Abstract
AIM: To investigate the effectiveness of mesenchymal stem cells (MSCs) in maxillary sinus augmentation (MSA), with various scaffold materials.
METHODS: MEDLINE, EMBASE and SCOPUS were searched using keywords such as sinus graft, MSA, maxillary sinus lift, sinus floor elevation, MSC and cell-based, in different combinations. The searches included full text articles written in English, published over a 10-year period (2004-2014). Inclusion criteria were clinical/radiographic and histologic/ histomorphometric studies in humans and animals, on the use of MSCs in MSA. Meta-analysis was performed only for experimental studies (randomized controlled trials and controlled trials) involving MSA, with an outcome measurement of histologic evaluation with histomorphometric analysis reported. Mean and standard deviation values of newly formed bone from each study were used, and weighted mean values were assessed to account for the difference in the number of subjects among the different studies. To compare the results between the test and the control groups, the differences of regenerated bone in mean and 95% confidence intervals were calculated.
RESULTS: Thirty-nine studies (18 animal studies and 21 human studies) published over a 10-year period (between 2004 and 2014) were considered to be eligible for inclusion in the present literature review. These studies demonstrated considerable variation with respect to study type, study design, follow-up, and results. Meta-analysis was performed on 9 studies (7 animal studies and 2 human studies). The weighted mean difference estimate from a random-effect model was 9.5% (95%CI: 3.6%-15.4%), suggesting a positive effect of stem cells on bone regeneration. Heterogeneity was measured by the I2 index. The formal test confirmed the presence of substantial heterogeneity (I2 = 83%, P < 0.0001). In attempt to explain the substantial heterogeneity observed, we considered a meta-regression model with publication year, support type (animal vs humans) and follow-up length (8 or 12 wk) as covariates. After adding publication year, support type and follow-up length to the meta-regression model, heterogeneity was no longer significant (I2 = 33%, P = 0.25).
CONCLUSION: Several studies have demonstrated the potential for cell-based approaches in MSA; further clinical trials are needed to confirm these results.
Keywords: Mesenchymal stem cells, Maxillary sinus, Sinus floor augmentation, Scaffolds, Bone regeneration
Core tip: Cell-based approaches, utilizing adult mesenchymal stem cells, may overcome the limitations of conventional bone augmentation procedures. The present review of the current literature aims to systematically review the available evidence on the characteristics and clinical effectiveness of cell-based maxillary sinus augmentation, compared to current evidence-based methods.
INTRODUCTION
Implant dentistry is a successful treatment procedure, as demonstrated by more than 20 years of clinical evidence[1-3].
However, the edentulous posterior maxilla is often characterized by a lack of bone because of severe post-extraction alveolar crest resorption coupled with age-linked sinus pneumatization[4,5]. This anatomic limitation often dictates the need for reconstructive osseous surgery to re-establish adequate bone volume for implant positioning[4,5].
Accordingly, different augmentation approaches have been introduced to obtain more maxillary bone volume, placing various grafting materials in the maxillary sinus[6-9]. Nowadays maxillary sinus augmentation (MSA) has become a reliable, commonly used procedure to increase bone volume in the posterior maxilla[4,5,9,10].
Autogenous bone (AB) is still the best grafting material in bone reconstructive surgery, and MSA has been originally carried out with it[10,11]. In fact, AB exhibits osteogenic and osteoconductive potential, since it contains living cells and growth factors[10-12]. However, additional surgical procedures are needed to harvest bone chips from other skeletal sites (intraorally or from the iliac crest); the available AB supply is limited, and morbidity at donor site is often a problem[11,12]. In addition, AB shows resorption patterns proportional to the quantity of harvested material, and this may results in a significant loss of grafted bone in large defect fillings over time[11,12].
To overcome these limitations, several osteoconductive materials have been used in MSA, such as autogenetic bone [allografts (AL)][13,14], xenografts [bovine bone mineral (BBM)][9,15,16], synthetic bone grafts [calcium phosphate ceramics (CPC)][4,17] or composite materials[18]. However, these bone grafting substitutes don’t contain living cells, so their healing times are longer than AB[12,19].
Bone tissue engineering (BTE) procedures may help lo overcome these limits[20]. According to BTE, a bone substitute should have biological and morphological features as similar as possible to AB, and the fabrication of the ideal bone graft requires the manipulation of three essential components: osteogenic cells, growth factors and osteoconductive scaffolds[20,21]. In particular, BTE aims to achieve bone augmentation without surgical AB harvesting from other donor sites, through the use of specific scaffolds seeded with osteogenic cells[12,19,21].
The aim of the present review was to evaluate the effectiveness of cell-based approaches in MSA, associated with various scaffold materials, in animals and humans.
MATERIALS AND METHODS
Study design
The protocol of this review is in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses[22], the Cochrane Collaboration[23] and CheckReview[24] checklists. It was developed a priori, covering rationale, design of the study, focused question, inclusion/exclusion criteria, search strategy, data synthesis. The protocol was examined and refereed by researchers with experience in systematic reviews. The focused questions was: “What is the effectiveness of cell-based approaches in MSA, with different materials?”.
Inclusion and exclusion criteria
Most important study designs to address the focused question were randomized controlled trials (RCTs) and controlled trials. Although this, both experimental and observational studies (RCTs, controlled trials, case series, case reports and prospective cohort trials) were included in this review. Inclusion criteria were studies of BTE methods in MSA using different scaffolds, in animals and humans, with analysis using either radiographic or histologic/histomorphometric approaches. Exclusion criteria were studies where there was no information about the surgical team or the location (private practice/hospital/university) where MSA was performed.
Search strategy
Systematic searches were performed in MEDLlNE, EMBASE and SCOPUS databases including full text articles published in English between October 2004 and October 2014, presenting either radiographic or histologic/histomorphometric evaluations.
Keywords such as sinus graft, MSA, maxillary sinus lift, sinus floor elevation, mesenchymal stem cell (MSC) and cell-based, in different combinations, were used. Titles and abstracts were examined and then expert reviewers (Mangano FG and Colombo M) evaluated full text of publications extrapolating relevant information. Data extraction procedures affected title, authors, year of publication, type of study, cells and scaffold used, design, randomization/blinding if present, number of procedures performed, treatment phase, follow-up, radiographic and/ or histologic/histomorphometric outcomes, statistical findings, conclusions. In addition, the reference lists of included studies were hand searched.
Statistical analysis
Experimental studies (RCTs/CTs) on MSA with an outcome measurement of histologic/histomorphometric evaluation were subjected to meta-analysis. Mean and standard deviation values of new bone formation were used; to overcome the differences of sample size between studies weighted mean was calculated. Test and the control groups values were compared by calculating the differences of bone gain in mean, and 95%CIs. Software package R version 2.14 (Package Metafor; Wolfgang Viechtbauer, Maastricht, The Netherlands) was used to perform all statistical analyses.
RESULTS
Result of search and included studies
Of the 325 eligible articles initially identified, 270 were excluded following assessment of the title and/or abstract. In total, 39 studies (18 animal studies and 21 human studies) were eligible for inclusion in the present literature review (Figure 1). All these publications were issued between 2004 and 2014, and were variable with respect to study type, design, follow-up, and results.
Figure 1.
Flow chart of manuscripts screened trough the review process.
MSCs
A stem cell is an undifferentiated cell with the capability to renew itself and to get a specific cell-phenotype if exposed to proper stimuli[25-28]. The more relevant stem cells types in clinical researches are embryonic stem cells (ESCs), typical of embryonic blastocyst, and adult stem cells, also defined as pluripotent MSCs[28-31].
ESCs have unlimited proliferation potential and are able, under appropriate inductive conditions, to produce all three germ layers cellular phonotypes[28-31]. ESCs can be cultured indefinitely in vitro for more than two years, with approximately 400 doubling cycles, without the loss of differentiation potential. They can be re-implanted into a host embryo giving rise to progenies that differentiate into all kind of tissues[29-31]. Although their clinical potentials several issues remain to be addressed with ESCs[30,31]. The use of these cells, in fact, presents the potential risks of immunorejection or teratomagenesis[30]. Moreover, despite the pluripotency of ESCs, moral and legal controversies concerning their use for therapeutic and clinical application have encouraged to find the reservoirs of progenitor cells in adult tissues[28,32,33].
Adult stem cells or pluripotent MSCs, derived from different adult tissues, have a wide self-renewal and proliferation capability, whereas if correctly stimulated have the ability to differentiate into specific cell-lines[25-31]. Although MSCs display a finite life span and get into senescence faster than ESCs, current in vitro techniques allow to expand them in sufficient number for clinical uses maintaining the undifferentiated phenotype[25-31]. MSCs lack immunogenic or tumorigenic features[26-28]; moreover, there is no ethical or legal concern for the clinical use of MSCs[32]. For all these reasons, these cells can be used in cell-based approaches in bone regeneration[25-31,33].
MSCs can be extracted from different tissues such as bone marrow [bone marrow stem cells (BMSCs)][25-31,34], periosteum (periosteal derived stem cells)[35], trabecular bone[36], adipose tissue [adipose stem cells (ADSCs)][37] or skeletal muscle[38], umbilical chord[39], amniotic fluid [amniotic fluid stem cells (AFSCs), and amniotic epithelial stem cells (AESCs)][40,41], skin [skin-derived stem cells (SDSCs)][42], dental pulp (dental pulp stem cells)[43,44], deciduous teeth [deciduous tooth stem cells (DTSCs)][45] and periodontal ligament [periodontal ligament stem cells (PDLSCs)][46].
The bone marrow aspirates (BMA), from the iliac crest of the pelvis, has always been considered the first source for MSCs[25-34]. The tibial and femoral marrow compartments are also available as alternative sources. Due to the morbidity and the operative difficulties of this procedure the possibility to harvest MSCs from other tissues, such as periosteum or maxillary tuberosity has become of interest[35,36]. At present, MSCs can be also achieved from adipose tissue by liposuction[37] or from the dental pulp[43,44]. The latter represents a very interesting option in the field of oral surgery[43,44].
As the number and the concentration of transplanted MSCs are critical to induce a significant clinical outcome, an adequate number of cells for cell culture/replication is needed. BMA represent a heterogeneous cell population; the amount of MSC is very small compared to that of hematopoietic cells and averaging 0.001%-0.01% of the total nucleated cells[47], thus requiring extensive in vitro separation steps and expansion. Moreover, the number of MSCs that can be collected is inversely correlated to patient age and to his/her systemic health state. Younger donors tend to provide higher yield of stem cells in the aspirate, and the age of the donor seems to be directly associated with detrimental effects in term of proliferation and differentiation, such as senescence[47]. Cell density varies with different skeletal sites of the donor: on average, human BMA yield 400 to 500 cells/mL with an estimated total volume of 600 cc in the iliac crest[48].
