Abstract
The best known cases of cell autotomy are the formation of erythrocytes and thrombocytes (platelets) from progenitor cells that reside in special niches. Recently, autotomy of stem cells and its enigmatic interaction with the niche has been reported from male germline stem cells (GSCs) in several insect species. First described in lepidopterans, the silkmoth, followed by the gipsy moth and consecutively in hemipterans, foremost the milkweed bug. In both, moths and the milkweed bug, GSCs form finger-like projections toward the niche, the apical cells (homologs of the hub cells in Drosophila). Whereas in the milkweed bug the projection terminals remain at the surface of the niche cells, in the gipsy moth they protrude deeply into the singular niche cell. In both cases, the projections undergo serial retrograde fragmentation with progressing signs of autophagy. In the gipsy moth, the autotomized vesicles are phagocytized and digested by the niche cell. In the milkweed bug the autotomized vesicles accumulate at the niche surface and disintegrate. Autotomy and sprouting of new projections appears to occur continuously. The significance of the GSC-niche interactions, however, remains enigmatic. Our concept on the signaling relationship between stem cell-niche in general and GSC and niche (hub cells and cyst stem cells) in particular has been greatly shaped by Drosophila melanogaster. In comparing the interactions of GSCs with their niche in Drosophila with those in species exhibiting GSC autotomy it is obvious that additional or alternative modes of stem cell-niche communication exist. Thus, essential signaling pathways, including niche-stem cell adhesion (E-cadherin) and the direction of asymmetrical GSC division - as they were found in Drosophila - can hardly be translated into the systems where GSC autotomy was reported. It is shown here that the serial autotomy of GSC projections shows remarkable similarities with Wallerian axonal destruction, developmental axon pruning and dying-back degeneration in neurodegenerative diseases. Especially the hypothesis of an existing evolutionary conserved “autodestruction program” in axons that might also be active in GSC projections appears attractive. Investigations on the underlying signaling pathways have to be carried out. There are two other well known cases of programmed cell autotomy: the enucleation of erythroblasts in the process of erythrocyte maturation and the segregation of thousands of thrombocytes (platelets) from one megakaryocyte. Both progenitor cell types - erythroblasts and megakaryocytes - are associated with a niche in the bone marrow, erythroblasts with a macrophage, which they surround, and the megakaryocytes with the endothelial cells of sinusoids and their extracellular matrix. Although the regulatory mechanisms may be specific in each case, there is one aspect that connects all described processes of programmed cell autotomy and neuronal autodestruction: apoptotic pathways play always a prominent role. Studies on the role of male GSC autotomy in stem cell-niche interaction have just started but are expected to reveal hitherto unknown ways of signal exchange. Spermatogenesis in mammals advance our understanding of insect spermatogenesis. Mammal and insect spermatogenesis share some broad principles, but a comparison of the signaling pathways is difficult. We have intimate knowledge from Drosophila, but of almost no other insect, and we have only limited knowledge from mammals. The discovery of stem cell autotomy as part of the interaction with the niche promises new general insights into the complicated stem cell-niche interdependence.
Keywords: Stem cell-niche interaction, Male germline stem cells, Spermatogenesis, Erythropoiesis, Stem cell autotomy, Thrombopoiesis
Core tip: A new mode of stem cell-niche interaction has been observed in insects. Male germline stem cells (GSCs) undergo autotomy by serial segregation of vesicles from finger-like projections. These vesicles either accumulate at the niche surface or are phagocytized by the niche cells. Autotomized projections are apparently replaced by newly sprouting ones. It is suggested that the unprecedented dynamics of GSC autotomy are involved in a not yet known form of information exchange between GSCs and niche. Apoptotic pathways and autodestruction programs could be involved in GSC autotomy.
EARLY OBSERVATIONS ON STEM CELL-NICHE RELATIONSHIPS PRIOR TO THE ESTABLISHMENT OF THE STEM CELL-NICHE HYPOTHESIS
When, in 1978, Schofield[1] put forward the hematopoietic stem cell (HSC)-“niche” hypothesis it was solely based on the assumed requirements the niche must fulfill, but he had no knowledge concerning the physical identity of the niche: “The location of the stem cell niche can, of course, only be a matter of speculation, although there are several items of data which suggest that they may well be in intimate association with the bone”. It is astonishing how accurate some of his predictions turned out to be. However, up to date the HSC niche is not fully understood and is apparently composed of a variety of different cell types: osteogenic cells, endothelial cells, perivascular mesenchymal cells and adipocytes[2,3]. Recently, Chasis et al[4] pointed out that the first description of a hematopoietic niche actually took place 20 years earlier when, in 1958, Bessis[5] described erythroblastic islands. These represent microenvironmental niches for erythropoiesis. Erythroblasts, which represent oligopotent progenitors derived from a small population of HSCs, are arranged rosette-like around a reticular cell (macrophage) where they proliferate and differentiate (Figure 1A). Erythroblastic islands offer striking structural similarities with another significant model system for research on stem cell niche-interactions, the male germline stem cells (GSCs) and its niche in insects (Figure 1B).
Figure 1.
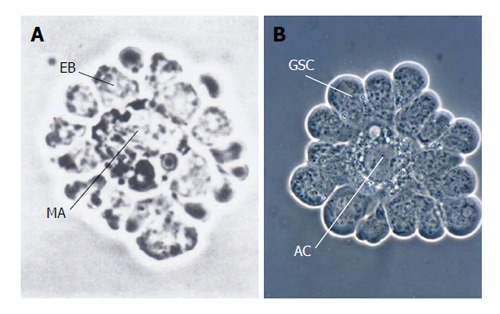
Simple systems of the stem cell/progenitor-niche interaction: isolated erythroblastic island and isolated apical complex from an insect exhibit strong resemblances. A: Erythroblastic island from dissociated rat bone marrow. A corona of erythroblasts (EB) has extensive cell-cell contact with their niche, the central macrophage (MA) (from Bessis et al[93] with kind permission of Springer Verlag, Berlin, Heidelberg); B: Isolated testicular apical complex from Locusta migratoria. Germline stem cells (GSC) surround the apical cell (AC), which represents the niche (besides the peripheral cyst stem cells, which were removed) (From Dorn et al[64]).
Due to its anatomical simplicity and the advantageous genetic access, the male GSC-niche system of Drosophila proved as an invaluable tool to study stem cell-niche interactions on the molecular level[6]. In insects the GSC niche is located in the apex of testicular follicles and consists of the somatic apical cells (ACs) (called hub cells in Drosophila) and the cyst stem cells (CySCs). As in the case of the erythroblastic islands, apical complexes were described long before Drosophila became the model system. Figure 2 demonstrates the testicular GSC-niche complex of butterflies published in 1889 by Verson[7] and in 1911 by Zick[8], respectively (The first record of apical complexes stems from Spichardt[9] published in 1886 from studies on butterflies). The drawings in Figure 2, based on light microscopical observations, indicate intricate physical relationships between AC and GSCs which could not be clearly resolved with the techniques available at that time. It is remarkable that several of the early investigators already suggested that the ACs might regulate the fate of the GSCs: Zick[8] in 1911 believed that the spermatogonial pathway could only be entered after detachment of the germ cells from the AC, and Buder[10] and Schneider[11] in 1915 postulated that ACs release an inhibitory factor which prevents the differentiation of GSCs into spermatogonia; Nelsen[12] in 1931 considered the ACs as an activation center which controls the mitotic activity of the GSCs. Thus, main characteristics of a niche were already hypothesized at the beginning of cell research. Since the early investigations on butterfly apical complexes developmental studies have ascertained the identity of GSCs and the role of ACs as their niche (together with CySCs). Electron microscopic studies revealed complex physical interactions between ACs and stem cells which points to a hitherto not elucidated communication between these cells. Figure 3D demonstrates the complicated structure of lepidopteran apical complexes. In fact, enigmatic physical relationships between male GSCs and their niche have been observed in a number of insect species. One of the most astonishing phenomenons is the controlled autotomy of GSC projections which are directed toward the ACs. Examples of these cases are examined for their functional significance in this review. They are compared with other stem cell-niche systems where cell autotomy takes place, such as enucleation of erythroblasts and megakaryocyte fragmentation are discussed in respect to the suggested “autodestruction program” of neurons.
Figure 2.
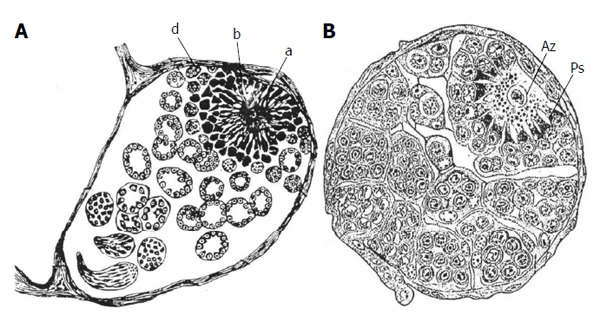
Early histological studies on testicular follicles in butterflies (Lepidoptera) that depict the complex structures of the apical complexes. A: Testicular follicle of the silkmoth Bombyx mori which includes the apical complex (a, b, d). The limited light microscopical resolution caused some misinterpretation concerning the identity of cell types: the central apical cell (a) was considered to be a “germ cell” (“Keimzelle”) with radial extensions. The germline stem cells were described as clumps of protoplasm with nuclei (b, d) (from Verson[7]); B: Testicular follicle of the cabbage white butterfly Pieris brassicae. The relationship between apical cell (Az) (also called Verson cell) and germline stem cells (Ps) is correctly described: The germline stem cells send projections toward the apical cell and their tips penetrate the apical cell. A layer of cells surround the germline stem cells, the cyst stem cell which, however, were not identified as such (from Zick[8]).
Figure 3.
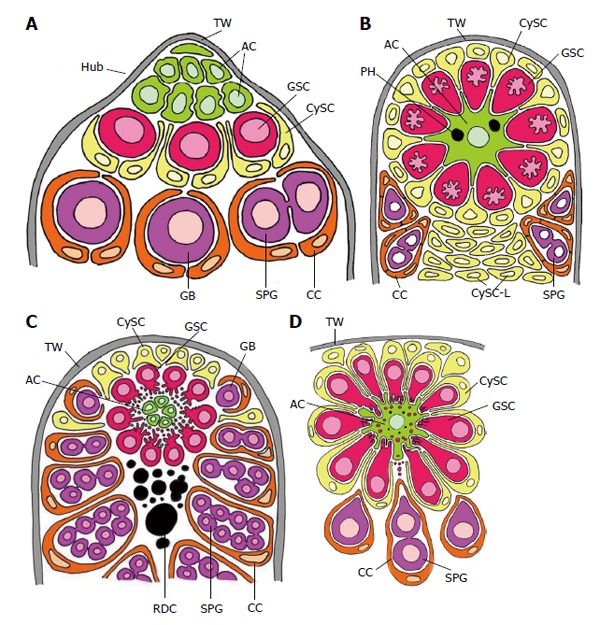
Schematized longitudinal sections through the apices of testicular follicles of four insect species. The order of the images from (A) to (D) is arranged according to increasing complexity of the structural relationships between germline stem cells (GSCs) and their niche. It should be noted that the order is not in congruence with the positions of the species in the natural insect system based on evolutionary progress. A: Drosophila melanogaster. The ACs (hub cells) are located in a small terminal appendix of the follicle, the hub (HUB), where many of them border the testicular wall (TW). (The testicular wall consists of an outer pigment layer resting on a basal lamina and a muscle cell layer sitting on an inner basal lamina.) The testicular wall does not provide a hemolymph-testis barrier. A spermatogonial is constituted by two CCs which protect the spermatogonia against hemolymph. Both GSCs and CySCs contact ACs (Adapted from Hardy et al[61]); B: Locusta migratoria. Depicted is the rosette-like arrangement of GSCs and CySCs with the single AC in the center. The AC of this example includes two phagocytized GSCs (see above). Besides GSCs also CySCs contact extensions of the star-like AC. A plug of CySC-like cells (CySC-L) is located below the apical rosette. The wall of the spermatogonial cysts is composed of numerous CCs (Adapted from Szöllösi et al[154] and Dorn et al[64]); C: Oncopeltus fasciatus. The cellular extensions of GSCs and segregated vesicles surround the surface of ACs. CySCs cover only the distal part of the apical rosette; they do not make contacts with the ACs. Proximal to the rosette, remnants of degraded GSCs and probably young cysts amass (RDC). Cysts form at the lateral parts of the rosette (Adapted from Schmidt et al[65]); D: Lymantria dispar. Note that the cellular extensions of GSCs protrude into the large singular AC. The extensions autotomize and the segregated vesicles are phagocytized by the AC. Each GSC is affiliated with one CySC. During early larval development the AC is attached to the TW [comparable to the situation in Drosophila, where ACs (i.e., hub cells) are lifelong attached to the TW], but separates with progressing development. The apical complex then adopts a spherical organization. (Adapted from Klein[66]). AC: Apical cell (often called hub cell in Drosophila) (green); CC: Cyst cell (orange); CySC: Cyst stem cell (yellow); GB: Gonialblast (purple); GSC: Germline stem cell (red); SPG: Spermatogonia (purple); TW: Testicular wall (grey).
GSC-NICHE INTERACTION IN THE TESTIS OF THE MODEL INSECT DROSOPHILA MELANOGASTER
The testis of Drosophila has become one of the most successful models for the exploration of molecular stem cell-niche interaction. Comprehensive reviews on this matter have been published recently[13,14]. A summary of the structural and molecular relationships within the apical complex of Drosophila is presented here and compared with observations in other insects.
Figure 3A shows the apex of a testicular follicle of Drosophila. A longitudinal section of the testicular follicle shows the three cell types that constitute the germinal proliferation center. A small cluster of somatic cells, the hub cells (i.e., ACs), is located in the follicular apex (the hub). Hub cells, together with CySCs, represent the niche for the row of bordering GSCs. Except for the region where hub cells and GSCs contact one another; each GSC is embraced by a pair of CySCs whose tips also contact the hub cells. CySCs are of somatic origin and, besides their niche function for GSCs, represent stem cells that generate the cyst cells by asymmetrical division. Hub cells only function as the CySC niche. Prior to an asymmetric division of a GSC (which produces a gonialblast that is directed toward the periphery of the apical complex and a daughter GSC that remains in contact with the niche) its two associated CySCs undergo a synchronized division resulting in a pair of daughter cells which encloses the forming gonialblast and becomes the cyst wall. The two cyst cells forming the wall no longer divide during subsequent spermatogenesis although the cysts enlarge considerably.
