Abstract
Recently, it has become clear that mild abnormal glucose tolerance increases the incidence of perinatal maternal-infant complications, and so the definition and diagnostic criteria of gestational diabetes mellitus (GDM) have been changed. Therefore, in patients with GDM and pregnant women with diabetes mellitus, even stricter glycemic control than before is required to reduce the incidence of perinatal maternal-infant complications. Strict glycemic control cannot be attained without an indicator of glycemic control; this review proposes a reliable indicator. The gold standard indicator of glycemic control in patients with diabetes mellitus is hemoglobin A1c (HbA1c); however, we have demonstrated that HbA1c does not reflect glycemic control accurately during pregnancy because of iron deficiency. It has also become clear that glycated albumin, another indicator of glycemic control, is not influenced by iron deficiency and therefore might be a better indicator of glycemic control in patients with GDM and pregnant women with diabetes mellitus. However, large-population epidemiological studies are necessary in order to confirm our proposal. Here, we outline the most recent findings about the indicators of glycemic control during pregnancy including fructosamine and 1,5-anhydroglucitol.
Keywords: Glycemic control; Hemoglobin A1c; Glycated albumin; 1,5-anhydroglucitol; Fructosamine; Gestational diabetes; Diabetes mellitus; Pregnancy
Core tip: In patients with gestational diabetes mellitus (GDM) and pregnant women with diabetes, stricter glycemic control is required to reduce the incidence of perinatal maternal-infant complications. We have demonstrated that hemoglobin A1c does not reflect glycemic control accurately during pregnancy because of iron deficiency. On the other hand, glycated albumin is not influenced by iron deficiency and therefore might be a better indicator of glycemic control in patients with GDM and pregnant women with diabetes. However, large-population epidemiological studies are necessary. Here, we outline the most recent findings about the indicators of glycemic control during pregnancy including fructosamine and 1,5-anhydroglucitol.
INTRODUCTION
The number of patients with diabetes mellitus has been steadily increasing worldwide[1] and diabetes mellitus has become a global health problem. This tendency is also observed in women of child-bearing age partly due to the change in diagnostic criteria of gestational diabetes mellitus (GDM) as mentioned later. The incidence of GDM in Japan has increased by 4.1 fold from 2.9% to 12.1%[2]. By detecting abnormal maternal glucose metabolism at an early stage of pregnancy and achieving excellent glycemic control during pregnancy, it is possible to prevent perinatal maternal-infant complications[3,4]. According to a report on a meta-analysis of 20 studies, the relative risk for patients with GDM of developing type 2 diabetes mellitus after delivery is 7.43 times higher than that of women who had normal glucose tolerance during pregnancy[5]. Therefore, it is important to follow up mothers after delivery. Moreover, the concept of developmental origins of health and diseases was proposed recently[6] and the long-term effects of mothers with abnormal glucose tolerance on fetuses after birth have been actively discussed. Thus, it is important to manage glycemic control of mothers appropriately not only because it helps to maintain the health of mothers and infants in the short term, but also helps to maintain the long-term health of mothers and the next generation. In the following sections, we outline the management of pregnant women with abnormal glucose metabolism. Finally, we propose a reliable indicator of glycemic control.
CHANGES IN GLUCOSE METABOLISM DURING PREGNANCY
During the early stage of pregnancy, increased secretion of progesterone and 17β-estrogen from the corpus luteum is observed; after the placenta is completed, it replaces the role of the corpus luteum. In humans, it is known that during pregnancy, the secretion of estrogen increases by about 30 fold and that of progesterone by about 10 fold compared with that during non-pregnancy. In addition, the secretion of prolactin and placental lactogen also increases gradually from week 12 of pregnancy. Placental lactogen is considered to be one of the typical hormones involved in the change in insulin sensitivity during pregnancy. Moreover, it has been shown that tumor necrosis factor-α secreted from macrophages which infiltrate into fat cells and villous cells is deeply involved in decreased insulin sensitivity[7]. It is known that high concentrations of estrogen and progesterone also induce decreased insulin sensitivity[8,9]. Because of the involvement of hormones and cytokines as mentioned above, there is a substantial change in glucose metabolism during pregnancy. In clinical researches conducted by Catalano et al[10-12], increased gluconeogenesis in the liver during the end stage of pregnancy demonstrated decreased insulin sensitivity in the liver. In addition, it has been shown that systemic insulin sensitivity decreases by about 50% to 60% during the end stage of pregnancy[13]. In pancreatic β-cells, increased β-cell volume and increased insulin secretion reaction take place to compensate for their insulin resistance. Regarding the mechanism of this phenomenon, it has been reported that serotonin is present downstream of the prolactin signal, which promotes pancreatic β-cell growth and greatly contributes to an increase in the cell volume[14]. It is considered that abnormal glucose tolerance develops in pregnant women when this compensatory effect is insufficient. In fact, the presence of pancreatic β-cell dysfunction in GDM has been demonstrated[15,16].
DEFINITION OF GDM
GDM, which was found or developed for the first time during pregnancy, is milder abnormal glucose metabolism than diabetes mellitus. It should be noted that GDM does not include overt diabetes in pregnancy[17].
It is not appropriate to apply the diagnostic criteria of diabetes mellitus during non-pregnancy as the diagnostic criteria of abnormal glucose tolerance during pregnancy for the following reasons. Firstly, the altered hormonal environment and the presence of the fetus during pregnancy cease at the time of delivery. Secondly, the diagnostic criteria of diabetes mellitus during non-pregnancy are established based on the incidence of diabetic complications (especially, diabetic retinopathy); on the other hand, the diagnostic criteria of GDM are established for the prevention of diabetes mellitus of mothers and the prevention of perinatal complications of mothers and infants. The diagnostic criteria of GDM of each country that were used in the past were established to prevent future onset of diabetes mellitus, and differed among countries. These differences in the definition of GDM caused various problems in international discussions. Accordingly, the International Association of Diabetes and Pregnancy Study Groups announced worldwide uniform diagnostic criteria of GDM[17] based on the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study[18] reported in 2008 (Table 1). The HAPO study was conducted in 25505 pregnant women at 15 facilities in nine countries worldwide to evaluate outcomes of mothers and infants; the data of the participants were opened to the primary physician only when fasting plasma glucose was not less than 105 mg/dL or plasma glucose at 2 h after meal was not less than 200 mg/dL; otherwise, the data were blinded to the primary physician. Primary outcomes included birth weight of not less than the 90 percentile, percentage of cesarean section, neonatal hypoglycemia, and cord serum C-peptide of not less than the 90 percentile; secondary outcomes included premature labor, shoulder dystocia/dystocia, hyperbilirubinemia, neonatal intensive care unit management, and pregnancy-induced hypertension syndrome. As a result, there was no threshold at which the incidence of primary outcomes showed a clear increase; plasma glucose level, whose odds ratio is 1.75 times higher than that in the lowest category, was adopted as the diagnostic cut-off value[17]. Regarding glycemic control indicators, there were no clear diagnostic threshold. Thus, the shift from the diagnostic criteria of GDM based on the future incidence of diabetes mellitus of mothers to diagnostic criteria of GDM for improving perinatal outcomes of mothers and infants during pregnancy was a major event.
Table 1.
