Abstract
Pathogens specifically target both the caspase 8-dependent apoptotic cell death pathway and the necrotic cell death pathway that is dependent on receptor-interacting protein 1 (RIP1; also known as RIPK1) and RIP3 (also known as RIPK3). The fundamental co-regulation of these two cell death pathways emerged when the midgestational death of mice deficient in FAS-associated death domain protein (FADD) or caspase 8 was reversed by elimination of RIP1 or RIP3, indicating a far more entwined relationship than previously appreciated. Thus, mammals require caspase 8 activity during embryogenesis to suppress the kinases RIP1 and RIP3 as part of the dialogue between two distinct cell death processes that together fulfil reinforcing roles in the host defence against intracellular pathogens such as herpesviruses.
Apoptotic and necrotic cell death pathways determine the fate of mammalian cells. Apoptosis follows well-defined pathways that centre around a caspase-dependent proteolytic cascade that coordinates cell-membrane blebbing, nuclear condensation and DNA fragmentation, while maintaining membrane integrity1,2. By contrast, necrosis is caspase-independent and involves cell rounding and cytoplasmic swelling, terminating with the loss of membrane integrity and cytoplasmic leakage3. Necrosis has long been associated with incidental (passive) death in damaged or diseased tissues; however, programmed necrotic death in specific contexts is orchestrated in a cell-autonomous manner via receptor-interacting protein 1 (RIP1; also known as RIPK1)4 and/or RIP3 (also known as RIPK3)5–7. The best-characterized form of programmed necrosis, known as necroptosis, requires the assembly of a RIP homotypic interaction motif (RHIM)-dependent8 signalling complex of RIP1 and RIP3 (REFS 5–7).
Diverse cell-intrinsic and cell-extrinsic signals converge on the activation of executioner caspases that mediate apoptosis. Particularly in the case of intracellular pathogens (such as viruses), apoptosis contributes to host defence by eliminating infected cells. Intrinsic apoptotic machinery exists in metazoan organisms to eliminate excess cells during embryonic development and to sustain tissue homeostasis, as well as to purge stressed, damaged or infected cells. By contrast, extrinsic death pathways evolved more recently and facilitate host defence against pathogens. Intrinsic apoptosis depends on mitochondrial outer membrane permeabilization by the pro-apoptotic B cell lymphoma 2 (BCL-2) family members BAX and BAK9,10. Following mitochondrial permeabilization, pro-apoptotic factors — such as cytochrome c and second mitochondrial activator of caspases (SMAC; also known as DIABLO) — are released into the cytosol, triggering the activation of caspase 9 and of downstream effector caspases, such as caspase 3 and caspase 7. These effector caspases dismantle the cell through the proteolytic destruction of vital substrates11,12. In contrast to intrinsic apoptosis, extrinsic apoptosis is initiated by ligands of the tumour necrosis factor (TNF) family that engage death receptors to activate caspase 8. Caspase 8 activation ultimately drives the activation of caspase 3 and/or caspase 7, either directly, or indirectly by initiating a mitochondrial amplification pathway via the pro-apoptotic BCL-2 family member BID13.
RIP1- and RIP3-dependent programmed necrosis (necroptosis) is unveiled when caspase 8 activity becomes compromised14. Investigators struggled for a decade to explain why mice with a germline disruption of the caspase 8 gene, the FAS-associated death domain protein (FADD) gene or the cellular FLICE-like inhibitory protein (cFLIP; also known as CFLAR) gene die during gestation at embryonic day 10 or 11 (see BOX 1). This pattern of death suggested a crucial non-apoptotic activity for caspase 8–FADD–cFLIP complexes15–21. Rescue of this embryonic lethality, as observed in either Casp8−/−Rip3−/− or Fadd−/−Rip1−/− mice, clarified the developmental role of caspase 8, strongly implicating this enzyme in the physiological suppression of necroptosis22–24. This interpretation was facilitated by evidence that death receptor-dependent signalling regulates the choice between caspase 8-directed apoptosis and the promotion of programmed necrosis by caspase inhibitors14,25,26. Moreover, accumulating evidence has shown that RIP1- and/or RIP3-dependent programmed necrosis can be initiated independently of death receptors of the TNF receptor (TNFR) superfamily during virus infection27 or following the activation of Toll-like receptors (TLRs)28,29, as well as in settings of genotoxic stress30.
Box 1. Lessons from mice deficient in FADD or caspase 8.