The fate of GSCs - maintenance, self-renewal (asymmetric and symmetric division), frequency of mitotic activity - is orchestrated by a multitude of factors and processes: (1) Short-range signaling between niche and GSCs. This was the first factor to be elucidated and proved to be exemplary for other stem cell-niche systems; (2) Niche-stem cell adhesion. Adherens junctions were found to play a crucial role in the regulation of signaling and asymmetric GSC division in addition to its physical adhesion function; (3) Cell intrinsic regulation. This has more recently come into focus; and (4) Systemic regulation. This may affect all aspects of niche-stem cell interaction, but is to date the least understood.
Short-range signaling
GSC maintenance and self-renewal are supported by a wide range of signals from the hub. The cytokine ligand unpaired (Upd), secreted by the hub cells activates Janus kinase-signal transducer and activator of transcription (JAK/STAT) signaling in GSCs and CySCs[15,16]. Gonialblast differentiation is caused by lower levels of Upd. In aging flies the number of GSCs and their proliferation rate declines in correlation with declining Upd levels in the hub cells. Upd secreted by hub cells also activates the JAK/STAT signaling in CySCs. Whereas JAK/STAT activation is sufficient for CySC maintenance and self-renewal, GSC self-renewal requires additional signals. Hub cells and CySCs both secrete glass bottom boat (Gbb) and decapentaplegic (Dpp). Both ligands activate the bone morphogenetic protein (BMP) signaling pathway in GSCs. BMP represses the transcription of the differentiation factor bag of marbles (Bam). Thus inhibition of differentiation to gonialblasts contributes to GSC self-renewal[17,18]. Hub cells also produce the ligand Hedgehog (Hh) that supports the self-renewal of CySCs in addition to JAK/STAT activation. GSC maintenance does not require Hh signaling[19,20]. Gbb and Dpp produced from CySCs contribute to the activation of BMP in GSCs (besides Gbb and Dpp signaling from the hub cells)[18]. They are conceivably activated by STAT and/or zinc-finger homeodomain protein 1 which is targeted by STAT as is chinmo (chronologically inappropriate morphogenesis)[21,22] (Figure 4).
Figure 4.
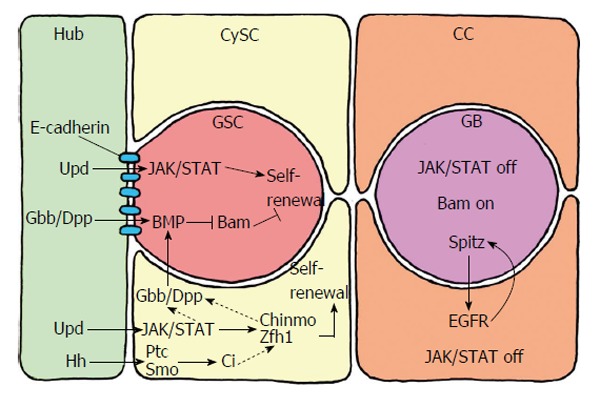
Short range signalling between the germline stem cells and their niche, which consists of the hub cells and the cyst stem cells. The hub cells secrete the ligand Upd, which activates JAK/STAT signalling in GSCs and CySCs. Whereas JAK/STAT activation is sufficient for CySC maintenance and self-renewal, GSC self-renewal requires additional signals. Hub cells and CySCs secrete the ligands Gbb and Dpp, which activate BMP in GSCs. BMP suppresses the transcription of Bam that inhibits the differentiation of GSCs to gonialblasts. BMP activation in GSCs is also supports by Gbb and Dpp produced by the CySCs. Gbb and Dpp in CySCs are conceivably activated by STAT and/or Zfh1 and Chinmo. Hub cells also secrete the ligand Hh (hedge hog), which supports CySC self-renewal in addition to, but independently of, JAK/STAT activity. Hh binds to the transmembrane receptor PTC (patched) of CySC, which releases Smo (smoothened) from repression. This leads to the activation of the transcription factor Ci (cubitus interruptus), which activates the transcription of target genes that support maintenance and self-renewal of CySCs besides JAK/STAT activity. The Hh signalling pathway in CySCs may also affect Zfh1 and, via Gbb/Dpp, influence BMP signalling in GSCs. Thus, Hh signalling in the testis niche apparently has a dual role. CC: Cyst cell; GSCs: Germline stem cells; CySCs: Cyst stem cells.
Niche-stem cell adhesion, adherens junctions, E-cadherin
GSCs and CySCs are both connected with hub cells via adherens junctions[23]. Hub cell-GSC connection plays are crucial role in GSC behavior. Tight contact of the GSCs with hub cells is correlated with high levels of E-cadherin and β-catenin at the interface (adherens junctions). Accumulation of both proteins at the interface is dependent upon guanine nucleotide exchange factor 26 (Gef26) for the Rap GTPase (Rap0-GEF)[24]. The intracellular domains of the cadherin molecules interact with cytoskeleton-associated proteins. JAK/STAT signaling is required in GSCs to maintain E-cadherin expression, niche anchorage and self-renewal and in CySCs to control BMP expression[25]. In addition, the leukocyte-antigen-related-like receptor tyrosine phosphatase has been proposed to regulate the attachment of GSCs to the hub cells[26]. It is responsible for the proper localization of tumor suppressor Adenomatous polyposis coli 2 (Apc2) and E-cadherin and the proper orientation of centrosomes in GSCs[23]. The BMP receptor complexes are localized to E-cadherin rich adherens junctions at the stem cell-niche junction, which might help restrict BMP signaling activity to the GSC niche interface[27]. Localized BMP signaling might be also affected by BMP signaling modulators that accumulate in the extracellular matrix such as the protein Magu (known to be involved in life span extension and late age female fecundity) which is transcribed in hub cells[28] and the heparin sulfate proteoglycans Dally (division abnormally delayed) and Dally-like[29]. Recently it was demonstrated that the actin-binding protein profilin is required cell autonomously to maintain GSCs, possibly facilitating localization or maintenance of E-cadherin to the GSC-hub cell interface[30].
The age dependent loss of GSC is accompanied by a decline in E-cadherin expression. Increased E-cadherin expression slows down GSC loss[31].
E-cadherin is also required in CySCs to maintain their adhesion to the hub. In addition, integrin-mediated adhesion exists between the hub and CySCs, and is limited by a negative regulator of STAT signaling[32].
Integrins: In the Drosophila testis, competition exists between GSCs and CySCs and among CySCs themselves for occupancy of the hub[33]. Interestingly, the CySCs with higher JAK/STAT signaling activity, which can be achieved experimentally by removing the function of the JAK/STAT negative regulator SOCS36E, can outcompete normal CySCs and can also push GSCs out of their niche. This JAK/STAT-regulated stem cell competition is dependent on the cell adhesion protein βPS integrin, but not E-cadherin. Integrin-mediated cell competition is thus thought to play a crucial role in balancing two stem cell populations in the same niche[33]. Integrins are also required for positioning the hub in the apical testis tip, but are dispensable for GSC or CySC anchorage to the niche[34]. The extracellular domains of integrins can bind directly to extracellular matrix (ECM) proteins, such as laminin, but there is no ECM between hub cells, GSCs and CySCs.
Gap junctions: The gap junction protein zero population growth is required for GSC maintenance and differentiation in Drosophila testes[35]. But it remains to be seen whether the function of gap junctions in the regulation of stem cell maintenance derives from their adhesion role, intercellular molecular transfer or electrical communication.
Cell intrinsic factors, hub cells
Upd levels in hub cells are regulated by IGF-II mRNA binding protein (Imp) that binds to upd mRNA and protects it from degradation caused by short interfering RNAs. Imp itself is repressed by let-7 microRNA (miRNA) that is expressed at higher levels in aging male GSCs[36]. epidermal growth factor (EGF) signaling negatively regulates GSC division frequency in adults, but not in larvae, and promotes gonialblast differentiation and enclosure of germ cells by somatic cyst cells. Stg (string, a Cdc 25 homolog-phosphatase) is essential for activating cyclin-dependent kinases and promoting the cell cycle and is therefore required for proliferation and maintenance of GSCs and CySCs. The transcriptional regulator lola (longitudinals lacking) is cell autonomously required for GSC (and CySC) maintenance[37]. MiRNAs control the stem cell differentiation pathway by regulating Bam[38]. Recently, the impact of epigenetic factors on male GSCs has been analyzed. Nucleosome remodeling factor promotes STAT expression while repressing Bam thus contributing to the maintenance of GSCs[39]. Additionally chromatin-associated proteins, such as no child left behind and PHD finger protein 7 are necessary for GSC maintenance[40,41]. CySCs. JAK/STAT signaling in CySCs is suppressed by suppressor of cytokine signaling at 36E (Socs36E) which may harmonize self-renewal of CySCs with that of GSCs[33,42]. Ken (ken and barbie, a transcriptional repressor) also promotes CySC identity[43]. Restriction of proliferation and maintenance of CySC identity are affected by polycomb repressive complex 1 genes posterior sex combs and suppressor of zeste two [Su(z)2][44]. The histone variant His2Av and the ATP-dependent chromatin-remodeling factor Domino are also required for GSC and CySC maintenance. Furthermore, in a recent review Zoller et al[45] listed about 50 genes that were found (or expected) to be involved in the direction of CySC specification, CySC self-renewing divisions, cyst cell differentiation, and soma-germline interactions.
Systemic factors
Numerous environmental factors, such as changing seasons or periods of drought and rain, nutritional conditions (irregular food availability or starvation), injury or illness all affect tissue homeostasis. As a consequence niche and stem cell activity must be adapted to these changing demands. This is primarily accomplished via systemic factors that may influence any of the regulatory entities of the stem cell-niche complex.
Best known is the support of GSCs maintenance by insulin signaling[46,47]. The source of some insulin-like peptides is the brain others are synthesized in the fat body and other tissues probably including the GSCs themselves. Effects of the nutritional status on GSC maintenance are apparently exerted by insulin signaling pathway[48]. In the Drosophila female it has been shown that the nutrient-sensing insulin/FOXO signaling directly controls Notch activation in the GSC niche which maintains the niche and GSC identity[49]. In maintaining embryonic stem cell pluripotency and the modulation of adult stem cell quiescence nutrient-sensing pathways play an important role. They maintain energy production by inhibition and stimulation of crucial processes like oxidative phosphorylation and glycolysis. “This interplay is key to the maintenance of stem-ness”[50].
Recently it has been found that day-night cycles and alterations in sleep can influence the daily dynamics of GSC divisions in male Drosophila[51]. The GSC division rate increases, when the sleep-promoting factor, Sleepless, is lacking. This is mediated, in part, by the GABAergic signaling pathway.
A systemic signal that presumably plays a decisive role in testis development and spermatogenesis is the steroid hormone ecdysone[52]. It is synthesized in testicular tissue of many insects: Heliothis virescens[53], Lymantria dispar[54], Ostrinia nubilalis[55], Spodoptera littoralis[56], Melanoplus sanguinipes[57]. However, its significance in the regulation of male GSC proliferation and self-renewal in Drosophila is not known.
Ecdysteroids play a role in female GSC regulation in Drosophila. Ecdysteroids are synthesized by developing follicles of ovarioles and regulate directly GSC maintenance, proliferation and self-renewal. Ecdysteroids interact with the intrinsic epigenetic factor ISWI, a chromatin remodeling factor[58]. The survival of ecdysone-producing follicles of ovarioles depends on the availability of food which points to an interaction of the hormonal with the nutrient-sensing signaling pathways[59].
INCREASING COMPLEXITY OF STRUCTURAL RELATIONSHIPS BETWEEN MALE GSCS AND THEIR NICHE IN DIFFERENT INSECT SPECIES: DROSOPHILA MELANOGASTER, LOCUSTA MIGRATORIA, ONCOPELTUS FASCIATUS, LYMANTRIA DISPAR, AND THE CASE OF LAMPYRIS NOCTILUCA
Insects with their long evolutionary history may be expected to present great variations on a theme, in this case the organization of GSCs and their niche. And in deed, the Figure 3 exhibits an increasing complication of the physical interactions of the cells of the apical complex. However, the complexity of the structures is not correlated with the systematic position of a given species. In fact, whereas the hemipteran Oncopeltus fasciatus (Figure 3C) shows an astonishingly irregular and dynamic anatomy of GSCs[60], another hemipteran, Corizus hyoscyami, has a relatively “simple” apical complex which harbors only one small AC, sparsely equipped with cell organelles, surrounded by a corona of pear-shaped GSCs (Klein personal communication). Nonetheless, it appears self-evident that the enigmatic structures expressed especially in the GSCs of Oncopeltus fasciatus and the lepidopteran Lymantria dispar (Figure 3D) - as well as in many other lepidopterans (Figure 2) - have a specific function (see below), in reverse to the principle: no function without structure. We will briefly describe the portrayed apical complexes and characterize the cell types.
Drosophila melanogaster
Figure 3A demonstrates the rather “ordinary” organization of the apical complex and Figure 5A the fine structural characteristics of its different components. The niche consists of 8-16 small hub cells, which richly interdigitate and are anchored to the testicular wall. They are characterized by a “light” appearance and scarcity of cell organelles. No mitoses were observed. The GSCs are, in contrast, rather “dark” due to many free ribosomes. They have a spheroidal shape but are flattened where they contact the hub cells. Their cytoplasm includes “spongy bodies”[61] which may represent nuage material that is typical for germ cells[62]. Mitoses of GSCs were rarely observed by the above authors, i.e., one mitotic GSC in 50 GSCs. E-cadherin-mediated adherens junctions (Figure 4A) attach GSCs to hub sells[26]. The CySCs are rather “light” and have inconspicuous organelle equipment. They also form adherens junctions with GSCs (Figure 5A). Among the 76 CySCs observed by Hardy et al[61] only two were in mitosis.
Figure 5.
