Abstract
Available data suggest a possible link between abnormal vitamin D level and abnormal glucose homeostasis, two of the most common chronic medical conditions. Both conditions are associated with inflammation, and the exact mechanism for role of either on the other is not well clear. Literature investigating the link between vitamin D and either pre-diabetic states or diabetes is reviewed. Vitamin D deficiency is detrimental to insulin synthesis and secretion in animal and human studies. In humans, it has been shown by majority of observational studies, that vitamin D is positively correlated with insulin sensitivity and its role is mediated both by direct mechanism through the availability of vitamin D receptors in several tissues and indirectly through the changes in calcium levels. Large number of, but not all, variable samples cross sectional human trials have demonstrated an inverse relation between vitamin D status and impaired glucose tolerance, insulin resistance or diabetes. To compliment this conclusively, evidence from intervention studies is critically warranted before we can frankly state that vitamin D plays a role in diabetes prevention or treatment. Absence of both sizable prospective observational trials utilizing 25(OH)D as the main variable and the non-availability of randomized studies specifically designed to assess the effects of vitamin D on pre-diabetes and diabetes states, are the main obstacles to draw solid and conclusive relationships.
Keywords: Vitamin D, Insulin resistance, Type 2 diabetes
Core tip: A potential role for abnormal vitamin D level in changes of glucose homeostasis has been described. It has been demonstrated that deficient vitamin D status is detrimental to the synthesis and secretion of insulin in animal and human studies. In several, but not all, human observational trials, an inverse correlation was seen between vitamin D with insulin insensitivity, pre-diabetic states and dysglycemia. However, evidence from randomized interventional studies assessing the effects of changes in vitamin D status on markers of dysglycemia and diabetes prevention is not available. Therefore, firm and true protective influence of vitamin D on glucose homeostasis remains to be defined.
INTRODUCTION
The widely common medical conditions, low vitamin D level and diabetes with its proceeding pre-diabetic state of insulin resistance, have become two of the most common chronic medical conditions diagnosed in modern years, both in developing and developed countries. There are around 387 million diabetic patients worldwide in year of 2014 and that number is projected to increase by 55% in 2035, as Africa is the highest projected region to have increased prevalence followed by the Middle East. The prevalence of impaired glucose tolerance, or insulin resistance, is even higher. Diabetes is leader in causing cardiovascular diseases and was responsible for 5.1 million deaths last year only[1].
Vitamin D deficiency is now recognized as a pandemic. Its prevalence varies according to geographic location, season, ethnicity and the standard laboratory value of what is considered normal, deficient and insufficient vitamin D. It was estimated that there were about 1 billion individuals with low vitamin D in 2008 and the number most likely is higher now[2]. Well-studied adverse outcomes of vitamin D deficiency are low bone density[3], non-vertebral fractures[4], increased risk of hip fracture[5] and slowed walking speed[6].
The link of vitamin D with insulin insensitivity or abnormal glucose metabolism gained much more scientific attention in the last decade. Several observations or associations were cited exploring the possible role for either altered vitamin D status and its metabolites or altered insulin sensitivity in the pathogenesis of the each disease. To gain more insight on the role of these variables, understanding of the metabolism of vitamin D and its relation to the pancreas is crucial.
VITAMIN D METABOLISM
In humans, vitamin D3 is mostly obtained from endogenous vitamin D resources as a result of skin exposure to ultraviolet B light and only a minor portion is extracted from meals containing fortified milk and dairy food resources, eggs, and wild oily sea fish[7] (Figure 1). It is crucial to note that vitamin D2 is the non-animal plant derived form of vitamin D and is called ergosterol. Vitamin D3 is a lipophilic precedent of the major circulating 25(OH) D3 metabolite which is hydroxylated, predominantly in the kidney, by a single enzyme 1 α-hydroxylase [1α(OH)lase; CYP27B1] into the most active vitamin D3 known as 1,25-dihydroxyvitamin D [1,25(OH)2D3], which may potentiate mineralization of bone via its role in the stimulation of calcium absorption in the intestine. Many immune cells also contain the machinery for the two-step conversion of vitamin D to 1,25(OH)2D3[8]. Moreover, 1,25(OH)2D3 can be produced locally in the pancreas from the main circulating form, 25(OH)D3, because 1 α-hydroxylase is present in islets[9]. 25(OH)D3 itself also has some biological activity, but the affinity of 1,25(OH)2D3 is about 1000-fold higher than 25(OH)D3 for the vitamin D receptor (VDR). All metabolites of vitamin D are circulating in the bloodstream bound to the vitamin D-binding protein that has a different affinity for the individual metabolites[10]. Seasonal factors, geographical variations, differences in skin color, age, and changes in lifestyle may make certain subjects more susceptible to develop vitamin D insufficiency [defined as 25(OH)D3 concentrations 20-30 ng/mL or 50-75 nmol/L], or vitamin D deficiency [25(OH)D3 concentration < 20 ng/mL or < 50 nmol/L][11] (Table 1).