The striking phenotypes that emerge when caspase 8 or FAS-associated death domain protein (FADD) are eliminated in specific mouse tissues must now be viewed with the understanding that a caspase 8–FADD complex controls receptor-interacting protein 1 (RIP1)- and RIP3-mediated necroptosis22–24. Caspase 8-mediated control of apoptosis is important for T cell homeostasis, as revealed in adult Casp8−/−Rip3−/− mice22,23, as well as in mice with a T cell-specific disruption of Casp8 or Fadd on a Rip3−/− background65,68. Moreover, the tissue-specific disruption of Casp8 or Fadd has revealed many examples of conditions under which necroptosis may be unveiled during life. Caspase 8 deficiency in TIE1+ endothelial cells results in a phenotype that parallels germline disruption15, reinforcing the conclusion that dysregulated RIP1–RIP3 underlies the vascular cell defects and embryonic death in Casp8-null mice. Interferon-inducible disruption of Casp8 or Fadd leads to the elimination of cells in various lineages, and this suppresses early and mid-stage haematopoietic development15,18,115. The disruption of Casp8 or Fadd in CD19+ B cells does not alter their response to antigens, although mutant cells fail to proliferate and die in response to Toll-like receptor 3 (TLR3) and TLR4 agonists that induce signalling through TIR domain-containing adaptor protein inducing IFNβ (TRIF)116–118. This provides a potential biological link to the recently identified ripoptosome29. The disruption of Casp8 in the epidermis results in atopic dermatitis during the cornification process and has been used to model chronic skin disease119–121. Furthermore, mice that express a catalytically inactive form of caspase 8 along with a single wild-type allele develop inflammation in internal organs and skin16. Hepatocyte-specific caspase 8 deficiency results in a strong inflammatory response following partial hepatectomy and impaired liver regeneration122, probably through the induction of necroptosis. Although a complete analysis is yet to be reported, Casp8−/−Rip3−/− mice continue through life without suffering any obvious deficits apart from abnormal T cell levels22,23. Remarkably, despite a pattern of midgestational death in Casp8−/− mice, the combined mutation of Casp8 and Rip3 results in embryos with functioning hearts, a correctly organized architecture of the yolk sac endothelia and normal levels of haematopoiesis. These mice appear normal and complete gestation to become fertile adults with no abnormal inflammation22,23, in a similar manner to Rip3−/− mice79. Fadd−/−Rip1−/− embryos appear normal throughout gestation but die soon after birth owing to the absence of RIP1 (REF. 24). The viability of Casp8−/−Rip3−/− mice establishes that caspase 8 is largely dispensable for mammalian development and tissue homeostasis. Therefore, the many settings in which this caspase seemed to be crucial will require re-examination. The recent demonstration that RIP3 deficiency rescues epithelial necroptosis32–34 reinforces this fact. Thus, severe inflammatory abnormalities that arise when caspase 8 (or FADD) is compromised15,32–34,119,120,122 are likely to be the consequence of unleashed necroptosis.
The choice between apoptotic and necrotic pathways following the ligation of death receptors has been extensively reviewed14,25,26. Likewise, a discussion of the inflammatory consequences of TNFR super family signalling and the roles of apoptosis and necrosis in inflammation are beyond the scope of this Review, although recent reports indicate an intimate association between inflammation and dysregulated programmed necrosis31–35. This Review focuses on the molecular pathways involved in the regulation of programmed necrosis, discusses the potential contribution of viral infections to the evolution of programmed necrosis as it is currently understood in mammals, and summarizes the latest evidence on the contribution of cell death pathways to immune homeostasis.
The players in death receptor signalling
Death receptors, including TNFR1 and FAS (also known as CD95), control three cellular responses in mice and humans: first, a pro-inflammatory cytokine response that is dependent on nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs); second, apoptosis; and third, necroptosis36–38. Activation of NF-κB transcription factors contributes to inflammation and the suppression of cell death39, and the interplay between the pro-inflammatory and the cell death processes that are under the control of death receptors probably contributes to disease pathology in many settings. Additional TNFR superfamily members drive the activation of NF-κB and inflammation without inducing cell death40. These, together with several classes of pattern recognition receptors (PRRs), sculpt inflammatory responses. These inflammatory responses contribute to the recognition and clearance of microbial infections through the activity of cytokines that initiate the elimination of infected cells via programmed cell death. Overlapping functions remain a major theme when considering pathogen recognition and death receptor signalling in host defence.
Caspase 8 activation
Ligation of TNFR superfamily death receptors promotes the assembly of a caspase 8- and FADD-containing complex that initiates apoptosis. Signalling via FAS, TRAIL receptor 1 (TRAILR1) or TRAILR2 results in interactions between the receptor death domains and FADD, leading to the formation of a receptor-associated death-inducing signalling complex (DISC)41–43. The DISC recruits caspase 8 via death effector domain (DED)-dependent interactions with FADD. Apoptosis ensues following caspase 8 homodimerization and self-cleavage, which unleashes the full activity of the enzyme44. The two isoforms of cFLIP — cFLIP long (cFLIPL) and cFLIP short (cFLIPS) — are non-catalytic paralogues of caspase 8 that heterodimerize with caspase 8 to suppress the self-processing that is necessary for the induction of apoptosis45 (FIG. 1).
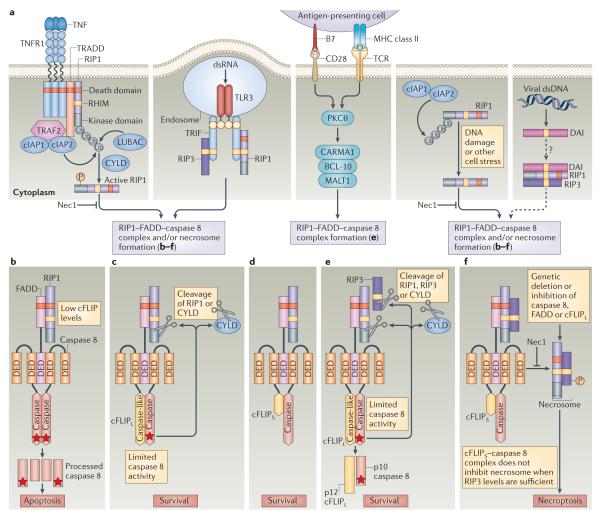
Figure 1. Caspase 8-mediated regulation of RIP1–RIP3 signalling pathways.
