publications from the first search (“endoscopy, gastrointestinal”, “endoscopy, digestive system” AND “sedation”, “conscious sedation”, “moderate sedation”, “deep sedation”, and “hypnotics and sedatives”; limits: publications in English, paediatric population
| Ref. | Methodology | Results | Limitations | Conclusions |
| Bedirli et al[3] | Study type: prospective, randomised, double-blinded Patients: N = 80; 1–16 yr; ASA I, II Procedure: upper GI endoscopy Drugs: baseline: propofol (1 mg/kg; additional 0.5–1 mg/kg as needed); intervention: fentanyl (2 μg/kg) vs tramadol (2 mg/kg) Intended sedation level: deep sedation Additional interventions: spray of lidocaine 10%; infusion of 10 lactated Ringer’s solution (10 mL/kg per hour); supplemental oxygen 3–4 L/min) Administered by: anesthesiologist Outcome measures: Adverse events: HR (change for 20% from the baseline), BP (change for 20% from the baseline), SpO2 (< 90% for more than 15 s), respiratory rate, agitation score Effectiveness: Ramsey sedation score, duration of endoscopy, Steward recovery score, endoscopist’s rating of ease of procedure, total propofol consumption | Adverse events: self-limited bradycardia and transient desaturation in age group 0–2 yr, more in the fentanyl group Effectiveness: lower sedation scores in tramadol group; no difference of gastroenterologist rating | Only one dosage of drugs instead of titrating them | Propofol with tramadol or propofol provided efficient sedation; significantly less adverse effects in the tramadol group |
| Brecelj et al[4] | Study type: randomized, controlled, single-blinded Patients: N = 201; 1–18 yr Procedure: gastroscopy, colonoscopy Drugs: ketamine (0.75 mg/kg with additions of 0.25 mg/kg up max. to 1.5 mg/kg; repeated after 10–15 min at 0.5 mg/kg as needed) Intervention: midazolam (0.1 mg/kg; max 2.5 mg; repeated after 30–60 min at 0.05 mg/kg as needed) vs no premedication Intended sedation level: deep sedation Additional interventions: none Administered by: dedicated nurse under supervision of endoscopist Outcome measures: Adverse events: respiration rate, HR, BP, SaO2 (any drop below 92%), adverse reactions Effectiveness: ease of procedure, total ketamine consumption | Adverse events: mild self-limited laryngospasm in 3%, high rate of desaturations (approx. in 40%), vomiting in 17%, regardless of study group; more emergence reactions in ketamine group during recovery (10 vs 2) Effectiveness: high rate of sedation adequacy | Study was not double-blinded | Ketamine starting dose should be at least 1 mg/kg; more emergence reactions without midazolam premedication; same frequency of other adverse reactions |
| Miqdady et al[5] | Study type: retrospective cohort study Patients: N = 301; 1 (more than 10 kg) –18 yr; ASA I, II Procedure: upper, lower or combined GI endoscopy Drugs: atropine (0.01–0.02 mg/kg per minute. 0.1 mg, max. 0.4 mg); midazolam (0.05–0.2 mg/kg); ketamine (0.5–1 mg/kg) Intended sedation level: deep sedation Additional interventions: none Administered by: endoscopist Outcome measures: Adverse events: respiration rate, HR, BP, SaO2 (any drop below 94%), side effects Effectiveness: the adequacy of sedation | Adverse events: desaturation in 12.3%, in 1.2% disruption of examination due to persistent desaturation; in 1.2% respiratory distress after examination Effectiveness: effective and uneventful sedation in 79.4% | Retrospective study | Midazolam and ketamine sedation is safe and effective for diagnostic GI endoscopies in children older than 1 yr weighting more than 10 kg without comorbidities |
| Motamed et al[6] | Study type: prospective, randomised, double-blinded Patients: N = 150; 1–18 yr; ASA I, II Procedure: upper GI endoscopy Drugs: main sedative: midazolam (0.1 mg/kg; if needed repeated doses up to 5 mg or 0.3 mg/kg); premedication 45 min before the procedure with oral placebo (normal saline), oral ketamin (5 mg/kg), or oral fentanyl (2 μg/kg) Additional interventions: spray of lidocaine 10%; additional oxygen trough nasal cannula at 2 L/min Administered by: registered nurse supervised by anaesthesiologist Outcome measures: Adverse events: respiration rate, HR (decrease by 30% from baseline), BP (decrease or increase by 20%), SaO2 (any drop below 90%) Effectiveness: total midazolam dose, modified Ramsey sedation score, procedure time, discharge time, ease of iv catheter placement, separation from parents agitation, the adequacy of sedation | Adverse events: in total in 26% of patients (hypoxia in 7.3%, hypotension in 6.7%, dizziness in 20%, nausea in 10%, vomiting in 17.6%); mild, easily managed Effectiveness: the total recovery and procedure duration time was shorter in the ketamine-midazolam group, inadequate sedation in 10.2% in placebo-midazolam and in 8% in fentanyl-midazolam vs in 3.9% in ketamine-midazolam group; the mean administered dose of midazolam was the lowest in ketamine-midazolam group; the iv line placement and separation from parents was easier in ketamine-midazolam group; only 27.4% of patients did not remember the procedure | Sedation with oral ketamine-iv midazolam is better than placebo-midazolam or oral fentanyl-iv midazolam | |
| Chiaretti et al[7] | Study type: retrospective (12 yr), multicentric Patients: N = 36516; 1 to > 10 yr; ASA I, II, III Procedure: different painful procedures Drugs: main sedative: propofol 2 mg/kg in children from 1 to 8 yr of age and 1 mg/kg in older children and in children younger than 1 yr; further doses of 0.5–1.0 mg/kg in the case of agitation or complain; premedication: atropine 0.010–0.015 mg/kg, ketamine (0.5 mg/kg) to avoid infusion pain in 2 centres (not in gastroscopy); additional oxygen trough nasal cannula at 6 L/min Intended sedation level: deep sedation Administered by: paediatrician (anaesthesiologist available in case of need) Outcome measures: mean arterial pressure, heart rate and SatO2, incidence, type and timing of adverse events (major and minor) and number of calls to the emergency team Effectiveness: total dosage of the sedative agents, level of sedation (Ramsay scale) | Adverse events: in 6 patients (0.02%) emergency team intervention (prolonged laryngospasm in 3 patients, bleeding in 1, intestinal perforation in 1, and 1 during lumbar puncture); milder adverse events: hypotension in 19 patients (0.05%), ventilation by face mask and additional oxygen in 128 patients (0.4%), laryngospasm in 78 patients (0.2%), bronchospasm in 15 patients (0.04%); minor complications more often in children who underwent gastroscopy; none of the children experienced severe side effects or prolonged hospitalisation. | Retrospective study | Propofol is safe and effective for paediatrician-administered procedural sedation in children; appropriate training for paediatricians is important |
| Gül et al[8] | Study type: randomized, controlled, double-blinded Patients: N = 64; 3-14 yr; ASA I Procedure: esophagogastroduodenoscopy Drugs: main sedative: propofol 2 mg/kg; analgesic: group R: remifentanil 0.25 μg/kg, group F: fentanyl 0.5 μg/kg; additional oxygen trough nasal cannula at 4 L/min Intended sedation level: deep sedation Administered by: anesthesiologist Outcome measures: MAP, HR, RR, and SpO2 Effectiveness: ease of gastroscopy, patient’s movements during procedure, additional doses of drugs; level of sedation (Ramsay scale); duration of PACU stay | Adverse events: prolonged apnoea in 14 (43.8%) children in group R and in 11 (33.3%) children in group F; none required endotracheal intubation; Effectiveness intraoperative respiratory rate, time to eye opening, opioid consumption, and duration of recovery were significantly shorter in group R duration of PACU stay were significantly shorter in group R than in group F | Remifentanil (combined with propofol) is an efficient and as safe as fentanyl propofol combination for esophagogastroduodenoscopy in children | |
| Long et al[9] | Study type: retrospective analysis of prospectively collected data Patients: N = 4904; 15-90 yr; ASA I-IV Procedure: esophagogastroduodenoscopy Drugs propofol 1-100 mg and/or midazolam 1-3 mg2 mg/kg Administered by: endoscopist Outcome measures: influence of pre-existing disease and ASA score on oxygen desaturation (SpO2) < 90% | Adverse events: hypoxemia in 245 patients (5%); risk factors: high BMI (30 kg/m2), hypertension, diabetes, gastrointestinal disease, heart disease ASA score was not predictive for hypoxemia | Retrospective study | Independent risk factors for hypoxemia were high BMI, hypertension, diabetes, gastrointestinal and heart diseases and combined gastro and colonoscopy |
| Agostoni et al[10] | Study type: retrospective analysis of prospectively collected data Patients: N = 17999 (17524 in older than 12 yr, 457 in < 12 yr); 4-74 yr; ASA I-IV Procedure: esophagogastroduodenoscopy and in some cases different procedures (mucosectomy, hemostatic clip, percutaneous endoscopic gastrostomy, …) Drugs: propofol induction (in children 1-2 mg/kg BW) then in continous infusion Intended sedation level: deep sedation Administered by: anesthesiologist Outcome measures: adverse events (hypotension, desaturation, bradycardia, hypertension, arrhythmia, aspiration, respiratory depression, vomiting, cardiac arrest, respiratory arrest, angina, hypoglycemia, and/or allergic reaction) | Adverse events: rare in children (2.6%) and in adults (4.5%), in children were more often only bradycardia (2.1%) and hypotension (0.44%) 3 adult patients died; no death case in children | Retrospective analysis, single centre data | Deep sedation with intravenous propofol for endoscopic procedures is safe in children and adults |
ASA: American Society for Anesthesiology; BP: Blood pressure; GI: Gastrointestinal; HR: Heart rate; SpO2: Oxygen saturation; BMI: Body mass index; RR: Respiratory rate.