Figure 1.
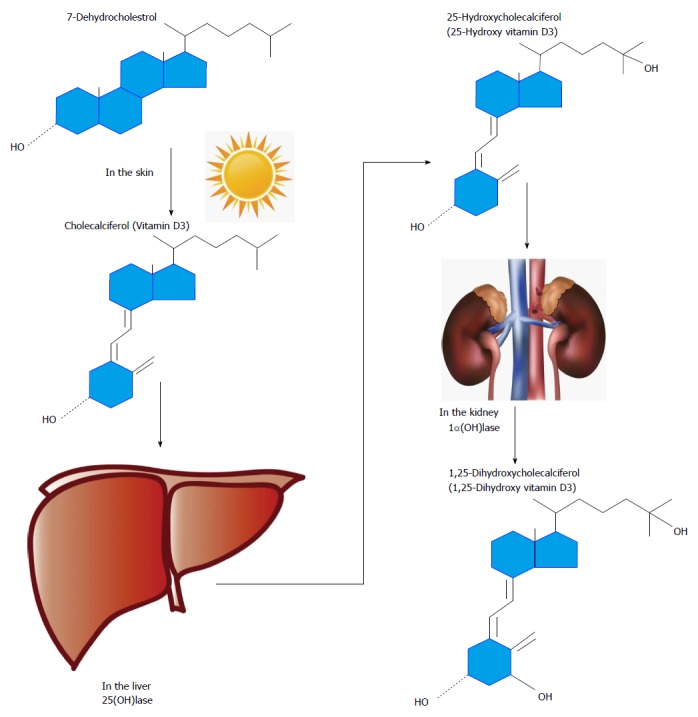
Schematic overview of the metabolism of vitamin D. Synthesis of vitamin D3 starts in the skin where 7-dehydrocholesterol is converted into vitamin D3 in response to UVB exposure. Vitamin D3 is hydroxylated by 25-hydroxylases in the liver. The resulting 25-hydroxyvitamin D3 is then hydroxylated in the kidney by 1α hydroxylase, to produce the final activated product, 1,25-dihydroxyvitamin D3.
Table 1.
Accepted cut-off values of 25 hydroxyvitamin D3 (25OHD3) that describe vitamin D status
| Vitamin D status | 25OHD3 (nmol/L)1 |
| Sufficient (optimal) | > 75 |
| Insufficient | 50-75 |
| Deficient | < 50 |
Conversion factor, 1 nmol/L = 2.5 ng/mL.
POSSIBLE MECHANISMS BY WHICH VITAMIN D MAY INFLUENCE GLUCOSE INTOLERANCE AND TYPE 2 DIABETES MELLITUS
The development of abnormal glucose tolerance and type 2 diabetes mellitus is always preceded by alterations in the function of pancreatic β-cells, insulin sensitivity, and systemic inflammation. Available data suggest that these mechanisms are influenced by vitamin D.
β-cell function of the pancreas
Responses of insulin to glucose load appears to be exclusively influenced by vitamin D. Vitamin D does not appear to affect basal insulin[12,13]. A positive role for vitamin D in the modification of the function of β-cells of the pancreas has been reported[14]. This role is mediated through several pathways, including direct stimulation of insulin secretion by vitamin D through the presence of vitamin D receptors (VDRs) in β-cells of the pancreas[14] and their expression of 1-α-hydroxylase enzyme[9]. Also, 1,25-(OH)2D is able to activate transcription of the gene of human insulin and thus play an essential role in insulin secretion[15]. In mice, it has been shown that insulin secretory response may be impaired if the functional VDRs were absent[13]. Several animal studies have also shown that when those were supplemented with vitamin D, they became able to restore their insulin secretion[12,16-19]. In human studies, introduction of vitamin D was associated with improvement in release of insulin in some[20-23], not all[21,22,24], limited-scale short-term studies.