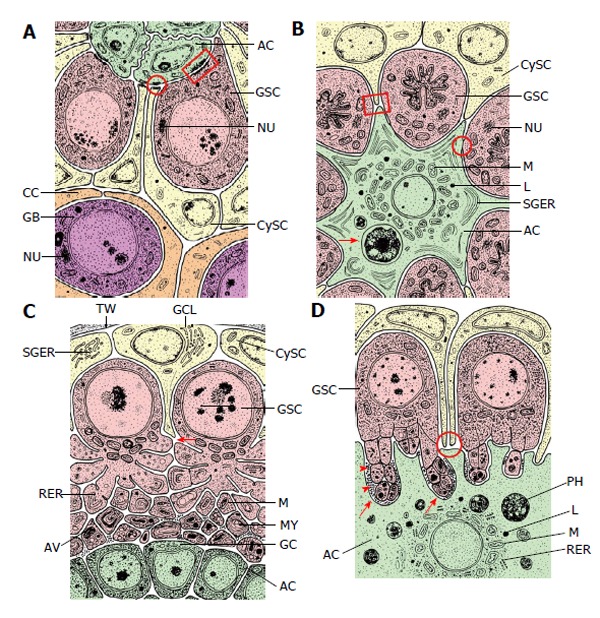
Fine structural organization (schematized) of the apical complexes (longitudinal sections) of four insect species. The order (A) to (D) is arranged according to the order in Figure 3. A: Drosophila melanogaster. ACs are “light” and interdigitate richly between themselves. Their organelle equipment is scarce. GSCs are “dark” due to many free ribosomes and characterized by “spongy bodies” which may represent nuage. Adherens junctions are expressed between ACs and GSCs (marked by a rectangle) and between ACs and CySCs (encircled). CySCs are “light” and include few cell organelles (Adapted from Hardy et al[61]); B: Locusta migratoria. The single apical cell of an apical complex is “light” and harbors a complex organelle equipment: in particular a ring of mitochondria (M) and lysosomes (L) around the nucleus, and stacks of sparsely granulated endoplasmic reticulum (SGER) at the cell periphery. Arrow marks a phagocytized and partly lysed GSC. ACs and GSCs are connected by gap-like junctions (encircled). The “dark” GSCs include extremely irregularly shaped nuclei and nuage-like material (NU). Extensions of the “light” CySCs reach to extensions of the star-shaped AC (marked by square) (Adapted from Dorn et al[64]); C: Oncopeltus fasciatus. The ACs are rather small and very “dark”. Cell organelles are inconspicuous besides Golgi complexes (GC) that face bordering GSC vesicles. The “dark” GSCs form cytoplasmic projections toward the ACs that undergo progressing autotomy. In the course of vesicle segregation rough endoplasmic reticulum (RER) and mitochondria (M) increase. Later autophagic vacuoles (AV) and myelin-like bodies (MY) are formed. Arrow points to a presumably newly sprouting cell projection. The “light” CySCs are characterized by extensive Golgi complex-like structures (GCL) that bare often associated with sparsely granulated endoplasmic reticulum (SGER) (Adapted from Dorn et al[60]); D: Lymantria dispar. The single AC of an apical complex is “light” and exhibits a spherical organization: mitochondria (M), lysosomes (L) and rough endoplasmic reticulum (RER) surround the nucleus: The cell periphery shows deep indentations caused by invading, autotomizing GSC projections (arrows). Segregated GSC vesicles are taken up by the AC, and phagosomes (PH) accumulate in the cytoplasm. The “dense” GSCs exhibit projection formation and projection autotomy that closely resembles that of Oncopeltus (see Figure 4C). GSC projections that indent the AC are often surrounded by extracellular granules (arrow heads). CySCs are “light”, and thin extensions reach the surface of the GSCs (encircled) (Adapted from Klein[66]). AC: Apical cell (green); CC: Cyst cell (orange); CySC: Cyst stem cell (yellow); GB: Gonialblast (purple); GSC: Germline stem cell (red); TW: Testicular wall (grey); AV: Autophagic vacuole; GC: Golgi complex; GCL: Golgi complex-like stricture; L: Lysosomes; M: Mitochondrion; MY: Myelin-like body; NU: Nuage; PH: Phagosomes; RER: Rough endoplasmic reticulum; SGER: Sparsely granulated endoplasmic reticulum.
Locusta migratoria
The longitudinal section through the follicular apex (Figure 3B) shows that the apical complex has the shape of a rosette with a singular AC in the center. The pear-shaped GSCs reach deeply into the large star-like AC. The CySCs are irregularly arranged around the GSCs and it is not clear how many CySCs are affiliated with a GSC. The ACs touch the protrusions of the CySCs between the GSCs. Remarkably, a plug of CySC-like cells is located beneath the rosette formed by AC, GSCs and CySCs. The CySC-like cells presumably participate in the formation of the cyst wall which consists of a higher number of cyst cells, up to 7 were counted on serial sections[63].
In contrast to the hub cells in Drosophila the large AC of Locusta (also “light”) shows a complex organelle equipment (Figure 5B). Around the large centrally located nucleus there is a broad ring of mitochondria that also includes lysosomal bodies. The periphery of the AC shows an abundance of sparsely granulated endoplasmic reticulum, often arranged in stacks and whorls that are also found in the cellular extensions that reach deeply between the basal parts of GSCs. The AC includes regularly one or two engulfed and more or less lysed GSCs (Figures 3B and 5B). The GSCs, “darker” than the ACs, show a polar organization. The nuclei are located in broader peripheral parts of the cells and present extremely irregular outlines. In the direction to the AC, mitochondria aggregate and fibrous nuage-like material is discernible. Free ribosomes are abundant. AC and GSCs form gap-like junctions (Figure 5B) but adherens junctions are not apparent[63]. No specific junctions seem to exist between CySCs and GSCs. CySCs and CySC-like cells have the same fine structural characteristics but only the CySCs contact the AC. Both cell types are rather “light”. Their cytoplasm is scant and shows no specifications. In mature males, many dividing GSCs, CySCs and CySC-like cells can be observed[64]. Both, asymmetrical and symmetrical divisions occur at the same time. Sometimes whole clusters of these cells are seen in mitosis.
Oncopeltus fasciatus
The longitudinal section through the follicular apex of Oncopeltus (Figure 3C) reveals an organization of the apical complex similar to that of Locusta (Figure 3B), however, with important cytological differences of the different cell types. The niche is represented by a small cluster of “dark” cells with a relatively thin cytoplasmic lining. Small areas of the cytoplasm include a Golgi complex in continuation with some strands of smooth endoplasmic reticulum which is directed toward the periphery where GSCs are bordering (Figure 5C). Specialized cell-cell contacts were not seen[60]. The GSCs reveal an extraordinary structure and astonishing dynamics. The GSCs have a polar structure, with a lobular perikaryon oriented toward the CySCs and prominent cytoplasmic projections toward the ACs (Figure 5C), which are reminiscent of neurons. The projections exhibit trabecular or septum-like ingrowths that are most advanced at their tips, next to the ACs. The process results in the segregation of free vesicles that amass around the niche. During the autotomy of the projection terminals, the number of mitochondria increases in these segments, lysosomal bodies and autophagic vacuole-like vesicles become abundant and rough endoplasmic reticulum is often arranged in whorls (Figure 5C). Degradation processes proceed, indicated by the presence of acid phosphatase and TPPase, resulting in myelin - and autophagosome-like bodies[60]. Autotomized vesicles aggregate at the surface of the ACs (Figure 6A). They finally rupture and release their content in the vicinity of the ACs. None of the debris is taken up by the ACs. Remarkably, intact-looking cytoplasm contains free ribosomes grouped in clusters. These clusters break up in slightly advanced stages of autotomy and the ribosomes are then evenly distributed. It is suggested that they give rise to electron dense granules of about 25 nm in diameter. These grana are sometimes enclosed in projection terminals; grana of lesser diameter occur free in the extracellular space between projection terminals and ACs (Figure 6A). This is of special interest, since morphologically similar grana were reported to be present at the same location in a number of apical complexes of different insect species (see Lymantria dispar, below). It is not clear whether or not these grana are taken up by ACs. After advanced autotomy of GSC projections new projections sprout at the “neck” of GSCs, where the projections arise from the perikaryon (Figure 5C). In this “neck” area of the cell there is an accumulation of mitochondria. CySCs surround only the apical part of the apical complex (Figure 3C). The ratio CySCs:GSCs is roughly 1:1, and only one cyst cell grows around a gonialblast. The cyst cell does not divide any more after its generation by division of a CySC but becomes highly polyploid as it enlarges during spermatogenesis[60]. Divisions of GSCs and CySCs are rarely observed. Asymmetrical divisions of GSCs, where the spindle axis is oriented perpendicularly to the niche, were never observed. During symmetrical GSC divisions, the cell projections are persistent. In the process of gonialblast formation one of the GSCs, that shows no structural difference to the remaining GSCs, moves away from the niche toward the periphery. It loses its projections as the cyst cell encloses it. The spermatogonial cysts now move proximally. Cyst cells, which are rather “light”, like the CySCs, develop striking organelles, composed of multiple complexes consisting of a meshwork of branching and anastomosing tubules and budding off vesicles which partly enclose electron-dense material. The Golgi complex-like structures (about 2.8 μm long and 1.1 μm in diameter) are often associated with sparsely granulated endoplasmic reticulum. The mitochondria are exceptionally long and branched. Cyst cells take up apoptotic spermatogonia[65]. Follicular apices include regularly extensive clusters of degenerating GSCs and spermatogonia (Figure 3C).
Figure 6.
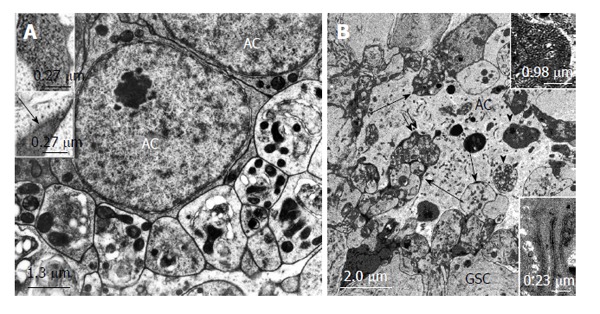
Structural relationships between apical cells and projections and autotomized vesicles of germline stem cells in Oncopeltus fasciatus and Lymantria dispar. A: Electron micrographs. Oncopeltus fasciatus. Vesicles that are segregated from GSC projections accumulate at the surface of ACs. The vesicles show signs of degeneration. Mitochondria are abundant and appear electron dense. (From Schmidt et al[65]). Upper inset: Intracellular granules in autotomizing GSC projections. Lower inset: Extracellular granules (arrow) between autotomizing GSC projections; B: Electron micrograph. Lymantria dispar. Numerous autotomized GSC projections protrude deeply into the AC (long arrows). Some of the segregated vesicles were apparently taken up by the AC and are being digested (arrow heads). Double arrow points to extracellular granules between GSC vesicles and the AC. Upper inset: Extracellular granules at higher magnification. Lower inset: Tubular indentations into the AC include electron dense material. (From Klein[66]). GSCs: Germline stem cells; AC: Apical cell.
Lymantria dispar
The niche of the apical complex consists of only one large AC (Figure 3D).The shape of the apical complex changes during development. During the first three larval stages the AC is attached to the envelope of the follicular apex, and only the distal part of it is contacted by GSCs[66]. From third to forth larval stage the AC detaches from the follicle envelope and moves somewhat distally. Then it adopts a concentric organization, and the GSCs attach from all sides (Figure 6A). Each GSC is accompanied by one CySC whose perikaryon covers the peripheral part of the GSC and sends delicate projections to the AC, separating neighboring GSCs. Whereas the symmetry of the apical complex changes during larval development (from bipolar to rotationally symmetrical), the intricate relationships between AC and GSCs are largely similar until the pupal stage when signs of senescence become apparent and cyst formation seizes[66].
The large, “light” AC includes a centrally located nucleus around which the cell organelles are concentrically arranged (at progressed developmental stages) (Figure 5D). Mitochondria, rough endoplasmic reticulum and lysosomes are especially abundant. As a rule the cell contains phagosomes, exceedingly numerous during early larval stages, with cell fragments of variable degree of degradation (Figure 5D and 6B). The periphery of the AC shows many Golgi complexes. The pear-shaped GSCs are “darker” than the AC apparently due to the presence of many free ribosomes. Striking are the cell projections of GSCs that deeply invade the AC. Similar to the GSC projections in Oncopeltus, GSC projections of Lymantria autotomize. But, unlike to Oncopeltus, the separated vesicles are phagocytized by the AC (Figure 6B). The GSC projections contain whirls of rough endoplasmic reticulum, many free ribosomes, mitochondria and multivesicular bodies. Phagosomes of the AC include similar cell organelles before they are digested indicating their origin from GSC projections. The electron micrograph Figure 6B demonstrates the extremely complex interactions between GSCs and AC. Extracellular space between GSC projections and AC regularly contains electron dense granules of 25-38 nm in diameter (Figure 6B inset 1). Tubular invaginations of the AC also contain electron dense material which often exhibits a fibrillar consistency (Figure 6B). A relationship with the dense granules is unclear. The large nucleus of the GSCs is located in the peripheral part of the cells. The organelles in the perinuclear cytoplasm are inconspicuous. Symmetrical as well as asymmetrical divisions have been observed (see below). CySCs are “light”. They divide apparently prior to the associated GSC[66].
Lampyris noctiluca
The glowworm represents a special case in as far as no niche cells (ACs) for the GSCs could be identified[67]. During early larval stages the gonadal follicles only include (“dark”) GSCs but no ACs and no CySCs; male and female gonads can not be differentiated. The onset of testis differentiation is marked by the appearance of “light” cyst progenitor cells (CPCs) segregated from the apical part of the follicle wall[67,68]. Whereas a cluster of theses cells is located and multiplies in the apex of the follicle, a cluster of dividing GSCs is located at the basal part of the follicle. The ratio of GSCs/CPCs is about 1/1. At that stage of development there are no associations between individual GSCs and individual CPCs. ACs are never observed. In later larval development, CPCs form cell projections, move toward and between the germ cells, which now may represent gonialblasts, contact and ensheathe them, thus forming spermatogonial cysts[67]. During transformation from CPCs to cyst cells, the cells develop conspicuous stacks and whorls of smooth endoplasmic reticulum. It was speculated that the cyst cells may produce hormones, i.e., juvenile hormone or ecdysone. Since all GSCs/gonialblasts and CPCs engage in cyst formation at approximately the same time, a precise temporal regulation of GSC division and gonialblast differentiation seems obsolete and the function of a niche therefore not necessary. The absence of ACs has also been reported from several other insect species[69].
In summary primarily electron microscopic studies revealed the enigmatic relationship between GSCs and their niche in numerous insects. Identification of the different cell types and their interactions were facilitated by the profound differences in electron density between the components of the apical complex: GSCs, ACs and CySCs. In almost all species studied, the ACs and the CySCs were “light” and the GSCs “dark”. Oncopeltus is an exception: here, ACs are “very dark” whereas GSCs are “dark” and the CySCs “light”. But, whereas the “light” and “dark” marking facilitates identification of the cell types, the functional significance of these characteristics remains unclear. In contrast, the intriguing processes of GSC autotomy and the interaction of autotomized GSC vesicles with niche cells (ACs) point to a to date unknown form of communication between GSCs and their niche. Next we will examine the process of GSC autotomy and compare it with morphologically similar processes in other systems: axon autodestruction, erythropoiesis and thrombopoiesis.