a ∣ Following tumour necrosis factor (TNF) binding, TNF receptor 1 (TNFR1) recruits receptor-interacting protein 1 (RIP1) via its death domain. When polyubiquitylated by the E3 ligases cellular inhibitor of apoptosis 1 (cIAP1), cIAP2 and linear ubiquitin chain assembly complex (LUBAC), RIP1 promotes the activation of nuclear factor-κB (NF- κB), which enhances cell survival by inducing the expression of cIAP1, cIAP2 and cellular FLICE-like inhibitory protein (cFLIP) (not shown). In the absence of RIP1 polyubiquitylation (for example, owing to insufficient cIAP levels or deubiquitylation by proteins such as cylindromatosis (CYLD)), RIP1 kinase activity downstream of TNFR1 facilitates the assembly of alternative signalling platforms comprising RIP1, FAS-associated death domain protein (FADD) and caspase 8. In an alternative scenario, the Toll-like receptor 3 (TLR3) and TLR4 adaptor protein TIR domain-containing adaptor protein inducing IFNβ (TRIF) binds to both RIP1 and RIP3 via RIP homotypic interaction motif (RHIM)-mediated interactions, bridging TLR3 signalling to the RIP1–FADD–caspase 8 complex (ripoptosome). Genotoxic damage or cell stress leads to the degradation of cIAP1 and cIAP2 and the formation of a ripoptosome. The cytosolic DNA sensor DAI (DNA-dependent activator of interferon regulatory factors) directly engages RIP1 and RIP3 in RHIM-dependent complexes to potentially drive the assembly and/or recruitment of a ripoptosome, in a similar manner to TRIF. The levels of cFLIP, the balance of cFLIP isoforms and the levels of caspase 8 activity determine whether apoptosis, necroptosis or cell survival ensues following the formation of the ripoptosome. b ∣ When the levels of cFLIP long (cFLIPL) or cFLIP short (cFLIPS) are limiting, caspase 8 homodimerization promotes enzymatic activity, and this leads to caspase 8 autoprocessing and the execution of apoptosis through BID and/or caspase 3. c,d ∣ In the presence of sufficient cFLIPL or cFLIPS, a caspase 8–cFLIPL or caspase 8–cFLIPS heterodimer forms, and this supports cell survival. Importantly, the caspase 8–cFLIPL heterodimer retains sufficient proteolytic activity to cleave substrates such as RIP1 and CYLD to prevent necroptosis without allowing caspase 8 to induce apoptosis. e ∣ T cell survival and proliferation following stimulation of the T cell receptor (TCR) and CD28 requires the inactivation of RIP1- and RIP3-mediated necroptosis by FADD–caspase 8–cFLIPL, and this probably occurs downstream of the CARMA1–BCL-10–MALT1 signalling complex. f ∣ When caspase 8 activity is blocked — under conditions of elevated cFLIPS levels or in the presence of a caspase 8 inhibitor — RIP1 binds to RIP3 to form a kinase-active necrosome to initiate necroptosis. RIP1 kinase activity drives the assembly of the cytosolic RIP1–FADD–caspase 8 signalling platform, as well as the necrosome. The RIP1 kinase inhibitor necrostatin 1 (Nec1) blocks both RIP1-dependent apoptosis and necroptosis. Stars indicate catalytically active caspase 8. DED, death effector domain; dsDNA, double-stranded DNA; dsRNA, double-stranded RNA; PKCθ, protein kinase Cθ; TRADD, TNFR1-associated death domain protein; TRAF2, TNFR-associated factor 2; Ub, ubiquitin.
TNFR1 signalling involves the recruitment of TNFR1-associated death domain protein (TRADD), together with RIP1 (REFS 46–48), into complex I. This drives the activation of NF-κB and MAPKs, which induce pro-survival genes49, including those encoding cFLIP and cellular inhibitor of apoptosis proteins (cIAP1 and cIAP2; collectively referred to as cIAP here). Within the TNFR1 signalling complex, cIAP and the linear ubiquitin chain assembly complex (LUBAC) polyubiquitylate RIP1 to promote the engagement of IκB kinase-β (IKKβ) by NF-κB essential modulator (NEMO; also known as IKKγ), which results in NF-κB-mediated gene transcription. Polyubiquitylated RIP1 does not support apoptosis50–54 or programmed necrosis6,55,56. When cIAP is inhibited — either naturally by SMAC or experimentally through the application of SMAC mimetics — or when the LUBAC component SHARPIN is absent, extrinsic death pathways that contribute to apoptosis and programmed necrosis become activated35,57. The deubiquitylation of RIP1 by enzymes such as cylindromatosis (CYLD) downregulates NF-κB activation and enables the kinase activity of RIP1 to direct the formation of a DISC-like cytosolic caspase 8-activating platform53 known as complex II, which is composed of caspase 8, FADD and cFLIP58 (FIG. 1).
When caspase 8 activity is compromised, programmed necrosis is initiated by a RIP1–RIP3 complex5–7 that has been called a necrosome14,26. Importantly, catalytically active but non-cleavable caspase 8 retains the ability to suppress programmed necrosis23 and can prevent the embryonic lethality caused by caspase 8 deficiency16. Caspase 8 orchestrates apoptosis and prevents necroptosis in association with its activator, FADD (FIG. 1b). Both isoforms of cFLIP inhibit caspase 8-induced apoptosis (FIG. 1c,d); however, in the presence of sufficient levels of RIP3, cFLIPS promotes RIP1- and RIP3-dependent necroptosis29 (FIG. 1f), whereas cFLIPL blocks necroptosis23. In contrast to a caspase 8–cFLIPS heterodimer, caspase 8–cFLIPL retains sufficient proteolytic activity59–62 to inactivate the key adaptors, RIP1 (REFS 63–65) and RIP3 (REF. 66), as well as the deubiquitylating enzyme CYLD67. This prevents RHIM-dependent oligomerization5–8 of RIP1 and RIP3 into a necrosome14,26 (FIG. 1e).