Through its regulatory role of the calcium pool of β-cell intracellularly and extracellularly, vitamin D insufficiency appears to affect normal release of insulin[25] particularly in reaction to a glucose intake since the secretion of insulin is mediated by a calcium dependent mechanism. Some[20,21,26-28], compared with other[22,29], studies of variable cohorts including diverse baseline status of vitamin D have reported a link between deficiency of vitamin D and impairment of release of glucose-induced insulin. Moreover, a role of calbindin-D28k (a calcium-buffering protein in pancreatic beta cell) in calcium regulation and modulation of insulin release has been described[25].
Insulin insensitivity
Improvement in action of insulin may be mediated by vitamin D directly through the presence of VDRs in skeletal muscles[30], stimulation of expression of insulin receptors in bone marrow cells[31] and through vitamin D activation of peroxisome proliferator activator receptor-δ[32], a transcription factor involved in the control of metabolism of fatty acids in adipose tissue and skeletal muscle[33]. The indirect role of vitamin D is via the regulation of pools of intracellular and extracellular calcium and control of normal influx of calcium through the membranes of cells. Some[27,34] studies have demonstrated a negative association of vitamin D with insulin insensitivity, but this was not shown by others[22].
Inflammation
In the state of systemic inflammation that T2DM can create based on wide range of clinical studies[35-37], altered function of β-cells triggered by apoptosis of β-cell can develop due to the presence of elevated cytokines that can also induce insulin resistance directly. Vitamin D can act to lower systemic inflammation in general by interacting with components in the region of promotion of cytokine genes interfering with generation and action of cytokines through impeding the role of factors involved in nuclear transcription[38-40].
Specifically to insulin insensitivity, vitamin D was demonstrated to under-regulate the activation of nuclear factor-κB[38,40,41], which plays a regulatory role for genes of cytokines of pro-inflammation implied in resistance of insulin[42]. On the other hand, data from human research with inconsistent outcome that have directly assessed the association of vitamin D or calcium status and systemic inflammation in relation to type 2 diabetes mellitus were reported[43-46].
EVIDENCE FOR VITAMIN D LINK WITH INSULIN RESISTANCE
Several trials have demonstrated an association between deficiency of vitamin D with increasing body mass index. One of those was a population trial from Norway with data from 10229 subjects, revealing an inverse association of 25(OH)D concentrations with BMI which was not only seen in summer, but also in winter months[47]. So levels of 25(OH)D may change in seasons, but not body mass index. Furthermore, the same trial reported results from 2656 studied subjects in a longitudinal study between 1994 until 2008, which showed a negative predictor role for the changes of BMI in levels of 25(OH)D in that a reduction of more than 1 kg/m2 in BMI, would result in an estimated increase of 2.8 ± 19.9 nmol/L in levels of 25(OH)D.
In a study of adults from North America by Devaraj et al[48], prediabetic state (state of a fasting plasma glucose concentration of 6.1-6.9 mmol/L, a 2 h glucose concentration of 7.8-11 mmol/L, or glycosylated hemoglobin of 5.7%-6.4%), a form of insulin resistance presentation, was noted to be associated with serum 25(OH)D in the first quartile in comparison with the fourth quartile in association with an adjusted odds ratio of 1.47[48]. The same study showed that in patients with metabolic syndrome, concentration of 25(OH)D was negatively associated with fasting glucose and homeostasis insulin resistance model of assessment.
Improvement in 25(OH)D status in T2DM patients was shown to be linked to some improvements in insulin sensitivity[27], but still, other parameters of insulin resistance like obesity did not change significantly with vitamin D supplementations in other studies. The data from the Norwegian study (The Tromsø study), also included an intervention arm where 93 subjects of varying BMI values received vitamin D at 40000 IU weekly for a year[47]. At the end of trial, increased vitamin D3 doses were not associated with significant decrease in weight. The intervention showed that individuals with obesity needed bigger vitamin D doses than lean ones to achieve similar concentration of 25(OH)D, and a similar outcome to this was demonstrated in another study by Lee et al[49]. At the end of the trial with non-significantly different 25(OH)D values at baselines and vitamin D treatment doses, subjects with higher BMIs had lower concentrations of 25(OH)D compared with those of lower BMIs indicating that possibly body composition and insulin resistance in higher BMI subjects have a regulatory influence on vitamin D absorption, metabolism and/or storage.