To diagnose gestational diabetes mellitus and cumulative proportion of Hyperglycemia and Adverse Pregnancy Outcome cohort equaling or exceeding those thresholds
| Glucose measure |
Glucose concentration threshold1 |
Above threshold (%) |
|
| mmol/L | mg/dL | cumulative | |
| FPG | 5.1 | 92 | 8.3 |
| 1-h plasma glucose | 10.0 | 180 | 14.0 |
| 2-h plasma glucose | 8.5 | 153 | 16.12 |
One or more of these values from a 75-g OGTT must be equaled or exceeded for the diagnosis of GDM;
In addition, 1.7% of participants in the initial cohort were unblinded because of FPG > 5.8 mmol/L (105 mg/dL) or 2-h OGTT values > 11.1 mmol/L (200 mg/dL), bringing the total to 17.8% (modified from Ref.[17]). FPG: Fasting plasma glucose; OGTT: Oral glucose tolerance test.
ADVERSE EVENTS DURING PREGNANCY OF GDM AND OVERT DIABETES MELLITUS
Because the definition of GDM is based on the incidence of perinatal complications of mothers and infants, we give an outline of perinatal complications once again. Maternal complications include pregnancy-induced hypertension syndrome, polyhydramnios, shoulder dystocia, and cesarean section. In pregnant women with diabetes mellitus, careful attention should also be paid to diabetic ketoacidosis, worsening of diabetic retinopathy and diabetic nephropathy, and hypoglycemia. For pregnancy-induced hypertension syndrome, 2% to 8% of all pregnant women are complicated with preeclampsia, which worsens perinatal outcomes. The late-onset form, which accounts for 80% of all cases of pregnancy-induced hypertension syndrome, is of maternal origin and is often accompanied by old age, obesity, diabetes mellitus, and chronic hypertension. That is to say, it is considered that abnormal glucose metabolism of mothers influences the onset of pregnancy-induced hypertension syndrome. Next, it has been reported that 0.5% to 0.7% of normal pregnant women and 2.0% to 2.1% of patients with GDM are complicated with polyhydramnios[19,20]. Polyhydramnios induces complications leading to perinatal death including premature labor, premature rupture of membranes, fetal malpresentation, weak labor, umbilical cord prolapse, premature separation of normally implanted placenta, and atonic hemorrhage after delivery. For pregnant women with diabetes mellitus, it has been reported that glucose concentration in amniotic fluid is related to maternal plasma glucose level[21] and that there is a positive correlation between amniotic fluid volume and glucose concentration in amniotic fluid[22]. Shoulder dystocia is a condition in which after the head of the infant is delivered in cephalic vaginal delivery, the shoulder of the infant is not delivered. It is a disease which may cause dystocia in both the mother and the infant. It is known that macrosomia is a risk factor for shoulder dystocia; on the other hand, it has been reported that pregnant women with abnormal glucose tolerance tend to experience shoulder dystocia regardless of the presence or absence of macrosomia[23]. For these reasons and also because of complications of fetuses as mentioned later, the percentage of cesarean section is obviously higher in pregnant women with abnormal glucose tolerance; the percentage is 10.7%-18.9% in normal pregnant women, compared with 19.3%-30.9% in pregnant women with GDM and 45.2% in pregnant women with diabetes mellitus[20,24-27].
Congenital anomaly is one of the complications of fetuses born from mothers with diabetes mellitus. According to a report in Japan, the incidence of congenital anomaly does not increase obviously when hemoglobin A1c (HbA1c) during the early stage of pregnancy is less than 7.4%; however, the incidence increases when HbA1c is 7.4% or more; the incidence is as high as 24.1% when HbA1c is 8.4% or more[28]. Macrosomia as a developmental anomaly is a fetal developmental anomaly unique to pregnancy in women with diabetes mellitus. The hyperglycemia-hyperinsulinemia hypothesis proposed by Pedersen[29] is that hyperglycemia of mothers induces hyperglycemia of fetuses, and hyperplasia of pancreatic β-cells of fetuses results in hypersecretion of insulin, leading to excessive growth of fetuses. Infants born from mothers with diabetes mellitus are called infants of diabetic mothers and are known as high-risk infants in whom multiple complications develop at a high incidence[30]. Such complications include hypoglycemia, polycythemia, hyperbilirubinemia, hypocalcemia, neonatal respiratory distress syndrome, and myocardial hypertrophy.
TREATMENT OF PREGNANT WOMEN WITH GDM AND OVERT DIABETIC MELLITUS
The basis of plasma glucose management during pregnancy is dietary therapy similar to that during non-pregnancy. As nutrition for fetuses during pregnancy, glucose, amino acids, and free fatty acids are supplied through the maternal placenta; the main energy source for fetuses is glucose. The main points of dietary therapy for pregnant women with abnormal glucose tolerance are to prevent ketosis of mothers resulting from insufficient carbohydrate intake and to perform strict glycemic control. In other words, energy intake at which the body weight of mothers before pregnancy does not decrease is determined as the basic food intake, and energy for fetuses according to the stage of pregnancy is added to it.
If the goal of glycemic control is not achieved in spite of dietary therapy, insulin therapy is selected. Currently, the types of insulin that can be used safely during pregnancy are human insulin as well as insulin aspart, insulin lispro, and insulin detemir; they are classified into the United States Food and Drug Administration (FDA) Pregnancy Category B. Insulin glargine and insulin glulisine are currently classified into the FDA Pregnancy Category C, and their potential risk cannot be ruled out. The principle of insulin therapy during pregnancy, which consists of supplementation of basal insulin secretion and supplementation of additional insulin secretion at the time of dietary intake, is the same as that during non-pregnancy. It should be noted that during the early stage of pregnancy, the insulin requirement decreases because of hyperemesis gravidarum, etc.; during and after the middle stage of pregnancy, insulin resistance increases; during the end stage of pregnancy, the insulin requirement increases to about two times that before pregnancy.
MEASUREMENT OF BLOOD GLUCOSE
In order to prevent perinatal complications of mothers and infants mentioned above, the goal of glycemic control during pregnancy should be to bring plasma glucose level as close to normal as possible without development of hypoglycemia. Strict glycemic control should be performed by self-monitoring of plasma glucose (SMBG) or continuous glucose monitoring (CGM).
SMBG
SMBG enables strict glycemic control. To achieve this, it is important to make patients understand why blood glucose should be measured, and to remind patients of the relationship between activity, events, meals, snacks, etc. and blood glucose levels. Because pregnant women tend to become anemic easily, this tendency should be taken into consideration. When hematocrit is low, plasma volume increases and plasma glucose level increases; conversely, when hematocrit is high, plasma volume decreases and plasma glucose level decreases. If insulin therapy is being performed, the insulin dose for glycemic control is adjusted based on the result of SMBG. The basic principle is to adjust basal insulin dose according to fasting blood glucose level and to adjust additional insulin dose according to postprandial blood glucose level. Langer et al[31] have reported that when fasting blood glucose is not less than 95 mg/dL in patients with GDM, the incidence of macrosomia significantly increases, and the incidence is decreased by insulin therapy. In addition, they have demonstrated that it is possible to decrease the incidence of both small-for-gestational-age and large-for-gestational-age by bringing mean blood glucose level to 87-104 mg/dL[32]. On the other hand, it has been reported that adjusting insulin therapy according to postprandial blood glucose level results in an improvement of glycemic control, a decrease in neonatal hypoglycemia, a decrease in macrosomia, and a decrease in cesarean section[33].