The ripoptosome
Recent investigations have examined the roles of caspase 8 and FADD in regulating necroptosis following stimulation via PRRs (such as TLR3)29 or the induction of genotoxic stress30. These studies have shown that TLR3-dependent or DNA damage-induced signals promote the formation of a high molecular weight complex called a ripoptosome29,30, which contains RIP1, FADD, caspase 8 and cFLIP. This complex regulates cell fate by functioning as a caspase 8 activation platform reminiscent of TNFR1-induced complex II, although it is assembled independently of death receptor signalling. In TLR3 signalling, TIR domain-containing adaptor protein inducing interferon-β (TRIF) recruits the ripoptosome through a RHIM-dependent interaction with RIP1. Here, as in death receptor pathways, the proteolytic activity of caspase 8–cFLIPL suppresses necroptosis. TRIF-mediated necroptosis in response to the stimulation of TLR3 or TLR4 may underlie the pathogenesis of acute bacterial inflammation under conditions in which caspase 8 activity is compromised33. Similarly, genotoxic stress can induce cIAP degradation, driving ripoptosome formation30. Growing evidence suggests that TCR stimulation also results in the formation of a ripoptosome-like complex65,68. Thus, a variety of extracellular and intra cellular stimuli appear to regulate ripoptosome formation, expanding the number of settings in which cell death choices are controlled by caspase 8 activity. Settings in which the caspase 8–cFLIPS hetero dimer dominates during infection or inflammation may be predicted to favour necroptosis (FIG. 1f).
The roles of caspase 8 in TCR signalling
Caspase 8- or FADD-deficient T cells die when stimulated by antigens. The successful rescue of T cell function through the deletion of Rip1 or Rip3 — as observed in Casp8−/−Rip3−/− mice22,23, in mice with Fadd−/−Rip1−/− haematopoietic cells24, and in mice with a T cell-specific caspase 8 or FADD deficiency on a Rip3−/− background65,68 — indicates that necroptosis underlies this T cell loss. Early evidence that linked NF-κB activation, as well as autophagy, with this type of T cell death is not supported by current data69. It has become very clear that TCR activation in caspase 8- or FADD-deficient T cells promotes RIP1- and RIP3-dependent necroptosis65,68–70. The CARMA1–BCL-10–MALT1 complex (FIG. 1a) activates NF-κB following antigen recognition via the TCR71–73, while also directing the formation of a complex containing caspase 8, cFLIPL and RIP1 (REFS 74,75). Despite the fact that FADD and caspase 8 are essential for T cell proliferation in response to antigens, T cell activation does not result in apoptosis. Instead, an explanation posited with experimental support in 2008 (REF. 70), that necroptosis follows antigen engagement in caspase 8- or FADD-deficient T cells, continues to implicate an inactivation of RIP1 and/or RIP3 mediated by a caspase 8–cFLIPL complex following TCR stimulation. It remains to be fully elucidated whether TCR stimulation induces necroptotic pathways in non-transgenic T cells or more physiological settings.
Programmed necrosis mediated by RIP1 and RIP3
RIP1- and RIP3-dependent programmed necrosis (necroptosis)
Necroptosis is observed when caspase 8 activity is compromised. In Drosophila melanogaster and zebrafish, the homologues of caspase 8 and FADD do not have a developmental role76–78. By contrast, the embryonic lethality in mice that have a germline mutation in Casp8 or Fadd indicates that a key developmental step in mammals requires caspase 8 activity15–20. Endothelial and haematopoietic cells are most affected in the absence of caspase 8, suggesting that normal development is predicated on the survival of these cells. A complex of FADD, caspase 8 (REF. 16) and cFLIPL23,29 restrains aberrant activation of RIP1–RIP3 during mammalian development, although the precise signals, cell-extrinsic or cell-intrinsic, that trigger caspase 8 and RIP1–RIP3 activation in these cell types remain to be defined (BOX 1). Although death receptor signalling may be involved, the recognition that ripoptosome formation follows cell-intrinsic as well as cell-extrinsic cues suggests new possible mechanisms for the activation of caspase 8 and the elimination of RIP1–RIP3 signalling (FIG. 1). The key players that promote embryonic lethality are clear; however, the downstream events in the execution of necroptosis and fetal loss remain vague. Several candidate targets of RIP1–RIP3 kinase activity have emerged from screens7, but these still need to be fitted into the cell death pathways14.
In addition to contributing to host defence during viral infections (see below)5,27,63, necroptosis is involved in bacterium-driven chronic inflammation in the intestine (as shown in mice that lack FADD or caspase 8 in intestinal epithelial cells33,34) and in the spontaneous inflammation that occurs in the skin of mice that have a keratinocyte-specific deficiency in FADD32. These findings suggest that necroptosis may underlie inflammatory diseases that affect the gut and skin of humans.
RIP1-independent, RIP3-dependent programmed necrosis
RIP3 is necessary for necroptosis, as well as for murine cytomegalovirus (MCMV)-induced programmed necrosis27, but does not contribute directly to TNFR- or TLR-induced NF-κB activation or apoptosis79. Despite their inability to support necroptosis5–7 or MCMV-induced programmed necrosis27, Rip3−/− mice lack obvious developmental or immunological abnormalities79. These animals show a full level of resistance to natural mouse pathogens, including MCMV27, murine hepatitis virus65 and lymphocytic choriomeningitis virus68. However, RIP3-deficient mice are remarkably susceptible to infection with vaccinia virus, a member of the poxvirus family5. Thus, RIP3-dependent pathways probably contribute to host defence against viral infection. However, these RIP3-dependent pathways may have evolved at a cost, as abnormal levels of necrosis may underlie sterile80, as well as pathogen-induced33,34, inflammatory diseases.
Although MCMV-induced programmed necrosis requires RIP3 kinase activity and RHIM-dependent interactions27, it is independent of RIP1 and thus distinct from necroptosis5–7. MCMV-induced necrotic death rapidly eliminates infected cells, thereby removing the infection before viral replication can occur; these findings reinforce the idea that this pathway has an important role in host defence27. Given that RIP1 and TRIF are not involved in this pathway27, RIP3 may either form a homotypic complex or interact with an additional cellular RHIM-containing protein. DNA-dependent activator of interferon regulatory factors (DAI; also known as ZBP1)81 remains an attractive candidate partner for RIP3 (FIG. 1a). DAI and RIP3 form a RHIM-dependent complex, and the MCMV-encoded viral inhibitor of RIP activation (vIRA) blocks this inter action82,83. DAI is a cytosolic DNA sensor81 that potentially contributes to host defence against human cytomegalo virus84. Although DAI-deficient mice lack any obvious developmental phenotype and retain the ability to recognize cytosolic DNA85, these mice will help to clarify the role of this adaptor in MCMV-induced programmed necrosis.