The negative association between body weight, together with evidence of increased adiposity and low adiponectin levels, and low 25(OH)D concentrations also were shown in different age groups including both children and adolescents. Deficiency of vitamin D was prevalent in young Norwegian subjects[50], African-American adolescents[51], in both black and Caucasian youth[52] and tropical locations like Malaysia and Colombia[53,54].
Those prevalence studies remain with low scientific evidence since they are only observational and it is difficult to draw a causality relationship from them due to multiple confounders. Despite attempts to control those confounders, still full causality cannot be achieved.
DATA ON VITAMIN D LINK WITH T2DM
Large number of human trials, mainly cross-sectional and some longitudinal, have demonstrated a negative correlation of vitamin D status with predominant hyperglycemia. This correlation was shown both in children and adults, in each gender, and in diverse backgrounds of ethnicity[48,55-61]. Also have been reported that seasonal variation in diabetes control being worse during winter months when vitamin D levels in their lowest[62]. Beside prevalence, incidence of T2DM with decreased level of vitamin D has also been shown in majority of longitudinal but observational trials. It is therefore worth to go through the available observational or intervention trials.
Observational studies for T2DM incidence in association with altered vitamin D
In the recent review of systematic analysis of observational studies, Song et al[63] described a reduction of 38% in relative risk in diabetes incidence for subjects with the highest compared with the lowest group of serum of 25(OH)D3 level. 21 prospective studies, largely population-based studies with white subjects, were included in the analysis involving about 70000 participants. The relations of 25(OH)D concentration and risk of diabetes were weakened but remained significant following corrections for hypertension and BMI. The association was not influenced by gender, sample size of study, period of follow up time, diagnostic criteria for diabetes, or method of 25(OH)D assay. About 4% reduction in T2DM risk was seen for each increment of 10 nmol/L (4 ng/mL) in serum 25(OH)D3 level. In similar review studies, but with smaller numbers, a meta-analysis found a higher relative risk of 50% for the development of T2DM in low vs high 25(OH)D concentrations[64]. In that last study, analyses stratified according to study design did not alter the appreciated association substantially.
Most of data analysis for risk of developing T2DM in relation to vitamin D status has used cutoffs categorizing vitamin D deficiency or insufficiency that is less clinically practiced in the last few years. In majority of trials, serum value of 25(OH)D above 50 nmol/L is considered sufficient while a large body of evidence supported by scientific agreement is considering a level above 75 nmol/L to be sufficient to execute its biological effect[11].
Even though serum level above 75 nmol/L of 25(OH)D could be beneficial to multiple physiological effects, its protective effect against developing T2DM compared to levels in the insufficient range (75-50 nmol/L) is doubtful or at least needs further investigation. In data from large number of participants of 9841 from The Copenhagen City Heart Study, a long-term prospective study with a median of 20 years follow up of the general population of Denmark, increased hazard ratios (HR) for T2DM with decreased concentrations of 25(OH)D by clinical severities and season quartiles were noted. For 25(OH)D less than 12.5 nmol/L compared with levels more than 50 nmol/L, the HR was 1.22, and was 1.35 for lowest compared with highest quartile[64]. This was not clinically significant when the values of more than 75 nmol/L is used as a sufficient level compared with values of 25(OH)D between 75-50 nmol/L (HR 0.91).
Serum vitamin D levels are influenced significantly by dietary habits, mainly consumption of dairy products. After a prospective follow up for 20 years, in a cohort of the Nurses Health Study, an inverse association between T2DM risk with total 25(OH)D and calcium intake was described[65]. The analysis showed that consumption of 3 or more vs only one daily dairy serving was associated with decreased risk of development of diabetes.