CGM
The CGM device can monitor blood glucose level every 5 min for up to 7 d. The device has demonstrated that there is a difference in circadian rhythm of blood glucose among non-diabetic pregnant women between normal-weight pregnant women and obese pregnant women[34]. That is, compared with normal-weight pregnant women, obese pregnant women have comparable fasting blood glucose and comparable mean blood glucose but higher postprandial blood glucose and lower nighttime blood glucose. Compared with SMBG, the CGM device can monitor glycemic excursion in greater detail, but continuous wearing of the device is neither economically viable nor suitable for continuous evaluation. However, the CGM device is a useful education tool for pregnant women with diabetes mellitus, and it has been reported that by performing CGM every 4 to 6 wk during pregnancy, glycemic control during the third trimester of pregnancy improved, and the risk of macrosomia decreased[35]. In addition, the ability to identify hypoglycemia is another noteworthy benefit of CGM. Rosenn et al[36] have demonstrated by CGM that 30% of pregnant women with type 1 diabetes mellitus experience at least three episodes of hypoglycemia during 2 wk. In such patients, it is possible to perform safer and more appropriate insulin therapy by performing CGM.
INDICATORS OF GLYCEMIC CONTROL
Plasma glucose measurement is important as part of glycemic control during pregnancy; however, it is actually difficult to measure blood glucose in all patients. Therefore, it is necessary to evaluate glycemic control condition using indicators of glycemic control such as HbA1c, glycated albumin (GA), fructosamine, and 1,5-AG. Each of these indicators of glycemic control has different characteristics as well as advantages and disadvantages[37,38]. In addition, there are both appropriate and inappropriate indicators of glycemic control during pregnancy. The following sections give an outline of these indicators of glycemic control.
HbA1c
HbA1c is a ketoamine formed from nonenzymatic reaction and binding between the aldehyde group of glucose and valine at the N-terminus of the hemoglobin β-chain. Because the life span of red blood cells is 120 d, HbA1c reflects glycemic control status during the past 1 to 2 mo. Specifically, the following findings have been reported: 50% reflect plasma glucose level during the past 1 mo; 25% reflect plasma glucose level during the past 1 to 2 mo; 25% reflect plasma glucose level during the past 2 to 4 mo[39]. Since the Diabetes Control and Complications Trial study[40], extensive evidence on the development and progress of complications has been gathered, and HbA1c has certainly become a gold standard indicator of glycemic control. Therefore, it is recommended to maintain excellent glycemic control in pregnant women with diabetes mellitus or patients with GDM using SMBG and HbA1c as indicators[41]. However, pregnant women are usually excluded from clinical studies of complications, and therefore little evidence has been obtained from pregnant women. Because chronic diabetic complications usually do not develop within a period as short as several months, there is no problem in using HbA1c as an indicator; however, there is little benefit in discussing glycemic control status during the past 1 to 2 mo of pregnancy. In addition, it is well known that HbA1c is influenced by the life span of red blood cells. Moreover, we have reported that for premenopausal women, HbA1c is significantly higher not only in women with iron deficiency anemia but also in women with iron deficiency compared with HbA1c in women without iron deficiency[42,43]. It is well known that the demand for iron increases during the end stage of pregnancy and that most mothers experience iron deficiency anemia; therefore, HbA1c may be higher relative to plasma glucose level during the end stage of pregnancy. The time course of HbA1c during pregnancy will be explained in detail later.
Fructosamine
Protein undergoes glycation reaction in accordance with plasma glucose concentration, and ketoamine, an early Maillard reaction product, is produced via aldimine. Because the side chain binding of ketoamine has the fructose structure, ketoamine is generically named fructosamine. Fructose-lysine (fructosamine), in which glucose is bound to lysine residue of protein, has a reducing ability under alkaline conditions; glycemic control is measured using this reducing ability. A large part of such measurement is made by the fructosamine method; glycemic control is measured by colorimetric determination by producing reduction color reaction using nitroblue tetrazolium as a substrate. Because 60% to 70% of serum protein is albumin, the main component of fructosamine is GA, but fructosamine contains other components such as glycated lipoprotein and glycation globulin. Fructosamine is not influenced by anemia or abnormal hemoglobin. In addition, albumin, which accounts for the majority of serum protein, has a faster turnover than hemoglobin; therefore, short-term glycemic control can be evaluated by measuring fructosamine[44]. In hyperthyroidism[45,46] and nephrotic syndrome[47] in which protein (albumin) metabolism is increased, fructosamine measured by this method is low; in hypothyroidism[45] in which protein (albumin) metabolism is delayed, fructosamine measured by this method is high.
HbA1c is a glycation product of hemoglobin (single protein) and GA is a glycation product of albumin (single protein); on the other hand, fructosamine is the generic name of all glycated proteins and lacks specificity. Because 60% to 70% of serum protein is albumin, the characteristics of fructosamine are similar to those of GA. However, this method measures other glycated proteins as well; therefore, there is a problem that in myeloma, fructosamine measured by this method is high[48]. In addition, it has been reported that fructosamine is associated with a larger intra-individual variability compared with HbA1c, and fructosamine is disadvantageous for detecting a significant change[49]. HbA1c is expressed as the ratio of hemoglobin and GA is expressed as the ratio of albumin; therefore, HbA1c and GA are not influenced by dilution of serum. On the other hand, fructosamine is expressed as reducing ability per 1 mL of serum; therefore, fructosamine is influenced by serum protein concentration, and in dilutional anemia, fructosamine measured by this method is apparently low. In this respect, fructosamine measured by this method is likely to be influenced by dilutional anemia which may develop during pregnancy. Because fructosamine is measured by colorimetric determination produced by reduction color reaction, it is influenced by substances with reducing ability such as bilirubin. It is considered that the effects of ascorbic acid and vitamin E are small; however, if they are consumed in large amounts, measurement of fructosamine may be influenced.
GA
GA is a ketoamine formed from nonenzymatic reaction and binding between four lysine residues of albumin and glucose. In other words, GA is an amadori compound similar to HbA1c, but it has been reported that the binding rate between albumin and glucose is 4.5 times higher than that between hemoglobin and glucose[50]. Because the half-life of albumin is about 14 d, GA is an indicator of glycemic control during a shorter period (during the past 2 to 3 wk) compared with HbA1c. In addition, it is known that GA reflects postprandial plasma glucose more accurately than HbA1c[37]. In the management of abnormal glucose metabolism during pregnancy, evaluation of mean plasma glucose level at a time point closer to the time of consultation with a doctor and evaluation of postprandial plasma glucose level are important, and GA is useful in this respect. Moreover, we have already reported that GA is not influenced by iron deficiency anemia or iron deficiency state[43]. It should be noted that because GA is influenced by albumin metabolism, evaluation of measured GA levels requires attention in conditions such as nephrotic syndrome[51] and abnormal thyroid function[52]. Unlike fructosamine, GA is not influenced by dilutional anemia during pregnancy. The time course of GA during pregnancy will be explained in detail later.
1,5-AG
1,5-AG is a polyol with a structure in which hydroxyl at the first position of glucose is reduced; it is contained in a wide variety of food, but is hardly metabolized in the body[53]. When plasma glucose is within the normal range, 1,5-AG is filtered in the kidney and then reabsorbed completely; therefore, serum 1,5-AG remains unchanged.