We found one guideline for pediatric GI endoscopy in English which addressed different aspects including sedation[15].
We expanded the search to guidelines for sedation for GI endoscopy performed by non-anesthesiologists in adult patients during the last 10 years. The search revealed 9 publications which are listed in Table 2[16-24].
Table 2.
Gastrointestinal endoscopy sedation guidelines for adults
| Organisation Ref. | Title | Year of publication |
| American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association Institute; American Society for Gastrointestinal Endoscopy; Society for Gastroenterology Nurses and Associates Vargo et al[16] | Multisociety sedation curriculum for GI endoscopy | 2012 |
| Task Force Members. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Dumonceau et al[17] | Guideline: Non-anesthesiologist administration of propofol for GI endoscopy | 2010 |
| Society of American Gastrointestinal Endoscopic Surgeons Heneghan et al[18] | Surgeons. Society of American Gastrointestinal Endoscopic Surgeons guidelines for office endoscopic services | 2009 |
| Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy Lichtenstein et al[19] | Sedation and anesthesia in GI endoscopy | 2008 |
| Training Committee of the American Society for Gastrointestinal Endoscopy Vargo et al[20] | Training in patient monitoring and sedation and analgesia | 2007 |
| Working Group on Endoscopy, Austrian Society of Gastroenterology and Hepatology (OGGH) Schreiber[21] | Austrian Society of Gastroenterology and Hepatology (OGGH)-guidelines on sedation and monitoring during GI endoscopy | 2007 |
| Training Committee American Society for Gastrointestinal Endoscopy[22] | Training guideline for use of propofol in gastrointestinal endoscopy | 2004 |
| American Society for Gastrointestinal Endoscopy, Standards of Practice Committee Waring et al[23] | Guidelines for conscious sedation and monitoring during GI endoscopy | 2003 |
| Standards Practice Committe American Society for Gastrointestinal Endoscopy Faigel et al[24] | Guidelines for the use of deep sedation and anesthesia for GI endoscopy | 2002 |
GI: Gastrointestinal.
The search for guidelines for pediatric procedural sedation published in English during the last 10 years revealed 10 publications. Two are general guidelines for sedation in children[25,26]. Another one, followed by an update published 7 years later, addresses specifically ketamine sedation for emergency departments[27,28]. Others are specifically developed for sedation for dental procedures in children. They are listed in Table 3[15,25,26,28-33].
Table 3.
Paediatric procedural sedation guidelines
| Organisation Ref. | Title | Year of publication |
| Green et al[28] | Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update | 2011 |
| National Clinical Guideline Centre (United Kingdom)[26] | Sedation in children and young people: Sedation for diagnostic and therapeutic procedures in children and young people | 2010 |
| American Academy on Pediatric Dentistry Clinical Affairs Committee-Sedation and General Anesthesia Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs[29] | Guideline on use of anesthesia personnel in the administration of office-based sedation/general anesthesia to the pediatric dental patient | 2009 |
| American Academy on Pediatrics; American Academy on Pediatric Dentistry[30] | Guideline for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures | 2009 |
| American Academy of Pediatrics; American Academy of Pediatric Dentistry Coté et al[25] | Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update | 2006 |
| American Academy on Pediatric Dentistry Clinical Affairs Committee-Sedation and General Anesthesia Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs[31] | Guideline on use of anesthesia care providers in the administration of in-office deep sedation/general anesthesia to the pediatric dental patient | 2005 |
| American Academy of Pediatric Dentistry | Guideline on the elective use of minimal, moderate, and deep sedation and general anesthesia for pediatric dental patients | 2005 |
| American Academy of Pediatric Dentistry Committee on Sedation and Anesthesia[15] | ||
| American Academy of Pediatric Dentistry[32] | Clinical guideline on the elective use of minimal, moderate, and deep sedation and general anesthesia for pediatric dental patients | 2004 |
| Green et al[27,28] | Clinical practice guideline for emergency department ketamine dissociative sedation in children | 2004 |
| UK National Clinical Guidelines in Pediatric Dentistry Hosey[33] | UK National Clinical Guidelines in Paediatric Dentistry. Managing anxious children: the use of conscious sedation in paediatric dentistry | 2002 |
Pre-requisites for safe and effective sedation by non-anesthesiologists
GI endoscopy must be discussed with the child if emotionally and intellectually competent enough and parent(s)/guardian(s). The pre-sedation assessment is listed in Table 4. Patients should be classified by physical status assessment as developed by the American Society for Anesthesiology (ASA) (Table 5). If the child’s ASA classification conforms to class I or II, sedation can be performed safely. If the child fits in ASA class III classification, the benefits of sedation must be carefully weighed against the risks and in the vast majority of cases anesthesiology will be preferable. Patients in ASA class IV and V must be anesthetized by anesthesiologists[28,34].
Table 4.
Preparation of a child for sedation for gastrointestinal endoscopy
| Preparation of the patient | Comments | |
| Planning of the investigation /procedure | Understanding of the investigation | Explanation of the examination: Aims of investigation Possible risks |
| Informed consent | Signed by parents and/or the child (depending on the age and legislation) | |
| Presedation assessment | Co-morbidity ASA score (Table 5) Medicines Bleeding tendency Previous undesirable effects of sedation/anesthesia Specific contraindications for the planned sedation Previous complications of investigations Allergies The need for antibiotic prophylaxis Laboratory investigation/consultation before the investigation/procedure (e.g., tests of hemostasis in case of bleeding tendency) Additional important data | |
| Preparation | Exact instructions (fasting time, colon cleansing etc.) | |
| On the day of examination | Focused history: Current health state Infectious diseases Epidemiologic situation Fasting Allergy Specific contraindications for the planned sedation | |
| Physical examination | Complete physical examination with the focus on respiratory and cardiovascular system | |
| Measurement of vital signs | Arterial blood pressure Heart rate Arterial oxygen saturation | |
| Laboratory investigations | If needed |
ASA: American Society for Anesthesiology.
Table 5.
American Society of Anesthesiologists physical status classification[24]
| Class | Description | Suitability for sedation |
| Class I | A normally healthy patient | Excellent |
| Class II | A patient with mild systemic disease (e.g., controlled asthma) | Generally good |
| Class III | A patient with severe systemic disease (e.g., a child who is actively wheezing) | Intermediate to poor |
| Class IV | A patient with severe systemic disease that is a constant | Poor |
| threat to life (e.g., a child with status asthmaticus) | ||
| Class V | A moribund patient who is not expected to survive without the operation (e.g., a patient with severe cardiomyopathy requiring heart transplantation | Extremely poor |
The depth of sedation is influenced by the procedure. If analgesia is needed together with sedation, as in the case of endoscopic-therapeutic procedures, the patient has to be anesthetized. The same is valid for emergency GI endoscopies such as removal of a foreign body from the upper GI tract and GI bleeding. Sedation necessitates that a team member assigned for observing the vital signs of the patient, since monitoring of pulse oximetry, heart rate and preferably also capnography are insufficient[8,12].
Equipment for resuscitation must be present in the endoscopy room. The team has to be trained in pediatric advanced live support techniques and has to be familiar with measures needed in any scenario of complications[1].
Sedatives and their combinations
Legislation and regulation regarding limitations of administration of different medications, such as inhalation anesthetics, differ from country to country. Therefore, limitations caused by local legislation should be carefully checked. In most countries, the administration of inhalation anesthetics is only authorized by anesthesiologists.
Premedication
Premedication with midazolam (oral or intra-nasal) lessens the stress for an intravenous (iv) catheter placement and other preparations for GI endoscopy before sedation or anesthesia. This procedure is effective and safe although intranasal administration may cause local discomfort. In order to decrease the stress and pain caused by a venepuncture, an eutectic mixture of the topical anesthetics lidocaine and prilocaine provides local anesthesia when applied with an occlusive dressing 30-60 min before venipuncture[35].