THE AUTOTOMY OF GSC PROJECTIONS: ARE THERE COMPARABLE PROCESSES IN OTHER CELL TYPES?
Autotomy of GSC projections in Oncopeltus fasciatus and Lymantria dispar
In both species the autotomy process follows an apparently standardized pattern (see above). Figure 7A gives a schematized view of the process in Oncopeltus. At the base of the cell projections the cytoplasm exhibits similar organelle equipment as the GSC perikaryon: scattered mitochondria, few strands of rough endoplasmic reticulum, many free ribosomes, often forming typical clusters and few lysosomes. The fractionation of the projections that leads to vesicle formation starts with the sequential ingrowth of the plasma membrane from the periphery (described by Schmidt et al[65] in Oncopeltus). With progressing vesiculation of a projection, mitochondria and rough endoplasmic reticulum that often form concentric whorls, become more abundant, clusters of ribosomes dissolve and the ribosomes are scattered evenly. Mitochondria and vacuoles accumulate. With progressing projection segregation mitochondria become swollen and show signs of degradation and the number of lysosomal bodies increase. Finally, autophagosomal activity becomes evident[60] and secondary lysosomes and myelin-like become abundant. The terminal vesicles then completely segregate from the GSC projections.
Figure 7.
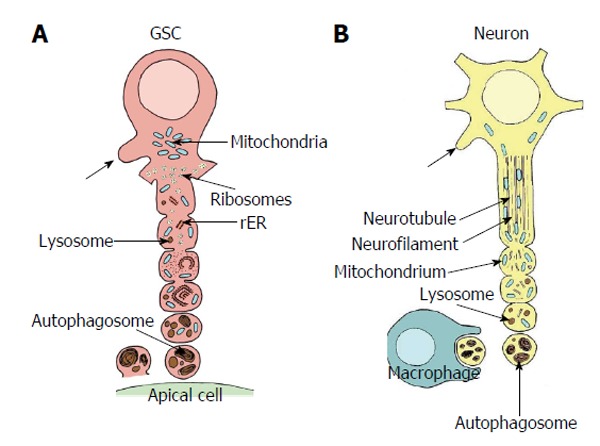
Comparison of autotomizing germline stem cell projection with autodestruction of an injured axon. A: Sequence of vesicle formation of a GSC projection in Oncopeltus fasciatus. The area of projection formation is characterized by an accumulation of mitochondria (blue). At the base of the projection ribosomes form small clusters (yellow), mitochondria are infrequent. Rough endoplasmic reticulum (rER, orange) and lysosomes (brown) are scarce. With progressing vesicle segregation mitochondria become more frequent and swollen, ribosomes form no longer clusters, rER becomes more prominent and lysosomal bodies increase. Segregated vesicles show many autophagosomes and myelin bodies. They accumulate at the surface of the apical cells (green) and disintegrate. Arrows point to newly sprouting projections (adapted from Dorn et al[60]); B: Sequence of progressive axonal fragmentation after injury. First neurotubules and neurofilaments break down. Then mitochondria accumulate and lysosomes (brown) become more abundant. Finally vesicles which mainly includ autophagosomes and myelin bodies are segregated and taken up by macrophages. Arrows points to newly sprouting axon (adapted from Lingor et al[155]; Beirowski et al[156]; Kerschensteiner et al[157]). GSC: Germline stem cell.
In the case of Oncopeltus the separated vesicles amass at the surface of the ACs, where they eventually rupture and release their contents. In the case of Lymantria the GSC projections are embedded in the AC and the segregated vesicles are phagocytized and digested by the AC (Figure 6B).
The process of projection segregation of GSCs exhibits remarkable similarities with degenerative processes of axons that take place either after injury, in neurodegenerative diseases or during developmental axon pruning. In the following it will be discussed whether an autodestruction program that may underlie the axonal destruction processes could possibly also be active in the process of GSC autotomy.
INJURY-INDUCED AXON DESTRUCTION (WALLERIAN DESTRUCTION) AND THE HYPOTHESIS OF AN AUTODESTRUCTION PROGRAM
Programmed cell autotomy seems to be a rather rare process. It is best known in axon degeneration that occurs after nerve injury in vertebrates and invertebrates (Wallerian degeneration), developmental neuron pruning and as pathological symptoms of neurodegenerative diseases (multiple sclerosis, Parkinson’s disease and others)[70]. Other examples of programmed cell autotomy are the formation of blood platelets and erythrocytes in mammals which are discussed later. Wallerian degeneration (Figure 7B) shares morphological similarities with axon degeneration during normal brain development (axon pruning) and with the “dying back” degeneration in neurodegenerative diseases.
Recent studies on the degeneration of injured axons strongly suggest that an active autodestruction program exists akin to apoptosis, and that the autodestruction pathway may be conserved between fly and human[71,72]. The sequence of progressing autodestruction of the stumps of transected giant nerve fibers in the cockroach Periplaneta americana, for instance, follows closely the segregation of GSC projection terminals in Oncopeltus (Figure 7): swelling of mitochondria, accumulation of myelin bodies, accumulation of lysosomal vacuoles and sequential segregation of vesicles[73]. Vesicle formation at the axon stump starts like in GSC projections with cell membrane ingrowths. Whereas the distal nerve stump degenerates, the proximal stump produces sprouts that have been interpreted as axonal regeneration. This resembles the expected outgrowth of new GSC projections after exhaustive vesicle segregation (Figures 5C and 7A).
Although still little is known concerning the signaling pathway directing the autodestruction program, some progress has been reported recently. The Wlds gene protects severed axons from degeneration. It encodes dNmnat (nicotinamide mononucleotide adenylyltransferase 1). Nmnat is a critical enzyme in the NAD+ biosynthesis pathway and is essential for many cellular processes[74]. Nmnat is likely essential for normal axon maintenance. Down regulation of Nmnat in the wing nerve of Drosophila leads to robust dying back fragmentation that markedly resembles Wallerian degeneration, whereas upregulation of Nmnat protects axon degeneration. The function of WldS/Nmnat may involve its essential role in NAD+ biosynthesis. The question arises if the mitochondria are the site of Nmnat-mediated action and mechanism[71]. Also the ubiquitin proteasome system (UPS) plays a crucial role in axon and dendrite maintenance and neuropathology, but the precise effects of WldS/Nmnat and the effect of the UPS vary depending upon the situation, an acute injury, developmental pruning or disease context (for review see Fang et al[71]).
The UPS is a potent regulatory mechanism used to control protein stability in numerous cellular processes, including neural development[75]. Many neurodegenerative diseases are featured by the accumulation of UPS-associated proteins, suggesting the UPS dysfunction may be crucial for pathogenesis. Recent experiments have highlighted the UPS as a key player during synaptic development. Recent discoveries center on the role of UPS in synapse remodeling and draw attention to the potential link between synaptic UPS dysfunction and the pathology of neurodegenerative diseases: Parkinson, Alzheimer, Huntington’s disease[75]. In Drosophila, the E3 ubiquitin ligase RPM-1 (disease resistance protein) targets DLK1 (delta homolog 1) which acts in the mitogen activated protein kinase (MAPK) cascade consisting of the MAPK MKK4 and the p38 kinase pMK3 or the MAPK c-Jun N-terminal kinase. Thereby RPM-1 regulates the organization and stabilization of presynaptic terminals and axon termination in mechano-sensory and motor neurons[76].
Regeneration of injured neurons can restore function, but most neurons regenerate poorly or not at all. The failure to regenerate in some cases is due to a lack of activation of cell-intrinsic regeneration pathways. These pathways might be targeted for the development of therapies that can restore neuron function after injury or disease. Hammarlund et al[77] showed that the DLK-1 MAPK pathway is essential for regeneration in Caenorhabditis elegans motor neurons. Loss of this pathway eliminates regeneration, whereas activating it improves regeneration. Further, these proteins also regulate the later steps of growth.
Osterloh et al[78] demonstrated that the ortholog genes sterile alpha and TIR motif-containing protein 1 (Sarm 1) in mouse and dSarm (sterile alpha/Armadillo/Toll-Interleukin receptor homology domain protein) in Drosophila promote cell autonomous axon destruction. The genes otherwise involved in innate immune response, are also players in a highly conserved axon destruction pathway. dSarm and Sarm 1 exhibit a punctate localization in neuronal cell bodies and a broad localization in neuritis of Drosophila and mouse respectively. An early event in the axon self-destruction pathway is the increase of intra-axonal calcium levels followed by a calcium-dependent cytoskeletal breakdown[72].
There is evidence that WldS enhances physiological functions of the mitochondria and that axonal mitochondria are required for WldS-dependent axon protection. WldS/Nmnat activity enhances mitochondrial motility and Ca2+ buffering and that the mitochondrion is an organelle necessary for WldS/Nmnat-mediated axonal protection[79,80].
DEVELOPMENTAL AXON PRUNING, DYING-BACK DEGENERATION AND NEURODEGENERATIVE DISEASES
Developmental axon pruning occurs at a large scale during metamorphosis of holometabolous insects, including Drosophila, where the process was studied in detail. Metamorphosis and axon pruning are controlled by ecdysone. Interestingly, in the mushroom body of the fly brain glia cells participate actively in axon pruning. Ecdysone stimulated axons extrinsically activate glial cells to infiltrate the axon branches and eliminate varicosities actively. They induce the fragmentation of axons, and engulf the fragments[81,82]. The process resembles the interaction of GSCs with the AC in Lymantria (Figure 6B). In both cases, the perikarya survive whereas the autotomy of cell projections/axons proceeds, and fragments are taken up by the AC and glia cells, respectively. In Oncopeltus, the GSC projections also autotomize but the severed and degrading vesicles are almost never phagocytized. It has been suggested that new projections sprout from the perikarya of Oncopeltus comparable to the sprouting of new neurites from pruned neurons. The neuron-glia interaction has an indispensable role in the pruning process of neurons in the mushroom body. The pruning proceeds in a neuron-autonomous manner. It resembles the interaction between phagocytes and apoptotic cells[81,82]. It was shown that dendrite-specific remodeling of Drosophila sensory neurons is controlled by two intracellular mechanisms: the ecdysone pathway and ubiquitin-proteasome system[83]. It should be noted that components of the ubiquitin-proteasome pathway has been linked to apoptosis[84].
A dying-back degeneration of axons can be induced in culture of mice neurons by removing nerve growth factor from the chamber. In the process, several distal ends of axons atrophy and undergo fragmentation while the neuronal somata survive. The controlling events are obviously confined to neurites and occur autonomously from the soma[85]. Many neurological deceases are accompanied by neurodegeneration. Although different factors might contribute to axon pathology in each case, what is clear is that the end result is always the same, the axon degenerates in a process that resembles Wallerian degeneration[86].
Wallerian degeneration is an evolutionary highly conserved process that is central to neurite autotomy occurring either regularly in developmental remodeling of neurons or pathologically in neurological diseases and experimental manipulations. The mechanism of serial autotomy of GSC projections in Oncopeltus and Lymantria (and probably butterflies in general) resembles closely axon autotomy described above. Molecular studies are necessary to uncover whether similar signaling pathways are involved.
CONSEQUENCES OF GSC AUTOTOMY ON SIGNAL EXCHANGE WITH THE NICHE AND GSC DIVISION (SYMMETRIC VS ASYMMETRIC DIVISION)
Until recently it was accepted that adult male GSCs in Drosophila only undergo asymmetric divisions[87]. GSCs attached to hub cells by adherens junctions provide a polarity cue that orients stem cells. The centrosome is oriented toward the hub cell-GSC interface throughout the cell cycle. The mother centrosome is always located close to the hub cells whereas the daughter centrosome moves to the opposite side. Consequently, GSC spindle orientation is predetermined during interphase[88]. The correct centrosome orientation toward the hub cells requires the adherens junction which is composed of E-cadherin and β-catenin, centrosomin, and Apc2. Apc2 is believed to connect astral microtubules to the adherens junction/actin cytoskeleton network formed between hub cells and GSCs thereby anchoring the centrosome[89]. Centrosome orientation prior to mitosis is accomplished by Par-1 (a serine/threonine kinase that regulates polarity in many systems) that regulates cyclin A localization[90]. Recently “symmetric renewal” of male GSCs in Drosophila has been observed[91]. In this process, GSC division starts like a typical asymmetric mitosis (the mitotic spindle is perpendicularly oriented toward the hub surface) but then the still interconnected pair of cells “swivel” such that both cells contact the hub. Studies on other insect testes revealed still different modes of GSC divisions and gonialblast differentiation.
Unique processes have been described in male GSCs of Lymantria (Figure 8). GSCs undergo either symmetrical divisions (the spindle is oriented parallel to the AC surface) or asymmetrical divisions (the spindle is oriented perpendicularly to the AC surface). Both types of mitosis can result in gonialblast formation but, surprisingly, after asymmetrical division GSC and daughter cell - interconnected by a fusome - “swivel”, comparable to the process in Drosophila described by Sheng et al[91] in 2011, and both contact the AC. Whereas the mother GSC maintains its intense interaction with the AC, the daughter and presumptive gonialblast does not form cell projections. Finally, the daughter cell moves further to the periphery and differentiates to a gonialblast. Alternatively, after symmetrical division the daughter cell is again characterized by the lack of cell projections, develops to a gonialblast in a similar fashion as in the case of asymmetric GSC division[66]. Each GSC is associated with one CySC. CySC division precedes GSC division and the mitotic spindle is always oriented parallel to the AC surface. The mechanism destining the centrosome location is not known and adherens junctions between GSCs and AC have not been described - and are not likely to exist, given the complex relationship between GSCs and AC. Consequently, the regulation of spindle orientation must differ in Lymantria from Drosophila.
Figure 8.