Usually, about 180 g of glucose is excreted from glomeruli daily, but almost 100% of the excreted glucose is reabsorbed by sodium glucose cotransporter 2 (SGLT2) which is a glucose-specific transporter and located in proximal tubules and SGLT1 which is a transporter for glucose and galactose and located downstream of SGLT2[54]. When diabetes mellitus develops, excretion of glucose increases; if excretion of glucose exceeds the reabsorptive capacity of SGLT2 and SGLT1, reabsorption of glucose by 1,5-AG/mannose/fructose cotransporter (sodium glucose cotransporter 4: SGLT4) which is present downstream from SGLT2 and SGLT1 takes place. Usually, there is no glucose in locations where SGLT4 is present; therefore, 99.9% of 1,5-AG is reabsorbed by SGLT4; however, because SGLT4 reabsorbs glucose as well, if the inflow of glucose into tubules increases, reabsorption of 1,5-AG is inhibited[55-57]. Specifically, if plasma glucose level exceeds 180 mg/dL, glucose is excreted in urine; therefore, 1,5-AG is also excreted in urine and serum 1,5-AG decreases.
Because of this mechanism, serum 1,5-AG reflects glycemic status during the past 24 h and is used as an indicator of very short-term glycemic control[58,59]. In addition, serum 1,5-AG is an indicator which reflects postprandial hyperglycemia more accurately than HbA1c[60,61]. It should be noted that in patients with marked hyperglycemia and a large urinary glucose excretion, even if glycemic control improves, serum 1,5-AG does not increase in a short period because the 1,5-AG pool in the body has decreased.
Because serum 1,5-AG is influenced by the threshold for urinary glucose excretion as well, serum 1,5-AG is low in renal glycosuria in which the threshold decreases. In chronic renal failure[62-64] in which reabsorption of 1,5-AG decreases, 1,5-AG is low because of transient glucosuria. In other conditions such as oxyhyperglycemia[65], patients receiving long-term hyperalimentation[66], and liver cirrhosis[67,68], serum 1,5-AG is abnormally low. On the other hand, one of the causes of abnormally high 1,5-AG levels is oral administration of Ninjin-yoei-to and Kami-kihi-to[69] which contain a large amount of 1,5-AG.
It has been reported that because the threshold for glucose in the kidney decreases during pregnancy, glucose tolerance may not change, and glucosuria may appear[70]. It has also been reported that serum 1,5-AG during pregnancy is low because of this mechanism[71]. Therefore, serum 1,5-AG during pregnancy does not reflect glycemic control accurately and is not an appropriate indicator of glycemic control.
CHANGE IN INDICATORS OF GLYCEMIC CONTROL DURING PREGNANCY
Change in indicators of glycemic control during normal pregnancy
In the past, Phelps et al[72] reported the time course of HbA1c during pregnancy in 377 non-diabetic pregnant women and the time course of plasma glucose level at 1 h after 50 g oral glucose loading in 1756 normal pregnant women. It was demonstrated that HbA1c shows a biphasic change with the trough level occurring at week 24 of pregnancy and that 1 h plasma glucose level also shows a biphasic change with the trough level occurring at week 20 of pregnancy. In a report of the Japanese Society of Diabetes and Pregnancy[73], similar tendencies were shown; according to an analysis of 574 normal pregnant women, HbA1c tends to decrease during the middle stage of pregnancy and increase during the end stage of pregnancy, and GA tends to decrease gradually toward the end stage of pregnancy (Figure 1). Judging from this report, the reference range in Japanese normal pregnant women was considered to be 4.4% to 5.7% for HbA1c and 11.5% to 15.7% for GA. In any case, there is undoubtedly a difference between the time course of HbA1c and GA during pregnancy, so which indicator of glycemic control is reliable? We investigated the effect of iron deficiency on HbA1c in 17 normal pregnant women[74]. HbA1c increased significantly from the middle stage of pregnancy (wk: 20-23) to the end stage of pregnancy (wk: 32-33) (4.7% ± 0.2% vs 5.1% ± 0.2%; P < 0.0001). On the other hand, GA showed no significant change. Mean corpuscular hemoglobin (MCH), transferrin saturation, and serum ferritin, which are indicators of iron deficiency, showed a decrease toward the end stage of pregnancy; there was a significant negative correlation between HbA1c and MCH, transferrin saturation, and serum ferritin (Figure 2). On the other hand, there was no significant correlation between GA and MCH, transferrin saturation, and serum ferritin. Based on the above findings, it is considered that in normal pregnant women, iron deficiency progresses during the end stage of pregnancy, and therefore HbA1c level increases. That is, HbA1c during pregnancy may not be a reliable indicator of glycemic control, especially during the end stage of pregnancy. However, if pregnant women take in a sufficient amount of iron during pregnancy, increase of HbA1c may not occur from the middle stage to the end stage of pregnancy as shown in Figure 1.
Figure 1.
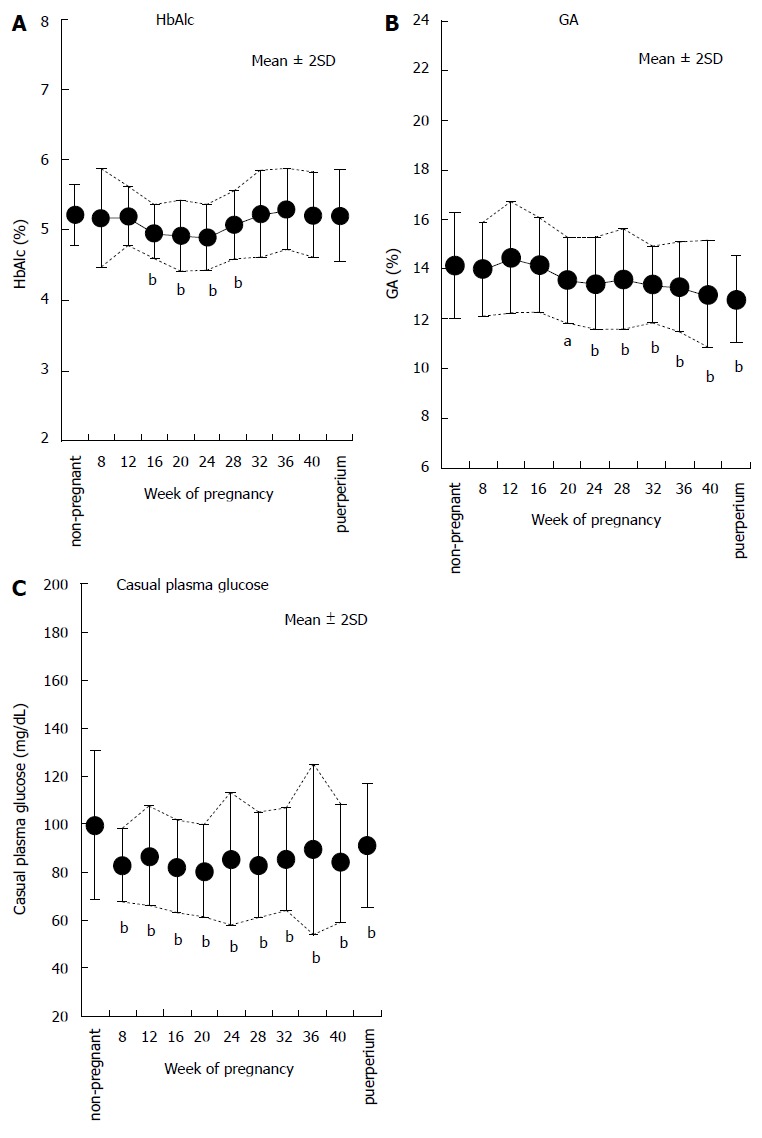
Time courses of indicators of glycemic control in normal pregnant women. The time courses of HbA1c (A), GA (B), and casual plasma glucose (C) in normal pregnant women are shown (modified from Ref.[73]). aP < 0.05, bP < 0.01 vs non-pregnant women. HbA1c: Hemoglobin A1c; GA: Glycated albumin.