An iv catheter provides the most effective way of delivering agents needed for sedation and analgesia. Inhalation, intramuscular or other sedation regimens are less well documented. An iv catheter is also important for emergency access in the case of adverse events occurring during sedation or the endoscopic procedure[25,36,37].
Mechanisms of action and the main undesirable effects of sedatives and adjuvant medicines are listed in Table 6[8,38-49]. Usual dosage regimens and the main contraindications are listed in Table 7.
Table 6.
Sedatives and adjuvant medicines for paediatric gastrointestinal endoscopy sedation
| Generic name | Mechanism(s) of action | Main undesirable effects | Comments | Ref. |
| Sedatives | ||||
| Fentanyl | Opioid receptors agonist; analgesia and sedation | Respiratory depression, hypotension | Due to analgesic effect only it should be combined with benzodiazepine; antagonist naloxone | [38-40] |
| Ketamine | Binds to the NmethylDaspartate (NMDA) receptors; anesthesia, analgesia, amnesia, sedation, immobilisation | Laryngospasm, hypertension, tachycardia, hypersalivation, vomiting, random movements, increase in intraocular pressure, emergence phenomena (floating sensations, vivid dreams, blurred vision, hallucinations, and delirium) | Beneficial respiratory properties and analgesic potency S(+) isomer has less adverse effects | [40-42] |
| Meperidine | Opioid receptors agonist; analgesia and sedation | Respiratory depression, pruritus, vomiting | Interaction with monoamine oxidase inhibitors | [38,43,44] [38-40] |
| Midazolam | GABA receptor agonist; anterograde amnesia, anxiolysis, sedation, hypnosis | Respiratory depression, hypotension, paradoxical agitation | Without analgesic effect; should be combined with analgesic (usually opioids) | |
| Concomitant use with opioid increases the risk of respiratory depression antagonist flumazenil | ||||
| Nitrous oxide | Inhalation anaesthetic | Vomiting, dizziness, voice change, euphoria, laughter | The need of scavenging system Use mostly limited to anaesthesiologists | [38,40,45] |
| Propofol | GABA receptor agonist; sedation, hypnosis, amnesia | Respiratory depression, apnoea, hypotension, painful injection | [38,40,46] | |
| Sevoflurane | Inhalation anaesthetic | Recovery agitation, bradycardia, hypotension, cough, vomiting, seizures | The need of scavenging system Use limited to anaesthesiologists | [47-49] |
| Antagonists | ||||
| Flumazenil | Benzodiazepine antagonist | Nausea, vomiting | Contraindicated in benzodiazepine dependence, seizure disorder, cyclic antidepressant overdose, elevated intracranial pressure in patients, and in patients taking medicines known to lower the seizure threshold | [40] |
| Naloxone | Opioid antagonist | Nausea, vomiting, tachycardia | [40] |
Table 7.
The list of sedatives/analgesic, adjuvant medicines and antagonists with usual dosage regimens, and main contraindications
| Medicine generic name | Route | Dose | Time to start sedation/analgesia (after iv application) | Sedation/analgesia duration | Repeating time and dose | Contraindications | Comments | Ref. |
| Sedative/analgesic | ||||||||
| Fentanyl | iv | 1–2 μg/kg (up to 50 μg) | 0.5 s | 20–40 min (30–60 min) | 3 min 1–1.25 μg/kg | Due to higher clearance younger children need frequent dosing | [38,40] | |
| Ketamine | iv slowly over 1 min; other routes have less predictive effects and different dosing – see the discussion | 1–1.5 mg/kg | 1–5 min | 15 min | 10 min | Severe cardiovascular disease, malignant hypertension, CSF obstructive states (controversial), intraocular pressure pathology; previous psychotic illness, hyperthyroidism or thyroid medicine use; porphyria | A single enantiomer S(+); | [8,40-42] |
| 0.5 mg/kg | the anesthetic management of seriously ill hypovolemic patients, it may be the agent of choice for managing children and burned patients; low cost | |||||||
| Meperidine | iv slowly over 1–2 min | 0.3–2 mg/kg | 3–6 min | 60–180 min | Simultaneous treatment with monoamine oxidase inhibitors | [38,43,44] | ||
| Midazolam | iv slowly over 2–3 min; other routes have less predictive effects and different dosing | 0.05–0.1 mg/kg in < 5 yr (max. 0.6 mg/kg); in 6–12 yr 0.025–0.05 mg/kg (max.0.4 mg/kg); in older than 12 yr 2–2.5 mg (in total not per kg BW) | 2–3 min | 45–60 min | Repeating doses every 2–5 min until desired effect; in children 6 mo–5 yr total dose up to 0.6 mg/kg or max. 6 mg; in 6–12 yr total dose up to 0.4 mg/kg or max. 10 mg; in older than 12 yr additional boluses of 1 mg until desired sedation | Respiratory depression, hypotension | Rarely used as a sole sedative; might be used to sedate the frightened child before iv catheter placement; mostly combined with opioids; paradoxical irritation in 1%–5% of patients | [38-40] |
| Nitrous oxide | Inhalation | Mostly the mixture of nitrous oxide (50%) and oxygen | 0.5–1 min | 5 min | Continuously or “on demand” | Pneumothorax, bowel obstruction, head injury, pregnancy | Its use limited to anaesthesiologists | [38,40,45] |
| Propofol | iv | 2 mg/kg in infants and young children (younger than 3 yr); 1 mg/kg in children older than 3 yr | 1–2 min | 5–15 min | 1 mg/kg (infants and children up to 3 yr); 0.5 mg/kg (children older than 3 yr) to reach the desired sedation; may be continuously infused at 100 μg/kg per min and increasing the speed of infusion by 50 μg/kg per min for prolonged procedures | Egg or soy allergy | For additional medication to alleviate infusion pain see text; alfentanil but not fentanyl increases propofol blood level; in many countries the use is limited to anaesthesiologists | [38,40,46] |
| Sevoflurane | Inhalation | Different concentrations according to the age | Duchenne’s muscular dystrophy, moderate to severe liver disease of unknown aetiology, history of malignant hyperthermia | Its use limited to anaesthesiologists | [47-49] | |||
| Antagonists | ||||||||
| Flumazenil | iv | 0.02 mg/kg (max. 1 mg) | 1–3 min | 30 min | 1 min; same dose | Chronic benzodiazepine use; ingestion of drugs that increase the risk for seizures development (e.g., cyclic antidepressants, cyclosporine, and others) | Due to its shorter duration of action than most of benzodiazepines (e.g., midazolam) repeated doses may be needed | [38,40] |
| Naloxone | iv or i.m. | 0.1 mg/kg (max. 2 mg) | 2 min | 20–40 min | 2 min; same dose | Hypersensitivity only | Due to its shorter duration of action than most of opioids (e.g., fentanyl) repeated doses may be needed | [38,40] |
Propofol
Propofol is a rapid onset and short acting anesthetic without analgesic properties and with a narrow therapeutic range. Its sedative properties result from agonistic action on gamma-aminobutyric acid (GABA) receptors. Propofol is contraindicated in infants younger than 1 mo bacasue of missing data on safety according to a Cochrane review[50]. The main undesirable effects include pain on injection, respiratory depression, bradycardia and hypotension[38,46].
van Beek and Leroy[2] reported failure to conduct a procedure due to incomplete sedation in only 0.0%–0.4% of cases, despite the fact that the sedation was performed in 88.1% by non-anesthesiologists[2]. The recovery time after propofol administration was shorter than after midazolam/meperidine[2]. Major respiratory complications occurred in 11/3883 propofol sedations (0.3%), but no I intubation and no sequelae were reported. The incidence of undesirable effects (e.g., temporary desaturation due to hypoventilation, laryngospasm) was comparable to other protocols and was more frequent in younger children, especially infants[2].
A randomized study in 90 adults undergoing colonoscopy showed that the satisfaction of patients was greater and there were less undesirable effects when they were sedated by an endoscopist than by an anesthesiologist[51]. A Scandinavian study tested a 6-wk educational program for registered nurses with excellent safety results[52].