At present, using standard cell culture techniques, MSCs can be isolated and expanded with great efficiency, inducing to grow into multiple lineages if exposed to the appropriate culture conditions[25]. For in vitro separation and expansion, cultured MSCs undergo successive passages. Cells are cultured in a medium supplemented with autogenous serum or fetal bovine serum and growth factors[49]. MSCs have fibroblastic morphological features in a monolayer culture and tend to adhere to the tissue culture substrate[49]; changing the medium, non-adherent hematopoietic cells are discharged[28,50]. MSCs are identified by their adhesion to plastic and their expression of peculiar membrane epitopes (CD73, CD90, CD105), with a lack of expression of human leucocyte antigen-DR and the hematopoietic markers (CD14, CD34, CD38, CD45)[49,50]. Unfortunately, MSCs display finite life spans. Recent studies have shown that extensive in vitro proliferation can affect MSCs replicative potential and differentiation capability: long-term culture and sequential passages affect the quality of MSCs in that their proliferation rate decreases and gradually lose their progenitor properties due to senescence and telomere shortening[51]. Since MSCs undergo limited mitotic divisions in vitro, the number of passages in in vitro expansion should not exceed five[51]. The addition of fibroblast growth factor-2 to the culture medium can stimulate the proliferation of MSCs maintaining their osteogenic potential, keeping the cells in a more immature state[52].
Usually, the osteogenic differentiation is initiated by supplementing the medium with dexamethasone, ascorbic acid and beta-glycerolphosphate[53]. In addition, soluble signals like bone morphogenetic proteins (BMPs) such as BMP-2, BMP-6 and BMP-9 can be used[53,54].
Scaffold
Cells are usually seeded onto a three dimensional (3D) scaffold that guides their growth and proliferation[55]. At present, there are at least 4 different strategies for the delivery of MSCs into the recipient site: MSCs can be replicated and differentiated in culture, then seeded on a 3D solid scaffold that can be implanted, after a stabilization time to obtain cell adhesion; MSCs can be replicated in culture and seeded into the 3D solid scaffold, then the cell-scaffold composite is placed in differentiation medium to stimulate the shift into the osteoblastic phenotype, ready to be implanted into the site; MSCs can be replicated and differentiated directly in the 3D scaffold in vitro, and the cell-scaffold composite can be implanted after a maturation time; finally MSCs can be replicated and differentiated in vitro, and the cell-scaffold composite can be incubated for a short time to prepare an injectable bone preparation.
In all these strategies, the scaffold has a key role providing a substrate into which bone cells migrate, proliferate, differentiate and make new bone[55,56]. The scaffold should be characterized by specific physical structure and chemical composition in order to mimic the hierarchical architecture and biological functions of native extracellular bone matrix[55,56]. Native extracellular matrix offers a physical substrate for cells, but also a biological environment for cell adhesion and chemotaxis through specific ligands. Providing specific growth factor, bone matrix regulates cellular proliferation and function[55,56]. First generation biomaterials were designed promoting mechanical resistance and stability over time, bioinertness or biocompatibility; nowadays new generation scaffold materials are developed with biologically-inspired approach; these new materials should incorporate signals into the scaffold, to modulate proliferation and differentiation[55-57]. Architectural characteristics define the ultimate shape of the new tissue. Highly porous structures with many interconnection and with large surface areas related to volumes, can aid cell ingrowth and their diffusion throughout the scaffold as well as the passage of nutrients and waste products[55,57].
Angiogenesis is a pre-requisite for osteogenesis[57]. Accordingly, pore sizes in the range of 200-800 μm have good results in these situations: in fact, they stimulate osteoprogenitor and endothelial cells to migrate into the matrix. Endothelial cells produce the vascular vessels for new bone nourishment[55-58]. Surface roughness, surface energy and the presence of cell attachment sites all influence specific proteins expression, quantity and structural conformation which adsorb onto materials surfaces, modulating cells behaviours[55,57]. Finally, substrate degradation by matrix enzymes[57] is critical. The ideal material should incorporate controlled resorption, and the regenerated tissue should assume function while the scaffold is slowly degraded[55,57].
Scaffolds and stem cells: Animal studies
In total, 18 animal studies evaluating the histologic/histomorphometric results obtained with MSCs combined with different scaffold materials were found in the literature[40-42,59-73]. Among these, one single study was on MSCs with allogeneic bone matrix (AL)[42], 6 studies were on MSCs with xenografts (BBM)[59-64], 10 studies were on MSCs with synthetic bone grafts (CPC)[40,41,65-71], and 2 studies were on MSCs with platelet-rich-plasma (PRP)[72,73].
AL (bone tissue from human cadavers), provides an osteoconductive scaffold which offers structural support for vascular and perivascular tissues growth, and for osteoprogenitor cells migration from the adjacent environment[13,14,42]. In a study on minipigs, Kang et al[42] evaluated in vivo osteogenesis of SDSCs with scaffolds composed by allogeneic demineralized bone (AL) and fibrin glue. The animals were allocated in two groups: in one group, MSA was performed with SDSCs + AL/fibrin glue (test), while in the other group the scaffold without cells was used[42]. They observed better trabecular bone formation and osteocalcin expression with scaffolds seeded with SDSCs compared with controls[42]. The authors found that autogenous SDSC grafting with a AL and fibrin glue scaffold can provide an adequate alternative to bone grafting in MSA procedures[42].
BBM associated with bone marrow aspirate concentrate (BMAC) could provide a substitute for AB to stimulate new bone formation[9,15,16,59-64]. Sununliganon et al[59] investigated the bone regeneration capacity of autologous BMAC mixed with BBM in MSA. Twenty-four white New Zealand rabbits were randomly subdivided into groups and when subjected to maxillary sinus floor elevation and augmentation with four different materials: saline solution, AB, BBM and BMAC + BBM[59]. Four MSA procedures were performed per each material. The animals were sacrificed at 2, 4 and 8 wk, and rates of new bone apposition in augmented surgical sites were evaluated; bone histomorphometry was also examined. Significant increase in the quantity of nucleated cells and colony forming unit-fibroblasts were confirmed in BMAC. MSCs in BMAC retained their in vitro multi-differentiation capability. BMAC + BBM showed a similar benefit to AB in term of acceleration, since higher (though not significantly different) rates of mineral appositions in the early period were detected in BMAC + BBM and AB than BBM alone[59]. Furthermore, graft volume/tissue volumes in BBM and BMAC + BBM resulted to be higher than in AB and saline solution. The results of this study suggest benefit in early bone formation in vivo using immediate autologous BMAC transplantation[59]. In a similar study, Yu et al[60] compared the potential of tissue-engineered bone derived from different stem cell sources for canine MSA. Bilateral MSA were performed in six beagle dogs and were randomly repaired with three graft types: BBM granules alone (n = 4), a complex of osteoblasts derived from BMSCs + BBM (n = 4), and a complex of osteoblasts derived from PDLSCs and BBM (n = 4). After 12 wk, the animals were sacrificed and fluorescent labeling, maxillofacial computed tomography (CT), scanning electron microscopy, and histologic/histomorphometric analyses were used to evaluate new bone deposition, mineralization, and healing processes in the augmented area[60]. At the end of the study, the osteogenic capacity was greater with BMSCs + BBM and PDLSCs + BBM than with BBM alone[60]. The level tended to be higher with PDLSCs than with BMSCs; however, the difference was not statistically significant[60]. Oshima et al[61] investigated the in vivo osteogenic potential of a novel gabapentin-lactam (GBP-L) in MSA. Bilateral MSA in 10 adult sheep were conducted. BBM and MSCs combined with novel GBP-L were placed into the test sinus of each sheep; MSCs + BBM alone served as the control on the contralateral side[61]. The animals were sacrificed after 8 and 16 wk, and the amount of newly formed bone was analysed using histology/histomorphometry. The histologic evaluation showed newly formed bone connected with the original bone in the control and test groups; however, the amount of newly formed bone was not significantly different between the test and control sites[61]. The authors concluded that the application of GBP-L did not induce faster new bone formation. However, GBP-L did not alter the multipotency of the MSCs or impair bone formation[61]. Jhin et al[62] evaluated the potential of BMP-2 gene-transduced BMSCs to facilitate osseous healing after MSA in rabbit. BMSCs derived from New Zealand white rabbits were cultured and some of these cells were transduced with BMP-2 (BMP-2/BMSCs) using an adenovirus vector. Then, BMSCs and BMP-2/BMSCs were seeded on a BBM scaffold. Twenty-seven animals were randomly allocated into three groups: MSA with BMSCs + BBM, MSA with BMP-2/BMSCs + BBM and MSA with BBM alone[62]. During all these procedures, a mini-implant was placed in the floor of each sinus[62]. Animals were sacrificed at 2, 4, and 8 wk after surgery, and new bone area and bone-to-implant contact (BIC) were evaluated histomorphometrically. The histomorphometric evaluation revealed that at 2 and 4 wk, the BMP-2/BMSC group showed more new bone and higher BIC than the other two groups; however, at 8 wk, there was no difference in new bone area or BIC among the three groups[62]. The authors concluded that BMP-2 delivery using BMSCs may result in earlier and increased bone formation in MSA; nevertheless, limitations in the stimulatory effect of BMP-2/BMSCs were evidenced in later healing stages[62]. Gutwald et al[63] compared the efficacy of mononuclear cells (MNCs, including MSCs) plus BBM with AB in MSA in sheep. Bilateral MSA were performed in 6 adult sheep. MNCs + BBM were mixed together and used into one sinus, AB in the other sinus[63]. After 8 and 16 wk, animals were sacrificed. Sites of augmentation were evaluated through radiographic and histological methods. After 8 wk, no difference in new bone formation was noticed between the two groups, but after 16 wk, sites grafted with MNCs + BBM showed 29% of newly formed bone vs 16% in sites grafted with AB[63]. The authors concluded that MNCs combined with BBM have the potential to stimulate new bone formation in MSA[63]. Finally, in a similar study, bilateral MSA were performed in 6 adult sheep by the same group of authors[64]. BBM and MSCs were used in test side and only BBM in the contra-lateral control side of each animal[64]. Animals were sacrificed after 8 and 16 wk. The regenerated areas were evaluated by CT, histology and histomorphometry. They observed that the newly formed bone was closely connected to BBM particles; furthermore its apposition was significantly faster in the test sides[64]. The authors concluded that mixture of BBM and MSCs could stimulate new bone deposition in MSA[64].