Viral control of programmed necrosis
Viruses are heavily reliant on the fate of infected cells and have evolved to encode suppressors of cell death that increase viral spread by preventing cell clearance86–88. The existence of these suppressors provides tangible evidence that both apoptotic and necrotic death pathways are a benefit to host defence. It is possible to appreciate the compendium of crucial biological pathways encountered by each viral pathogen through the suppressors that the pathogen encodes. Programmed necrosis can occur when caspase 8 activity is compromised, suggesting that the abundance of viral caspase 8 inhibitors (TABLE 1) may have driven the evolution of programmed necrosis as a counteradaptation for host defence27,63,89. Few viral inhibitors of programmed necrosis have been identified (FIG. 2; TABLE 1), which at first glance may suggest a potential recent emergence. However, as programmed necrosis has only recently joined the ranks of bona fide host defence pathways, additional mechanisms and inhibitors of programmed necrosis may soon be recognized.
Table 1.
Selected viral inhibitors of apoptosis and necrosis
| Type of inhibitor | Inhibitor | Virus | Known targets | Mechanism | Gene ID or accession number |
|---|---|---|---|---|---|
| Inhibitors of apoptosis and programmed necrosis | |||||
| cFLIP homologue | MC159 | MCV | Caspase 8, FADD | Inhibits oligomerization |
1487017 |
| cFLIP homologue | K13 | KSHV | Caspase 8 | Prevents activation | 4961494 |
| cFLIP homologue | E8 | EHV-1 | Caspase 8 | – | 1461076 |
| RHIM inhibitor | vIRA | MCMV | RIP1, RIP3, TRIF, DAI |
Inhibits RHIM- mediated interactions |
CAP08092.1 |
| Inhibitors of apoptosis | |||||
| Caspase 8 inhibitor | vICA | CMV | Caspase 8 | Prevents activation | 3077442 |
| Caspase 8 inhibitor | BORFE2 | BHV-4 | Caspase 8 | – | 1684940 |
| Caspase 8 inhibitor | E3 14.7 kDa | Adenovirus | Caspase 8 | Prevents activation | 1460862 |
| Caspase 8 inhibitor | UL39 | HSV-1, HSV-2 | Caspase 8 | Prevents activation | 2703361, 1487325 |
| Serpin | CrmA | Cowpox virus | Caspases 1, 4, 5, 8 and 10, granzyme B |
Inhibits activity | 1486086 |
| Serpin | B13R | Vaccinia virus | Caspases | – | 3707572 |
| Serpin | Serp2 | Myxoma virus | Caspases | – | 932102 |
| Other | E6 | HPV-16 | Caspase 8, FADD | Inhibits oligomerization, degrades |
1489078 |
| Other | p35 | Baculovirus | Caspases | Inhibits activity | 1403968 |
BHV-4, bovine herpesvirus 4; CMV, cytomegalovirus; DAI, DNA-dependent activator of interferon regulatory factors; EHV-1, equine herpesvirus 1; FADD, FAS-associated death domain protein; HPV-16, human papillomavirus 16; HSV, herpes simplex virus; KSHV, Kaposi’s sarcoma-associated herpesvirus; MCMV, murine cytomegalovirus; MCV, molluscum contagiosum virus; RHIM, RIP homotypic interaction motif; RIP, receptor-interacting protein; TRIF, TIR domain-containing adaptor protein inducing IFNβ; vICA, viral inhibitor of caspase 8 activation; vIRA, viral inhibitor of RIP activation.
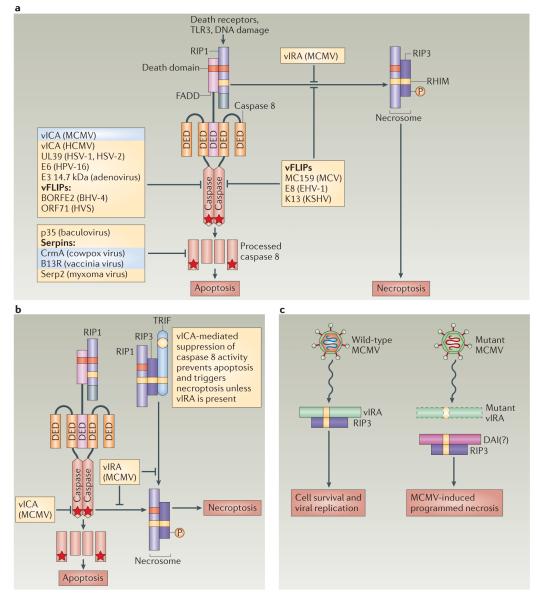
Figure 2. Viral modulation of cell death signals mediated by caspase 8 activation and RIP1–RIP3 pathways.