The largest multicenter prospective study of propofol sedation for different pediatric procedures outside an operating theatre was published by the international (United States and Canada) Pediatric Sedation Research Consortium. They analysed the data of 49836 propofol sedation episodes and showed that propofol-based sedation is amongst the safest sedation practice for children[53]. Cardio-respiratory resuscitation was necessary in two cases. Pulmonary aspiration of gastric fluid secondary to vomiting during sedation occurred in four patients. Less serious respiratory adverse events were: desaturation in 154/10000 procedures; central apnea or upper airway obstruction in 124/10000; stridor in 10/10000; laryngospasm in 20/10000; excessive salivation in 73/10000; and vomiting in 10/10000 cases. The authors of this report estimate propofol sedation safe in children. Interestingly there were no differences in adverse effects between anesthesiologists and non-anesthesiologist. However, it should be pointed out that this report did not focus on upper GI endoscopy specifically, in which a shared airway is an important consideration, especially as attempting esophageal intubation may have the potential for induction of laryngospasm. However, it is stressed by the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Endoscopy Working Group that the advice of the Pediatric Sedation Research Consortium, including institutions with highly motivated and well organized sedation/anesthesia teams, is only to be considered when anesthetic teams are not available, and that priority should go to actions to obtain these anesthetic teams.
Chiaretti et al[7] published a retrospective study on pediatric procedural sedation with propofol over a 12-year period in three Italian hospitals[7]. They analyzed 36516 procedural sedations for different painful procedures. Deep sedation was achieved in all patients. None of the children experienced severe side effects or needed a prolonged hospitalization. In six patients (0.02%) emergency team had to intervene (prolonged laryngospasm in three patients, bleeding in one, intestinal perforation in one, and one during lumbar puncture). But milder adverse events were more often: hypotension in 19 patients (0.05%), ventilation by face mask and additional oxygen in 128 patients (0.4%), laryngospasm in 78 patients (0.2%), bronchospasm in 15 patients (0.04%). Minor complications were more often in children who underwent gastroscopy.
The usual loading dose of propofol is 2 mg/kg in infants and young children (younger than 3 years) and 1 mg/kg in older children and teenagers. Subsequent boluses of 1 mg/kg for younger, or 0.5 mg/kg for older children, may be added to ensure the appropriate level of sedation. For longer procedures propofol may be administered in a continuous infusion[38].
For painful procedures an analgesic must be added as propofol has no analgesic properties[38]. Bedirli et al3] showed that the addition of tramadol or fentanyl to propofol provided efficient sedation, with less adverse events in the tramadol group (less desaturation, hypotension, and bradycardia; but more vomiting in fentanyl group)[3]. According to Gül et al[8] there was no difference in safety and efficacy between remifentanil and fentanyl co-administration with propofol.
The pain of propofol injection can be reduced by choosing a larger vein such as the antecubital site, or alternatively the injection of lidocaine[54]. A possible flow chart of propofol sedation for pediatric GI endoscopy is presented in Figure 1.
Figure 1.
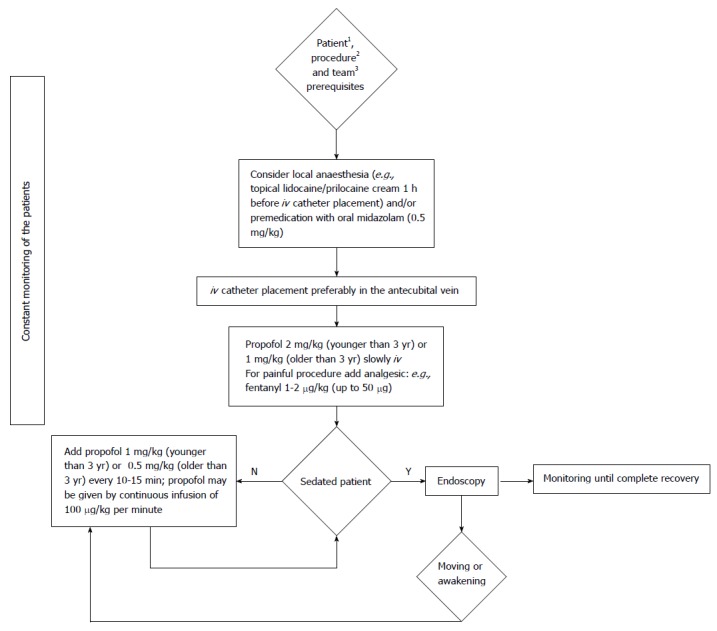
Flow chart of propofol sedation protocol for paediatric gastrointestinal endoscopy. 1Older than 1 mo, without contraindications (egg or soy allergy); 2Diagnostic endoscopy or procedure for which no endotracheal intubation is needed; 3The team qualified for paediatric sedation for gastrointestinal endoscopy.
Generally, one cannot extrapolate data from adult practice to children. However, four different European Societies (of Gastrointestinal Endoscopy, of Gastroenterology, of Endoscopy Nurses and Associates, and of Anesthesiology) jointly issued guidelines for propofol sedation of adults for GI endoscopy by non-anaesthesiologists[16]. It is interesting that although the Board of Directors of the European Society of Anesthesiology (ESA) decided unanimously to endorse these guidelines, a majority of the national societies of the ESA did not support them. Consequently ESA retracted the endorsement[55]. The Danish training program for nurses includes training on how to administer propofol for GI endoscopic procedures in adults[52].
Ketamine
Ketamine is a dissociative anesthetic and analgesic. It is an N-methyl-D-aspartate channel antagonist and depresses sensory association areas of the cortex, limbic system and thalamus. It has been used for a long time for sedation and analgesia in emergency pediatrics due to its association with a preserve gag reflex and lack of respiration depression and hypotension[41]. Despite its good safety profile, the significant association with laryngospasm (especially with gastroscopy), emergence phenomena such as hallucinations, excitation, nightmares, delirium, recurrent illusions or “flashbacks”, vomiting, and hypersalivation limit ketamine’s broader use[27,38,41].
When used as a sedative, ketamine must be administered by slow iv injection at a dosage of 1-2 mg/kg initially. The sedative effect lasts 10-15 min. Repeated doses of 0.5 mg/kg prolong its action (Figure 2)[27,38].
Figure 2.
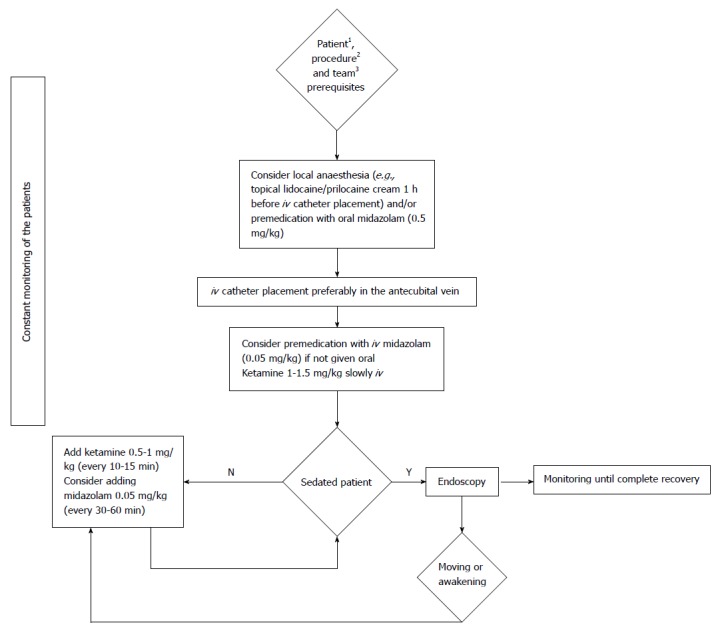
Flow chart of ketamine sedation protocol for paediatric gastrointestinal endoscopy. 1Older than 3 mo, without contraindications (severe cardiovascular disease, malignant hypertension, CSF obstructive states, intraocular pressure pathology, psychotic illness, hyperthyroidism or thyroid medicines use, and porphyria); 2Diagnostic endoscopy or procedure for which no endotracheal intubation is needed; 3The team qualified for paediatric sedation for gastrointestinal endoscopy.
The most frequent undesirable effects are vomiting, hypersalivation, nystagmus, hypertension, tachycardia, skin erythema, and emergence phenomena. Laryngospasm, which is potentially of greatest danger, is uncommon. The use of ketamine is contraindicated in infants younger than 3 mo, patients with psychosis, uncontrollable hypertension or hyperthyroidism, and as it increases intracranial and intraocular pressure. Ketamine should not be used after a head or eye trauma, or surgery, although some data advocate against these precautions[27,38].
The concomitant use of midazolam with ketamine decreases the frequency of emergence phenomena, although this remains controversial[56]. Two randomized double-blind studies performed in pediatric emergency departments did not find sufficient evidence to support the addition of midazolam for this purpose[57,58]. However, a randomized study using midazolam in co-administration with ketamine for pediatric sedation for GI endoscopy suggests that midazolam does prevent emergence phenomena[4]. Other co-administered medicines might lessen some undesirable effects of ketamine but their use is not supported by sufficient evidence. Anticholinergics may prevent hypersalivation[59], but this has also been contradicted[60]. The anti-emetic ondansetron prevents vomiting in some patients[61].