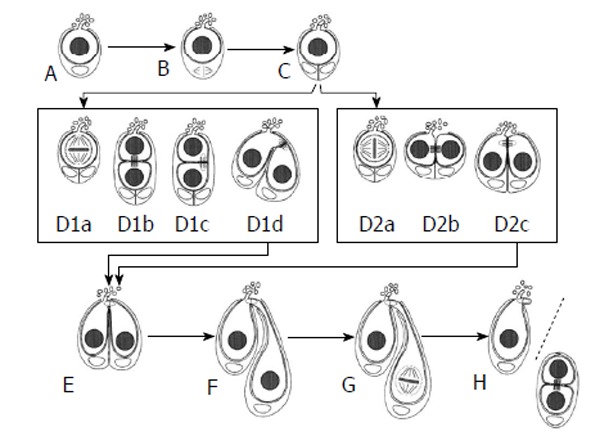
Symmetric and asymmetric germline stem cell division in Lymantria dispar. Both symmetric and asymmetric GSC divisions occur in this insect species. Both are preceded by the division of the single cyst stem cell that is associated with a GSC (A to C). Asymmetric division is depicted in D1a to D1d. One of the daughter cells is oriented toward the AC and still forms projections, whereas the other daughter cell has no contact with the AC and is devoid of projections (D1b). Then this daughter cell “swivels” round toward the AC (D1d) and adopts a similar position as daughter cells have after a symmetric GSC division (D2a to D2c). In each case, the daughter cell that doesn’t form projections becomes the gonialblast (E to H) (from Klein[66]). GSCs: Germline stem cells; AC: Apical cell.
Also in Oncopeltus adherens junctions could not be identified in electron microscopic studies[92]. In this species, only symmetrical divisions of GSCs have been observed. GSC projections persist during division. Gonialblasts are formed when GSCS migrate toward the periphery of the apical complex. Its projections elongate, become thin and gradually degenerate before one cyst cell encloses the gonialblast[92]. Locusta shows both symmetrical and asymmetrical divisions[64]. But again, besides tight junction-like connections, no adherens junctions have been detected between GSCs and AC. It should be mentioned, however, that GSCs and AC are extremely difficult to separate, either enzymatically or mechanically, in Locusta[64].
In summary, the mechanism for spindle orientation appears variable in insect male GSCs. But up to date, Drosophila is the main species studied in this respect in the evolutionary highly diversified group of insects.
Interactions between GSCs and ACs in the testes of various insects are diverse and it is evident that the mode of short range signaling between stem cell and niche differ in distinct species from that in Drosophila. In Oncopeltus, ACs are surrounded by vesicles segregated from GSC projections (Figure 6A). This poses the questions: how is the communication and information exchange organized between GSCs and AC? and how is the sequential projection autotomy, progressing degradation, and sprouting of new projections programmed? There are no studies on the molecular level concerning these questions. However, the ancient conserved axon destructing Wallerian pathway bears many similarities with GSC projection autotomy. Further, the outgrowth of new projections near the perikaryon of Oncopeltus GSCs parallels the outgrowth of new neuritis after Wallerian axon destruction. As described above, developmental axon pruning and neurite dying-back in neuronal diseases all appear to be governed by a related axon-autonomous program that carries characteristics of apoptotic processes. Extensive fragmentation of megakaryocyte projections takes place during platelet formation as described below. Also in this case apoptotic processes take place, i.e., fragmentation of the nucleus. The segregation of platelets, however, appears to follow a different pattern than Wallerian axon fragmentation and GSC projection autotomy. In the latter cases, concentrically transverse ingrowing plasma membrane that finally fuses, cuts off vesicular fragments. Pinching off platelets apparently involves transverse microtubule arrangement and vesicle widening at the constriction zone (see below).
In Lymantria, segregation of GSC projection terminals resembles that in Oncopeltus. Thus, the underlying cell autotomy program is expected to be similar as in Oncopeltus. But in Lymantria and several other butterflies (see above) the AC embraces the GSC projections and engulfs and digests separated vesicles. In Locusta, ACs almost constantly include one or two phagocytized GSCs. The interactions of GSCs and ACs (their niche) are puzzling in Oncopeltus as well as in butterflies. We speculate that the AC sends signals that promote GSC autotomy and that in return the AC receives information concerning the surrounding GSC population. In response to that information the AC may regulate GSC self-renewal and maintenance. Unfortunately, none of these aspects have yet been tested. In about all insect apical complexes studied by electron microscopy, “dark” granules have been described in the interface between AC and GSCs (Figure 6). In Lymantria, the AC shows tubular invaginations filled with “dark” material of unknown fate. Due to the phagocytic processes and material exchange early investigators suggested a trophic role of ACs[92]. We believe that these processes are part of the information exchange and signaling pathways. In erythroblastic islands, after release of the reticulocyte, the pyrenocyte is phagocytized by the central macrophage. This is mandatory for continued erythropoiesis (see below). This indicates that the involvement of phagocytic processes in stem cell-niche interaction is existent but needs further investigation.
STEM CELL/PROGENITOR AUTOTOMY IN ERYTHROCYTE AND PLATELET FORMATION
Erythroblastic islands and enucleation of erythroblasts
In vivo, erythropoiesis occurs in specific units, the erythroblastic islands in the bone marrow of mammals[93]. Erythroblastic islands were also described in the spleen, yolk sac and fetal liver. They harbor a central macrophage that arises from a resident monocyte precursor with a unique immunophenotypic signature[94]. The central macrophage, representing the niche, is surrounded by one or more synchronously maturing cohorts of erythroid cells that undergo four or five divisions between proerythroblast and orthochromatic erythroblast stage. In their fine structural study Allen et al[95] describe gap junction-like contacts between the macrophage and erythroblasts and possible reciprocal vesicular activity. Several molecules indicate adhesive interactions within the erythroblastic islands[4]: (1) Erythroblast macrophage protein (Emp) forms macrophage/erythroblast attachments via hemophilic binding; (2) α4β1 integrin in erythroblasts and vascular cell adhesion molecule-1 in the central macrophage mediate receptor/counter receptor cell-cell interactions; (3) Macrophage α integrin and erythroid intercellular adhesion molecule-4 are expected to contribute to the island integrity; and (4) Other macrophage adhesion glycoproteins, i.e., CD69 and CD 163, have been detected[96] although their erythroid binding partners are unknown. It is expected that adhesive connections between erythroblasts and macrophages play a crucial role in signaling pathways as they do in Drosophila testes. The central macrophage secretes soluble factors, cytokines, that promote proliferation and maturation of erythroblasts (insulin-like growth factor-1 and others) and also negative regulatory factors [transforming growth factor-β1 (TGF-β), TNF-α, ILG and others][4] (Figure 9).
Figure 9.
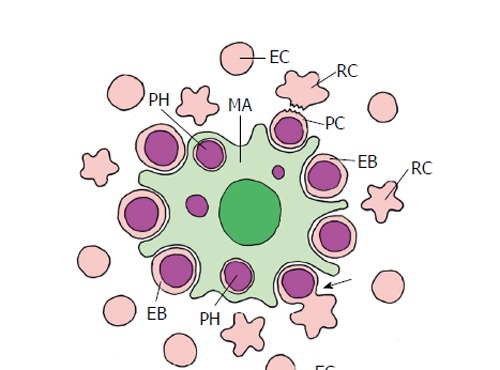
Erythropoiesis in mammal bone marrow (schematized). The erythroblastic island consists of a central macrophage (MA) that functions as the niche, and the peripheral erythroblasts (EB), that represent stem/progenitor cells. Erythroblasts undergo an enucleation process (arrow) that results in the pyrenocyte (PC) that mainly consists of the erythroblast nucleus, and the nucleus-free reticulocyte (RC). The pyrenocyte is phagocytized by the macrophage (PH, phagosome), whereas the reticulocyte develops to the erythrocyte (EC) (adapted from Chasis et al[4]; Keerthivasan et al[98]).
The most striking event in mammalian erythrocyte maturation is the enucleation of orthochromatic erythroblasts at the last stage of erythroblast differentiation. It results in multilobulated non-nuclear reticulocytes and pyrenocytes which mainly consist of the nucleus and the enwrapping plasma membrane. Enucleation is regulated by retinoblastoma protein (Rb) but other signaling molecules, e.g., p38 MAPK (p38) and Rac-1 GTPase, have been found to be involved in the enucleation[97]. Interestingly, sorting of protein and vesicle trafficking in the orthochromatic erythroblast in concert with nuclear positioning are essential for the enucleation process[98]. The pyrenocytes express phosphatidylserine, a recognition signal similar to apoptotic cells, on their surface that signals macrophages to engulf and digest them. The taken up DNA is digested by DNase II from the macrophage[99]. The lack of DNase II in DNase II knockout mice is lethal in utero due to embryonic anemia. Digestion of engulfed pyrenocyte nuclei is vital for continued erythropoiesis[100].
Already in the 1990ties Hanspal et al[101,102] found that the interaction between erythroblasts and macrophages is needed for normal erythroblast proliferation and for enucleation. The authors showed that this interaction is mediated by Emp that prevents apoptosis of developing erythrocytes. Nonetheless, erythroblasts cultured in vitro in the absence of macrophages undergo complete differentiation including nuclear extrusion[103,104]. Although erythropoietin was used to start erythroblast amplification (and other factors were eventually added) erythropoiesis proceeded apparently normal without macrophages, however, at a much lower pace as it does in erythroblastic islands[93]. Thus the question arises: have macrophages merely a trophic function? As mentioned above, genetic manipulations of macrophage activity resulted in lethality due to anemia. It may be speculated that in these cases erythropoiesis was (only) insufficient (but not completely) suppressed. The “true” function of island macrophages is to optimize and accelerate erythrocyte production allowing effective erythrocyte supply and rapid adjustment to the actual need. Erythrocyte homeostasis might be largely regulated by systemic factors that convey the need or surplus of erythrocytes and affect short-range signaling within the erythroblast island which determines the pace of erythrocyte production.
We suggest that spermatocyte production in insect testes is regulated in a similar way. The example of Lampyris shows that no ACs are needed for GSC differentiation per se. The apparent reason: all GSCs differentiate at the same time and the process does not need any temporal regulation. Onset of GSC differentiation and production of CC progenitors occurs during larval development of Lampyris and is expectedly put in motion by the release of morphogenetic hormones, e.g., ecdysone and juvenile hormone, representing systemic signals[67].
Another conspicuous interaction shared by the niche of erythroblastic islands and the niche of butterfly testes are spectacular phagocytic processes. In the case of erythroblastic islands, macrophages engulf the pyrenocytes; in the case of butterflies, ACs phagocytise large vesicles segregated from GSC projections. DNA taken up by macrophages plays a role in signaling, as described above. And there are also vesicle interactions with expected receptor exchange. In butterflies, the vivid autotomic activity of GSCs and the phagocytotic uptake of the autotomized vesicles are not understood. We propose that it represents an interaction/communication between niche and stem cell hitherto unknown. Noteworthy, whereas autotomy of GSC projections also takes place in Oncopeltus the segregated vesicles are not taken up by ACs, but degenerate in a distinct pattern as described above. We suggest that the degenerating vesicles provide specific signals that are recognized by the AC. Interestingly, the ACs of Locusta include, as a rule, one or two phagocytized GSCs (see above). Phagocytosis here may play a similar role as it does in Lymantria. The variations in stem cell-niche relation seem highly variable in insect testes, and the analysis of species beyond Drosophila might provide new insights.
Platelet formation by megakaryocyte fragmentation
The probably most spectacular case of programmed cell autotomy is the shedding of 5000-10000 platelets from one megakaryocyte. Platelets are characterized by the absence of a nucleus and by the accumulation of three types of granules: (1) Dense (or delta) granules, with a diameter of 150 nm, contain ADP or ATP, Ca and Serotonin. They are secreted to recruit other platelets; (2) Alpha-granules, with a diameter of 200-400 nm, contain P-selectin, platelet factor 4, TGF-β1, platelet-derived growth factor, fibronectin, B-thromboglobulin, von Willebrand factor (VWF), fibrinogen, coagulation factors V and XIII. They are responsible for adhesion and healing processes; and (3) Lambda granules, with a diameter of 175-250 nm, resemble lysosomes. They contain several hydrolytic enzymes that are able to eliminate circulating platelet aggregates (for review see Rendu et al[105]) (Figure 10).
Figure 10.
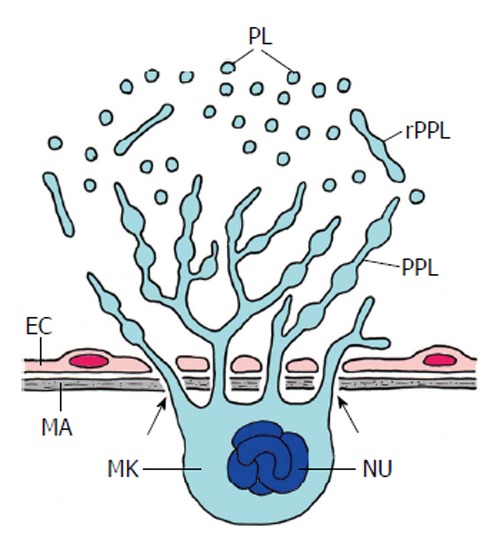
Thrombopoiesis (platelet formation) in mammal bone marrow (schematized). Platelets are produced by a most spectacular form of cell autotomy performed by the megakaryocyte (MK). Maturation and fragmentation of the megakaryocyte is orchestrated by the vascular niche which consists of the endothelial cells (EC) of sinusoids and the extracellular matrix (MA) of the endothelium. The megakaryocyte is located at the outer surface of the sinusoid epithelium and sends pseudopods through the pores of the endothelium into the lumen of the sinusoids (arrows). The pseudopods branch and form proplatelets (PPL). These are released into the sinusoids (rPPL) followed by the release of platelets (PL). Nu, polyploidy nucleus of the megakaryocyte (Adapted from Patel et al[115]).
Megakaryocytes mature from megakaryoblasts via promegakaryocytes in the bone marrow. Megakaryoblasts reside in the osteoblastic niche where osteoblasts secrete the cytokine thrombopoietin (TPO) that regulates megakaryopoiesis and thrombopoiesis, i.e., platelet formation, as well[106]. Stromal cell derived factor-1 from stromal fibroblasts and fibroblast growth factor-4 direct megakaryocyte interaction with the bone marrow stroma and regulate cytokine-independent megakaryocyte maturation[107]. A number of cytokines [interleukin-3 (IL-3), IL-6, IL-11, IL-13, leukemia inhibiting factor (LIF), stem cell factor, and others] affect megakaryopoiesis which was mostly tested in in vitro systems[108,109]. In the process of maturation, the precursors of the megakaryocytes migrate from the osteoblastic to the vascular niche along an oxygen gradient to the higher oxygen of the vascularized compartment of the bone marrow[110]. During maturation megakaryocyte progenitors undergo endomitosis, up to 128 times. Reaching the endothelium of the marrow sinusoids the strongly enlarged megakaryocytes form cytoplasmic projections that protrude through endothelial pores into the lumina of the sinusoids. There, the projections presumably (as inferred from in vitro observations) branch repeatedly forming formidable trees of proplatelets which segregate and release the platelets into the lumina of the sinusoids. The vascular niche that promotes platelet formation and shedding consists of the endothelial cells and the extracellular matrix of the endothelium[111].