a ∣ Viral proteins that directly target receptor-interacting protein 1 (RIP1), FAS-associated death domain protein (FADD) and/or caspase 8 suppress signalling pathways that are activated by death receptors, pattern recognition receptors (PRRs; such as Toll-like receptor 3 (TLR3)) or cell stress (such as DNA damage). Many virus-encoded proteins block caspase 8-dependent apoptosis by interfering with caspase 8, FADD and/or RIP1 (see also TABLE 1). Several poxvirus proteins of the serpin family bind directly to fully processed caspase 8 to prevent apoptosis, whereas other viral inhibitors of caspase 8 — such as vICA (viral inhibitor of caspase 8 activation), CrmA and B13R (all highlighted in blue) — sensitize cells to death receptor-induced necroptosis by disrupting caspase 8-mediated suppression of RIP1–RIP3 activity. A subset of viral FLICE-like inhibitory proteins (vFLIPs) — including MC159, E8 and the murine cytomegalovirus (MCMV) protein vIRA (viral inhibitor of RIP activation) — block both apoptosis and RIP1- and RIP3-mediated necrosis. b ∣ The MCMV protein vICA prevents caspase 8 activation, and this sensitizes cells to death receptor-induced necroptosis. In addition, the MCMV protein vIRA blocks necroptosis and MCMV-induced programmed necrosis by inhibiting RHIM (RIP homotypic interaction motif)-dependent interactions. vIRA also inhibits the activation of caspase 8 by RIP1 or TIR domain-containing adaptor protein inducing IFNβ (TRIF). c ∣ A mutant MCMV that encodes vIRA with a mutant RHIM domain triggers programmed necrosis in cells with sufficient levels of RIP3. MCMV-induced programmed necrosis is independent of RIP1. The cellular RHIM-containing cytosolic sensor of double-stranded DNA DAI (DNA-dependent activator of interferon regulatory factors) may promote RIP3-dependent programmed necrosis during infection with mutant MCMV. Stars indicate catalytically active caspase 8. BHV-4, bovine herpesvirus 4; DED, death effector domain; EHV-1, equine herpesvirus 1; HCMV, human cytomegalovirus; HPV-16, human papillomavirus 16; HSV, herpes simplex virus; HVS, herpesvirus saimiri; KSHV, Kaposi’s sarcoma-associated herpesvirus; MCV, molluscum contagiosum virus.
The activation of caspase 8 is prevented by various pathogen-encoded molecules, including the baculovirus p35 protein90, the adenovirus E3 14.7 kDa protein91, several poxvirus serpins and vFLIPs (viral FLIPs)92, the gammaherpesvirus vFLIPs93 and the cytomegalovirus protein vICA (viral inhibitor of caspase 8 activation)94 (FIG. 2). Vaccinia virus-infected cells become susceptible to death receptor-induced necroptosis owing to inhibition of caspase 8 by a viral caspase inhibitor95 in host cells that have sufficient levels of RIP3 (REF. 5). These features help to explain the susceptibility of RIP3-deficient mice to lethal vaccinia virus infection5, as well as why necroptosis can be viewed as a trap door to eliminate cells when caspase 8 activity is compromised.
Cytomegaloviruses are evolutionarily ancient herpesviruses that give rise to a complex pathogenesis and can persist in targeted host epithelial, myeloid and endothelial cells96. Like other mammalian viruses, herpesviruses differ in pathogenicity and virulence in large part owing to their expression of immune modulators that facilitate infection by undermining host innate and adaptive immunity. The presence in all mammalian cytomegaloviruses of well-conserved inhibitors of caspase 8 activation and of mitochondrial inhibitors of apoptosis that target the activation of BAX and BAK demonstrate that apoptotic cell death pathways contribute to viral clearance94,97–100.
By targeting caspase 8, cytomegalovirus-derived vICA reduces the impact of extrinsic, death receptor-associated host control over the virus. Moreover, MCMV counteracts necrotic death by encoding vIRA, which is a product of the M45 gene that blocks RHIM-dependent signalling pathways, including death receptor-induced apoptosis and necroptosis27,83,101, TRIF-dependent apoptosis27 and TRIF- or DAI-dependent NF-κB activation82,83,102. The region of M45 encoding the RHIM domain, which is crucial for vIRA function, is not present in primate cytomegaloviruses, even though the gene is otherwise conserved. Rat cytomegalovirus encodes a close homologue of vIRA and, interestingly, an evolutionarily distant orthologue may be carried by herpes simplex virus 2 (REF. 103).
An understanding of the role of vIRA in viral pathogenesis emerged from studies of a precise mutation that affects only the RHIM domain27,101. Wild-type MCMV replicates in an equivalent manner in both wild-type and Rip3−/− mice, demonstrating that RIP3 pathways make little difference during infection by a fully armed herpesvirus that has a full complement of immune modulators. By contrast, MCMV with the specific M45 mutation is completely attenuated by RIP3-dependent pathways in C57BL/6 mice. Importantly, the replication and pathogenesis of the mutant virus are completely normalized in RIP3-deficient mice, a fact that establishes the direct relationship between RIP3 as the target and vIRA as a RHIM-disrupting suppressor of cell death.
The evolutionary adaptation of necrotic death into a host defence pathway may have driven the acquisition of vIRA by a progenitor of MCMV (and rat cytomegalovirus), even though this acquisition has not apparently affected primate cytomegaloviruses. Once a primordial vertebrate evolved to execute programmed necrosis in response to pathogen-encoded caspase inhibitors, the arms race was heightened while the pathogen evolved to counteract the additional death pathway. Each pathogen–host pairing reaches détente, but solutions vary. The fact that MCMV vICA targets caspase 8 (REFS 98,104) and that this sensitizes cells to MCMV-induced necrosis, which can be suppressed by vIRA27, fits with such an evolutionary scenario. Cycles of pathogen–host adaptation and counteradaptation play out through evolution105 and, in the case of herpesviruses, the survival of both the pathogen and the host involves a continually changing battle over the lifetime of an individual, as well as a war that wages over evolutionary time in populations. In addition to vIRA, several vFLIPs — namely, MC159 from molluscum contagiosum virus, E8 from equine herpesvirus 1 and K13 from Kaposi’s sarcoma-associated herpesvirus (also known as human herpesvirus 8) — also have the capacity to block both apoptosis and necroptosis26,63. These vFLIPs are structurally most similar to the cFLIPS isoform and likewise inhibit the enzymatic activity of caspase 8; thus, the biochemical mechanisms by which these viral inhibitors prevent necroptosis remain to be established. Given that only two viral antinecrotic strategies have been described, the absence of a RHIM-dependent inhibitor similar to vIRA in primate cytomegaloviruses may be due to an independent adaptation, such as a mechanism that suppresses RIP3 kinase activity, and this is worth exploring.