Benzodiazepines and opioids
Midazolam is a short-acting benzodiazepine which is widely used for sedation but is generally considered to be insufficient as a monotherapy. It has anxiolytic, amnesic, sedative, hypnotic, muscle relaxant, and anticonvulsant properties which result from GABA receptor activation[38,39]. The major undesirable effects are respiratory depression and hypotension, which are avoidable with appropriate dosing and are reversed by the antagonist flumazenil[38]. Other undesirable effects such as paradoxical agitation are reported in up to 15% of children[38].
Midazolam may be administered orally as an anxiolytic before the placement of an iv cannula but its effect is less predictive orally than when administered iv The usual starting dose is 0.1 mg/kg iv as a pre-medication but may be titrated to the desired effect by incremental doses of 0.05 mg/kg[39].
Opioids are potent analgesics which express their activity via different opioid receptors. The most suitable for sedation is fentanyl due to its rapid onset and short action. As it has no sedation properties it must be combined with benzodiazepines but the combination increases the risk of respiratory depression[38]. Other undesirable effects are itching, hypotension and vomiting but those are less pronounced than in histamine-releasing opioids such as morphine and meperidine[38]. Naloxone is an opioid receptor antagonist and is administered intravenously at 0.1 mg/kg[38].
Meperidine was the first synthetic opioid agent. It acts mainly as an antagonist of μ and κ receptors and has an analgesic potency ten times greater than that of morphine[62]. Like other opioid drugs, meperidine causes nausea, vomiting, urinary retention and respiratory depression. Its property of acting on nerve fibers, similar to those of local anesthetics, allows its use as an alternative for anesthetic blockade and differentiates it from other opioids. An iv route has been used for treating moderate to severe pain, for regional anaesthesia, for pre-medication and for analgesia during anesthesia. The combination of midazolam and meperidine can be used to achieve sedation and analgesia during colonoscopy[63]. There are few studies that have compared the efficacy of midazolam alone to midazolam and meperidine. According to Ozel et al[64], there were no significant differences in oxygen saturation/blood pressure but a better patient compliance was observed in the combined sedation group[64]. Cinar et al[65] showed that in respect of the recovery and procedure time there were no significant differences between the midazolam and the midazolam/meperidine group[65]. In a randomized trial comparing the efficacy and recovery time of two sedation regimens consisting of midazolam in combination with either meperidine or fentanyl, it was found that the fentanyl combination with midazolam resulted in a significantly faster recovery, without any apparent loss of analgesic effect[66]. Again, these are adult studies, and extrapolation to pediatrics is not necessarily appropriate.
Meperidine is administered intravenously at 1 mg/kg[64]. A possible flow chart of benzodiazepine and opioid sedation for pediatric GI endoscopy is presented in Figure 3.
Figure 3.
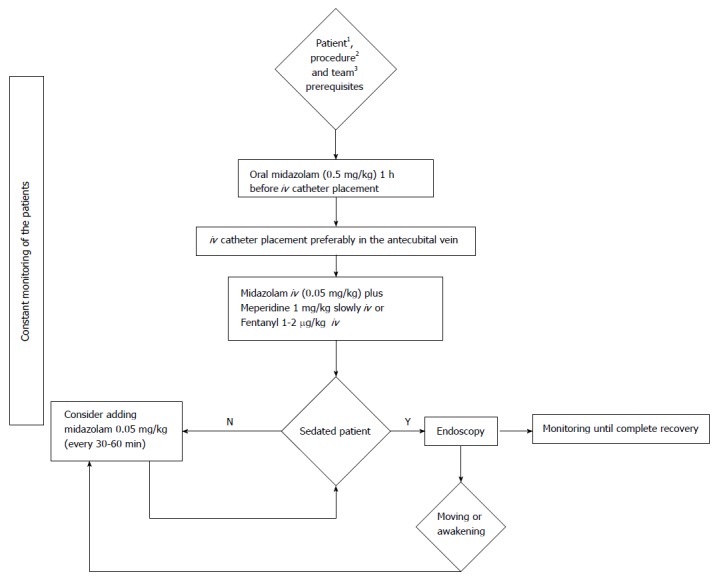
Flow chart of opioid and benzodiazepine sedation protocol for paediatric endoscopy. 1Patient without contraindications (not being simultaneously treated with monoamine oxidase inhibitors); 2Diagnostic endoscopy or procedure for which no endotracheal intubation is needed; 3The team qualified for paediatric sedation for gastrointestinal endoscopy.
Fentanyl is usually administered at 1–2 μg/kg. The analgesic effect lasts 20-40 min[38].
van Beek and Leroy[2]’s analysis found opioid and benzodiazepine sedation protocols suboptimal. These protocols were inferior in comparison to general anaesthesia. The comparison of midazolam/fentanyl with propofol sedation by Lightdale et al[67] addressed mainly procedure duration and discharge times which were similar for both groups, but the endpoint of this study was not to compare safety or efficacy.
Inhalation anesthetics
In most countries, legislation limits the administration of inhalation anesthetics to anesthesiologists.
Sevoflurane: Sevoflurane is an inhalational anesthetic with a very good safety profile (low incidence of airway hypersecretion, respiratory depression or cardiovascular events)[47]. When used for paediatric sedation for endoscopies it was characterized by a shorter recovery time and earlier discharge. Sevoflurane can only be administered by an anesthesiologist. The insertion of an iv catheter may not be needed. The use of inhaled anesthetics requires waste gas scavenging to prevent anesthetic gases being released into the ambient air[47].
There are no recently published studies on sevoflurane sedation for pediatric GI endoscopies.
Nitrous oxide: Nitrous oxide is an inert gas which has analgesic, sedative and amnesic properties of short duration. Michaud et al[68] reported a good experience with 50% nitrous oxide for gastroscopies and procto-sigmoidoscopies in children. They did not evaluate it for ileo-colonoscopy nor compare this type of sedation to other protocols[68]. There are no newer studies on nitrous oxide sedation for GI endoscopy in children.
In adults nitrous oxide has been used successfully for proctoscopies and colonoscopies. In a systematic review Welchman et al[45] analyzed in a systematic review 11 studies including 623 patients. Continuous nitrous oxide inhalation provided comparable analgesia to iv sedation for colonoscopies. There was no difference in procedural pain between on-demand nitrous oxide and no sedation for colonoscopies. The recovery time was shorter in the nitrous oxide groups[45].
Nitrous oxide is often more used as an anxiolytic before iv catheter placement if the face mask does not agitate the patient. However, most anesthesiologists would suggest that age-appropriate calming of a patient by engagement would have a similar result. Vomiting occurs in up to 10%. It is contraindicated in bowel obstruction and should not be administered if any of the team members is pregnant[38]. Its routine use in pediatric GI endoscopy is not ratified.
Adjuvant medicines and antagonists
Anti-cholinergics: As discussed in the section on ketamine, anti-cholinergics (e.g., atropine or glycopirolate) decrease the hypersalivatory effect which may influence airway patency[59]. However, importantly, it should be noted that available evidence does not support this practice and anti-cholinergics are no longer routinely recommended[26,60].
Anti-emetics: Many sedative/analgesic agents (e.g., ketamin, fentanyl), with the exception of propofol, provoke vomiting[50]. Ondansetron reduced the incidence of vomiting in a double-blind, randomized, placebo-controlled study in 255 children in an emergency department sedated by ketamine[61].
Flumazenil: Flumazenil is an antagonist used to reverse the undesirable effects of benzodiazepines such as respiratory depression. It is delivered iv at 0.1 mg/kg up to a maximum of 2 mg and has a rapid onset of action in 1-3 min. The half-life of flumazenil is shorter than that of other benzodiazepines (e.g., midazolam) making close monitoring essential and reapplication sometimes needed[38,40].
Naloxone: Naloxone reverses opioid effects and results in normal respiration within 1-2 min of application of 0.1 mg/kg (up to 2 mg) iv or intramuscular. Its duration of action is around 20-40 min hence repeated doses might be needed as the duration of action of most opioids (e.g., fentanyl) is longer[38,40].
DISCUSSION
Effective and safe sedation for pediatric endoscopic procedures is a non-negotiable pre-requisite and an important factor for lowering patient distress. In principle, total iv anesthesia should be performed by anesthesiologists. However, it has to be recognized that in many countries, including a majority of European countries and in parts of the United States, the limited availability of anesthesiology teams and limited organizational considerations represents a medical dilemma. In many European countries anesthesia departments cannot cope with the increasing demands[37]. Therefore, a shortage of anesthetic teams may force pediatric endoscopists to conduct sedation without anesthetic teams applying guidelines adapted according to national regulations and institutional practices[4]. However, this situation is not optimal and requires consequent actions to increase the number of anesthesiologists.