The dynamic interactions of megakaryocytes with different extracellular matrix proteins seem to orchestrate their maturation in specific sites[111]. In the vascular niche such proteins include collagen type IV, fibronectin, laminin, fibrinogen and VWF. (VWF is secreted by endothelial cells and megakaryocytes into the blood and has a function in adhesion and aggregation of platelets.) In mice (in vitro) fibrinogen binding to the fibrinogen receptor αIIbβ3, which is expressed in megakaryocytes, is essential for proplatelet formation. However, the role of the interaction in humans is not fully understood[112]. Astonishingly, in vitro studies have shown that the cytokine TPO alone is required for thrombopoiesis and that extracellular matrix and other cytokines are not essential, although they may have regulatory functions in vivo that accommodates platelet homeostasis[113]. This is reminiscent of the niche function in erythroblastic islands and testes of insects where stem cell differentiation can proceed without niche but is adjusted to the actual need by niche interaction.
The process of proplatelet formation and platelet shedding is highly complicated and still not fully understood[114,115]. Electron microscopic studies on cultured megakaryocytes demonstrated that prior to proplatelet formation mature megakaryocytes have already a well-developed demarcation membrane system[116]. This elaborate membrane system is - as commonly believed - formed by invaginations of the plasma membrane, shows open cisternae and is at first randomly distributed throughout the cytoplasm. Randomly scattered α-granules and some dense granules are also present. Preparing for active platelet shedding, the peripheral demarcation membranes dilate and align at the cell periphery. Cytoplasmic sheets unfold and cell projections extend. The extensions display a beaded appearance with constriction points separating discrete platelet-like territories. A bundle of longitudinal microtubules runs through the center of the extensions. At the constriction zone transverse microtubules are observed near the longitudinal microtubules. Also, a vacuole of increasing size is formed at the constriction zone which may lead to the detachment of the platelet fragment. These observations of Cramer et al[116] suggest that vesiculation and microtubule force attribute to autotomy. Other studies emphasize even more the role of microtubules in platelet segregation[115,117]. Before proplatelet formation, microtubules align into bundles beneath the surface of megakaryocytes and, at projection formation, fill the cortex of outgrowing cones. Cell organelles are in direct contact with microtubules and are transported along these elements. Microtubules coil at the end of the projections, but their exact role in platelet segregation remains unresolved.
It should be mentioned that in an opposing view the membrane boundaries of platelets are not provided by involutions of the megakaryocyte plasma membrane but by vesicles from Golgi complexes. So-called proplatelets constitute within the megakaryocyte whose plasma membrane finally ruptures and releases the platelets. Extensions of the megakaryocyte with proplatelets and segregation of terminal platelets are considered as artifacts by some authors[114,118,119].
It is, however, the prevailing view that platelets segregate from ends of the megakaryocytes extensions that offer a beaded structure, in as much platelet-sized and platelet-structured proplatelets are connected by string-like connections with longitudinally running microtubules (Figure 10). Interestingly, the process of platelet assembly is accompanied by some characteristics associated with apoptosis: cytoskeletal reorganization, membrane condensation and chromatin condensation. Microtubules and F-actin play supposedly a major role in proplatelet formation and fragmentation. The kinetics of platelet release in vitro corresponds to the onset of apoptosis in the megakaryocyte. Maximal platelet production and megakaryocyte apoptosis are closely related events[115,120,121]. Molecular evidence of apoptotic processes in megakaryocytes provided the detection of caspase 3. Before the platelet formation caspase 3 shows a punctuate cytoplasmic distribution (in a presumably inactive state) and a diffuse staining pattern (in a presumably active state) in senescent megakaryocytes. It was concluded, that proplatelet formation is regulated by caspase activation limited to only cellular compartments[122]. Further evidence for an involvement of apoptotic processes in platelet formation comes from the presence of the antiapoptotic protein BclxL which is upregulated during megakaryocyte differentiation but absent during late megakaryopoiesis. Bclxl overexpression causes a strong decrease in proplatelet formation[123]. Other apoptotic-related genes such as TGF-β1 and SMAD proteins are expressed during thrombopoiesis which supports the significance of apoptotic signaling in the process[124]. Besides, NO in conjunction with TPO facilitates platelet production[125]. Nagata et al[126] report that estradiol synthesized in megakaryocytes triggers proplatelet formation by autocrine action. Few transcription factors were reported to play major roles in thrombopoiesis. GATA-1, which interacts with friend of GATA-1 controls proliferation during megakaryopoiesis, and NF-E2 regulates platelet biosynthesis[127-129].
Cell autotomy in the process of erythrocyte formation (i.e., enucleation) and platelet generation (by megalokaryocyte fragmentation) appear to follow quite different strategies. However, in both cases apoptotic processes play a prominent role. This offers parallels to the autodestructive processes in neurons (see above). Concerning GSC autotomy, no attempts have been made to demonstrate apoptotic processes in GSC projections. Schmidt et al[92] described apoptosis of GSCs and spermatogonia in Oncopeltus but its significance - apart from removal of surplus spermatogonia - remains obscure. Dying germ cells in Drosophila revealed mixed morphologies of apoptosis and necrosis that may indicate an alternative developmental cell death pathway[130]. The role of the cytoskeleton in cell autotomy is little understood although it may play a major role in all cases. Concerning megakaryocyte fragmentation, the cytoskeleton is obviously decisively involved in platelet segregation. Neurofilaments and microtubules are the first cell organelles that break down in axonal autodestruction following intracellular calcium increase after nerve injury. The role of the cytoskeleton in GSC autotomy is not known but is conceivably important and should be analyzed.
COMPARISON OF THE INSECT AND MAMMAL SPERMATOGENESIS
Testes of insects offer a rather simple architecture. One testis is composed of one to many testicular follicles, blind ending tubules that join into a common seminal duct. GSCs and niche exhibit a globular arrangement, mostly in the form of a rosette at the apex of a testicular follicle, representing the apical complex. Spermatogonial cysts move distally during spermatogenesis. The follicular epithelium that envelopes the apical complex and cysts is mostly thin and has, so far, not been considered as part of the niche for GSCs. However, as pointed out above, in a number of species, the envelope synthesizes and releases the steroid ecdysone at some point of development[53]. Although effects of ecdysone on spermatogenesis have been reported, a specific function or signaling pathway has not been elucidated. Nonetheless, it parallels the production of steroids, androgens, in Leydig cells of the testes of mammals.
Mammalian testes exhibit a complex epithelial organization (for review see Yoshida[131]). The long seminiferous tubules are convoluted and both ends open into the rete testis. Figure 11 shows the organization of a seminiferous tubule. The high epithelium of the tubules consists of the larger Sertoli cells, and the smaller spermatogonia and spermatocytes. The epithelium rests on a basement membrane, and below it stretches peritubular myoid cells. Located in the interstitial between the seminiferous tubules are Leydig cells, macrophages, lymphoid epithelial cells and connective tissue. Blood vessels form a network around the tubules and run in the interstitial spaces. Besides the germ cells all mentioned cell types and structures may be part of the GSC niche. The complexity of the niche, which doesn’t offer spatial specifications, is reflected by the difficulty to define GSCs. They represent obviously a small population of spermatogonia. According to de Rooij et al[132] and Russell et al[132,133], spermatogenesis progresses uniformly all over the inner surface of the seminiferous epithelium, and stem cells are scattered all over. But how are GSCs identified? First, they are located in the basal compartment of the epithelium. All neighboring Sertoli cells form tight junctions at a distinct height separating a basal compartment which has contact with blood vessels and an adluminal compartment without blood contact. Thus, a blood-testis-barrier is installed at the level of tight junctions. Located in the basal compartment are “undifferentiated spermatogonia” (Aundiff), comprising singly located spermatogonia (As) and such that have undergone up to four mitotic divisions. The mitotic spermatogonia form syncytia due to incomplete cytokinesis. As have contact with the basement membrane and a subpopulation of them might represent yet-to-be-identified GSCs. At the transition from mitotic to meiotic divisions, spermatogonia move to the adluminal compartment and are now called spermatocytes. From there on spermatogenesis takes place behind the blood-testis-barrier. Round spermatids move to the luminal surface where they elongate. The accumulation of stags of spermatogonia and spermatocytes among the Sertoli cell epithelium results in a multilayered organization of the seminiferous tubules.
Figure 11.
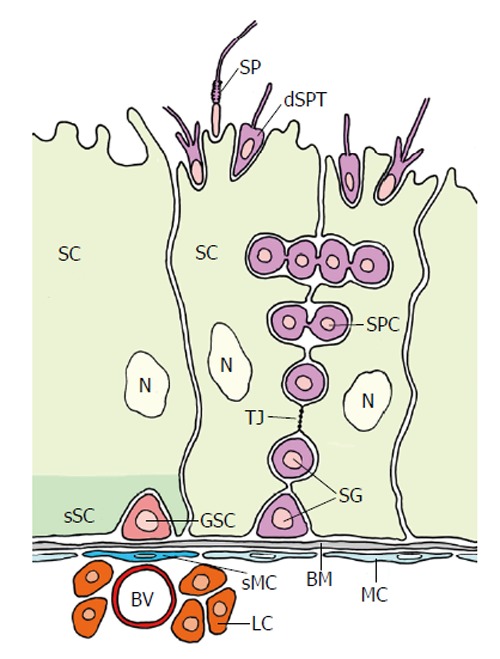
Organization of the mammalian testicular germline stem cell niche (adapted from Yoshida[131]). The seminiferous tubules exist of large sertoli cells (SC) and spermatocytes (SPC). The putative germline stem cell (GSC) is located at the basement membrane (BM) at an interstitium. It is in close vicinity of a blood vessel (BV) and leydig cells (LC). Myoid cells (MC) that line the BM are thought to be “specialized” MC (sMC) below the GSC. The basal part of the SC that surrounds the GSC presumably performs a specialized “domain” acting as (the main) part of the complex niche. The niche allegedly consists of several additional entities besides the specialized SC domain: the BM, the sMC, the BV, the LC and probably others (see text). Tight junctions (arrow) between SCs establish a blood-testis barrier: GSCs and spermatogonia are exposed to the vascular system, whereas primary spermatocytes (SPC) and the following stages of Spermatogenesis proceed behind the barrier. dSPT: Differentiating spermatid; SP: Spermatozoon; N: Nucleus of SC; Red: GSC; Light green: SC; Dark green: sSC domain surrounding the GSC; Purple: Spermatogonia and different stages of spermatogenesis; Dark blue: sMC; Light blue: MC; Light red: BV; Orange: LC; Grey: BM.
Since As are spread all over the tubules the question arises: which mechanisms provide uneven features within the basal compartment to specify the niche microenvironment for stem cells? Yoshida et al[100] demonstrated that Aundiff (and possibly GSCs) are preferentially localized to the area adjacent to the interstitium at branching points of blood vessels of medium thickness. Yoshida et al[131] suggests that Sertoli cells and myoid cells in this region might be “specialized”. Theoretically, all parts of the niche could control stem cells directly by short-range signaling. Systemic signals could arrive via blood directly at the GSCs or indirectly by modulating the short-range signaling of other parts of the niche. The niche region might not be fixed.
Several signaling factors are known to have effects on spermatogenic cells (Figure 12). Most important is glial cell line-derived neurotrophic factor (GDNF), a member of the TGF-β superfamily[134]. The ligand GDNF is expressed in Sertoli cells and its receptor c-Ret and co-receptor GFRα1 is expressed in the least mature subsets of Aundiff, the putative GSC[135,136]. Thus, via GDNF signaling Sertoli cells control self-renewal, survival, and maintenance of GSCs. Another receptor highly expressed in GSCs is colony stimulating factor 1 receptor. Its ligand is expressed in Leydig cells of the interstitium and a subset of myoid cells[137]. It is suggested that CSF1 may cooperate with GDNF in supporting the self maintenance of GSCs. The tyrosine kinase receptor c-kit and its ligand KitL, which is expressed in Sertoli cells, have been recently shown to be involved in proliferation, survival and migration of spermatogonia. However, only KIT(-) spermatogonia have stem cell activity. Several factors have effects on GSCs in vitro: FGF, EGF and LIF, in the presence of GDNF, support the proliferation of GSCs[138]. LIF is probably also involved in the maturation of gonocytes into spermatogonia[139]. But a possible function in vivo is uncertain. The transcriptional regulator Ets related molecule has been detected in nuclei of Sertoli cells of adult testes. It is assumed that it regulates Sertoli cell function that mediates germ cell self-renewal[140].
Figure 12.
Short range signalling between germline stem cell and its niche. GDNF is the most important signal produced by the “specialized” basal compartment of SC. Its receptor - c-RET (transmembrane tyrosine kinase receptor) and co-receptor GFRα1, which functions as a glycosylphosphatidylinositol-anchored docking receptor that is needed for GDNF to bind with c-RET - is expressed in GSC, and SFK and Akt pathway are initiated. They control self-renewal and maintenance of GSCs. GSCs also express CSF1R (colony stimulating factor 1 receptor). Its ligand CSF1 is synthesized in “specialized” MCs (sMC) and in leydig cells (LC). Thus, CSF1 may cooperate with GDNF in supporting self-maintenance. Integrins attach the GSCs to the BM and may also contribute to signalling. Red: GSC; Light green: SC; Dark green: sSC domain surrounding the GSC; Purple: Spermatogonium (SG); Dark blue: sMC; Light blue: MC; Light red: BV; Orange: LC; Grey: BM.
Stem cells and spermatogonial populations express α6 and β1-integrin[136] which mediates the attachment to the basement membrane via binding of laminins, probably as a heterocomplex with α6-integrin[141]. Its significance in signaling in vivo is not known; GSCs lacking β1-integrins fail to develop spermatogenic colonies after transplantation[142]. E-cadherin is expressed in Aundiff but is dispensable for the normal functioning of stem cells[143].