Synthetic CPC such as porous hydroxyapatite (HA), beta-tricalcium phosphate (beta-TCP) and biphasic combinations of these two are excellent bone alternatives[40,41,65-71]. CPC are considered biocompatible, non-immunological, osteoconductive (they act as scaffolds witch allow internal growth of vessels from neighbouring bone tissue)[40,41,65-71]. Zhao et al[65] investigated the effect of MSA with engineered bone constructs from DTSCs and CPC in goats. Eighteen bilateral maxillary sinuses of nine goats were randomly assigned into three groups (6 sinuses per group). In the first group, MSA was performed with DTSCs + CPC, while in the second and in the third group MSA was performed with CPC alone and AB, respectively[65]. All augmentation sites were analysed using CT, histology and histomorphometry. After 12 wk of healing, CT analysis evidenced that the volume of new bone with DTSCs + CPC was greater than that in the other two groups[65]. In addition, the histological/histomorphometrical evaluation indicated that the DTSCs + CPC compound significantly stimulated new bone formation and mineralization, when compared with CPC and AB alone[65]. The authors concluded that DTSCs can stimulate new bone formation and maturation in the maxillary sinus of goats, and that the engineered mixture of DTSCs and CPC should represent a possible substitute for MSA procedures[65]. Zhang et al[66] compared the bone formation capacity of ADSCs and BMSCs in a canine MSA model. Bilateral MSA were performed in nine beagle dogs using randomly three graft material combinations: BMSCs + CPC (n = 9), ADSCs + CPC (n = 9) and CPC alone (n = 9)[66]. After 6 wk, the animals were sacrificed and the histological/histomorphometric evaluation suggested that BMSCs might be more advantageous than ADSCs for fast bone regeneration in MSA[66]. Barboni et al[41] evaluated the bone regenerative property of AESCs seeded on a CPC synthetic bone substitute (fabricated using rapid prototyping techniques) in MSA in sheep. Two blocks of CPC, engineered with ovine AESCs or alone, were grafted bilaterally into maxillary sinuses of six adult sheep[41]. The sheep were randomly divided into two groups and sacrificed at 45 and 90 d after surgery. Micro-CT, morphological, biochemical and morphometric analyses were performed to evaluate tissue regeneration in the sinus explants. AESCs seem to influence positively scaffold integration and new bone deposition[41]. Engineered scaffolds, derived from explanted sinuses grafted with AESCs, displayed an accelerated process of angiogenesis; moreover, AESCs significantly promoted osteogenesis[41]. These results confirmed those of a previous study by the same group of authors[40] in which bilateral MSA was performed on eight adult sheep, in order to evaluate the bone regeneration process at 6 and 12 wk after implantation of AFSCs combined with a magnesium-enriched HA/collagen-based scaffold (test) with the scaffold alone (control). In fact, the use of AFSCs increased bone apposition and promoted a faster angiogenesis[40]. The authors concluded that AFSCs may be a new, easily accessible source of MSCs to develop cell-based therapy for oral augmentation procedures: in fact, the osteoinduction of a biomimetic commercial scaffold may be significantly enhanced by these cells[40].
Zeng et al[67] evaluated the efficacy of BMSCs seeded on CPC, magnesium phosphate cement (MPC), and a calcium-MPC (CMPC), in MSA in rabbit. In test groups augmentation procedures were performed using BMSCs in addition to CPC, MPC, and CMPC; the same materials (CPC, MPC and CMPC), without cells, were used for surgical procedures as control[67]. In each group new bone formation was investigated histologically and by fluorochrome labeling at weeks 2 and 8 after MSA[67]. The authors found that CMPC cement could better help new bone formation and mineralization than CPC or MPC cements, and that the addition of BMSCs could further promote its osteogenic capacity significantly[67].
Zou et al[68] assessed the potential of BMSCs when combined with CPC in MSA in goats. They randomly allocated nine goats in three different groups: the first group received BMSCs + CPC, the second CPC alone and the third AB. Each animal underwent a bilateral MSA procedure. Implants were also placed in order to evaluate BIC[68]. After 12 wk, the histological/histomorphometric evaluations showed that the BMSCs + CPC composite could foster earlier bone formation and mineralization, and could preserve more volume and height after MSA[68]. In addition, BIC was significantly higher in the BMSCs + CPC group than in the other two groups. The authors concluded that BMSCs + CPC seems to be a good graft material for MSA, allowing faster healing in augmented sites and a better stability of implants[68]. In a study by Xia et al[69] 36 rabbits were randomly allocated in 4 groups, to test CPC scaffolds associated with recombinant BMP-2 and BMSCs, in different combinations. Although the authors found no significant difference among groups for augmented height, histomorphometric analysis showed significantly less residual graft material and more new bone formation and mineralization in BMP-2/BMSCs + CPC than in other groups[69]. Based on these outcomes, they suggested that combining BMP-2/BMSCs with CPC could enhance new bone formation and maturation as compared with BMP-2 + CPC or BMSCs + CPC[69]. In two different studies, Sun et al[70,71] evaluated the outcome of MSA with CPC and BMSCs in rabbits. In the first study[70] 16 MSA were performed bilaterally in 8 animals and randomly grafted by BMSCs + CPC, CPC alone, AB and blood clot (4 sites per group). The animals were sacrificed 2, 4 and 8 wk after the surgery and studied histologically and histomorphometrically[70]. After 8 wk, the authors observed a significantly higher amount of new bone in the test groups (BMSCs + scaffold and scaffold alone) than in control groups (AB and blood clot). An increase in bone height along time was found for the test groups, while control groups showed a constant decrease of augmented height[70]. Surgical sites augmented with BMSCs + CPC showed more bone than areas grafted with CPC alone, but this difference was not statistically significant. These results suggested that CPC could be used as a bone graft substitute in bone augmentation procedures and that adding BMSCs to this material could successfully promote new bone formation in maxillary sinus elevation[70]. In a second, similar study[71] the same authors evaluated the effects of MSA by a tissue engineered bone composite with BMP-2 and EGFP gene modified BMSCs and CPC. In this study, eight rabbits were allocated in two groups (four rabbits per group), and subjected to bilateral MSA with two different materials: BMP-2/BMSCs + CPC (test) and EGFP/BMSCs + CPC (control)[71]. Histological/histomorphometric evaluation was performed 2 and 4 wk after surgery. The vertical bone gain was maintained, over all the experimental period, for both groups, while new bone volume increased over time for test group[71]. Four weeks after surgery, bone area in test group was significantly more than that in control group[71]. The authors concluded that BMSCs modified with BMP-2 gene can stimulate new bone formation in MSA in rabbit animal model, and that CPC scaffold can be a valid vector for gene improved BTE[71].
Finally, in a split-mouth controlled study on eight minipigs, Pieri et al[72] investigated whether BMSCs and PRP loaded on a HA substrate can influence bone formation and BIC in MSA, when compared to HA scaffold alone. Bilateral MSA procedures were performed in eight minipigs: each animal received BMSCs + PRP + HA in one sinus (test) and HA alone in the other (control)[72]. In addition, distal to the augmented site, one endosseous implant was placed per sinus, to evaluate BIC. The animals were sacrificed 12 wk after surgical procedures. Block sections of the implant sites were extracted and prepared for histologic/histomorphometric analysis. The histomorphometric observations revealed statistically significant increase both in bone quantity and in the BIC for the test sites[72]. This study showed that the use of BMSCs and PRP with a HA scaffold can significantly promote bone growth in MSA techniques, and can enhance the osseointegration of endosseous dental implants positioned in the augmented sites, in comparison with HA alone[72]. In another, similar study, Ohya et al[73] evaluated the outcomes of BMSCs associated with PRP vs particulate cancellous bone and marrow (PCBM) with PRP. Bilateral MSA procedures were performed in 18 adult Japanese white rabbits. Each rabbit received BMSCs + PRP in one sinus (test) and PCBM + PRP in the other (control)[73]. The animals were sacrificed at 2, 4, and 8 wk after surgery, and histological/histomorphometric evaluation was executed. Both test and control sites displayed newly formed bone and neovascularization at 2 and 4 wk in histological preparations; at 8 wk, authors observed large areas of fatty marrow inside lamellar bone in both sites. The analysis of bone volume and augmented height did not highlight any significant differences between BMSCs + PRP and PCBM + PRP groups. On the other hand, significant differences in bone volume and augmented height between 2 and 8 wk in PCBM + PRP or BMSCs + PRP groups were found, as well as in bone volume between 4 and 8 wk in the PCBM + PRP group[73]. The authors concluded that the use of a BMSCs + PRP compose could give good outcomes in osteogenesis and bone volume gain comparable to that achieved by particulate cancellous bone in MSA[73].
The histologic/histomorphometric results of all these animal studies are reported in Table 1.
Table 1.