In this situation, the intention of the authors is not to encourage such practice. This paper summarizes the evidence for sedation schemes which could be safely and efficiently performed by non-anesthesiologists. Sedation protocols have to be adapted to international, national and local legislation and institutional practice. The national institutions must organize multidisciplinary teams for education, licensing and supervision of non-anesthesiologists and registered nurses involved in sedation practices as long as there is a shortness of anesthesiologists. An efficient system of quality control is a paramount.
The choice of medicines for procedural sedation is wide, but none has the properties of an ideal sedative, which are: predictable dose dependent level of sedation with rapid onset; broad therapeutic window; anxiolytic effect with anterograde amnesia for the duration of the procedure; absence of respiratory, cardiovascular and other undesirable effects; and a smooth post-procedural recovery without side effects[34]. Another important problem in pediatrics is the off-label use of many medicines, which was recently addressed for medicines prescribed for outpatients in pediatric gastroenterology[69]. The investigators found that in 33.2% of the prescriptions, medicines were used “off-label” and that 47.3% of the patients had at least 1 medicine described as an “off-label” medication. Sedatives and other iv medicines were not covered by this study. The legal risk of a prescribing doctor is greater when using “off-label” medicines or indications. Parents should be informed of the “off-label” use. A solution of this problem is to motivate the pharmaceutical companies to register medicines for pediatric use, as has happened in the majority of the EU Countries under the jurisdiction of the European Medical Agency for new medicines.
Propofol is probably the most promising and controversial sedative/anesthetic at present. It is stated that only those trained in anesthesia should use it, a position that anesthesiologists and their societies strongly defend[70]. On the other hand, there are studies of safe and efficient use of propofol for sedation for GI endoscopic investigations in pediatric and adult gastroenterology[2,3,7,8,51,71]. The administration of propofol by non-anesthesiologists is “off-label” in most cases and, therefore, every adverse event might have medico-legal consequences.
Therefore, these data could not be simply extrapolated to every sedation/analgesia practice. According to the review by Havidich et al[72] the evidence of the safety of sedation by non-anesthesiologists for procedures outside operating theatres is growing, especially for propofol. Despite the drawbacks listed above, published data justify propofol use in certain circumstances[2].
Ketamine-based sedation is safe and effective in otherwise healthy infants older than 3 mo[27]. Ketamine has dissociative anesthetic and analgesic properties with a wide safety margin and is frequently used in pediatric emergency departments[27,28]. Emergence reactions are observed in adults in up to 28%, but seem less prevalent in paediatric studies and not influenced by the addition of midazolam to ketamine[56-58]. Guidelines advised against routine benzodiazepine pre-medication[27,28]. Data from larger studies are needed as one recent study found less emergence reactions when midazolam was routinely administered as a pre-medication[4]. Another major limitation of ketamine-based sedation for endoscopy is laryngospasm. In general, the laryngospasm resolves without consequences rapidly after removal of the endoscope and administration of oxygen[73]. Another study reports transient laryngospasm manageable with simple measures in 3% of gastroscopies[4]. Therefore, the ketamine-based sedation regime for GI endoscopy is an acceptable option when sedation with propofol is not feasible.
Midazolam is most likely the most widely used drug for sedation in everyday endoscopic work. The duration of action of midazolam is dependent on the duration of its administration. The sedative and amnestic effects of benzodiazepines sometimes do not provide adequate patient comfort during colonoscopic procedures[74]. Opioids are often added and meperidine is commonly used[75]. The value of adding analgesics to sedatives has well evaluated in large number of prospective, randomized and placebo-controlled studies[76]. Sedation with midazolam/meperidine is safely and can be administrated under adequate monitoring[77].
These recommendations review and discuss sedation practices for pediatric GI endoscopy which can be safely and efficiently performed by non-anesthesiologists, but only when the necessary pre-requisites regarding patient assessment, team composition and experience, medicines and equipment are met.
ACKNOWLEDGMENTS
The authors reviewed the literature and made practical recommendations for effective and safe sedation for endoscopic procedures in children. However, the authors decline every legal responsibility for the proposed algorithms. Legislation and regulation regarding limitations of administration of different medications, such as inhalation anesthetics, differ from country to country. Therefore, limitations caused by local legislation should be carefully checked.
COMMENTS
Background
Anesthesia is by preference performed by anesthesiologists.
Research frontiers
The creation of sedation teams led by non-anesthesiologists and a careful selection of anesthetic drugs may offer an alternative, but should be in line with national legislation and institutional regulations.
Innovations and breakthroughs
The intention of this review is to offer effective and safe alternatives for non-anesthesiologists.
Peer-review
The present paper was well organized and well investigated. This paper will give us important information about the anesthesia during endoscopy especially in children.
Footnotes
Conflict-of-interest statement: None of the authors reported a conflict of interest related to this article. There was no funding. Rok Orel has participated as a clinical investigator or speaker with Medis, Nutricia, Ewopharma, Biogaia, United Pharmaceuticals, Danone, Abbvie, and MSD. Jernej Brecelj has participated as a speaker for MSD and has received travel grants from Abbvie, MSD and Dr. Falk Foundation. Jorge Amil Dias received honoraria for lectures from MJN, Danone, MSD, Abbvie, Falk, and participated in Advisory boards for MSD, Abbvie, Receptos. Claudio Romano did not report any potential conflict of interests. Fernanda Barros has been a clinical investigator for MSD and speaker for B-Braun. Mike Thomson has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for Danone/Nutricia, Mead Johnson, Movetis, Nestle, Norgine, Reckitt-Benckieser and Sandhill Scientific. Yvan Vandenplas has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for Abbott Nutrition, Aspen, Biogaia, Biocodex, Danone, Hero, Nestle Nutrition Institute, Nutricia, Mead Johnson Nutrition, Merck, Orafti, Phacobel, Sari Husada, United Pharmaceuticals, Wyeth and Yakult.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 8, 2015
First decision: April 27, 2015
Article in press: June 19, 2015
P- Reviewer: Aisa AP, Lee CL, Mentes O, Naito Y, Shih SC S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.Lee KK, Anderson MA, Baron TH, Banerjee S, Cash BD, Dominitz JA, Gan SI, Harrison ME, Ikenberry SO, Jagannath SB, et al. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc. 2008;67:1–9. doi: 10.1016/j.gie.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 2.van Beek EJ, Leroy PL. Safe and effective procedural sedation for gastrointestinal endoscopy in children. J Pediatr Gastroenterol Nutr. 2012;54:171–185. doi: 10.1097/MPG.0b013e31823a2985. [DOI] [PubMed] [Google Scholar]
- 3.Bedirli N, Egritas O, Cosarcan K, Bozkirli F. A comparison of fentanyl with tramadol during propofol-based deep sedation for pediatric upper endoscopy. Paediatr Anaesth. 2012;22:150–155. doi: 10.1111/j.1460-9592.2011.03707.x. [DOI] [PubMed] [Google Scholar]
- 4.Brecelj J, Trop TK, Orel R. Ketamine with and without midazolam for gastrointestinal endoscopies in children. J Pediatr Gastroenterol Nutr. 2012;54:748–752. doi: 10.1097/MPG.0b013e31824504af. [DOI] [PubMed] [Google Scholar]
- 5.Miqdady MI, Hayajneh WA, Abdelhadi R, Gilger MA. Ketamine and midazolam sedation for pediatric gastrointestinal endoscopy in the Arab world. World J Gastroenterol. 2011;17:3630–3635. doi: 10.3748/wjg.v17.i31.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motamed F, Aminpour Y, Hashemian H, Soltani AE, Najafi M, Farahmand F. Midazolam-ketamine combination for moderate sedation in upper GI endoscopy. J Pediatr Gastroenterol Nutr. 2012;54:422–426. doi: 10.1097/MPG.0b013e3182323c75. [DOI] [PubMed] [Google Scholar]