Despite the profound differences between the organization of the insect Drosophila and the mammal mouse testis, several important common principles can be observed. In both cases, the stem cell-niche complex is exposed to blood: in insects hemolymph freely surrounds the testicular follicles whose envelope allows the passage of larger molecules[144]. In mammals blood vessels run through the niche of GSCs and release molecules in the vicinity of GSCs. In several adult stem cell-niche systems blood vessels and endothelial cells are an integral part of the niche: the HSC niche and the vascular niche of platelet producing megakaryocytes (see above), the neural stem cell (NSC) niche the B1 NSCs within the ventricular-subventricular zone sends out a basal process ending in a specialized end-foot that contacts blood vessels; blood-borne factors and endothelial-derived factors may act on B1 cells in this domain[145], intestinal stem cell and probably other niches[146,147]. Recently, it has been reported that an important function of endothelial cells in glioblastoma multiforme is to create a niche that helps to promote self-renewal in cancer stem-like cells[148]. In liver regeneration, endothelial cells establish an instructive vascular niche, which through elaboration of paracrine trophogenes stimulates organ regeneration, in a manor similar to endothelial-cell-derived angiocrine factors that support hematopoiesis[149].
Meiotic divisions of spermatogonia in mammals and all mitotic and meiotic divisions after gonialblast formation in insects take place behind the blood-testis-barrier. Whereas tight junctions between neighboring Sertoli cells establish the barrier in mammals, it is the cyst cells that isolate the developing germ cells from the hemolymph in insects. There is little information on the necessity for a compartment in which spermatogenesis is protected from blood-borne factors.
Mouse and Drosophila differentiating germ cells share another astonishing potency: they can generate GSCs, in vivo. In mice “spermatogonial progenitors committed to differentiation” can generate functional GSCs that can repopulate germ cell-depleted testes when transplanted into adult males. GDNF and FGF2 are able to reprogram in vitro spermatogonial progenitors for reverse differentiation[150,151]. Amazingly, posttransplantation homing GSCs have to perform multiple steps: attachment to the Sertoli cell surface, retrograde translocation to the basal compartment across tight junctions, migration to the presumptive stem cell niche, survival, proliferation, and self-renewal within the niche, expansion of the transient amplifying spermatogonia and differentiation into sperm[131]. β1-intergin, which is expressed in spermatogonial cells, plays an essential role in GSC homing[142]. In Drosophila testes spermatogonia undergoing transit-amplifying divisions can be reverted to stem cell identity by conditionally manipulating Jak-STAT signaling[152]. In the process, the spermatogonia, which are enclosed in cysts and interconnected by fusomes, have to break up their connections, and the cyst wall has to disintegrate in order to release the dedifferentiating germ cells. These germ cells populate the orphaned niche and reestablish normal spermatogenesis. Spermatogonial dedifferentiation can be genetically induced by conditional loss of STAT or misexpression of the differentiation factor Bam within the testes. This causes the differentiation of all GSCs and free niche space. If normal signaling is restored differentiating spermatogonia revert to stem cells, as described above, adhere to hub cells and function normally[14,152]. Remarkably, dedifferentiation of oogonia has also been shown in the Drosophila ovary[153].
CONCLUSION
This review summarizes the current knowledge of a novel mode of interaction between GSCs and their niche, the ACs, in insects. In several insect species (Oncopeltus, Lymantria and other moths) male GSCs undergo autotomy of cell projections, which are directed toward the ACs. The segregated GSC vesicles degrade at the surface of the niche cells or are phagocytized by them. This unique stem cell-niche relationship has been compared with known examples of stem cell/progenitor autotomy (i.e., erythrocyte and thrombocyte formation) and autotomy of neurons in developmental axon pruning or neurodegenerative processes. In all the cases described, apoptotic signalling is involved. Studies on injury-induced axon destruction (Wallerian degeneration) suggest that an active autodestruction program exists akin to apoptosis and that the autodestructive pathway maybe conserved between fly and human[71,72]. We propose that this pathway also exists and is active in male GSCs of Oncopeltus, Lymantria and other species. The analysis of signal exchange between autotomized GSC vesicles and niche cells is expected to reveal a new mechanism of stem cell-niche interaction.
Footnotes
P- Reviewer: Li SC, Song GB S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: David C Dorn has not received fees for serving as a speaker. David C Dorn has only received research funding from the state and his home institution. David C Dorn is solely an employee of his home institution. David C Dorn owns no stocks and/or shares. David C Dorn owns patents to: (1) Synthesis of epothilones, intermediates thereto, analogues and uses thereof, patent number: 7384964; (2) Migrastatin analogs in the treatment of cancer, patent number: 8957056; (3) Isomigrastatin analogs in the treatment of cancer, patent number: 8188141.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 20, 2014
First decision: October 14, 2014
Article in press: May 27, 2015
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park D, Sykes DB, Scadden DT. The hematopoietic stem cell niche. Front Biosci (Landmark Ed) 2012;17:30–39. doi: 10.2741/3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessis M. [Erythroblastic island, functional unity of bone marrow] Rev Hematol. 1958;13:8–11. [PubMed] [Google Scholar]
- 6.Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3:931–940. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- 7.Verson E. Zur spermatogenesis. Zool Anz. 1889;12:100–103. [Google Scholar]
- 8.Zick K. Beitrage zur kenntnis der postembryonalen entwicklungs-geschichte der genitalorgane bei lepidopteren. Zeitschrift fuer Wissenschaftliche Zoologie Leipzig. 1911;98:430–477. [Google Scholar]
- 9.Spichardt C. Beitrag zur entwicklung der männlichen genitalien und ihrer ausführungsgänge bei lepidopteren. Verh Naturhist Verein Rheinland Bonn. 1886;43:1–43. [Google Scholar]
- 10.Buder JE. Die spermatogenese von deilephila euphorbiae l. Archiv f Zellforschung. 1915;14:27–78. [Google Scholar]
- 11.Schneider K. Die entwicklung des eierstockes und eies von deilephila euphorbiae. Archiv f Zellforschung. 1915;14:79–143. [Google Scholar]
- 12.Nelsen OE. Life cycle, sex differentiation, and testis development in melanoplus differentialis (acrididae, orthoptera) Jour Morph and Physiol. 1931;51:509–511. [Google Scholar]
- 13.Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–265. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matunis EL, Stine RR, de Cuevas M. Recent advances in Drosophila male germline stem cell biology. Spermatogenesis. 2012;2:137–144. doi: 10.4161/spmg.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 16.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- 19.Amoyel M, Sanny J, Burel M, Bach EA. Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development. 2013;140:56–65. doi: 10.1242/dev.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel M, Kupinski AP, Raabe I, Bökel C. Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development. 2012;139:2663–2669. doi: 10.1242/dev.075242. [DOI] [PubMed] [Google Scholar]
- 21.Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, Banerjee U, Bach EA. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18:556–568. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba M, Yuan H, Salzmann V, Fuller MT, Yamashita YM. E-cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS One. 2010;5:e12473. doi: 10.1371/journal.pone.0012473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Singh SR, Zheng Z, Oh SW, Chen X, Edwards K, Hou SX. Rap-GEF signaling controls stem cell anchoring to their niche through regulating DE-cadherin-mediated cell adhesion in the Drosophila testis. Dev Cell. 2006;10:117–126. doi: 10.1016/j.devcel.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan S, Mahowald AP, Fuller MT. The receptor tyrosine phosphatase Lar regulates adhesion between Drosophila male germline stem cells and the niche. Development. 2012;139:1381–1390. doi: 10.1242/dev.070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel M, Raabe I, Kupinski AP, Pérez-Palencia R, Bökel C. Local BMP receptor activation at adherens junctions in the Drosophila germline stem cell niche. Nat Commun. 2011;2:415. doi: 10.1038/ncomms1426. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Q, Wang Y, Vargas E, DiNardo S. magu is required for germline stem cell self-renewal through BMP signaling in the Drosophila testis. Dev Biol. 2011;357:202–210. doi: 10.1016/j.ydbio.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi Y, Kobayashi S, Nakato H. Drosophila glypicans regulate the germline stem cell niche. J Cell Biol. 2009;187:473–480. doi: 10.1083/jcb.200904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shields AR, Spence AC, Yamashita YM, Davies EL, Fuller MT. The actin-binding protein profilin is required for germline stem cell maintenance and germ cell enclosure by somatic cyst cells. Development. 2014;141:73–82. doi: 10.1242/dev.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Voog J, D’Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–1136. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilboa L, Forbes A, Tazuke SI, Fuller MT, Lehmann R. Germ line stem cell differentiation in Drosophila requires gap junctions and proceeds via an intermediate state. Development. 2003;130:6625–6634. doi: 10.1242/dev.00853. [DOI] [PubMed] [Google Scholar]
- 36.Toledano H, D’Alterio C, Czech B, Levine E, Jones DL. The let-7-Imp axis regulates ageing of the Drosophila testis stem-cell niche. Nature. 2012;485:605–610. doi: 10.1038/nature11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies EL, Lim JG, Joo WJ, Tam CH, Fuller MT. The transcriptional regulator lola is required for stem cell maintenance and germ cell differentiation in the Drosophila testis. Dev Biol. 2013;373:310–321. doi: 10.1016/j.ydbio.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eun SH, Stoiber PM, Wright HJ, McMurdie KE, Choi CH, Gan Q, Lim C, Chen X. MicroRNAs downregulate Bag of marbles to ensure proper terminal differentiation in the Drosophila male germline. Development. 2013;140:23–30. doi: 10.1242/dev.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cherry CM, Matunis EL. Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome-remodeling factor NURF. Cell Stem Cell. 2010;6:557–567. doi: 10.1016/j.stem.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casper AL, Baxter K, Van Doren M. no child left behind encodes a novel chromatin factor required for germline stem cell maintenance in males but not females. Development. 2011;138:3357–3366. doi: 10.1242/dev.067942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang SY, Baxter EM, Van Doren M. Phf7 controls male sex determination in the Drosophila germline. Dev Cell. 2012;22:1041–1051. doi: 10.1016/j.devcel.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh SR, Zheng Z, Wang H, Oh SW, Chen X, Hou SX. Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. J Cell Physiol. 2010;223:500–510. doi: 10.1002/jcp.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Issigonis M, Matunis E. The Drosophila BCL6 homolog Ken and Barbie promotes somatic stem cell self-renewal in the testis niche. Dev Biol. 2012;368:181–192. doi: 10.1016/j.ydbio.2012.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morillo Prado JR, Chen X, Fuller MT. Polycomb group genes Psc and Su(z)2 maintain somatic stem cell identity and activity in Drosophila. PLoS One. 2012;7:e52892. doi: 10.1371/journal.pone.0052892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoller R, Schulz C. The Drosophila cyst stem cell lineage: Partners behind the scenes? Spermatogenesis. 2012;2:145–157. doi: 10.4161/spmg.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the drosophila insulin receptor and insulin-like peptides in growth control. Current biology: CB. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 47.Ueishi S, Shimizu H, Hi Y. Male germline stem cell division and spermatocyte growth require insulin signaling in drosophila. Cell structure and function. 2009;34:61–69. doi: 10.1247/csf.08042. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, McLeod CJ, Jones DL. Regulation of adult stem cell behavior by nutrient signaling. Cell Cycle. 2011;10:2628–2634. doi: 10.4161/cc.10.16.17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang SA, Wang WD, Chen CT, Tseng CY, Chen YN, Hsu HJ. FOXO/Fringe is necessary for maintenance of the germline stem cell niche in response to insulin insufficiency. Dev Biol. 2013;382:124–135. doi: 10.1016/j.ydbio.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Ochocki JD, Simon MC. Nutrient-sensing pathways and metabolic regulation in stem cells. J Cell Biol. 2013;203:23–33. doi: 10.1083/jcb.201303110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tulina NM, Chen WF, Chen JH, Sowcik M, Sehgal A. Day-night cycles and the sleep-promoting factor, Sleepless, affect stem cell activity in the Drosophila testis. Proc Natl Acad Sci USA. 2014;111:3026–3031. doi: 10.1073/pnas.1316552111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Loof A. Ecdysteroids: The overlooked sex steroids of insects? Males: The black box. Insect Science. 2006;13:325–338. [Google Scholar]
- 53.Loeb MJ, Woods CW, Brandt EP, Boakovec AB. Larval testes of the tobacco budworm: a new source of insect ecdysteroids. Science. 1982;218:896–898. doi: 10.1126/science.218.4575.896. [DOI] [PubMed] [Google Scholar]
- 54.Loeb MJ, Brandt EP, Woods CW, Bell RA. Secretion of ecdysteroid by sheaths of testes of the gypsy moth, lymantria dispar, and its regulation by testis ecdysiotropin. J Exp Zool. 1988;248:94–100. [Google Scholar]
- 55.Gelman DB, Woods CW, Loeb MJ, Borkovec AB. Ecdysteroid synthesis by testes of 5th instars and pupae of the european corn borer, ostrinia nubilalis (hubner) Invertebr Reprod Dev. 1989;15:177–184. [Google Scholar]
- 56.Jarvis TD, Earley FGP, Rees HH. Ecdysteroid biosynthesis in larval testes of spodoptera littoralis. Insect Biochem Mol Biol. 1994;24:531–537. [Google Scholar]
- 57.Gillott C, Ismail PM. In vitro synthesis of ecdysteroid by the male accessory reproductive glands, testis and abdominal integument of the adult migratory grasshopper, melanoplus sanguinipes. Invertebr Reprod Dev. 1995;27:65–71. [Google Scholar]
- 58.Ables ET, Drummond-Barbosa D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell. 2010;7:581–592. doi: 10.1016/j.stem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gancz D, Gilboa L. Hormonal control of stem cell systems. Annu Rev Cell Dev Biol. 2013;29:137–162. doi: 10.1146/annurev-cellbio-101512-122331. [DOI] [PubMed] [Google Scholar]
- 60.Dorn DC, Dorn A. Structural polarity and dynamics of male germline stem cells in an insect (milkweed bug Oncopeltus fasciatus) Methods Mol Biol. 2008;450:71–94. doi: 10.1007/978-1-60327-214-8_5. [DOI] [PubMed] [Google Scholar]