Histomorphometric results of the animal studies included in the review
| Ref. | Animal model | Study design | Histomorphometry (% of newly formed bone) |
| Kang et al[42] | Minipig | SDSCs + AL | NR |
| AL alone | NR | ||
| Sununliganon et al[59] | Rabbit | BMSCs + BBM | 59.2 ± 2.1 (4 wk) |
| 55.9 ± 3.6 (8 wk) | |||
| BBM alone | 54.3 ± 2.8 (4 wk) | ||
| 51.5 ± 2.6 (8 wk) | |||
| AB | NR | ||
| Saline solution | NR | ||
| Yu et al[60] | Dog | BMSCs + BBM | NR |
| PDLSCs + BBM | NR | ||
| BBM alone | NR | ||
| Oshima et al[61] | Sheep | BMSCs + GBP-L + BBM | 17.0 ± NR (8 wk) |
| 23.0 ± NR (16 wk) | |||
| BMSCs + BBM | 18.0 ± NR (8 wk) | ||
| 23.0 ± NR (16 wk) | |||
| Jhin et al[62] | Rabbit | BMP-2/BMSCs + BBM | 12.6 ± 2.8 (2 wk) |
| 29.3 ± 4.6 (4 wk) | |||
| 25.7 ± 3.8 (8 wk) | |||
| BMSCs + BBM | 6.2 ± 2.6 (2 wk) | ||
| 25.7 ± 2.8 (4 wk) | |||
| 27.7 ± 8.2 (8 wk) | |||
| BBM alone | 4.2 ± 2.0 (2 wk) | ||
| 15.1 ± 2.9 (4 wk) | |||
| 22.7 ± 3.3 (8 wk) | |||
| Gutwald et al[63] | Sheep | MNCs + BBM | 19.0 ± 11 (8 wk) |
| 29.0 ± 12 (16 wk) | |||
| AB | 20.0 ± 13 (8 wk) | ||
| 16.0 ± 6 (16 wk) | |||
| Sauerbier et al[64] | Sheep | MSCs + BBM | NR |
| BBM alone | NR | ||
| Zhao et al[65] | Goat | DTSCs + CPC | 41.8 ± 6.2 (12 wk) |
| CPC alone | 30.1 ± 8.0 (12 wk) | ||
| AB | 23.0 ± 10.2 (12 wk) | ||
| Zhang et al[66] | Dog | BMSCs + CPC | NR |
| ADSCs + CPC | NR | ||
| CPC alone | NR | ||
| Barboni et al[41] | Sheep | AESCs + CPC | NR |
| CPC alone | NR | ||
| Berardinelli et al[40] | Sheep | AFSCs + MgHA/collagen | NR |
| MgHA/collagen alone | NR | ||
| Zeng et al[67] | Rabbit | BMSCs + CPC | 11.7 ± 1.8 (2 wk) |
| 25.4 ± 3.4 (8 wk) | |||
| CPC alone | 5.9 ± 1.4 (2 wk) | ||
| 20.5 ± 3.6 (8 wk) | |||
| BMSCs + MPC | 5.1 ± 1.7 (2 wk) | ||
| 13.5 ± 3.5 (8 wk) | |||
| MPC alone | 4.0 ± 1.2 (2 wk) | ||
| 6.5 ± 2.0 (8 wk) | |||
| BMSCs + CMPC | 12.7 ± 1.9 (2 wk) | ||
| 30.9 ± 3.1 (8 wk) | |||
| CMPC alone | 6.8 ± 1.3 (2 wk) | ||
| 25.5 ± 4.1 (8 wk) | |||
| Zou et al[68] | Goat | BMSCs + CPC | 35.6 ± 9.4 (12 wk) |
| CPC alone | 22.4 ± 4.2 (12 wk) | ||
| AB | 28.2 ± 8.0 (12 wk) | ||
| Xia et al[69] | Rabbit | BMSCs + rhBMP-2/CPC | 17.9 ± 4.3 (2 wk) |
| 30.5 ± 5.7 (4 wk) | |||
| 42.2 ± 4.0 (8 wk) | |||
| BMSCs + CPC | 13.9 ± 2.5 (2 wk) | ||
| 21.8 ± 4.4 (4 wk) | |||
| 28.7 ± 3.7 (8 wk) | |||
| rhBMP-2/CPC | 12.8 ± 3.0 (2 wk) | ||
| 18.9 ± 2.6 (4 wk) | |||
| 31.1 ± 4.5 (8 wk) | |||
| CPC alone | 8.0 ± 2.0 (2 wk) | ||
| 12.2 ± 3.1 (4 wk) | |||
| 22.7 ± 5.7 (8 wk) | |||
| Sun et al[70] | Rabbit | BMSCs + CPC | 21.0 ± 2.6 (2 wk) |
| 23.4 ± 3.0 (4 wk) | |||
| 35.3 ± 10.5 (8 wk) | |||
| CPC alone | 19.2 ± 2.2 (2 wk) | ||
| 22.9 ± 2.1 (4 wk) | |||
| 19.5 ± 2.4 (8 wk) | |||
| AB | 34.7 ± 7.1 (2 wk) | ||
| 28.7 ± 5.8 (4 wk) | |||
| NR (8 wk) | |||
| Blood clot | NR (2 wk) | ||
| 17.5 ± 3.3 (4 wk) | |||
| 13.8 ± 4.0 (8 wk) | |||
| Sun et al[71] | Rabbit | BMP-2/BMSCs + CPC | 21.2 ± 2.1 (2 wk) |
| 31.9 ± 2.2 (4 wk) | |||
| EGFP/BMSCs + CPC | 18.9 ± 1.9 (2 wk) | ||
| 23.19 ± 1.9 (4 wk) | |||
| Pieri et al[72] | Minipig | BMSCs + PRP + HA | 42.5 ± 7.0 (12 wk) |
| HA alone | 18.9 ± 0.9 (12 wk) | ||
| Ohya et al[73] | Rabbit | BMSCs + PRP | 29.1 ± 4.4 (2 wk) |
| 24.1 ± 3.6 (4 wk) | |||
| 20.9 ± 4.1 (8 wk) | |||
| PCBM + PRP | 35.0 ± 5.2 (2 wk) | ||
| 28.6 ± 3.4 (4 wk) | |||
| 20.6 ± 4.0 (8 wk) |
SDSCs: Skin-derived stem cells; AL: Allograft; NR: Not reported; BMSCs: Bone marrow stem cells; BBM: Bovine bone mineral; AB: Autogenous bone; PDLSCs: Periodontal ligament stem cells; GBP-L: Gabapentin-lactam; MNCs: Mononuclear cells; DTSCs: Deciduos tooth stem cells; CPC: Calcium phosphate ceramics; AESCs: Amniotic epithelial stem cells; AFSCs: Amniotic fluid stem cells; Mg/HA: Magnesium/hydroxyapatite; MPC: Magnesium phosphate cement; CMPC: Calcium-magnesium phosphate cement; Rh-BMP-2: Recombinant human bone morphogenetic protein 2; PRP: Platelet rich in plasma; HA: Hydroxyapatite; PCBM: Particulate cancellous bone and marrow; ADSC: Adipose stem cell.
Scaffolds and stem cells: Human studies
In total, 21 human studies evaluating the histologic/histomorphometric results obtained in MSA with MSCs combined with different scaffold materials were found in the literature[74-95]. Among these, 2 studies were on MSCs with allogeneic bone matrix (AL)[74,75], 9 studies were on MSCs with xenografts (BBM)[77-85], 2 were on MSCs with synthetic grafts (CPC)[86,87], 6 were on MSCs with synthetic polymers [polylactid-co-glycolic acid, (PLGA)][88-93], and 2 were on MSCs with PRP[94,95].
A commercially available AL containing native MSCs has been clinically used for MSA in 2 different studies[74,75]. McAllister et al[74] evaluated the bone formation following MSA using an AL containing native stem cells. After a healing period of 4 mo, biopsy and histologic evaluation were performed. The histologic and histomorphometric evaluation for the five cases reported revealed a high percentage of vital bone content, after a relatively short healing period[74]. A recent clinical study by Gonshor et al[75] evaluated bone formation in MSA sites using either an AL cellular bone matrix containing MSCs or a conventional AL. Statistically significant difference were found between the two groups, in terms of vital and residual bone content, using histomorphometric comparison[75]. In AL, cryopreservation is used to maintain cell viability and multipotential characteristics[75]. This might help the healing process stimulating bone formation directly from within the graft material, allowing earlier and larger quantity of available vital bone[74,75]. However, there is a high level of uncertainty around the possibility of iatrogenic transmission of prion or viral infections with AL, due to a lack of evidence-based research on this problem[76].
BBM has excellent osteoconductive properties[9,15,16] and the encouraging results emerged from pre-clinical studies[59-64] have led to the clinical use of this material in MSA, with the cell-based approach[77-85]. Unfortunately, however, human studies[77-85] on MSCs + BBM have not confirmed the excellent outcomes originally emerged from animal studies. In a recent clinical study on implant survival after MSA, Duttenhoefer et al[77] concentrated MSCs with either Ficoll (control group, n = 6 sinus) or BMAC (test group, n = 12 sinus) and transplanted in combination with BBM. 50 dental endosseous implants were positioned with other surgical procedures (17 Ficoll/33 BMAC) and loaded after 4 mo. At the end of the study, implant survival of the Ficoll group was 100% compared with 93.4% survival of the BMAC group; however, both cell isolation methods were found to be efficient because the difference between the groups was not statistically significant[77]. In a randomized, controlled, split-mouth design study by Rickert et al[78], a bilateral MSA procedure was executed in 12 edentulous patients. At random, one side was treated with BMSCs + BBM (test side) and the other with BBM mixed with AB (control side)[78]. Three to four months after MSA, 66 implants were placed. Implant survival, plaque, gingival, and bleeding indices, probing depth, and peri-implant radiographic bone levels were assessed at baseline and 1 year after functional loading. During osseointegration, 3 implants failed on the test side and no implants failed on the control side, resulting in 3-mo survival rates of 91% and 100%, respectively. No other implants were lost after 1 year of functional loading[78]. Even if the two reconstructive techniques were reliable in providing new bone for implant placement in the posterior maxilla, a higher implant failure rate was reported in MSA procedures with BMSCs + BBM[78]. In a radiographic study, Kühl et al[79] investigated BMA and BMAC influence on graft materials stability when added to BBM within the first 6 mo after MSA. Using a 3D reconstruction software, CT data of 13 patients undergoing bilateral MSA in a split-mouth study, were processed to evaluate graft volumes 2 wk after the sinus lift procedure and 6 mo later[79]. The comparison between volumes at 2 wk and 6 mo showed a statistically significant decrease in all single groups between 15% and 21%. However, changes in volumes between the different groups were not statistically significant[79]. Since an evident decrease in graft volume over the first 6 mo of healing has to be expected, over-augmentation of the sinus is recommended with this cell-based approach[79]. In an interesting split-mouth design study, Wildburger et al[80] evaluated early bone formation in BBM sinus grafts using also BMSCs in test group, after 3 and 6 mo. Seven patients, with a posterior maxilla characterized by atrophic bone, were included in this study[80]. In test side, augmentation procedures were performed with BMSCs mixed to BBM; control sides were grafted using pure BBM. At 3 and 6 mo, biopsies of augmented sites were taken[80]. The histologic/histomorphometric evaluation found no significant difference in new bone formation between the test and control group[80]. These results confirmed those of a previous multicentric, controlled study by Sauerbier et al[81]; they found that BMAC + BBM or a mixture of AB + BBM, used in sinus augmentation, give similar new bone formation values, after 3-4 mo of healing. However, these outcomes are partially in contrast with those of a previous histologic/histomorphometric study[82], where adding MSCs to BBM leaded to more new bone formation compared with BBM combined with AB. In this study, Rickert et al[82], in fact, described how BMSCs seeded on BBM particles could bring sufficient volume of new bone to allow clinicians to place endosseous implants with a comparable timing regarding to the use of AB or a mixture of AB + BBM. The slow resorption rate of BBM permits an adequate bone integration before scaffold resorption[81-83]; however, the rate of non-mineralized material is generally high, even 6 mo after augmentation procedure[81-83]. Finally, Fuerst et al[84] examined the 12-mo histologic/histomorphometric and radiologic outcomes after MSA with autogenous culture-expanded bone cells and BBM. In total, 22 sinuses of 12 patients were grafted with AB cells seeded on BBM. Six months after MSA, during endosseous implants (n = 82) placement procedures, a biopsy was taken from each sinus[84]. The percent newly formed bone was determined on undecalcified histologic preparations. Graft stability was estimated using dental CT scans after MSA (CT 1), after implant insertion (CT 2) and after implant uncover (CT 3)[84]. Despite a considerable reduction of the graft volume along time, AB cells and BBM provided an adequate bone volume, which permitted implant placement and tolerated functional loading[84].