The role of cell death pathways in immunity
In addition to its role in natural host defence through the elimination of infected cells, programmed cell death plays an important part in shaping host immune responses106. Although the general consensus is that apoptosis is immunologically tolerogenic and necrosis is immunogenic, questions remain as to how the different modes of death, particularly programmed necrosis, influence adaptive immune responses. Despite the absence of caspase 8-dependent and RIP3-dependent death pathways, Casp8−/−Rip3−/− mice mount immune responses during viral infections22, so these mice provide a tool for understanding the contributions of extrinsic apoptosis and of danger signals that result from necrosome-dependent cell death107 to innate and adaptive immunity. In addition to the control of MCMV infection by Casp8−/−Rip3−/− mice22, RNA virus infections can be controlled by mice reconstituted with Fadd−/− Rip1−/− haematopoietic progenitors24 and by Rip3−/− mice in which T cells express a dominant-negative mutant of FADD65 or lack caspase 8 (REF. 68). These observations indicate that basic innate and adaptive immune responses to natural pathogens are sustained in these mice despite the absence of extrinsic apoptosis and programmed necrosis pathways. In Casp8−/−Rip3−/− mice, intrinsic apoptosis remains intact. A combination of intrinsic death and inflammatory responses appears to be adequate for the elimination of infected cells, antiviral interferon action and the cross-presentation of antigens. These events are sufficient for the initiation of an adaptive immune response and the elimination of pathogen-infected cells by activated T cells, despite the absence of extrinsic apoptosis and programmed necrosis. Moreover, intrinsic apoptosis appears to be sufficient for the contraction phase of the immune response, as well as for immune memory. It will be important to determine the precise apoptotic and inflammatory responses that contribute to immune control of infections and immune regulation in the absence of both caspase 8-dependent apoptosis and RIP3-dependent necrosis, given the potential for either pathway to influence adaptive immunity86,108.
T cells naturally exhibit the greatest proliferative capacity of any mammalian cell type. Cell death pathways have been implicated in the homeostasis, activation and contraction of T cell populations. FAS-dependent apoptosis mediates the elimination of excess T cells that accumulate over the course of life109. The importance of FAS signalling in homeostatic T cell control has been evident since lymphadenopathy, splenomegaly and abnormal CD3+CD4−CD8−B220+ lymphocytes were first observed in ageing lpr/lpr mice (which are deficient in FAS)110 and gld/gld mice (which are deficient in FAS ligand)111. A deficit in caspase 8 or FADD alone in T cells does not recapitulate this phenotype69, although modest lymphoproliferation is sometimes noted112. Progressive abnormal T cell accumulation similar to that observed in mice deficient in FAS signalling is observed in Casp8−/− Rip3−/− mice22,23, presumably because caspase 8 activation lies directly downstream of FAS in the apoptotic pathway109. Similar defects are observed in mice that have a T cell-specific disruption of caspase 8 or FADD on a Rip3−/− background65,68. These defects do not compromise T cell responses towards a variety of viral infections22,65,68, but they clearly indicate that caspase 8 has a crucial role in T cell homeostasis downstream of FAS.
RIP1- and RIP3-dependent necroptosis underlies mouse T cell loss in caspase 8- or FADD-deficient settings69,70. The T cell response is rescued when necrosome components are eliminated22–24,65,68, suggesting that necroptosis may regulate the antigen-specific T cell response. It is tempting to extrapolate these findings to human biology, where the presence of caspase 10 — a caspase 8 paralogue not present in mice — contributes to outcomes along with caspase 8. Interestingly, the T cell defects in individuals with mutant caspase 8 are different from those found in individuals with mutant caspase 10. Casp8 mutations result in immunodeficiency113, suggesting a role in inhibiting necroptosis in lymphocytes, whereas caspase 10 deficiency results in lymphadenopathy, splenomegaly and auto immunity114, which is most aligned with a role downstream of FAS in apoptosis. Moreover, human caspase 8 and caspase 10 may have only partially overlapping functions during human embryonic development, as individuals with mutations in either gene have different outcomes; caspase 8 is predicted to control apoptosis and necroptosis, whereas caspase 10 is possibly restricted to the control of apoptosis68. The contributions that the two human orthologues make to apoptotic and necrotic death during development, host defence, cancer, immunity and disease require closer comparative evaluation.
Conclusions
It is now clear that programmed necrosis and apoptosis have complementary roles in host defence against pathogens. One form of programmed necrosis, necroptosis, is dependent on RIP1 and RIP3 and is triggered via death receptors, PRRs, the TCR or genotoxic stress, as well as during midgestational development, in settings when caspase 8 activity is compromised. Necroptosis has a role in host defence during infection with intra-cellular pathogens that encode caspase 8 inhibitors, but it also contributes to disease pathogenesis in acute or chronic bacterium-induced inflammation. By contrast, MCMV-induced programmed necrosis is independent of RIP1 but dependent on RIP3. MCMV encodes not only a caspase 8 inhibitor that sensitizes infected cells to programmed necrosis, but also a RHIM-dependent inhibitor of necroptosis and MCMV-induced programmed necrosis. The identification of MCMV-induced programmed necrosis implicates viral infection — and viral suppressors of cell death — in the evolution of programmed necrosis as an alternative mechanism of host defence.
Acknowledgements
The authors thank D. Livingston-Rosanoff and L. Daley-Bauer for their contributions, L. Roback and A. L. McCormick for helping to facilitate this research and C. Benedict for discussions. The research was supported by grants from the US National Institutes of Health (AI030363 and AI020212) and the Georgia Cancer Coalition. We apologize to investigators whose contributions were not cited more extensively owing to space limitations.