Figure 2.
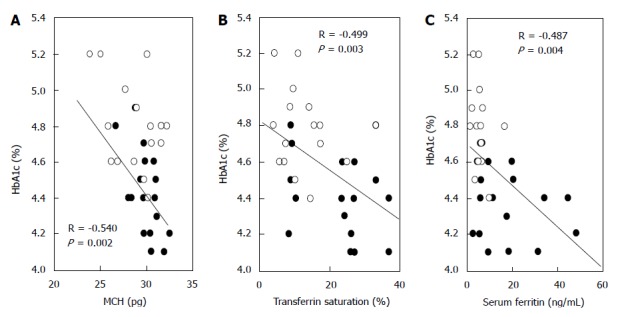
Correlations between hemoglobin A1c and indicators of iron deficiency in normal pregnant women. The correlations between HbA1c and mean corpuscular hemoglobin (MCH) (A), transferrin saturation (B), and serum ferritin (C) in normal pregnant women are shown (modified from Reference[74]). ●: Middle stage of pregnancy (wk: 20-23); ○: End stage of pregnancy (wk: 32-33); HbA1c: Hemoglobin A1c.
Change in indicators of glycemic control of women with GDM and overt diabetes mellitus
Glycemic control status is important in pregnant women with diabetes mellitus and patients with GDM. For the time courses of HbA1c and GA in pregnant women with diabetes mellitus and patients with GDM as well, the GA Study Group of the Japanese Society of Diabetes and Pregnancy has issued a detailed report[75]. According to this report, in 193 pregnant women with diabetes mellitus and patients with GDM, HbA1c decreased during the middle stage of pregnancy and then increased during the end stage of pregnancy, as in the case of normal pregnant women. On the other hand, GA decreased as the gestational age advanced (Figure 3). However, gestational diabetes was not distinguished from pregnancy complicated with preexisting diabetes in this report. The time courses of HbA1c and GA were similar to those observed in normal pregnant women; as expected, there was a difference between time courses of different indicators of glycemic control. Therefore, we made a similar investigation in 11 pregnant women with diabetes mellitus (7 patients with type 1 diabetes mellitus and 4 patients with type 2 diabetes mellitus) and 6 patients with GDM[76]. As in the case of normal pregnant women, HbA1c increased significantly from the middle stage of pregnancy (wk: 20-23) to the end stage of pregnancy (wk: 32-35) (5.8% ± 0.7% vs 6.1% ± 0.6%; P < 0.05), whereas GA showed no significant change. During the end stage of pregnancy, MCH, transferrin saturation, and serum ferritin level decreased, and there was a significant positive correlation between transferrin saturation and the GA/HbA1c ratio. These results show that in pregnant women with diabetes mellitus and patients with GDM, iron deficiency progresses, and HbA1c increases during the end stage of pregnancy.
Figure 3.
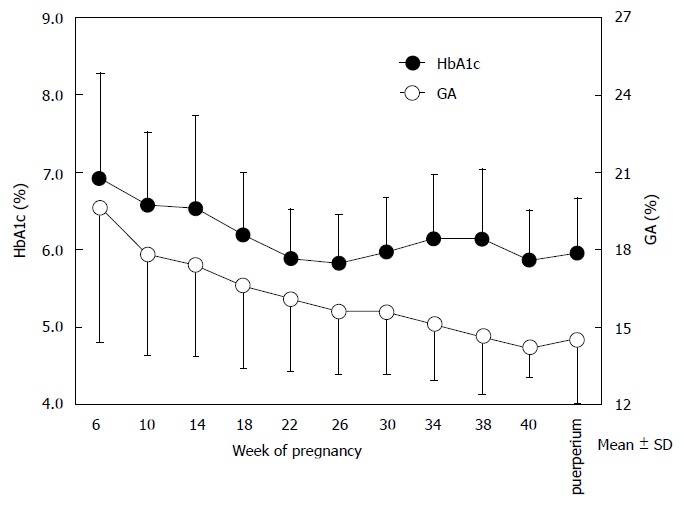
Time courses of hemoglobin A1c and glycated albumin in pregnant women with diabetes mellitus and patients with gestational diabetes mellitus. The time courses of HbA1c (closed circles) and GA (open circles) in pregnant women with diabetes mellitus and patients with gestational diabetes mellitus are shown (modified from Hiramatsu et al[73]). HbA1c: Hemoglobin A1c. GA: Glycated albumin.
We introduce a typical pregnant woman with diabetes mellitus (36-year-old woman) as one of our patients. She was not obese before pregnancy (BMI before pregnancy was 22.7 kg/m2) and treatment with insulin therapy was started before pregnancy. As the gestational age advanced, HbA1c increased from 6.2% (wk 18) to 7.3% (wk 37), which suggested worsening of glycemic control status. However, GA was stable between 17.2% (wk 18) and 16.6% (wk 37). In contrast, serum ferritin decreased from 22.8 ng/mL (wk 18) to 7.5 ng/mL (wk 37) during this period (Figure 4A). Because the time course of HbA1c and serum ferritin formed a mirror image, it was considered that the increase in HbA1c during the end stage of pregnancy was not due to poor glycemic control status but due to iron deficiency anemia (iron deficiency state). We emphasize that HbA1c (R = -0.935, P < 0.001), but not GA (R = 0.322, P = 0.534), was negatively correlated with serum ferritin (Figure 4B). In conclusion, GA is not influenced by iron deficiency and so is a reliable indicator of glycemic control.
Figure 4.
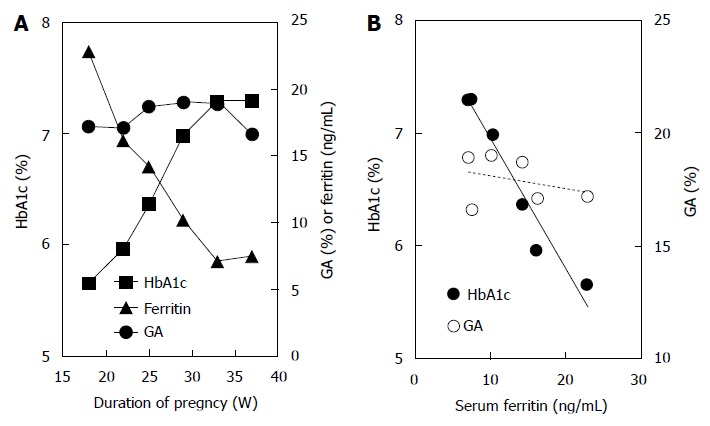
Time courses of hemoglobin A1c and glycated albumin and correlations between serum ferritin and hemoglobin A1c or glycated albumin in a pregnant woman with diabetes mellitus. A: The time courses of HbA1c (closed squares), GA (closed circles), and serum ferritin (closed triangles) in a pregnant woman with diabetes mellitus (a 36-year-old woman with type 2 diabetes mellitus receiving insulin therapy), are shown; B: Correlations between serum ferritin and HbA1c (R = -0.975, P < 0.001) or GA (R = 0.322, P = 0.534) in a pregnant woman with diabetes mellitus are shown. HbA1c: Hemoglobin A1c; GA: Glycated albumin.