Synthetic porous CPC ceramics can support new bone apposition by MSCs in vivo[86,87]. Osteoconductive scaffolds such as HA and beta-TCP have the ability to attract fibronectin and vitronectin, which are ligands for the integrin family of cell adhesion receptors; these proteins mediates adhesion of MSCs and osteoblast precursors[20,21,86,87]. In addition, HA/beta-TCP degradation products may favour an alkaline microenvironment and provide calcium and phosphate ions requested for the mineralization of extracellular matrix phases during ossification process[20,21,86,87]. Finally, microporosity of synthetic CPC (given by pores with a controlled size, communicating through interconnections) supports angiogenesis[20,21,86,87]. Blood vessels carry cells and soluble signals that promote new bone apposition[86,87]. In a recent study by Shayesteh et al[86], CPC has been used in combination with BMSCs in MSA. Six patients underwent MSA with BMSCs + HA/beta-TCP. Three and twelve months after MSA, a radiographic evaluation was performed[86]. In total, 30 fixtures were inserted and a biopsy was taken from each implant site. Prosthetic rehabilitation were delivered after 4 mo. Clinical successful implant rate was 93% (28 implants over 30)[86]. Histologic evaluation showed several areas of osteoid and bone formation without any inflammatory cell infiltration. Mean bone regenerate was 41.34%. No complications were clinically observed. Mean bone height was 12.0 mm, 10.0 mm, 3 and 12 mo after MSA, respectively[86]. The authors concluded that sinus grafting with HA/beta-TCP seeded with BMSCs can offer reliable results[86]. A previous study by Smiler et al[87] evaluated the effect of bone marrow aspirate added to xenografts or alloplast graft matrix scaffold (beta-TCP) to enhance bone formation in MSA. Clinical procedures involved harvesting four cc of bone marrow aspirate from the anterior iliac crest; these materials were seeded on matrix scaffold before sinus augmentation operations. Seven graft sites were evaluated in five patients; various MSA techniques were performed such as particulate onlay graft of the maxilla via a tunneling procedure, and particulate onlay graft of the maxilla stabilized with titanium mesh. Biopsies at 4 mo showed, in beta-TCP scaffolds, 40% a newly formed bone completely vital[87]; there was 57% of interstitial material and 3% of residual graft scaffold[87]. With these histological preparations, the authors presented evidence that stem cells aspirated from bone marrow and seeded onto beta-TCP scaffolds can be a useful method to obtain new bone in augmentation procedures[87].
Synthetic polymeric materials are an interesting category of materials. PLGA copolymer and its homopolymer derivatives have been considered for potential use in bone reconstruction procedures. MSCs have been associated to PLGA in MSA[88-93]. MSCs were isolated from periosteum, re-suspended and cultured; the suspension was soaked in polymer fleeces and the cell-polymer association were used in MSA[88-93]. Under specific conditions, periosteum-derived, tissue engineered bone grafts showed typical osteogenic differentiation characteristics such as: expression of alkaline phosphatase activity, bone gene expression and mineralization[93]. Trautvetter et al[88] performed MSA with simultaneous dental implant placement; they used an autologous tissue-engineered periosteal bone grafts based on bioresorbable PLGA scaffolds. Ten patients were radiologically assessed 5 years after MSA; in addition, histologic evaluation was performed[88]. The authors reported excellent outcomes after only 4 mo from surgical procedure; they observed significantly greater bone height over the 5-years follow-up observation period. Furthermore histological preparation from bone biopsies of two patients six months after surgery showed trabecular bone with osteocytes and active osteoblasts[88]. Accordingly, the authors concluded that the use of autologous periosteal bone grafts with simultaneous dental endosseous implants placement is a valid procedure, with excellent clinical, radiographic and histologic outcomes[88]. Although this clinical study[88] and those of previous researches[89,93] have reported that the newly formed bone provided by augmentation procedures, using tissue engineered bone grafts, allowed proper initial stability for dental implant placement[89,90], the degradation rate of the PLGA scaffold may be too fast to maintain an optimal substrate to support bone formation[90-92]. This was evidenced by a recent MSA study, where AB transplants from the iliac crest were compared with tissue engineered grafts (BMSCs loaded on PLGA scaffolds): this research showed considerable graft resorption (approximately 90%, in a 3 mo observation period) and less mineralization density in the sites augmented with tissue engineered bone[92]. Two other comparative studies revealed that coral-derived HA[90] and AB[91] show greater volume maintenance than PLGA scaffolds cultured with MSCs. These studies showed that the significant resorption of the PLGA grafts may be an important problem in the clinical scenario, with potential failure of augmentation particularly in large areas[90,91]. The fast resorption rate of the PLGA, in fact, represents an unfavourable factor for bone regeneration, making it impossible to provide mechanical stability to MSCs transplanted in the augmentation site. Osteoblasts must adhere to a stable structure to produce a new bone matrix that will be interested by consequent mineralization and maturation processes. In this way, a too fast and extended degradation of the supporting scaffold determines an instability of augmented area and then the probably failure of bone regeneration because of the collapse of newly formed, immature bone matrix[90,91]. Supply of oxygen and nutrients is essential to cells embedded within large cell-polymer constructs, in order to sustain their survival and proliferation[89-92]. In addition, PLGA resorption generates a low pH that is detrimental to osteoblasts[89-92].
Finally, two different clinical studies used an injectable tissue engineered bone, composed by BMSCs and PRP, to conduct MSA[94,95]. In a recent study, Yamada et al[94] evaluated the effects of an injectable tissue engineered bone on osteotome technique with simultaneous implant placement. Injectable bone, composed of BMSCs and PRP, was used as bone graft in 23 cases of MSA[94]. The osteotome technique was used[7]: after dental implant sites were pre-prepared with pilot drills and/or using the osteotomes, the injectable bone was inserted and then endosseous implants were placed. The bone regeneration technique was effective, as the lift-up bone height by injectable bone using BMSCs showed an increase of 6.1 ± 1.5 mm; the authors concluded that the application of injectable bone using osteotome technique can stably predict the success of bone formation and dental implants, providing also minimally invasive cell therapy[94]. These results confirmed those of a previous study by Ueda et al[95] in which the height of mineralized tissue after 2 years showed a mean gain of 8.8 mm compared to pre-operative values. However, more studies are needed to understand the efficacy of injectable bone as a graft for MSA: this material has poor compressive and tensile strength[94].
The histologic/histomorphometric results of all these human studies are reported in Table 2.
Table 2.
Histomorphometric results of the human studies included in the review
| Ref. | Patients | Study design | Histomorphometry (% of newly formed bone) |
| McAllister et al[74] | 5 | 5 MSA: MSCs + AL | 33.0 ± NR (16 wk) |
| Gonshor et al[75] | 18 | 18 MSA: MSCs + AL | 32.5 ± 6.8 (12 wk) |
| 8 MSA: AL | 18.3 ± 10.6 (12 wk) | ||
| Duttenhoefer et al[77] | 11 | 12 MSA: BMAC + BBM | NR |
| 6 MSA: Ficoll + BBM | |||
| Rickert et al[78] | 12 | 12 MSA: BMSCs + BBM | NR |
| 12 MSA: AB + BBM | |||
| Kühl et al[79] | 13 | 13 MSA: BMA + BBM | NR |
| 13 MSA: BMAC + BBM | |||
| Wildburger et al[80] | 7 | 7 MSA: BMSCs + BBM | 7.4 ± 4.1 (12 wk) |
| 13.5 ± 5.4 (24 wk) | |||
| 7 MSA: BBM | 11.8 ± 6.2 (12 wk) | ||
| 13.9 ± 8.5 (24 wk) | |||
| Sauerbier et al[81] | 26 | 34 MSA: BMAC + BBM | 12.6 ± 1.7 (12 wk) |
| 11 MSA: AB + BBM | 14.3 ± 1.8 (12 wk) | ||
| Rickert et al[82] | 12 | 12 MSA: BMSCs + BBM | 17.7 ± 7.3 (14 wk) |
| 12 MSA: AB + BBM | 12.0 ± 6.6 (14 wk) | ||
| Schmelzeisen et al[83] | 1 | 2 MSA: BMAC + BBM | 26.9 ± NR (12 wk) |
| Fuerst et al[84] | 12 | 22 MSA: BMSCs + BBM | 17.9 ± 4.6 (24 wk) |
| Beaumont et al[85] | 3 | 6 MSA: PDSCs + BBM | NR |
| Shayesteh et al[86] | 6 | 6 MSA: BMSCs + HA/β-TCP | 41.3 ± NR (24 wk) |
| Smiler et al[87] | 4 | 2 MSA: BMSCs + β-TCP | 40.0 ± NR (16 wk) |
| 1 MSA: BMSCs + BBM | 13.0 ± NR (16 wk) | ||
| 1 MSA: BMSCs + HA | 31.0 ± NR (16 wk) | ||
| Trautvetter et al[88] | 10 | 10 MSA: PDSCs + PLGA | NR |
| Mangano et al[89] | 1 | 1 MSA: PDSCs + PLGA | 28.8 ± NR |
| Voss et al[91] | 35 | 50 MSA: PDSCs + PLGA | NR |
| 63 MSA: AB | |||
| Mangano et al[90] | 5 | 5 MSA: PDSCs + PLGA | 37.3 ± 19.5 (24 wk) |
| 5 MSA: HA | 54.6 ± 21.1 (24 wk) | ||
| Zizelmann et al[92] | 20 | 14 MSA: PDSCs + PLGA | NR |
| 17 MSA: AB | |||
| Schimming et al[93] | 27 | 27 MSA: PDSCs + PLGA | NR |
| Yamada et al[94] | 23 | 23 MSA: BMSCs + PRP | NR |
| Ueda et al[95] | 6 | 6 MSA: BMSCs + PRP | NR |
MSA: Maxillary sinus augmentation; MSCs: Mesenchymal stem cells; AL: Allograft; NR: Not reported; BMAC: Autologous bone marrow aspirate concentrate; BBM: Bovine bone mineral; BMSCs: Bone marrow stem cells; AB: Autogenous bone; BMA: Bone marrow aspirate; PDSCs: Periosteal derived stem cells; HA/β-TCP: Hydroxyapatite/beta-tricalcium phosphate; PLGA: Polylactid-co-glycolic acid; PRP: Platelet rich in plasma.
In vivo experiment meta-analysis
A meta-analysis of 9 studies (7 animal and 2 human studies) was performed to give a quantitative estimate of the mean difference of the newly formed bone between the two groups (stem cells + scaffold vs scaffold alone). We considered the mean difference in % newly formed bone at 12 wk as it was the most frequently reported time period; the latest examination period was at 8 wk in 4 studies, all of them on animals. All the analyses were conducted using Package Metafor (R version 2.14). The weighted mean difference estimate from a random-effect model was 9.5% (95%CI: 3.6%-15.4%), suggesting a positive effect of stem cells on the bone re-growth (Figure 2). Heterogeneity was measured by the I2 index. The formal test confirmed the presence of substantial heterogeneity (I2 = 83%, P < 0.0001). In an attempt to explain the substantial heterogeneity observed, we considered a meta-regression model with publication year, support type (animals vs humans) and follow-up length (8 or 12 wk) as covariates. After adding publication year, support type and follow-up length to the meta-regression model, heterogeneity was no longer significant (I2 = 33%, P = 0.25).
Figure 2.
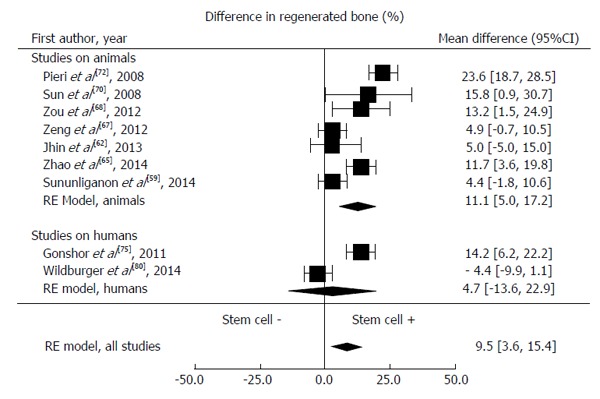
Differences in regenerated bone between test and control groups from meta-analysis. RE: Reference.