- 7.Chiaretti A, Benini F, Pierri F, Vecchiato K, Ronfani L, Agosto C, Ventura A, Genovese O, Barbi E. Safety and efficacy of propofol administered by paediatricians during procedural sedation in children. Acta Paediatr. 2014;103:182–187. doi: 10.1111/apa.12472. [DOI] [PubMed] [Google Scholar]
- 8.Gül R, Hizli S, Kocamer B, Koruk S, Sahin L, Kilinçaslan H, Sariçek V. The safety and efficacy of remifentanil compared to fentanyl in pediatric endoscopy. Turk J Med Sci. 2013;43:611–616. [Google Scholar]
- 9.Long Y, Liu HH, Yu C, Tian X, Yang YR, Wang C, Pan Y. Pre-existing diseases of patients increase susceptibility to hypoxemia during gastrointestinal endoscopy. PLoS One. 2012;7:e37614. doi: 10.1371/journal.pone.0037614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agostoni M, Fanti L, Gemma M, Pasculli N, Beretta L, Testoni PA. Adverse events during monitored anesthesia care for GI endoscopy: an 8-year experience. Gastrointest Endosc. 2011;74:266–275. doi: 10.1016/j.gie.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Waxman DA, Main R, Mattke S. Utilization of anesthesia services during outpatient endoscopies and colonoscopies and associated spending in 2003-2009. JAMA. 2012;307:1178–1184. doi: 10.1001/jama.2012.270. [DOI] [PubMed] [Google Scholar]
- 12.Yen YH, Lin TF, Lin CJ, Lee YC, Lau HP, Yeh HM. Sex differences in conscious sedation during upper gastrointestinal panendoscopic examination. J Formos Med Assoc. 2011;110:44–49. doi: 10.1016/S0929-6646(11)60007-7. [DOI] [PubMed] [Google Scholar]
- 13.Vadlamudi N, Alkhouri N, Mahajan L, Lopez R, Shen B. Ileoscopy via stoma after diverting ileostomy: a safe and effective tool to evaluate for Crohn’s recurrence of neoterminal ileum. Dig Dis Sci. 2011;56:866–870. doi: 10.1007/s10620-010-1332-0. [DOI] [PubMed] [Google Scholar]
- 14.Siwiec RM, Dua K, Surapaneni SN, Hafeezullah M, Massey B, Shaker R. Unsedated transnasal endoscopy with ultrathin endoscope as a screening tool for research studies. Laryngoscope. 2012;122:1719–1723. doi: 10.1002/lary.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Academy of Pediatric Dentistry; American Academy of Pediatric Dentistry Committee on Sedation and Anesthesia. Guideline on the elective use of minimal, moderate, and deep sedation and general anesthesia for pediatric dental patients. Pediatr Dent 2005- 2006;27:110–118. [PubMed] [Google Scholar]
- 16.Vargo JJ, DeLegge MH, Feld AD, Gerstenberger PD, Kwo PY, Lightdale JR, Nuccio S, Rex DK, Schiller LR. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastroenterology. 2012;143:e18–e41. doi: 10.1053/j.gastro.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JT, Ortmann M, Paspatis G, Ponsioen CY, Racz I, Schreiber F, et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anaesthesiologist administration of propofol for GI endoscopy. Eur J Anaesthesiol. 2010;27:1016–1030. doi: 10.1097/EJA.0b013e32834136bf. [DOI] [PubMed] [Google Scholar]
- 18.Heneghan S, Myers J, Fanelli R, Richardson W. Society of American Gastrointestinal Endoscopic Surgeons (SAGES) guidelines for office endoscopic services. Surg Endosc. 2009;23:1125–1129. doi: 10.1007/s00464-009-0410-x. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, Dominitz JA, Fanelli RD, Gan SI, Harrison ME, Ikenberry SO, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:815–826. doi: 10.1016/j.gie.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Vargo JJ, Ahmad AS, Aslanian HR, Buscaglia JM, Das AM, Desilets DJ, Dunkin BJ, Inkster M, Jamidar PA, Kowalski TE, et al. Training in patient monitoring and sedation and analgesia. Gastrointest Endosc. 2007;66:7–10. doi: 10.1016/j.gie.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber F. Austrian Society of Gastroenterology and Hepatology (OGGH)--guidelines on sedation and monitoring during gastrointestinal endoscopy. Endoscopy. 2007;39:259–262. doi: 10.1055/s-2007-966254. [DOI] [PubMed] [Google Scholar]
- 22.Training Committee. American Society for Gastrointestinal Endoscopy. Training guideline for use of propofol in gastrointestinal endoscopy. Gastrointest Endosc. 2004;60:167–172. doi: 10.1016/s0016-5107(04)01699-2. [DOI] [PubMed] [Google Scholar]
- 23.Waring JP, Baron TH, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Faigel DO. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58:317–322. doi: 10.1067/s0016-5107(03)00001-4. [DOI] [PubMed] [Google Scholar]
- 24.Faigel DO, Baron TH, Goldstein JL, Hirota WK, Jacobson BC, Johanson JF, Leighton JA, Mallery JS, Peterson KA, Waring JP, et al. Guidelines for the use of deep sedation and anesthesia for GI endoscopy. Gastrointest Endosc. 2002;56:613–617. doi: 10.1016/s0016-5107(02)70104-1. [DOI] [PubMed] [Google Scholar]
- 25.Coté CJ, Wilson S. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118:2587–2602. doi: 10.1542/peds.2006-2780. [DOI] [PubMed] [Google Scholar]
- 26.National Clinical Guideline Centre (UK) Sedation in children and young people: Sedation for diagnostic and therapeutic procedures in children and young people [Internet] London: Royal College of Physicians (UK); 2010. Available from: http: //www.ncbi.nlm.nih.gov/books/NBK82237/ [PubMed] [Google Scholar]
- 27.Green SM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation in children. Ann Emerg Med. 2004;44:460–471. doi: 10.1016/S0196064404006365. [DOI] [PubMed] [Google Scholar]
- 28.Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57:449–461. doi: 10.1016/j.annemergmed.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 29.American Academy on Pediatric Dentistry Clinical Affairs Committee-Sedation and General Anesthesia Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on use of anesthesia personnel in the administration of office-based sedation/generral anesthesia to the pediatric dental patient. Pediatr Dent. 2008;30:160–162. [PubMed] [Google Scholar]
- 30.American Academy of Pediatrics; American Academy on Pediatric Dentistry. Guideline for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatr Dent. 2008;30:143–159. [PubMed] [Google Scholar]
- 31.American Academy of Pediatric Dentistry Council on Clinical Affairs--Sedation and General Anesthesia Subcommittee; American Academy of Pediatric Dentistry Council on Clinical Affairs--Sedation and Anesthesia Subcommittee. Guideline on use of anesthesia care providers in the administration of in-office deep sedation/general anesthesia to the pediatric dental patient. Pediatr Dent 2005- 2006;27:119–121. [PubMed] [Google Scholar]
- 32.American Academy of Pediatric Dentistry. Clinical guideline on the elective use of minimal, moderate, and deep sedation and general anesthesia for pediatric dental patients. Pediatr Dent. 2004;26:95–103. [PubMed] [Google Scholar]
- 33.Hosey MT. UK National Clinical Guidelines in Paediatric Dentistry. Managing anxious children: the use of conscious sedation in paediatric dentistry. Int J Paediatr Dent. 2002;12:359–372. doi: 10.1046/j.1365-263x.2002.03792.x. [DOI] [PubMed] [Google Scholar]
- 34.Tolia V, Peters JM, Gilger MA. Sedation for pediatric endoscopic procedures. J Pediatr Gastroenterol Nutr. 2000;30:477–485. doi: 10.1097/00005176-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber S, Ronfani L, Chiaffoni GP, Matarazzo L, Minute M, Panontin E, Poropat F, Germani C, Barbi E. Does EMLA cream application interfere with the success of venipuncture or venous cannulation? A prospective multicenter observational study. Eur J Pediatr. 2013;172:265–268. doi: 10.1007/s00431-012-1866-6. [DOI] [PubMed] [Google Scholar]
- 36.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 37.Ramaiah R, Bhananker S. Pediatric procedural sedation and analgesia outside the operating room: anticipating, avoiding and managing complications. Expert Rev Neurother. 2011;11:755–763. doi: 10.1586/ern.11.52. [DOI] [PubMed] [Google Scholar]
- 38.Sahyoun C, Krauss B. Clinical implications of pharmacokinetics and pharmacodynamics of procedural sedation agents in children. Curr Opin Pediatr. 2012;24:225–232. doi: 10.1097/MOP.0b013e3283504f88. [DOI] [PubMed] [Google Scholar]
- 39.Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310–324. [PubMed] [Google Scholar]
- 40.Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet. 2006;367:766–780. doi: 10.1016/S0140-6736(06)68230-5. [DOI] [PubMed] [Google Scholar]