ASSOCIATION BETWEEN INDICATORS OF GLYCEMIC CONTROL AND COMPLICATIONS IN THE PERINATAL PERIOD
The GA Study Group of the Japanese Society of Diabetes and Pregnancy has analyzed the association between outcomes (neonatal complications and birth weight) and indicators of glycemic control (HbA1c and GA)[75]. The analysis was made considering the upper limits in normal pregnant women (HbA1c: 5.7%; GA: 15.7%); for neonatal complications, the incidences of neonatal hypoglycemia, polycythemia, and respiratory disorder were found to be significantly higher in the group of women with GA of more than 15.7% (Figure 5). In addition, it was reported that the incidence of large-for gestational age was also significantly higher in the group of women with GA of more than 15.7% compared with the group of women with GA of 15.7% or less. On the other hand, it was reported that there was no significant increase in the incidence in the group of women with HbA1c of more than 5.7% compared with the group of women with HbA1c of 5.7% or less. Although a more accurate judgment should be made by ROC analysis for different cut-offs, GA is superior to HbA1c for prediction of perinatal complications. Furthermore, appropriate regression analysis is necessary to see if the indicator remains significant after eliminating the iron factors. As we demonstrated in our patients, if HbA1c is apparently high during the end stage of pregnancy, it may be misinterpreted that glycemic control has worsened and excessive insulin therapy may be performed, leading to hypoglycemia and increased incidence of perinatal complications of mothers and infants. Hence, management based on GA is essential during pregnancy also from the viewpoint of perinatal complications.
Figure 5.
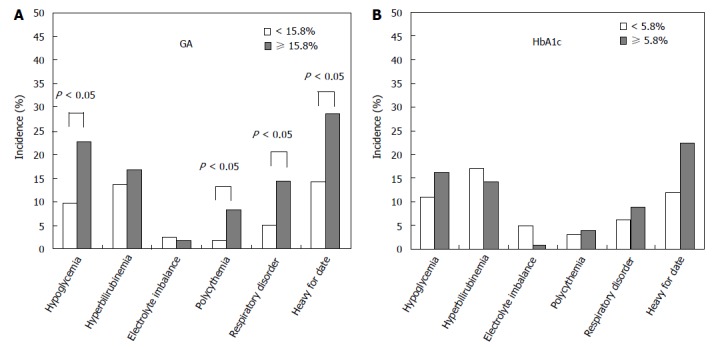
Comparison between glycated albumin and hemoglobin A1c during pregnancy and the incidence of neonatal complications. For GA (A) and HbA1c (B) measured during the end stage of pregnancy, the incidence of neonatal complications was compared between the group of women whose GA or HbA1c was within the reference range (GA < 15.8%; HbA1c < 5.8%) and the group of women whose GA or HbA1c exceeded the reference range (GA ≥ 15.8%; HbA1c ≥ 5.8%) (modified from Shimizu et al[75]). HbA1c: Hemoglobin A1c; GA: Glycated albumin.
CONCLUSION
We outlined indicators of glycemic control in abnormal glucose metabolism during pregnancy. As explained, it is insufficient during pregnancy to use HbA1c as an indicator of glycemic control; glycemic control using GA is recommended. It is necessary to measure HbA1c to enable comparison with the large amount of data accumulated so far; the goal of management of abnormal glucose metabolism during pregnancy might be to maintain GA within the normal range (15.7% or less). However, because little data from clinical studies is available, large-population epidemiological studies would be necessary in order to confirm our proposal.
Footnotes
Conflict-of-interest statement: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 29, 2014
First decision: January 20, 2015
Article in press: May 6, 2015
P- Reviewer: García Mayor R, Guerrero-Romero F, Masaki T S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 6th ed. [accessed 2013 November] Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf.
- 2.Sugiyama T, Kusaka H, Sagawa N, Toyoda N. Universal screening tests for gestational diabetes mellitus in Japan. Diabetes and Pregnancy. 2006;6:7–12. [Google Scholar]
- 3.Evers IM, de Valk HW, Mol BW, ter Braak EW, Visser GH. Macrosomia despite good glycaemic control in Type I diabetic pregnancy; results of a nationwide study in The Netherlands. Diabetologia. 2002;45:1484–1489. doi: 10.1007/s00125-002-0958-7. [DOI] [PubMed] [Google Scholar]
- 4.Lauenborg J, Mathiesen E, Ovesen P, Westergaard JG, Ekbom P, Mølsted-Pedersen L, Damm P. Audit on stillbirths in women with pregestational type 1 diabetes. Diabetes Care. 2003;26:1385–1389. doi: 10.2337/diacare.26.5.1385. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 6.Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 7.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, Kalhan SC, Catalano PM. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 8.Nagira K, Sasaoka T, Wada T, Fukui K, Ikubo M, Hori S, Tsuneki H, Saito S, Kobayashi M. Altered subcellular distribution of estrogen receptor alpha is implicated in estradiol-induced dual regulation of insulin signaling in 3T3-L1 adipocytes. Endocrinology. 2006;147:1020–1028. doi: 10.1210/en.2005-0825. [DOI] [PubMed] [Google Scholar]
- 9.Wada T, Hori S, Sugiyama M, Fujisawa E, Nakano T, Tsuneki H, Nagira K, Saito S, Sasaoka T. Progesterone inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2010;298:E881–E888. doi: 10.1152/ajpendo.00649.2009. [DOI] [PubMed] [Google Scholar]
- 10.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165:1667–1672. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 11.Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, Sims EA. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264:E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 13.Landon MB, Catalano PM, Gabbe SG. Diabetes mellitus complicating pregnancy. In: Gabbe SG, Niebl JR, Galan HL, Jauniaux ERM, Landon MB, et al., editors. Obstetrics: Normal and problem pregnancies, 6th ed. Philadelphia, Saunders; 2012. pp. 887–921. [Google Scholar]
- 14.Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Cianni G, Seghieri G, Lencioni C, Cuccuru I, Anichini R, De Bellis A, Ghio A, Tesi F, Volpe L, Del Prato S. Normal glucose tolerance and gestational diabetes mellitus: what is in between? Diabetes Care. 2007;30:1783–1788. doi: 10.2337/dc07-0119. [DOI] [PubMed] [Google Scholar]
- 16.Saisho Y, Miyakoshi K, Tanaka M, Shimada A, Ikenoue S, Kadohira I, Yoshimura Y, Itoh H. Beta cell dysfunction and its clinical significance in gestational diabetes. Endocr J. 2010;57:973–980. doi: 10.1507/endocrj.k10e-231. [DOI] [PubMed] [Google Scholar]
- 17.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson JD, Cousins L. A population-based study of maternal and perinatal outcome in patients with gestational diabetes. Am J Obstet Gynecol. 1989;161:981–986. doi: 10.1016/0002-9378(89)90767-9. [DOI] [PubMed] [Google Scholar]
- 20.Goldman M, Kitzmiller JL, Abrams B, Cowan RM, Laros RK. Obstetric complications with GDM. Effects of maternal weight. Diabetes. 1991;40 Suppl 2:79–82. doi: 10.2337/diab.40.2.s79. [DOI] [PubMed] [Google Scholar]