Several genetic studies have identified a relationship of circulating 25(OH)D with presence of polymorphisms of single nucleotide in six genetic regions[66-68]. In an observational study of Buijsse et al[69] where eight of those polymorphisms of single nucleotides, most strongly associated with 25(OH)D, were tested in relation to levels of 25(OH)D and T2DM incidence in an observational prospective case-control manner. In that study, it was found that in a population with relatively low serum values of 25(OH)D, 25(OH)D was inversely associated with T2DM risk, for concentrations of 25(OH)D below 45 nmol/L only (compared with higher levels), after controlling for measures of general and abdominal adiposity. After being adjusted for age, gender, center, and month of the year blood drawn, HR of T2DM per 5 nmol/L higher 25(OH)D was 0.92. But this study also found that genetically determined 25(OH)D was not related to T2DM across the entire 25(OH)D range or below 45 nmol/L. This latter finding argues against a strong causal relationship of 25(OH)D with T2DM but requires further investigation in larger research groups.
Caution has to be applied when making conclusions from observational studies due to possible multiple confounding factors affecting vitamin D status like age, race, dietary habits and level of activity, which are also known to play a role in increased risk for development of diabetes. Table 2 shows summary of selected epidemiological studies on the association of vitamin D with markers of insulin insensitivity, pre-diabetic resistance to insulin and T2DM is displayed in Table 2.
Table 2.
Data of selected epidemiological studies on the relation between vitamin D level and markers of insulin resistance (A), insulin resistance (B) and type 2 diabetes (C)
| Ref. | No. of subjects | Age, mean or range | Trial outcome |
| (A) | |||
| Jorde et al[47] | 10229 | 58 | 25OHD negatively associated with BMI |
| Lee et al[49] | 95 | 68, 47-91 | 25OHD negatively related to BMI |
| Lagunova et al[50] | 102 | 8-19 | ↑ prevalence of Vit D Def. (19%) and insuff. (> 50%) in obese |
| Suijder et al[60] | 453 | > 65 | ↑ BMI is associated with ↓ 25OHD |
| (B) | |||
| Chiu et al[27] | 125 | 26 | +ve relation between 25OHD and insulin sensitivity |
| Nunlee-Bland et al[51] | 34 | 10-20 | ↓ 25OHD is associated with insulin resistance |
| Shankar et al[61] | 12719 | > 20 | ↓ 25OHD is associated with pre-diabetes state |
| (C) | |||
| Song et al[63] | 76220 | meta-analysis | inverse relation between 25OHD and risk for T2DM |
| Afzal et al[64] | 9841 | 48-65 | ↓ 25OHD is associated with ↑ risk for T2DM |
| Pittas et al[65] | 95243 | meta-analysis | ↓ incidence of T2DM in highest vs lowest 25OHD |
| Buijsse et al[69] | 53088 | 50.9 | HR of T2DM is ↓ with ↑ in 25OHD |
T2DM:Type 2 diabetes mellitus; BMI: Body mass index.
Randomized intervention trials for the relation of vitamin D and T2DM
Direct evidence of a role for vitamin D in diabetes prevention and treatment is critically needed from interventional randomized studies before any conclusion can be made. Those needed intervention trials, ideally large-scale randomized trials, are lacking today. What is available are either data of scattered small-scale trials or some of post hoc data from analyses of somewhat larger studies on the influence of supplementation of vitamin D on parameters related to diabetes and those were either inconsistent or inconclusive, though vitamin D is known to have certain advantageous effects in subjects with increased diabetes risk[43,64,70,71]. There are several limitations that can make it difficult to draw conclusions of solid nature from the limited available-to-date small interventional trials. Some of those trials were intended mainly to assess outcomes on glycemia and the majority of them were underpowered. Also, there was a variation in the dosing of vitamin D for replacement, some of these studies used supraphysiological vitamin D doses at infrequent manner, while others used daily doses and this would potentially cause different pharmacokinetic effect on concentration of 25(OH)D and pharmacotherapeutic effects on target cells.
In description of some of those trials, data analysis of the well known randomized trial of Women’s Health Initiative[72], in which about 50% of the women had 25(OH)D concentration less than 45 nmol/L, revealed no effect of administration of vitamin D3 at 400 IU and calcium at 1000 mg daily for 7 years on risk of diabetes (HR 1.01). Two smaller randomized trials tested the glycemic effect of applying vitamin D3 in subjects with impaired fasting glucose; the first one found that people using vitamin D3 daily at 700 IU and calcium at 500 mg for 3 years had a less rapid worsening of glycemia than those on placebo[43]. The second trial supplemented with vitamin D3 to get 25(OH)D levels between 150 and 225 nmol/L[73]. After 1 year, no effect was seen on incident diabetes. Together with the mixed findings of short-term trials on the influences of vitamin D on release of insulin and its sensitivity[73,74], the experimental evidence today is inconsistent.