- 41.Aroni F, Iacovidou N, Dontas I, Pourzitaki C, Xanthos T. Pharmacological aspects and potential new clinical applications of ketamine: reevaluation of an old drug. J Clin Pharmacol. 2009;49:957–964. doi: 10.1177/0091270009337941. [DOI] [PubMed] [Google Scholar]
- 42.Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;(182):313–333. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- 43.Devlin JW, Roberts RJ. Pharmacology of commonly used analgesics and sedatives in the ICU: benzodiazepines, propofol, and opioids. Anesthesiol Clin. 2011;29:567–585. doi: 10.1016/j.anclin.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675–701. doi: 10.1053/j.gastro.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Welchman S, Cochrane S, Minto G, Lewis S. Systematic review: the use of nitrous oxide gas for lower gastrointestinal endoscopy. Aliment Pharmacol Ther. 2010;32:324–333. doi: 10.1111/j.1365-2036.2010.04359.x. [DOI] [PubMed] [Google Scholar]
- 46.Vanlersberghe C, Camu F. Propofol. Handb Exp Pharmacol. 2008;(182):227–252. doi: 10.1007/978-3-540-74806-9_11. [DOI] [PubMed] [Google Scholar]
- 47.Montes RG, Bohn RA. Deep sedation with inhaled sevoflurane for pediatric outpatient gastrointestinal endoscopy. J Pediatr Gastroenterol Nutr. 2000;31:41–46. doi: 10.1097/00005176-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Kuratani N, Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trials. Anesthesiology. 2008;109:225–232. doi: 10.1097/ALN.0b013e31817f5c18. [DOI] [PubMed] [Google Scholar]
- 49.Lerman J. Inhalation agents in pediatric anaesthesia - an update. Curr Opin Anaesthesiol. 2007;20:221–226. doi: 10.1097/ACO.0b013e32811e16e7. [DOI] [PubMed] [Google Scholar]
- 50.Shah PS, Shah VS. Propofol for procedural sedation/anaesthesia in neonates. Cochrane Database Syst Rev. 2011;(3):CD007248. doi: 10.1002/14651858.CD007248.pub2. [DOI] [PubMed] [Google Scholar]
- 51.Poincloux L, Laquière A, Bazin JE, Monzy F, Artigues F, Bonny C, Abergel A, Dapoigny M, Bommelaer G. A randomized controlled trial of endoscopist vs. anaesthetist-administered sedation for colonoscopy. Dig Liver Dis. 2011;43:553–558. doi: 10.1016/j.dld.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Slagelse C, Vilmann P, Hornslet P, Hammering A, Mantoni T. Nurse-administered propofol sedation for gastrointestinal endoscopic procedures: first Nordic results from implementation of a structured training program. Scand J Gastroenterol. 2011;46:1503–1509. doi: 10.3109/00365521.2011.619274. [DOI] [PubMed] [Google Scholar]
- 53.Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108:795–804. doi: 10.1213/ane.0b013e31818fc334. [DOI] [PubMed] [Google Scholar]
- 54.Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, Pace NL, Apfel CC. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ. 2011;342:d1110. doi: 10.1136/bmj.d1110. [DOI] [PubMed] [Google Scholar]
- 55.Pelosi P. Retraction of endorsement: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2012;44:302; author reply 302. doi: 10.1055/s-0031-1291648. [DOI] [PubMed] [Google Scholar]
- 56.Sener S, Eken C, Schultz CH, Serinken M, Ozsarac M. Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med. 2011;57:109–114.e2. doi: 10.1016/j.annemergmed.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Sherwin TS, Green SM, Khan A, Chapman DS, Dannenberg B. Does adjunctive midazolam reduce recovery agitation after ketamine sedation for pediatric procedures? A randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2000;35:229–238. doi: 10.1016/s0196-0644(00)70073-4. [DOI] [PubMed] [Google Scholar]
- 58.Wathen JE, Roback MG, Mackenzie T, Bothner JP. Does midazolam alter the clinical effects of intravenous ketamine sedation in children? A double-blind, randomized, controlled, emergency department trial. Ann Emerg Med. 2000;36:579–588. doi: 10.1067/mem.2000.111131. [DOI] [PubMed] [Google Scholar]
- 59.Heinz P, Geelhoed GC, Wee C, Pascoe EM. Is atropine needed with ketamine sedation? A prospective, randomised, double blind study. Emerg Med J. 2006;23:206–209. doi: 10.1136/emj.2005.028969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown L, Christian-Kopp S, Sherwin TS, Khan A, Barcega B, Denmark TK, Moynihan JA, Kim GJ, Stewart G, Green SM. Adjunctive atropine is unnecessary during ketamine sedation in children. Acad Emerg Med. 2008;15:314–318. doi: 10.1111/j.1553-2712.2008.00074.x. [DOI] [PubMed] [Google Scholar]
- 61.Langston WT, Wathen JE, Roback MG, Bajaj L. Effect of ondansetron on the incidence of vomiting associated with ketamine sedation in children: a double-blind, randomized, placebo-controlled trial. Ann Emerg Med. 2008;52:30–34. doi: 10.1016/j.annemergmed.2008.01.326. [DOI] [PubMed] [Google Scholar]
- 62.Manickam P, Kanaan Z, Zakaria K. Conscious sedation: a dying practice? World J Gastroenterol. 2013;19:4633–4634. doi: 10.3748/wjg.v19.i28.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen PH, Wu TC, Chiu CY. Pediatric gastrointestinal endoscopic sedation: a 2010 nationwide survey in Taiwan. Pediatr Neonatol. 2012;53:188–192. doi: 10.1016/j.pedneo.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Ozel AM, Oncü K, Yazgan Y, Gürbüz AK, Demirtürk L. Comparison of the effects of intravenous midazolam alone and in combination with meperidine on hemodynamic and respiratory responses and on patient compliance during upper gastrointestinal endoscopy: a randomized, double-blind trial. Turk J Gastroenterol. 2008;19:8–13. [PubMed] [Google Scholar]
- 65.Cinar K, Yakut M, Ozden A. Sedation with midazolam versus midazolam plus meperidine for routine colonoscopy: a prospective, randomized, controlled study. Turk J Gastroenterol. 2009;20:271–275. doi: 10.4318/tjg.2009.0025. [DOI] [PubMed] [Google Scholar]
- 66.Hayee B, Dunn J, Loganayagam A, Wong M, Saxena V, Rowbotham D, McNair A. Midazolam with meperidine or fentanyl for colonoscopy: results of a randomized trial. Gastrointest Endosc. 2009;69:681–687. doi: 10.1016/j.gie.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 67.Lightdale JR, Mahoney LB, Schwarz SM, Liacouras CA. Methods of sedation in pediatric endoscopy: a survey of NASPGHAN members. J Pediatr Gastroenterol Nutr. 2007;45:500–502. doi: 10.1097/MPG.0b013e3180691168. [DOI] [PubMed] [Google Scholar]
- 68.Michaud L, Gottrand F, Ganga-Zandzou PS, Ouali M, Vetter-Laffargue A, Lambilliotte A, Dalmas S, Turck D. Nitrous oxide sedation in pediatric patients undergoing gastrointestinal endoscopy. J Pediatr Gastroenterol Nutr. 1999;28:310–314. doi: 10.1097/00005176-199903000-00018. [DOI] [PubMed] [Google Scholar]
- 69.Ruíz-Antorán B, Piñeiro R, Avendaño C, Román E, Cilleruelo ML, Gutiérrez-Junquera C, Centeno G, Cilleruelo MJ. Drug utilization and off-label drug use in Spanish pediatric gastroenterology outpatients. J Pediatr Gastroenterol Nutr. 2013;56:173–177. doi: 10.1097/MPG.0b013e3182566d92. [DOI] [PubMed] [Google Scholar]
- 70.Triantafillidis JK, Merikas E, Nikolakis D, Papalois AE. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol. 2013;19:463–481. doi: 10.3748/wjg.v19.i4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rex DK, Deenadayalu VP, Eid E, Imperiale TF, Walker JA, Sandhu K, Clarke AC, Hillman LC, Horiuchi A, Cohen LB, et al. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137:1229–1237; quiz 1518-1519. doi: 10.1053/j.gastro.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 72.Havidich JE, Cravero JP. The current status of procedural sedation for pediatric patients in out-of-operating room locations. Curr Opin Anaesthesiol. 2012;25:453–460. doi: 10.1097/ACO.0b013e32835562d8. [DOI] [PubMed] [Google Scholar]
- 73.Green SM, Klooster M, Harris T, Lynch EL, Rothrock SG. Ketamine sedation for pediatric gastroenterology procedures. J Pediatr Gastroenterol Nutr. 2001;32:26–33. doi: 10.1097/00005176-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Patel NC, Heckman MG, Palmer WC, Cangemi D, DeVault KR. A comparison of patient satisfaction with sedation between fentanyl/midazolam and meperidine/midazolam in patients undergoing endoscopy. Am J Gastroenterol. 2014;109:772–774. doi: 10.1038/ajg.2014.31. [DOI] [PubMed] [Google Scholar]
- 75.Fredette ME, Lightdale JR. Endoscopic sedation in pediatric practice. Gastrointest Endosc Clin N Am. 2008;18:739–751, ix. doi: 10.1016/j.giec.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Lightdale JR. Sedation and analgesia in the pediatric patient. Gastrointest Endosc Clin N Am. 2004;14:385–399. doi: 10.1016/j.giec.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Cohen LB, Wecsler JS, Gaetano JN, Benson AA, Miller KM, Durkalski V, Aisenberg J. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol. 2006;101:967–974. doi: 10.1111/j.1572-0241.2006.00500.x. [DOI] [PubMed] [Google Scholar]