- 21.Spellacy WN, Buhi WC, Bradley B, Holsinger KK. Maternal, fetal and amniotic fluid levels of glucose, insulin and growth hormone. Obstet Gynecol. 1973;41:323–331. [PubMed] [Google Scholar]
- 22.Dashe JS, Nathan L, McIntire DD, Leveno KJ. Correlation between amniotic fluid glucose concentration and amniotic fluid volume in pregnancy complicated by diabetes. Am J Obstet Gynecol. 2000;182:901–904. doi: 10.1016/s0002-9378(00)70343-7. [DOI] [PubMed] [Google Scholar]
- 23.McFarland MB, Trylovich CG, Langer O. Anthropometric differences in macrosomic infants of diabetic and nondiabetic mothers. J Matern Fetal Med. 1998;7:292–295. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<292::AID-MFM7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Cousins L. Obstetric complications. In: Reece EA, Coustan DR, editors. Diabetes mellitus in pregnancy. Churchill Livingstone, New York; 1995. pp. 287–302. [Google Scholar]
- 25.Hanson U, Persson B. Outcome of pregnancies complicated by type 1 insulin-dependent diabetes in Sweden: acute pregnancy complications, neonatal mortality and morbidity. Am J Perinatol. 1993;10:330–333. doi: 10.1055/s-2007-994754. [DOI] [PubMed] [Google Scholar]
- 26.Nordlander E, Hanson U, Persson B. Factors influencing neonatal morbidity in gestational diabetic pregnancy. Br J Obstet Gynaecol. 1989;96:671–678. doi: 10.1111/j.1471-0528.1989.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 27.Suhonen L, Teramo K. Hypertension and pre-eclampsia in women with gestational glucose intolerance. Acta Obstet Gynecol Scand. 1993;72:269–272. doi: 10.3109/00016349309068036. [DOI] [PubMed] [Google Scholar]
- 28.Suehara S, Waguri M, Wakabayashi K, Nakanishi I. Frequency and congenital anomaly of pregnant women with diabetes mellitus and gestational diabetes mellitus. Diabetes and Pregnancy. 2010;10:104–108. [Google Scholar]
- 29.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954;16:330–342. doi: 10.1530/acta.0.0160330. [DOI] [PubMed] [Google Scholar]
- 30.Nold JL, Georgieff MK. Infants of diabetic mothers. Pediatr Clin North Am. 2004;51:619–637, viii. doi: 10.1016/j.pcl.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Langer O, Berkus M, Brustman L, Anyaegbunam A, Mazze R. Rationale for insulin management in gestational diabetes mellitus. Diabetes. 1991;40 Suppl 2:186–190. doi: 10.2337/diab.40.2.s186. [DOI] [PubMed] [Google Scholar]
- 32.Langer O, Levy J, Brustman L, Anyaegbunam A, Merkatz R, Divon M. Glycemic control in gestational diabetes mellitus--how tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol. 1989;161:646–653. doi: 10.1016/0002-9378(89)90371-2. [DOI] [PubMed] [Google Scholar]
- 33.de Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, Evans AT. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–1241. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 34.Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191:949–953. doi: 10.1016/j.ajog.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 35.Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K, Fowler D, Campbell PJ, Temple RC. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680. doi: 10.1136/bmj.a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenn BM, Miodovnik M, Holcberg G, Khoury JC, Siddiqi TA. Hypoglycemia: the price of intensive insulin therapy for pregnant women with insulin-dependent diabetes mellitus. Obstet Gynecol. 1995;85:417–422. doi: 10.1016/0029-7844(94)00415-A. [DOI] [PubMed] [Google Scholar]
- 37.Koga M. Glycated albumin; clinical usefulness. Clin Chim Acta. 2014;433:96–104. doi: 10.1016/j.cca.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Koga M. 1,5-Anhydroglucitol and glycated albumin in glycemia. Adv Clin Chem. 2014;64:269–301. doi: 10.1016/b978-0-12-800263-6.00007-0. [DOI] [PubMed] [Google Scholar]
- 39.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440–447. doi: 10.2337/diacare.18.4.440. [DOI] [PubMed] [Google Scholar]
- 40.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 41.Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, Yogev Y. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4227–4249. doi: 10.1210/jc.2013-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koga M, Morita S, Saito H, Mukai M, Kasayama S. Association of erythrocyte indices with glycated haemoglobin in pre-menopausal women. Diabet Med. 2007;24:843–847. doi: 10.1111/j.1464-5491.2007.02161.x. [DOI] [PubMed] [Google Scholar]
- 43.Koga M, Saito H, Mukai M, Matsumoto S, Kasayama S. Influence of iron metabolism indices on glycated haemoglobin but not glycated albumin levels in premenopausal women. Acta Diabetol. 2010;47 Suppl 1:65–69. doi: 10.1007/s00592-009-0123-6. [DOI] [PubMed] [Google Scholar]
- 44.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153–2163. [PubMed] [Google Scholar]
- 45.Ford HC, Lim WC, Crooke MJ. Hemoglobin A1 and serum fructosamine levels in hyperthyroidism. Clin Chim Acta. 1987;166:317–321. doi: 10.1016/0009-8981(87)90435-9. [DOI] [PubMed] [Google Scholar]
- 46.Sako Y, Umeda F, Hashimoto T, Haji M, Nawata H. Serum fructosamine in assessment of diabetic control and relation to thyroid function. Horm Metab Res. 1989;21:669–672. doi: 10.1055/s-2007-1009316. [DOI] [PubMed] [Google Scholar]
- 47.Constanti C, Simo JM, Joven J, Camps J. Serum fructosamine concentration in patients with nephrotic syndrome and with cirrhosis of the liver: the influence of hypoalbuminaemia and hypergammaglobulinaemia. Ann Clin Biochem. 1992;29(Pt4):437–442. doi: 10.1177/000456329202900412. [DOI] [PubMed] [Google Scholar]
- 48.Montagna MP, Laghi F, Cremona G, Zuppi C, Barbaresi G, Castellana ML. Influence of serum proteins on fructosamine concentration in multiple myeloma. Clin Chim Acta. 1991;204:123–130. doi: 10.1016/0009-8981(91)90223-y. [DOI] [PubMed] [Google Scholar]
- 49.Howey JE, Bennet WM, Browning MC, Jung RT, Fraser CG. Clinical utility of assays of glycosylated haemoglobin and serum fructosamine compared: use of data on biological variation. Diabet Med. 1989;6:793–796. doi: 10.1111/j.1464-5491.1989.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 50.Ueda Y, Matsumoto H. Recent topics in chemical and clinical research on glycated albumin. J Diabetes Sci Technol. 2015;9:177–182. doi: 10.1177/1932296814567225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okada T, Nakao T, Matsumoto H, Nagaoka Y, Tomaru R, Iwasawa H, Wada T. Influence of proteinuria on glycated albumin values in diabetic patients with chronic kidney disease. Intern Med. 2011;50:23–29. doi: 10.2169/internalmedicine.50.4129. [DOI] [PubMed] [Google Scholar]