- 61.Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of drosophila melanogaster. J Ultra Res. 1979;69:180–190. doi: 10.1016/s0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- 62.Jaglarz MK, Kloc M, Jankowska W, Szymanska B, Bilinski SM. Nuage morphogenesis becomes more complex: two translocation pathways and two forms of nuage coexist in Drosophila germline syncytia. Cell Tissue Res. 2011;344:169–181. doi: 10.1007/s00441-011-1145-2. [DOI] [PubMed] [Google Scholar]
- 63.Zahn J, Doormann P, Dorn A, Dorn DC. Apoptosis of male germ-line stem cells after laser ablation of their niche. Stem Cell Res. 2007;1:75–85. doi: 10.1016/j.scr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Dorn DC, Dorn A. Structural characterization and primary in vitro cell culture of locust male germline stem cells and their niche. Stem Cell Res. 2011;6:112–128. doi: 10.1016/j.scr.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt ED, Sehn E, Dorn A. Differentiation and ultrastructure of the spermatogonial cyst cells in the milkweed bug, oncopeltus fasciatus. Invert Reprod Develop. 2002;42:163–178. [Google Scholar]
- 66.Klein C. Die postembryonale gonadenentwicklung und gametogenese bei lymantria dispar l. (lepidoptera) mit besonderer berücksichtigung des gonadensomas. Licht- und elektronenmikroskopische untersuchungen. Thesis, Universität des Saarlandes. 2000 [Google Scholar]
- 67.Balles S, Maas U, Sehn E, Dorn A. Testis differentiation in the glowworm, Lampyris noctiluca, with special reference to the apical tissue. J Morphol. 2002;251:22–37. doi: 10.1002/jmor.1072. [DOI] [PubMed] [Google Scholar]
- 68.Naisse J. [Endocrine control of sexual differentiation in the insect Lampyris noctiluca (Coleoptera Malacoderma Lampyridae). I. Androgenic role of the testes] Arch Biol (Liege) 1966;77:139–201. [PubMed] [Google Scholar]
- 69.Roosen-Runge EC. The process of spermatogenesis in animals. CUP Archive: 1977. [Google Scholar]
- 70.Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang Y, Bonini NM. Axon degeneration and regeneration: insights from Drosophila models of nerve injury. Annu Rev Cell Dev Biol. 2012;28:575–597. doi: 10.1146/annurev-cellbio-101011-155836. [DOI] [PubMed] [Google Scholar]
- 72.Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meiri H, Dormann A, Spira ME. Comparison of ultrastructural changes in proximal and distal segments of transected giant fibers of the cockroach Periplaneta americana. Brain Res. 1983;263:1–14. doi: 10.1016/0006-8993(83)91195-2. [DOI] [PubMed] [Google Scholar]
- 74.Jayaram HN, Kusumanchi P, Yalowitz JA. NMNAT expression and its relation to NAD metabolism. Curr Med Chem. 2011;18:1962–1972. doi: 10.2174/092986711795590138. [DOI] [PubMed] [Google Scholar]
- 75.Ding M, Shen K. The role of the ubiquitin proteasome system in synapse remodeling and neurodegenerative diseases. Bioessays. 2008;30:1075–1083. doi: 10.1002/bies.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wakatsuki S, Saitoh F, Araki T. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nat Cell Biol. 2011;13:1415–1423. doi: 10.1038/ncb2373. [DOI] [PubMed] [Google Scholar]
- 77.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avery MA, Rooney TM, Pandya JD, Wishart TM, Gillingwater TH, Geddes JW, Sullivan PG, Freeman MR. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr Biol. 2012;22:596–600. doi: 10.1016/j.cub.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang Y, Soares L, Teng X, Geary M, Bonini NM. A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr Biol. 2012;22:590–595. doi: 10.1016/j.cub.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–677. doi: 10.1016/j.cub.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr Biol. 2004;14:678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 83.Kuo CT, Jan LY, Jan YN. Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc Natl Acad Sci USA. 2005;102:15230–15235. doi: 10.1073/pnas.0507393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orlowski RZ. The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Differ. 1999;6:303–313. doi: 10.1038/sj.cdd.4400505. [DOI] [PubMed] [Google Scholar]
- 85.Deckwerth TL, Johnson EM. Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Dev Biol. 1994;165:63–72. doi: 10.1006/dbio.1994.1234. [DOI] [PubMed] [Google Scholar]
- 86.Coleman MP, Perry VH. Axon pathology in neurological disease: A neglected therapeutic target. Trends in neurosciences. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- 87.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 88.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pereira G, Yamashita YM. Fly meets yeast: checking the correct orientation of cell division. Trends Cell Biol. 2011;21:526–533. doi: 10.1016/j.tcb.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan H, Chiang CY, Cheng J, Salzmann V, Yamashita YM. Regulation of cyclin A localization downstream of Par-1 function is critical for the centrosome orientation checkpoint in Drosophila male germline stem cells. Dev Biol. 2012;361:57–67. doi: 10.1016/j.ydbio.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheng XR, Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138:3367–3376. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt ED, Papanikolaou A, Dorn A. The relationship between germline cells and apical complex in the testes of the milkweed bug throughout postembryonic development. Invertebr Reprod Dev. 2001;39:109–126. [Google Scholar]
- 93.Bessis M, Mize C, Prenant M. Erythropoiesis: comparison of in vivo and in vitro amplification. Blood Cells. 1978;4:155–174. [PubMed] [Google Scholar]
- 94.Manwani D, Bieker JJ. The erythroblastic island. Curr Top Dev Biol. 2008;82:23–53. doi: 10.1016/S0070-2153(07)00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allen TD, Dexter TM. Ultrastructural aspects of erythropoietic differentiation in long-term bone marrow culture. Differentiation. 1982;21:86–94. doi: 10.1111/j.1432-0436.1982.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 96.Crocker PR, Werb Z, Gordon S, Bainton DF. Ultrastructural localization of a macrophage-restricted sialic acid binding hemagglutinin, SER, in macrophage-hematopoietic cell clusters. Blood. 1990;76:1131–1138. [PubMed] [Google Scholar]
- 97.Ji P, Lodish HF. Rac GTPases play multiple roles in erythropoiesis. Haematologica. 2010;95:2–4. doi: 10.3324/haematol.2009.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keerthivasan G, Wickrema A, Crispino JD. Erythroblast enucleation. Stem Cells Int. 2011;2011:139851. doi: 10.4061/2011/139851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Porcu S, Manchinu MF, Marongiu MF, Sogos V, Poddie D, Asunis I, Porcu L, Marini MG, Moi P, Cao A, et al. Klf1 affects DNase II-alpha expression in the central macrophage of a fetal liver erythroblastic island: a non-cell-autonomous role in definitive erythropoiesis. Mol Cell Biol. 2011;31:4144–4154. doi: 10.1128/MCB.05532-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 101.Hanspal M, Hanspal JS. The association of erythroblasts with macrophages promotes erythroid proliferation and maturation: a 30-kD heparin-binding protein is involved in this contact. Blood. 1994;84:3494–3504. [PubMed] [Google Scholar]
- 102.Hanspal M, Smockova Y, Uong Q. Molecular identification and functional characterization of a novel protein that mediates the attachment of erythroblasts to macrophages. Blood. 1998;92:2940–2950. [PubMed] [Google Scholar]
- 103.Kang JA, Zhou Y, Weis TL, Liu H, Ulaszek J, Satgurunathan N, Zhou L, van Besien K, Crispino J, Verma A, et al. Osteopontin regulates actin cytoskeleton and contributes to cell proliferation in primary erythroblasts. J Biol Chem. 2008;283:6997–7006. doi: 10.1074/jbc.M706712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24:1255–1256. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- 105.Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets. 2001;12:261–273. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 106.Yu M, Cantor AB. Megakaryopoiesis and thrombopoiesis: an update on cytokines and lineage surface markers. Methods Mol Biol. 2012;788:291–303. doi: 10.1007/978-1-61779-307-3_20. [DOI] [PubMed] [Google Scholar]
- 107.Schulze H, Shivdasani RA. Mechanisms of thrombopoiesis. J Thromb Haemost. 2005;3:1717–1724. doi: 10.1111/j.1538-7836.2005.01426.x. [DOI] [PubMed] [Google Scholar]
- 108.Ellis MH, Avraham H, Groopman JE. The regulation of megakaryocytopoiesis. Blood Rev. 1995;9:1–6. doi: 10.1016/0268-960x(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 109.Matsumura I, Kanakura Y. Molecular control of megakaryopoiesis and thrombopoiesis. Int J Hematol. 2002;75:473–483. doi: 10.1007/BF02982109. [DOI] [PubMed] [Google Scholar]
- 110.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J. 2001;81:685–696. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pallotta I, Lovett M, Rice W, Kaplan DL, Balduini A. Bone marrow osteoblastic niche: a new model to study physiological regulation of megakaryopoiesis. PLoS One. 2009;4:e8359. doi: 10.1371/journal.pone.0008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bury L, Malara A, Gresele P, Balduini A. Outside-in signalling generated by a constitutively activated integrin αIIbβ3 impairs proplatelet formation in human megakaryocytes. PLoS One. 2012;7:e34449. doi: 10.1371/journal.pone.0034449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaushansky K, Drachman JG. The molecular and cellular biology of thrombopoietin: the primary regulator of platelet production. Oncogene. 2002;21:3359–3367. doi: 10.1038/sj.onc.1205323. [DOI] [PubMed] [Google Scholar]
- 114.Kosaki G, Kambayashi J. Thrombocytogenesis by megakaryocyte; Interpretation by protoplatelet hypothesis. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:254–273. doi: 10.2183/pjab.87.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patel SR, Hartwig JH, Italiano JE. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cramer EM, Norol F, Guichard J, Breton-Gorius J, Vainchenker W, Massé JM, Debili N. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood. 1997;89:2336–2346. [PubMed] [Google Scholar]
- 117.Italiano JE, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147:1299–1312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kosaki G. In vivo platelet production from mature megakaryocytes: does platelet release occur via proplatelets? Int J Hematol. 2005;81:208–219. doi: 10.1532/IJH97.04177. [DOI] [PubMed] [Google Scholar]
- 119.Kosaki G. Platelet production by megakaryocytes: protoplatelet theory justifies cytoplasmic fragmentation model. Int J Hematol. 2008;88:255–267. doi: 10.1007/s12185-008-0147-7. [DOI] [PubMed] [Google Scholar]
- 120.Falcieri E, Bassini A, Pierpaoli S, Luchetti F, Zamai L, Vitale M, Guidotti L, Zauli G. Ultrastructural characterization of maturation, platelet release, and senescence of human cultured megakaryocytes. Anat Rec. 2000;258:90–99. doi: 10.1002/(SICI)1097-0185(20000101)258:1<90::AID-AR10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 121.Kaluzhny Y, Ravid K. Role of apoptotic processes in platelet biogenesis. Acta Haematol. 2004;111:67–77. doi: 10.1159/000074487. [DOI] [PubMed] [Google Scholar]
- 122.De Botton S, Sabri S, Daugas E, Zermati Y, Guidotti JE, Hermine O, Kroemer G, Vainchenker W, Debili N. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 123.Kaluzhny Y, Yu G, Sun S, Toselli PA, Nieswandt B, Jackson CW, Ravid K. BclxL overexpression in megakaryocytes leads to impaired platelet fragmentation. Blood. 2002;100:1670–1678. doi: 10.1182/blood-2001-12-0263. [DOI] [PubMed] [Google Scholar]
- 124.Kim JA, Jung YJ, Seoh JY, Woo SY, Seo JS, Kim HL. Gene expression profile of megakaryocytes from human cord blood CD34(+) cells ex vivo expanded by thrombopoietin. Stem Cells. 2002;20:402–416. doi: 10.1634/stemcells.20-5-402. [DOI] [PubMed] [Google Scholar]
- 125.Battinelli E, Willoughby SR, Foxall T, Valeri CR, Loscalzo J. Induction of platelet formation from megakaryocytoid cells by nitric oxide. Proc Natl Acad Sci USA. 2001;98:14458–14463. doi: 10.1073/pnas.241427398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagata Y, Yoshikawa J, Hashimoto A, Yamamoto M, Payne AH, Todokoro K. Proplatelet formation of megakaryocytes is triggered by autocrine-synthesized estradiol. Genes Dev. 2003;17:2864–2869. doi: 10.1101/gad.1128003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Crispino JD. GATA1 in normal and malignant hematopoiesis. Semin Cell Dev Biol. 2005;16:137–147. doi: 10.1016/j.semcdb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 128.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 130.Yacobi-Sharon K, Namdar Y, Arama E. Alternative germ cell death pathway in Drosophila involves HtrA2/Omi, lysosomes, and a caspase-9 counterpart. Dev Cell. 2013;25:29–42. doi: 10.1016/j.devcel.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 131.Yoshida S. Stem cell niche system in mouse spermatogenesis. Male Germline Stem Cells: Developmental and Regenerative Potential Stem Cell Biology and Regenerative Medicine; 2011. [Google Scholar]
- 132.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 133.Russell LD, Ettlin RA, Hikim APS, Clegg ED. Histological and histopathological evaluation of the testis. Int J Androl. 1993;16:83–83. [Google Scholar]
- 134.Paratcha G, Ledda F. The GTPase-activating protein Rap1GAP: a new player to modulate Ret signaling. Cell Res. 2011;21:217–219. doi: 10.1038/cr.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tokuda M, Kadokawa Y, Kurahashi H, Marunouchi T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod. 2007;76:130–141. doi: 10.1095/biolreprod.106.053181. [DOI] [PubMed] [Google Scholar]
- 137.Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136:1191–1199. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 139.Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Yoshida S, Toyokuni S, Lee J, Ogura A, Shinohara T. Leukemia inhibitory factor enhances formation of germ cell colonies in neonatal mouse testis culture. Biol Reprod. 2007;76:55–62. doi: 10.1095/biolreprod.106.055863. [DOI] [PubMed] [Google Scholar]
- 140.Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fässler R, Shinohara T. Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell. 2008;3:533–542. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 143.Lassalle B, Bastos H, Louis JP, Riou L, Testart J, Dutrillaux B, Fouchet P, Allemand I. ‘Side Population’ cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development. 2004;131:479–487. doi: 10.1242/dev.00918. [DOI] [PubMed] [Google Scholar]
- 144.Szollosi A, Marcaillou C. Electron microscope study of the blood-testis barrier in an insect: Locusta migratoria. J Ultrastruct Res. 1977;59:158–172. doi: 10.1016/s0022-5320(77)80076-2. [DOI] [PubMed] [Google Scholar]
- 145.Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 147.Mills JC, Gordon JI. The intestinal stem cell niche: there grows the neighborhood. Proc Natl Acad Sci USA. 2001;98:12334–12336. doi: 10.1073/pnas.231487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061–6072. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, Allemand I, Riou L, Fouchet P. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 151.Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- 153.Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Cold Spring Harb Perspect Biol. 2011;3:a002642. doi: 10.1101/cshperspect.a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Szöllösi A, Marcaillou C. The apical cell of the locust testis: an ultrastructural study. J Ultrastruct Res. 1979;69:331–342. doi: 10.1016/s0022-5320(79)80051-9. [DOI] [PubMed] [Google Scholar]
- 155.Lingor P, Koch JC, Tönges L, Bähr M. Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res. 2012;349:289–311. doi: 10.1007/s00441-012-1362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6. doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]