DISCUSSION
The finding that adult MSCs can be manipulated in vitro and subsequently form bone in vivo provides new therapeutic strategies for bone regeneration in dentistry.
Several researches have demonstrated that MSCs can be used in MSA: controlled experimental and clinical studies showed higher bone regeneration applying MSCs compared with controls. However, further clinical trials, which clearly demonstrate benefits of cell-based approach compared to conventional treatments are still needed: these studies should evaluate patient-based outcomes, including the time and cost-effectiveness of cell-based approaches.
In the future, the use of stem cells seeded on appropriated scaffold materials will dictates advances in bone regeneration: as a consequence, improvements upon current therapeutic strategies will depend on innovations in material science. It will be mandatory to search for appropriate scaffolds for MSCs, with adequate resorption rate and osteoconductive surface, over which new bone formation can occur.
The merger between these two disciplines - stem cell research and scaffold engineering - will draw the future of regenerative medicine.
ACKNOWLEDGMENTS
Dr. Francesco Mangano is a fellow of the PHD program in Biotechnology Biosciences and Surgical Technologies at the School in Biological/Medical Science, University of Insubria, Varese, Italy.
COMMENTS
Background
Adult mesenchymal stem cells (MSCs) that can be obtained from several tissues represents the new frontier for bone regeneration, due to their proven ability to differentiate into functional osteoblast, capable to produce new bone. Maxillary sinus augmentation (MSA) enables rehabilitation with oral implants in the posterior maxilla. Fresh autogenous bone (AB) has been always considered the gold-standard for MSA, but limited availability and donor-site morbidity reduce its application. Several osteoconductive scaffolds, such as allografts, bovine bone mineral and synthetic bone grafts (calcium phosphate ceramics) have been used in MSA with clinically successful results, but these materials do not have cells and require more time for healing. The science of bone tissue engineering (BTE) aims to overcome this problem, as it promises to obtain bone regeneration through the use of scaffolds seeded with osteogenic cells, without harvesting AB from other anatomical sites.
Research frontiers
According to BTE, a bone substitute should possess the same biological and structural properties as native bone, and the fabrication of the ideal bone graft requires the manipulation of three essential elements: scaffold, growth factors and osteogenic cells. BTE is a multidisciplinary science, based on harvesting of living cells that are expanded and differentiated in laboratory, then seeded on an appropriate scaffold, capable to mimic the structures and physiological behaviour of natural tissues. Ultimately, these “engineered scaffold” are implanted in patients. In this context, cells are the basic unit for the regeneration strategy. Several cell types have been investigated for their application in bone regeneration: MSCs can be a suitable for this aim. The authors’ present review aimed to investigate the effectiveness of MSCs in MSA, with differents scaffold materials, in animals and humans.
Innovations and breakthroughs
In total, 39 studies (18 animal studies, 21 human studies) published over a 10-year period (between 2004 and 2014) were included in our present review; these studies were variables with respect to type, study design, follow-up, and results. Meta-analysis was performed on 9 studies (7 animal studies and 2 human studies): the weighted mean difference estimate from a random-effect model was 9.5% (95%CI: 3.6%-15.4%), suggesting a positive effect of MSCs on bone regeneration. These results are similar to those of previous reviews of the literature on the same topic, where a positive influence of MSCs on bone regeneration was evidenced.
Applications
The use of scaffolds seeded with MSCs seems to represent a safe and successful treatment procedure to achieve bone regeneration in MSA. In the coming years there will be a further, huge flood of new studies on MSA with MSCs. Accordingly, dental professionals/surgeons need to change the way they think and work, to adapt to a new challenging scenario that is increasingly driven by the fascinating concepts of BTE. BTE will change the world of dentistry, changing patients’ expectations towards dental treatments: waiting to adopt or integrate these new techniques would leave oral surgeons decades behind.
Terminology
MSA is a surgical procedure to increase the amount of bone in the posterior maxilla by sacrificing some of the volume of the maxillary sinus. In case of tooth/teeth loss (due to caries, periodontal disease or traumatic injury) the alveolar process undergoes remodelling, usually losing both height and width; in addition, the floor of the sinus gradually becomes lower. This represents a problem for the correct placement of dental implants, which rely on osseointegration. The aim of MSA is to graft extra bone into the maxillary sinus, in order to support dental implant. BTE is a new science that combines cells, materials and growth factors, with the aim to obtain bone regeneration in the clinical field. While most definitions of tissue engineering cover a broad range of applications, in practice the term is closely associated with applications that repair or replace portions of or whole tissues (i.e., bone, BTE: science that aims to achieve bone regeneration, without harvesting AB from other anatomical sites, through the use in combination of scaffolds, osteogenic cells and soluble signals).
Peer-review
Several studies have reported the potential for cell-based approaches in MSA; however, most of these are animal studies. Accordingly, further clinical studies, which clearly demonstrate benefits of cell-based approach compared to conventional treatments are still needed.
Footnotes
P- Reviewer: Guo ZK, Minana MD, Wang LS S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: The authors declare that they have no financial relationship with any commercial firm that may pose a conflict of interests regarding the publication of this study. No grants, equipment, or other sources of support were provided.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at Francesco Mangano (francescomangano1@mclink.net). Participants gave informed consent for data sharing even though are anonymized and the risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 26, 2014
First decision: December 27, 2014
Article in press: May 6, 2015
References
- 1.Mangano C, Iaculli F, Piattelli A, Mangano F. Fixed restorations supported by Morse-taper connection implants: a retrospective clinical study with 10-20 years of follow-up. Clin Oral Implants Res. 2014:Epub ahead of print. doi: 10.1111/clr.12439. [DOI] [PubMed] [Google Scholar]
- 2.Chappuis V, Buser R, Brägger U, Bornstein MM, Salvi GE, Buser D. Long-term outcomes of dental implants with a titanium plasma-sprayed surface: a 20-year prospective case series study in partially edentulous patients. Clin Implant Dent Relat Res. 2013;15:780–790. doi: 10.1111/cid.12056. [DOI] [PubMed] [Google Scholar]
- 3.Mangano F, Macchi A, Caprioglio A, Sammons RL, Piattelli A, Mangano C. Survival and complication rates of fixed restorations supported by locking-taper implants: a prospective study with 1 to 10 years of follow-up. J Prosthodont. 2014;23:434–444. doi: 10.1111/jopr.12152. [DOI] [PubMed] [Google Scholar]
- 4.Mangano C, Sinjari B, Shibli JA, Mangano F, Hamisch S, Piattelli A, Perrotti V, Iezzi G. A Human Clinical, Histological, Histomorphometrical, and Radiographical Study on Biphasic HA-Beta-TCP 30/70 in Maxillary Sinus Augmentation. Clin Implant Dent Relat Res. 2015;17:610–618. doi: 10.1111/cid.12145. [DOI] [PubMed] [Google Scholar]
- 5.Mangano C, Iaculli F, Piattelli A, Mangano F, Shibli JA, Perrotti V, Iezzi G. Clinical and histologic evaluation of calcium carbonate in sinus augmentation: a case series. Int J Periodontics Restorative Dent. 2014;34:e43–e49. doi: 10.11607/prd.1832. [DOI] [PubMed] [Google Scholar]
- 6.Tatum H. Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986;30:207–229. [PubMed] [Google Scholar]
- 7.Summers RB. A new concept in maxillary implant surgery: the osteotome technique. Compendium. 1994;15:152, 154–156, 158 passim; quiz 162. [PubMed] [Google Scholar]
- 8.Kfir E, Kfir V, Goldstein M, Mazor Z, Kaluski E. Minimally invasive subnasal elevation and antral membrane balloon elevation along with bone augmentation and implants placement. J Oral Implantol. 2012;38:365–376. doi: 10.1563/AAID-JOI-D-10-00129. [DOI] [PubMed] [Google Scholar]
- 9.Mangano F, Zecca P, Pozzi-Taubert S, Macchi A, Ricci M, Luongo G, Mangano C. Maxillary sinus augmentation using computer-aided design/computer-aided manufacturing (CAD/CAM) technology. Int J Med Robot. 2013;9:331–338. doi: 10.1002/rcs.1460. [DOI] [PubMed] [Google Scholar]
- 10.Duttenhoefer F, Souren C, Menne D, Emmerich D, Schön R, Sauerbier S. Long-term survival of dental implants placed in the grafted maxillary sinus: systematic review and meta-analysis of treatment modalities. PLoS One. 2013;8:e75357. doi: 10.1371/journal.pone.0075357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rickert D, Slater JJ, Meijer HJ, Vissink A, Raghoebar GM. Maxillary sinus lift with solely autogenous bone compared to a combination of autogenous bone and growth factors or (solely) bone substitutes. A systematic review. Int J Oral Maxillofac Surg. 2012;41:160–167. doi: 10.1016/j.ijom.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Mangano FG, Tettamanti L, Sammons RL, Azzi L, Caprioglio A, Macchi A, Mangano C. Maxillary sinus augmentation with adult mesenchymal stem cells: a review of the current literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:717–723. doi: 10.1016/j.oooo.2012.09.087. [DOI] [PubMed] [Google Scholar]
- 13.Xavier SP, Dias RR, Sehn FP, Kahn A, Chaushu L, Chaushu G. Maxillary sinus grafting with autograft vs. fresh frozen allograft: a split-mouth histomorphometric study. Clin Oral Implants Res. 2014:Epub ahead of print. doi: 10.1111/clr.12404. [DOI] [PubMed] [Google Scholar]