CONCLUSION
In conclusion, data from non-interventional observational trials have shown a negative relationship between the status of vitamin D and parameters of insulin insensitivity and incidence of T2DM. A biological active position for vitamin D in both insulin secretion and action, and in the function of β-cells has been considered. However, definitive conclusion for a causative link for vitamin D with T2DM can not be drawn due to the missing of large-sized prospective observational investigations that use 25(OH)D as the target variable and the absence of randomized trials particularly designed to assess the influence of vitamin D on diabetes. Similar randomized prospective trials are needed to correctly explain the outcome of vitamin D administration as an interventional agent for preventing and managing diabetes. We anticipate that these future well designed randomized prospective trials to answer several important questions. Firstly, whether the daily interventional utilization of vitamin D in the pre-diabetic states works as a strong defensive tool against progression to type 2 diabetes? Secondly, whether the daily intake of vitamin D will be accompanied with significant glycemic improvement? And finally, whether supplementation of vitamin D to diabetics will delay or prevent some of the adverse diabetic complications or have positive effects on cardiometabolic outcomes in long term.
Footnotes
Conflict-of-interest statement: No conflict of interest of any kind.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 9, 2014
First decision: January 8, 2015
Article in press: May 6, 2015
P- Reviewer: Charoenphandhu N, Makishima M S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. Available from: http://www.idf.org/diabetesatlas. [Google Scholar]
- 2.James WP. 22nd Marabou Symposium: the changing faces of vitamin D. Nutr Rev. 2008;66:286–290. doi: 10.1111/j.1753-4887.2008.00034.x. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, Orav JE, Li R, Spiegelman D, Dietrich T, Willett WC. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res. 2009;24:935–942. doi: 10.1359/JBMR.081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 5.Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149:242–250. doi: 10.7326/0003-4819-149-4-200808190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annweiler C, Schott AM, Montero-Odasso M, Berrut G, Fantino B, Herrmann FR, Beauchet O. Cross-sectional association between serum vitamin D concentration and walking speed measured at usual and fast pace among older women: the EPIDOS study. J Bone Miner Res. 2010;25:1858–1866. doi: 10.1002/jbmr.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolden-Kirk H, Overbergh L, Christesen HT, Brusgaard K, Mathieu C. Vitamin D and diabetes: its importance for beta cell and immune function. Mol Cell Endocrinol. 2011;347:106–120. doi: 10.1016/j.mce.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Baeke F, Gysemans C, Korf H, Mathieu C. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol. 2010;25:1597–1606. doi: 10.1007/s00467-010-1452-y. [DOI] [PubMed] [Google Scholar]
- 9.Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, Hewison M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90:121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 10.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 11.Al-Shoumer KAS. Vitamin D: A Methodological Update. United Updates. 2011;4:12–17. [Google Scholar]
- 12.Bourlon PM, Billaudel B, Faure-Dussert A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. J Endocrinol. 1999;160:87–95. doi: 10.1677/joe.0.1600087. [DOI] [PubMed] [Google Scholar]
- 13.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17:509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol. 1994;267:E356–E360. doi: 10.1152/ajpendo.1994.267.3.E356. [DOI] [PubMed] [Google Scholar]
- 15.Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3) Cell Biochem Funct. 2002;20:227–232. doi: 10.1002/cbf.951. [DOI] [PubMed] [Google Scholar]
- 16.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Seino Y, Ishida M, Yamaoka K, Yabuuchi H, Ishida H, Seino S, Seino Y, Imura H. Effect of vitamin D3 on the pancreatic secretion of insulin and somatostatin. Acta Endocrinol (Copenh) 1984;105:528–533. doi: 10.1530/acta.0.1050528. [DOI] [PubMed] [Google Scholar]