- 52.Koga M, Murai J, Saito H, Matsumoto S, Kasayama S. Effects of thyroid hormone on serum glycated albumin levels: study on non-diabetic subjects. Diabetes Res Clin Pract. 2009;84:163–167. doi: 10.1016/j.diabres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Yamanouchi T, Tachibana Y, Akanuma H, Minoda S, Shinohara T, Moromizato H, Miyashita H, Akaoka I. Origin and disposal of 1,5-anhydroglucitol, a major polyol in the human body. Am J Physiol. 1992;263:E268–E273. doi: 10.1152/ajpendo.1992.263.2.E268. [DOI] [PubMed] [Google Scholar]
- 54.Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280:F10–F18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]
- 55.Yamanouchi T, Ogata N, Tagaya T, Kawasaki T, Sekino N, Funato H, Akaoka L, Miyashita H. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet. 1996;347:1514–1518. doi: 10.1016/s0140-6736(96)90672-8. [DOI] [PubMed] [Google Scholar]
- 56.Yamanouchi T, Shinohara T, Ogata N, Tachibana Y, Akaoka I, Miyashita H. Common reabsorption system of 1,5-anhydro-D-glucitol, fructose, and mannose in rat renal tubule. Biochim Biophys Acta. 1996;1291:89–95. doi: 10.1016/0304-4165(96)00050-5. [DOI] [PubMed] [Google Scholar]
- 57.Tazawa S, Yamato T, Fujikura H, Hiratochi M, Itoh F, Tomae M, Takemura Y, Maruyama H, Sugiyama T, Wakamatsu A, et al. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose, 1,5-anhydro-D-glucitol, and fructose. Life Sci. 2005;76:1039–1050. doi: 10.1016/j.lfs.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5:355–363. doi: 10.1089/152091503765691839. [DOI] [PubMed] [Google Scholar]
- 59.Dungan KM. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8:9–19. doi: 10.1586/14737159.8.1.9. [DOI] [PubMed] [Google Scholar]
- 60.Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29:1214–1219. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
- 61.Stettler C, Stahl M, Allemann S, Diem P, Schmidlin K, Zwahlen M, Riesen W, Keller U, Christ E. Association of 1,5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care. 2008;31:1534–1535. doi: 10.2337/dc08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimizu H, Shouzu A, Nishikawa M, Omoto S, Hayakawa T, Miyake Y, Yonemoto T, Inada M. Serum concentration and renal handling of 1,5-anhydro-D-glucitol in patients with chronic renal failure. Ann Clin Biochem. 1999;36(Pt 6):749–754. doi: 10.1177/000456329903600608. [DOI] [PubMed] [Google Scholar]
- 63.Emoto M, Tabata T, Inoue T, Nishizawa Y, Morii H. Plasma 1,5-anhydroglucitol concentration in patients with end-stage renal disease with and without diabetes mellitus. Nephron. 1992;61:181–186. doi: 10.1159/000186868. [DOI] [PubMed] [Google Scholar]
- 64.Kim WJ, Park CY, Lee KB, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW. Serum 1,5-anhydroglucitol concentrations are a reliable index of glycemic control in type 2 diabetes with mild or moderate renal dysfunction. Diabetes Care. 2012;35:281–286. doi: 10.2337/dc11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murai J, Koga M, Saito H, Mukai M, Kasayama S. Serum 1,5-anhydroglucitol is low in gastrectomized men. Acta Diabetol. 2014;51:337–338. doi: 10.1007/s00592-011-0354-1. [DOI] [PubMed] [Google Scholar]
- 66.Yamanouchi T, Minoda S, Ogata N, Tachibana Y, Sekino N, Miyashita H, Akaoka I. Prolonged hyperalimentation as a possible cause of renal tubular dysfunction: evaluation of 1,5-anhydro-D-glucitol resorption and N-acetylglucosaminidase excretion in humans. Clin Sci (Lond) 1995;88:203–210. doi: 10.1042/cs0880203. [DOI] [PubMed] [Google Scholar]
- 67.Yamagishi S, Ohta M. Serum 1,5-anhydro-D-glucitol levels in liver cirrhosis. Acta Diabetol. 1998;35:65–66. doi: 10.1007/s005920050104. [DOI] [PubMed] [Google Scholar]
- 68.Koga M, Murai J, Saito H, Mukai M, Toya D, Tanaka N, Kanehara H, Bando Y, Kasayama S. 1,5-Anhydroglucitol levels are low irrespective of plasma glucose levels in patients with chronic liver disease. Ann Clin Biochem. 2011;48:121–125. doi: 10.1258/acb.2010.010053. [DOI] [PubMed] [Google Scholar]
- 69.Kawasaki T, Yamanouchi T, Kashiwabara A, Inoue T, Yoshimura T, Fujimori S, Tanabe T, Aiso Y. The influence of traditional Chinese herbal drugs on serum 1, 5-anhydroglucitol levels. Diabetes Res Clin Pract. 2000;50:97–101. doi: 10.1016/s0168-8227(00)00167-4. [DOI] [PubMed] [Google Scholar]
- 70.Davison JM, Hytten FE. The effect of pregnancy on the renal handling of glucose. Br J Obstet Gynaecol. 1975;82:374–381. doi: 10.1111/j.1471-0528.1975.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 71.Tetsuo M, Hamada T, Yoshimatsu K, Ishimatsu J, Matsunaga T. Serum levels of 1,5-anhydro-D-glucitol during the normal and diabetic pregnancy and puerperium. Acta Obstet Gynecol Scand. 1990;69:479–485. doi: 10.3109/00016349009013322. [DOI] [PubMed] [Google Scholar]
- 72.Phelps RL, Honig GR, Green D, Metzger BE, Frederiksen MC, Freinkel N. Biphasic changes in hemoglobin A1c concentrations during normal human pregnancy. Am J Obstet Gynecol. 1983;147:651–653. doi: 10.1016/0002-9378(83)90443-x. [DOI] [PubMed] [Google Scholar]
- 73.Hiramatsu Y, Shimizu I, Omori Y, Nakabayashi M. Determination of reference intervals of glycated albumin and hemoglobin A1c in healthy pregnant Japanese women and analysis of their time courses and influencing factors during pregnancy. Endocr J. 2012;59:145–151. doi: 10.1507/endocrj.k10e-410. [DOI] [PubMed] [Google Scholar]
- 74.Hashimoto K, Noguchi S, Morimoto Y, Hamada S, Wasada K, Imai S, Murata Y, Kasayama S, Koga M. A1C but not serum glycated albumin is elevated in late pregnancy owing to iron deficiency. Diabetes Care. 2008;31:1945–1948. doi: 10.2337/dc08-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimizu I, Hiramatsu Y, Omori Y, Nakabayashi M. Glycated albumin reflects maternal and perinatal outcome in a multicenter study in Japan. Diabetes and Pregnancy. 2010;10:27–31. [Google Scholar]
- 76.Hashimoto K, Osugi T, Noguchi S, Morimoto Y, Wasada K, Imai S, Waguri M, Toyoda R, Fujita T, Kasayama S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care. 2010;33:509–511. doi: 10.2337/dc09-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]