- 14.Sbordone C, Toti P, Guidetti F, Califano L, Pannone G, Sbordone L. Volumetric changes after sinus augmentation using blocks of autogenous iliac bone or freeze-dried allogeneic bone. A non-randomized study. J Craniomaxillofac Surg. 2014;42:113–118. doi: 10.1016/j.jcms.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt CM, Moest T, Lutz R, Neukam FW, Schlegel KA. Anorganic bovine bone (ABB) vs. autologous bone (AB) plus ABB in maxillary sinus grafting. A prospective non-randomized clinical and histomorphometrical trial. Clin Oral Implants Res. 2014:Epub ahead of print. doi: 10.1111/clr.12396. [DOI] [PubMed] [Google Scholar]
- 16.Mangano C, Scarano A, Perrotti V, Iezzi G, Piattelli A. Maxillary sinus augmentation with a porous synthetic hydroxyapatite and bovine-derived hydroxyapatite: a comparative clinical and histologic study. Int J Oral Maxillofac Implants. 2007;22:980–986. [PubMed] [Google Scholar]
- 17.Mangano C, Scarano A, Iezzi G, Orsini G, Perrotti V, Mangano F, Montini S, Piccirilli M, Piattelli A. Maxillary sinus augmentation using an engineered porous hydroxyapatite: a clinical, histological, and transmission electron microscopy study in man. J Oral Implantol. 2006;32:122–131. doi: 10.1563/796.1. [DOI] [PubMed] [Google Scholar]
- 18.Mendonça-Caridad JJ, Nunez M, Juiz-Lopez P, Pita-Fernandez S, Seoane J. Sinus floor elevation using a composite graft: clinical outcome of immediate implant placement. Int J Oral Maxillofac Implants. 2013;28:252–260. doi: 10.11607/jomi.2379. [DOI] [PubMed] [Google Scholar]
- 19.Park JB. Use of cell-based approaches in maxillary sinus augmentation procedures. J Craniofac Surg. 2010;21:557–560. doi: 10.1097/SCS.0b013e3181d02577. [DOI] [PubMed] [Google Scholar]
- 20.Tevlin R, McArdle A, Atashroo D, Walmsley GG, Senarath-Yapa K, Zielins ER, Paik KJ, Longaker MT, Wan DC. Biomaterials for craniofacial bone engineering. J Dent Res. 2014;93:1187–1195. doi: 10.1177/0022034514547271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferretti C, Ripamonti U, Tsiridis E, Kerawala CJ, Mantalaris A, Heliotis M. Osteoinduction: translating preclinical promise into clinical reality. Br J Oral Maxillofac Surg. 2010;48:536–539. doi: 10.1016/j.bjoms.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. [Updated 2011 March].The Cochrane Collaboration. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 24.Chambrone L, Faggion CM, Pannuti CM, Chambrone LA. Evidence-based periodontal plastic surgery: an assessment of quality of systematic reviews in the treatment of recession-type defects. J Clin Periodontol. 2010;37:1110–1118. doi: 10.1111/j.1600-051X.2010.01634.x. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez JM, Bai Q, Dijon-Grinand M, Assou S, Gerbal-Chaloin S, Hamamah S, De Vos J. Human pluripotent stem cells: from biology to cell therapy. World J Stem Cells. 2010;2:24–33. doi: 10.4252/wjsc.v2.i2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripamonti U. Soluble and insoluble signals sculpt osteogenesis in angiogenesis. World J Biol Chem. 2010;1:109–132. doi: 10.4331/wjbc.v1.i5.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosso F, Marino G, Giordano A, Barbarisi M, Parmeggiani D, Barbarisi A. Smart materials as scaffolds for tissue engineering. J Cell Physiol. 2005;203:465–470. doi: 10.1002/jcp.20270. [DOI] [PubMed] [Google Scholar]
- 28.Marolt D, Knezevic M, Novakovic GV. Bone tissue engineering with human stem cells. Stem Cell Res Ther. 2010;1:10. doi: 10.1186/scrt10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Handschel J, Wiesmann HP, Depprich R, Kübler NR, Meyer U. Cell-based bone reconstruction therapies--cell sources. Int J Oral Maxillofac Implants. 2006;21:890–898. [PubMed] [Google Scholar]
- 30.Bifari F, Pacelli L, Krampera M. Immunological properties of embryonic and adult stem cells. World J Stem Cells. 2010;2:50–60. doi: 10.4252/wjsc.v2.i3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Razzouk S, Schoor R. Mesenchymal stem cells and their challenges for bone regeneration and osseointegration. J Periodontol. 2012;83:547–550. doi: 10.1902/jop.2011.110384. [DOI] [PubMed] [Google Scholar]
- 32.Daar AS, Sheremeta L. The science of stem cells: ethical, legal and social issues. Exp Clin Transplant. 2003;1:139–146. [PubMed] [Google Scholar]
- 33.Mahalakshmi S. Potentials of stem cell research and the implications of legislation. J Stem Cells Regen Med. 2006;1:37–39. doi: 10.46582/jsrm.0101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao HT, Chen CT. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6:288–295. doi: 10.4252/wjsc.v6.i3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferretti C, Mattioli-Belmonte M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J Stem Cells. 2014;6:266–277. doi: 10.4252/wjsc.v6.i3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cicconetti A, Sacchetti B, Bartoli A, Michienzi S, Corsi A, Funari A, Robey PG, Bianco P, Riminucci M. Human maxillary tuberosity and jaw periosteum as sources of osteoprogenitor cells for tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:618.e1–618.12. doi: 10.1016/j.tripleo.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014;6:312–321. doi: 10.4252/wjsc.v6.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosch P, Musgrave DS, Lee JY, Cummins J, Shuler T, Ghivizzani TC, Evans T, Robbins TD. Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18:933–944. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- 39.Zeddou M, Relic B, Malaise MG. Umbilical cord fibroblasts: Could they be considered as mesenchymal stem cells? World J Stem Cells. 2014;6:367–370. doi: 10.4252/wjsc.v6.i3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berardinelli P, Valbonetti L, Muttini A, Martelli A, Peli R, Zizzari V, Nardinocchi D, Vulpiani MP, Tetè S, Barboni B, et al. Role of amniotic fluid mesenchymal cells engineered on MgHA/collagen-based scaffold allotransplanted on an experimental animal study of sinus augmentation. Clin Oral Investig. 2013;17:1661–1675. doi: 10.1007/s00784-012-0857-3. [DOI] [PubMed] [Google Scholar]
- 41.Barboni B, Mangano C, Valbonetti L, Marruchella G, Berardinelli P, Martelli A, Muttini A, Mauro A, Bedini R, Turriani M, et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS One. 2013;8:e63256. doi: 10.1371/journal.pone.0063256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang EJ, Byun JH, Choi YJ, Maeng GH, Lee SL, Kang DH, Lee JS, Rho GJ, Park BW. In vitro and in vivo osteogenesis of porcine skin-derived mesenchymal stem cell-like cells with a demineralized bone and fibrin glue scaffold. Tissue Eng Part A. 2010;16:815–827. doi: 10.1089/ten.TEA.2009.0439. [DOI] [PubMed] [Google Scholar]
- 43.Mangano C, Paino F, d’Aquino R, De Rosa A, Iezzi G, Piattelli A, Laino L, Mitsiadis T, Desiderio V, Mangano F, et al. Human dental pulp stem cells hook into biocoral scaffold forming an engineered biocomplex. PLoS One. 2011;6:e18721. doi: 10.1371/journal.pone.0018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez Lozano FJ, Moraleda JM. Mesenchymal dental pulp stem cells: a new tool in sinus lift. J Craniofac Surg. 2011;22:774–775. doi: 10.1097/SCS.0b013e318208ba61. [DOI] [PubMed] [Google Scholar]
- 45.Behnia A, Haghighat A, Talebi A, Nourbakhsh N, Heidari F. Transplantation of stem cells from human exfoliated deciduous teeth for bone regeneration in the dog mandibular defect. World J Stem Cells. 2014;6:505–510. doi: 10.4252/wjsc.v6.i4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohta S, Yamada S, Matuzaka K, Inoue T. The behavior of stem cells and progenitor cells in the periodontal ligament during wound healing as observed using immunohistochemical methods. J Periodontal Res. 2008;43:595–603. doi: 10.1111/j.1600-0765.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- 47.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 48.McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655–2661. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda M, Tohnai I, Nakai H. Tissue engineering research in oral implant surgery. Artif Organs. 2001;25:164–171. doi: 10.1046/j.1525-1594.2001.025003164.x. [DOI] [PubMed] [Google Scholar]
- 51.Ly H. Telomere dynamics in induced pluripotent stem cells: Potentials for human disease modeling. World J Stem Cells. 2011;3:89–95. doi: 10.4252/wjsc.v3.i10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 53.Park BW, Hah YS, Kim DR, Kim JR, Byun JH. Vascular endothelial growth factor expression in cultured periosteal-derived cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:554–560. doi: 10.1016/j.tripleo.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 54.Fakhry M, Hamade E, Badran B, Buchet R, Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells. 2013;5:136–148. doi: 10.4252/wjsc.v5.i4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuboki Y, Jin Q, Kikuchi M, Mamood J, Takita H. Geometry of artificial ECM: sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect Tissue Res. 2002;43:529–534. doi: 10.1080/03008200290001104. [DOI] [PubMed] [Google Scholar]
- 56.Kuboki Y, Takita H, Kobayashi D, Tsuruga E, Inoue M, Murata M, Nagai N, Dohi Y, Ohgushi H. BMP-induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: topology of osteogenesis. J Biomed Mater Res. 1998;39:190–199. doi: 10.1002/(sici)1097-4636(199802)39:2<190::aid-jbm4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 57.Murphy CM, O’Brien FJ, Little DG, Schindeler A. Cell-scaffold interactions in the bone tissue engineering triad. Eur Cell Mater. 2013;26:120–132. doi: 10.22203/ecm.v026a09. [DOI] [PubMed] [Google Scholar]
- 58.Logeart-Avramoglou D, Anagnostou F, Bizios R, Petite H. Engineering bone: challenges and obstacles. J Cell Mol Med. 2005;9:72–84. doi: 10.1111/j.1582-4934.2005.tb00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sununliganon L, Peng L, Singhatanadgit W, Cheung LK. Osteogenic efficacy of bone marrow concentrate in rabbit maxillary sinus grafting. J Craniomaxillofac Surg. 2014;42:1753–1765. doi: 10.1016/j.jcms.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Yu BH, Zhou Q, Wang ZL. Comparison of tissue-engineered bone from different stem cell sources for maxillary sinus floor augmentation: a study in a canine model. J Oral Maxillofac Surg. 2014;72:1084–1092. doi: 10.1016/j.joms.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 61.Oshima T, Duttenhoefer F, Xavier S, Nelson K, Sauerbier S. Can mesenchymal stem cells and novel gabapentin-lactam enhance maxillary bone formation? J Oral Maxillofac Surg. 2014;72:485–495. doi: 10.1016/j.joms.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 62.Jhin MJ, Kim KH, Kim SH, Kim YS, Kim ST, Koo KT, Kim TI, Seol YJ, Ku Y, Rhyu IC, et al. Ex vivo bone morphogenetic protein-2 gene delivery using bone marrow stem cells in rabbit maxillary sinus augmentation in conjunction with implant placement. J Periodontol. 2013;84:985–994. doi: 10.1902/jop.2012.120221. [DOI] [PubMed] [Google Scholar]