- 18.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 19.Clark SA, Stumpf WE, Sar M. Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. Diabetes. 1981;30:382–386. doi: 10.2337/diab.30.5.382. [DOI] [PubMed] [Google Scholar]
- 20.Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38:1239–1245. doi: 10.1007/BF00422375. [DOI] [PubMed] [Google Scholar]
- 21.Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57:258–261. [PubMed] [Google Scholar]
- 22.Al-Shoumer KA, Al-Asoosi AA, Ali AH, Nair VS. Does insulin resistance in type 2 diabetes alter vitamin D status? Prim Care Diabetes. 2013;7:283–287. doi: 10.1016/j.pcd.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Inomata S, Kadowaki S, Yamatani T, Fukase M, Fujita T. Effect of 1 alpha (OH)-vitamin D3 on insulin secretion in diabetes mellitus. Bone Miner. 1986;1:187–192. [PubMed] [Google Scholar]
- 24.Nyomba BL, Auwerx J, Bormans V, Peeters TL, Pelemans W, Reynaert J, Bouillon R, Vantrappen G, De Moor P. Pancreatic secretion in man with subclinical vitamin D deficiency. Diabetologia. 1986;29:34–38. doi: 10.1007/BF02427278. [DOI] [PubMed] [Google Scholar]
- 25.Sooy K, Schermerhorn T, Noda M, Surana M, Rhoten WB, Meyer M, Fleischer N, Sharp GW, Christakos S. Calbindin-D(28k) controls [Ca(2+)](i) and insulin release. Evidence obtained from calbindin-d(28k) knockout mice and beta cell lines. J Biol Chem. 1999;274:34343–34349. doi: 10.1074/jbc.274.48.34343. [DOI] [PubMed] [Google Scholar]
- 26.Baynes KC, Boucher BJ, Feskens EJ, Kromhout D. Vitamin D, glucose tolerance and insulinaemia in elderly men. Diabetologia. 1997;40:344–347. doi: 10.1007/s001250050685. [DOI] [PubMed] [Google Scholar]
- 27.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 28.Gedik O, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia. 1986;29:142–145. doi: 10.1007/BF02427083. [DOI] [PubMed] [Google Scholar]
- 29.Lind L, Pollare T, Hvarfner A, Lithell H, Sørensen OH, Ljunghall S. Long-term treatment with active vitamin D (alphacalcidol) in middle-aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Res. 1989;11:141–147. [PubMed] [Google Scholar]
- 30.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882–8891. [PubMed] [Google Scholar]
- 31.Maestro B, Campión J, Dávila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47:383–391. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 32.Dunlop TW, Väisänen S, Frank C, Molnár F, Sinkkonen L, Carlberg C. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol. 2005;349:248–260. doi: 10.1016/j.jmb.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 33.Luquet S, Gaudel C, Holst D, Lopez-Soriano J, Jehl-Pietri C, Fredenrich A, Grimaldi PA. Roles of PPAR delta in lipid absorption and metabolism: a new target for the treatment of type 2 diabetes. Biochim Biophys Acta. 2005;1740:313–317. doi: 10.1016/j.bbadis.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 35.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 36.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 37.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 38.Riachy R, Vandewalle B, Kerr Conte J, Moerman E, Sacchetti P, Lukowiak B, Gmyr V, Bouckenooghe T, Dubois M, Pattou F. 1,25-dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: implication of the antiapoptotic protein A20. Endocrinology. 2002;143:4809–4819. doi: 10.1210/en.2002-220449. [DOI] [PubMed] [Google Scholar]
- 39.Gysemans CA, Cardozo AK, Callewaert H, Giulietti A, Hulshagen L, Bouillon R, Eizirik DL, Mathieu C. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146:1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 40.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 41.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004;89:447–452. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 43.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 44.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29:722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 45.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 46.Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 47.Jorde R, Sneve M, Emaus N, Figenschau Y, Grimnes G. Cross-sectional and longitudinal relation between serum 25-hydroxyvitamin D and body mass index: the Tromsø study. Eur J Nutr. 2010;49:401–407. doi: 10.1007/s00394-010-0098-7. [DOI] [PubMed] [Google Scholar]
- 48.Devaraj S, Jialal G, Cook T, Siegel D, Jialal I. Low vitamin D levels in Northern American adults with the metabolic syndrome. Horm Metab Res. 2011;43:72–74. doi: 10.1055/s-0030-1268485. [DOI] [PubMed] [Google Scholar]
- 49.Lee P, Greenfield JR, Seibel MJ, Eisman JA, Center JR. Adequacy of vitamin D replacement in severe deficiency is dependent on body mass index. Am J Med. 2009;122:1056–1060. doi: 10.1016/j.amjmed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Lagunova Z, Porojnicu AC, Lindberg FA, Aksnes L, Moan J. Vitamin D status in Norwegian children and adolescents with excess body weight. Pediatr Diabetes. 2011;12:120–126. doi: 10.1111/j.1399-5448.2010.00672.x. [DOI] [PubMed] [Google Scholar]
- 51.Nunlee-Bland G, Gambhir K, Abrams C, Abdul M, Vahedi M, Odonkor W. Vitamin D deficiency and insulin resistance in obese African-American adolescents. J Pediatr Endocrinol Metab. 2011;24:29–33. doi: 10.1515/jpem.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J Clin Endocrinol Metab. 2011;96:1560–1567. doi: 10.1210/jc.2010-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khor GL, Chee WS, Shariff ZM, Poh BK, Arumugam M, Rahman JA, Theobald HE. High prevalence of vitamin D insufficiency and its association with BMI-for-age among primary school children in Kuala Lumpur, Malaysia. BMC Public Health. 2011;11:95. doi: 10.1186/1471-2458-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert-Diamond D, Baylin A, Mora-Plazas M, Marin C, Arsenault JE, Hughes MD, Willett WC, Villamor E. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. Am J Clin Nutr. 2010;92:1446–1451. doi: 10.3945/ajcn.2010.29746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elsammak MY, Al-Wosaibi AA, Al-Howeish A, Alsaeed J. Vitamin d deficiency in Saudi Arabs. Horm Metab Res. 2010;42:364–368. doi: 10.1055/s-0030-1248296. [DOI] [PubMed] [Google Scholar]
- 56.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–2135. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 57.Hey H, Stokholm KH, Lund B, Lund B, Sørensen OH. Vitamin D deficiency in obese patients and changes in circulating vitamin D metabolites following jejunoileal bypass. Int J Obes. 1982;6:473–479. [PubMed] [Google Scholar]
- 58.Meier DE, Luckey MM, Wallenstein S, Clemens TL, Orwoll ES, Waslien CI. Calcium, vitamin D, and parathyroid hormone status in young white and black women: association with racial differences in bone mass. J Clin Endocrinol Metab. 1991;72:703–710. doi: 10.1210/jcem-72-3-703. [DOI] [PubMed] [Google Scholar]
- 59.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43:199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 60.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–4123. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 61.Shankar A, Sabanayagam C, Kalidindi S. Serum 25-hydroxyvitamin d levels and prediabetes among subjects free of diabetes. Diabetes Care. 2011;34:1114–1119. doi: 10.2337/dc10-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–1428. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59:381–391. doi: 10.1373/clinchem.2012.193003. [DOI] [PubMed] [Google Scholar]
- 65.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hyppönen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE - a significant but nonlinear relationship. Allergy. 2009;64:613–620. doi: 10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 69.Buijsse B, Boeing H, Hirche F, Weikert C, Schulze MB, Gottschald M, Kühn T, Katzke VA, Teucher B, Dierkes J, et al. Plasma 25-hydroxyvitamin D and its genetic determinants in relation to incident type 2 diabetes: a prospective case-cohort study. Eur J Epidemiol. 2013;28:743–752. doi: 10.1007/s10654-013-9844-5. [DOI] [PubMed] [Google Scholar]
- 70.von Hurst PR, Stonehouse W, Matthys C, Conlon C, Kruger MC, Coad J. Study protocol--metabolic syndrome, vitamin D and bone status in South Asian women living in Auckland, New Zealand: a randomised, placebo-controlled, double-blind vitamin D intervention. BMC Public Health. 2008;8:267. doi: 10.1186/1471-2458-8-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94:486–494. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, Larson JC, Manson JE, Margolis KL, Siscovick DS, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31:701–707. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davidson MB, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36:260–266. doi: 10.2337/dc12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alvarez JA, Ashraf A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351–385. doi: 10.1155/2010/351385. [DOI] [PMC free article] [PubMed] [Google Scholar]