- 63.Gutwald R, Haberstroh J, Kuschnierz J, Kister C, Lysek DA, Maglione M, Xavier SP, Oshima T, Schmelzeisen R, Sauerbier S. Mesenchymal stem cells and inorganic bovine bone mineral in sinus augmentation: comparison with augmentation by autologous bone in adult sheep. Br J Oral Maxillofac Surg. 2010;48:285–290. doi: 10.1016/j.bjoms.2009.06.226. [DOI] [PubMed] [Google Scholar]
- 64.Sauerbier S, Stubbe K, Maglione M, Haberstroh J, Kuschnierz J, Oshima T, Xavier SP, Brunnberg L, Schmelzeisen R, Gutwald R. Mesenchymal stem cells and bovine bone mineral in sinus lift procedures--an experimental study in sheep. Tissue Eng Part C Methods. 2010;16:1033–1039. doi: 10.1089/ten.TEC.2009.0734. [DOI] [PubMed] [Google Scholar]
- 65.Zhao W, Lu JY, Hao YM, Cao CH, Zou DR. Maxillary sinus floor elevation with a tissue-engineered bone composite of deciduous tooth stem cells and calcium phosphate cement in goats. J Tissue Eng Regen Med. 2014:Epub ahead of print. doi: 10.1002/term.1867. [DOI] [PubMed] [Google Scholar]
- 66.Zhang W, Zhang X, Wang S, Xu L, Zhang M, Wang G, Jin Y, Zhang X, Jiang X. Comparison of the use of adipose tissue-derived and bone marrow-derived stem cells for rapid bone regeneration. J Dent Res. 2013;92:1136–1141. doi: 10.1177/0022034513507581. [DOI] [PubMed] [Google Scholar]
- 67.Zeng D, Xia L, Zhang W, Huang H, Wei B, Huang Q, Wei J, Liu C, Jiang X. Maxillary sinus floor elevation using a tissue-engineered bone with calcium-magnesium phosphate cement and bone marrow stromal cells in rabbits. Tissue Eng Part A. 2012;18:870–881. doi: 10.1089/ten.TEA.2011.0379. [DOI] [PubMed] [Google Scholar]
- 68.Zou D, Guo L, Lu J, Zhang X, Wei J, Liu C, Zhang Z, Jiang X. Engineering of bone using porous calcium phosphate cement and bone marrow stromal cells for maxillary sinus augmentation with simultaneous implant placement in goats. Tissue Eng Part A. 2012;18:1464–1478. doi: 10.1089/ten.TEA.2011.0501. [DOI] [PubMed] [Google Scholar]
- 69.Xia L, Xu Y, Chang Q, Sun X, Zeng D, Zhang W, Zhang X, Zhang Z, Jiang X. Maxillary sinus floor elevation using BMP-2 and Nell-1 gene-modified bone marrow stromal cells and TCP in rabbits. Calcif Tissue Int. 2011;89:53–64. doi: 10.1007/s00223-011-9493-1. [DOI] [PubMed] [Google Scholar]
- 70.Sun XJ, Zhang ZY, Wang SY, Gittens SA, Jiang XQ, Chou LL. Maxillary sinus floor elevation using a tissue-engineered bone complex with OsteoBone and bMSCs in rabbits. Clin Oral Implants Res. 2008;19:804–813. doi: 10.1111/j.1600-0501.2008.01577.x. [DOI] [PubMed] [Google Scholar]
- 71.Sun XJ, Xia LG, Chou LL, Zhong W, Zhang XL, Wang SY, Zhao J, Jiang XQ, Zhang ZY. Maxillary sinus floor elevation using a tissue engineered bone complex with BMP-2 gene modified bMSCs and a novel porous ceramic scaffold in rabbits. Arch Oral Biol. 2010;55:195–202. doi: 10.1016/j.archoralbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Pieri F, Lucarelli E, Corinaldesi G, Iezzi G, Piattelli A, Giardino R, Bassi M, Donati D, Marchetti C. Mesenchymal stem cells and platelet-rich plasma enhance bone formation in sinus grafting: a histomorphometric study in minipigs. J Clin Periodontol. 2008;35:539–546. doi: 10.1111/j.1600-051X.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 73.Ohya M, Yamada Y, Ozawa R, Ito K, Takahashi M, Ueda M. Sinus floor elevation applied tissue-engineered bone. Comparative study between mesenchymal stem cells/platelet-rich plasma (PRP) and autogenous bone with PRP complexes in rabbits. Clin Oral Implants Res. 2005;16:622–629. doi: 10.1111/j.1600-0501.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 74.McAllister BS, Haghighat K, Gonshor A. Histologic evaluation of a stem cell-based sinus-augmentation procedure. J Periodontol. 2009;80:679–686. doi: 10.1902/jop.2009.080345. [DOI] [PubMed] [Google Scholar]
- 75.Gonshor A, McAllister BS, Wallace SS, Prasad H. Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int J Oral Maxillofac Implants. 2011;26:123–131. [PubMed] [Google Scholar]
- 76.Tyshenko MG, ElSaadany S, Oraby T, Darshan S, Aspinall W, Cooke R, Catford A, Krewski D. Expert elicitation for the judgment of prion disease risk uncertainties. J Toxicol Environ Health A. 2011;74:261–285. doi: 10.1080/15287394.2011.529783. [DOI] [PubMed] [Google Scholar]
- 77.Duttenhoefer F, Hieber SF, Stricker A, Schmelzeisen R, Gutwald R, Sauerbier S. Follow-up of implant survival comparing ficoll and bone marrow aspirate concentrate methods for hard tissue regeneration with mesenchymal stem cells in humans. Biores Open Access. 2014;3:75–76. doi: 10.1089/biores.2014.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rickert D, Vissink A, Slot WJ, Sauerbier S, Meijer HJ, Raghoebar GM. Maxillary sinus floor elevation surgery with BioOss® mixed with a bone marrow concentrate or autogenous bone: test of principle on implant survival and clinical performance. Int J Oral Maxillofac Surg. 2014;43:243–247. doi: 10.1016/j.ijom.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Kühl S, Payer M, Kirmeier R, Wildburger A, Wegscheider W, Jakse N. The influence of bone marrow aspirates and concentrates on the early volume stability of maxillary sinus grafts with deproteinized bovine bone mineral - first results of a RCT. Clin Oral Implants Res. 2014;25:221–225. doi: 10.1111/clr.12101. [DOI] [PubMed] [Google Scholar]
- 80.Wildburger A, Payer M, Jakse N, Strunk D, Etchard-Liechtenstein N, Sauerbier S. Impact of autogenous concentrated bone marrow aspirate on bone regeneration after sinus floor augmentation with a bovine bone substitute--a split-mouth pilot study. Clin Oral Implants Res. 2014;25:1175–1181. doi: 10.1111/clr.12228. [DOI] [PubMed] [Google Scholar]
- 81.Sauerbier S, Rickert D, Gutwald R, Nagursky H, Oshima T, Xavier SP, Christmann J, Kurz P, Menne D, Vissink A, et al. Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: a controlled, randomized, single-blinded clinical and histological trial--per-protocol analysis. Tissue Eng Part A. 2011;17:2187–2197. doi: 10.1089/ten.TEA.2010.0516. [DOI] [PubMed] [Google Scholar]
- 82.Rickert D, Sauerbier S, Nagursky H, Menne D, Vissink A, Raghoebar GM. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res. 2011;22:251–258. doi: 10.1111/j.1600-0501.2010.01981.x. [DOI] [PubMed] [Google Scholar]
- 83.Schmelzeisen R, Gutwald R, Oshima T, Nagursky H, Vogeler M, Sauerbier S. Making bone II: maxillary sinus augmentation with mononuclear cells--case report with a new clinical method. Br J Oral Maxillofac Surg. 2011;49:480–482. doi: 10.1016/j.bjoms.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 84.Fuerst G, Strbac GD, Vasak C, Tangl S, Leber J, Gahleitner A, Gruber R, Watzek G. Are culture-expanded autogenous bone cells a clinically reliable option for sinus grafting? Clin Oral Implants Res. 2009;20:135–139. doi: 10.1111/j.1600-0501.2008.01624.x. [DOI] [PubMed] [Google Scholar]
- 85.Beaumont C, Schmidt RJ, Tatakis DN, Zafiropoulos GG. Use of engineered bone for sinus augmentation. J Periodontol. 2008;79:541–548. doi: 10.1902/jop.2008.070255. [DOI] [PubMed] [Google Scholar]
- 86.Shayesteh YS, Khojasteh A, Soleimani M, Alikhasi M, Khoshzaban A, Ahmadbeigi N. Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:203–209. doi: 10.1016/j.tripleo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Smiler D, Soltan M, Lee JW. A histomorphogenic analysis of bone grafts augmented with adult stem cells. Implant Dent. 2007;16:42–53. doi: 10.1097/ID.0b013e3180335934. [DOI] [PubMed] [Google Scholar]
- 88.Trautvetter W, Kaps C, Schmelzeisen R, Sauerbier S, Sittinger M. Tissue-engineered polymer-based periosteal bone grafts for maxillary sinus augmentation: five-year clinical results. J Oral Maxillofac Surg. 2011;69:2753–2762. doi: 10.1016/j.joms.2011.02.096. [DOI] [PubMed] [Google Scholar]
- 89.Mangano C, Piattelli A, Tettamanti L, Mangano F, Mangano A, Borges F, Iezzi G, d’Avila S, Shibli JA. Engineered bone by autologous osteoblasts on polymeric scaffolds in maxillary sinus augmentation: histologic report. J Oral Implantol. 2010;36:491–496. doi: 10.1563/AAID-JOI-D-09-00028. [DOI] [PubMed] [Google Scholar]
- 90.Mangano C, Piattelli A, Mangano A, Mangano F, Mangano A, Iezzi G, Borges FL, d’Avila S, Shibli JA. Combining scaffolds and osteogenic cells in regenerative bone surgery: a preliminary histological report in human maxillary sinus augmentation. Clin Implant Dent Relat Res. 2009;11 Suppl 1:e92–102. doi: 10.1111/j.1708-8208.2009.00227.x. [DOI] [PubMed] [Google Scholar]
- 91.Voss P, Sauerbier S, Wiedmann-Al-Ahmad M, Zizelmann C, Stricker A, Schmelzeisen R, Gutwald R. Bone regeneration in sinus lifts: comparing tissue-engineered bone and iliac bone. Br J Oral Maxillofac Surg. 2010;48:121–126. doi: 10.1016/j.bjoms.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 92.Zizelmann C, Schoen R, Metzger MC, Schmelzeisen R, Schramm A, Dott B, Bormann KH, Gellrich NC. Bone formation after sinus augmentation with engineered bone. Clin Oral Implants Res. 2007;18:69–73. doi: 10.1111/j.1600-0501.2006.01295.x. [DOI] [PubMed] [Google Scholar]
- 93.Schimming R, Schmelzeisen R. Tissue-engineered bone for maxillary sinus augmentation. J Oral Maxillofac Surg. 2004;62:724–729. doi: 10.1016/j.joms.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Yamada Y, Nakamura S, Ueda M, Ito K. Osteotome technique with injectable tissue-engineered bone and simultaneous implant placement by cell therapy. Clin Oral Implants Res. 2013;24:468–474. doi: 10.1111/j.1600-0501.2011.02353.x. [DOI] [PubMed] [Google Scholar]
- 95.Ueda M, Yamada Y, Kagami H, Hibi H. Injectable bone applied for ridge augmentation and dental implant placement: human progress study. Implant Dent. 2008;17:82–90. doi: 10.1097/ID.0b013e31815cd591. [DOI] [PubMed] [Google Scholar]