Glossary
- Apoptosis
The most common form of developmental cell death that regulates cell numbers, drives morphogenesis, deletes structures and eliminates unneeded and harmful cells. Apoptosis is a cell-autonomous death pathway mediated by caspases that dismantles the cell but maintains membrane integrity and is characterized by cell shrinkage, membrane blebbing and DNA fragmentation.
- RIP1
(Receptor-interacting protein 1; also known as RIPK1). A signalling adaptor and protein kinase that regulates the activation of gene expression via the NF-κB and MAPK pathways and controls the initiation of necroptosis and apoptosis via death domain- and RHIM-dependent complexes.
- RIP3
(Receptor-interacting protein 3; also known as RIPK3). A RHIM-containing signalling adaptor and protein kinase that mediates necroptosis and MCMV-induced necrosis.
- Programmed necrosis
A cell-autonomous, regulated cell death pathway that is characterized by cell swelling, membrane rupture and cytoplasmic leakage, but not by membrane blebbing.
- Necroptosis
A form of programmed necrosis that is executed by the kinase activities of RIP1 and RIP3. This pathway is inhibited by RIP1 kinase inhibitors, such as necrostatin 1 (5-(1H-indol-3-ylmethyl)-3-methyl-2-thioxo-4-imidazolidinone).
- RIP homotypic interaction motif
(RHIM). A protein–protein interaction motif containing the core sequence (I/V/L)-(Q/M)-(I/V/L)-G that mediates homophilic interactions between four cellular proteins (namely, RIP1, RIP3, TRIF and DAI) and one viral protein (MCMV vIRA).
- Death receptors
A subset of receptors belonging to the TNF receptor superfamily that transmit cell death signals initiated by their cognate ligands. These receptors include TNFR1, FAS, DR3, TRAILR1 and TRAILR2.
- Toll-like receptors
(TLRs). A family of pattern recognition receptors that recognize unique structures derived from microorganisms. TLR signalling promotes inflammatory and cytokine responses, as well as cell proliferation or cell death pathways.
- FAS
Also known as CD95). A death receptor of the TNF receptor superfamily. FAS ligand binding induces cell death. FAS signalling controls the homeostatic elimination of T cells.
- Death-inducing signalling complex
(DISC). A death receptor-bound complex that contains FADD and caspase 8 (or caspase 10). The DISC assembles following ligand binding and drives autocatalytic caspase 8 (or caspase 10) activation.
- Complex I
A TNFR1-bound complex that contains TRADD, TRAF2 or TRAF5, cIAP and RIP1. This complex drives the activation of gene expression via the NF-κB and MAPK pathways.
- Cellular inhibitor of apoptosis proteins
(cIAP1 and cIAP2; collectively referred to as cIAP here). Members of a family of functionally and structurally related E3 ubiquitin ligases that regulate canonical and non-canonical activation of NF-κB, as well as MAPK activation by receptors of the TNF receptor superfamily. cIAP polyubiquitylates RIP1 to prevent the formation of the ripoptosome or TNFR1-dependent complex II.
- Complex II
A TNFR1- and RIP1-dependent cytosolic complex that contains caspase 8, FADD, cFLIP and RIP1. Within this complex, caspase 8 and cFLIP regulate programmed cell death pathways.
- Necrosome
An inducible cytosolic complex that contains oligomerized RIP1 and RIP3. This complex drives RIP1- and RIP3-dependent necroptosis.
- Ripoptosome
A RIP1-dependent cytosolic complex that is similar in composition to TNFR1-dependent complex II and that controls programmed cell death pathways.
- TIR domain-containing adaptor protein inducing interferon-β
(TRIF). A adaptor protein for TLR3 and TLR4 that organizes downstream signalling cascades leading to IRF3 and NF-κB activation, or cell death. TRIF mediates signalling through a TIR domain, TRAF-binding sites and RHIM-mediated interactions with RIP1 and RIP3.
- CARMA1–BCL-10–MALT1 complex
A PKC-dependent specialized signalling complex that is formed during TCR-dependent antigen recognition and that triggers NF-κB activation.
- DNA-dependent activator of interferon regulatory factors
(DAI; also known as ZBP1). A cytosolic, RHIM-containing sensor of double-stranded DNA that activates IRF3 and NF-κB.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu. Rev. Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 3.Yuan J, Kroemer G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010;24:2592–2602. doi: 10.1101/gad.1984410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]; A seminal study that demonstrated that the kinase RIP1 is a mediator of necrosis induced by the death receptor FAS and that caspase 8 is a crucial negative regulator of the pathway.
- 5.Cho YS, et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]; References 5–7 demonstrated that a RHIM-dependent RIP1–RIP3 kinase complex mediates necrosis induced by death receptors.
- 8.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J. Biol. Chem. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 9.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol. Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 11.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 12.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J. Biol. Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantari C, Walczak H. Caspase-8 and Bid: caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature Rev. Mol. Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 15.Kang TB, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J. Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 16.Kang TB, et al. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J. Immunol. 2008;181:2522–2532. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]; This study formally demonstrated that the role of caspase 8 in mediating extrinsic apoptosis is distinct from its essential role during mammalian embryogenesis.
- 17.Sakamaki K, et al. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 2002;9:1196–1206. doi: 10.1038/sj.cdd.4401090. [DOI] [PubMed] [Google Scholar]
- 18.Varfolomeev EE, et al. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 19.Yeh WC, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 21.Yeh WC, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that mice lacking both Casp8 and Rip3 are viable, fertile and immunocompetent, and sustain antiviral T cell immunity. Thus, dysregulation of RIP3 underlies the embryonic lethality, as well as the defects in T cell function, in caspase 8-deficient mice. Like FAS-deficient mice, Casp8−/−Rip3−/− mice fail to eliminate excess T cells as they age.
- 23.Oberst A, et al. Catalytic activity of the caspase-8–FLIPL complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that mice lacking both Casp8 and Rip3 develop normally into adults but fail to eliminate excess T cells as they age, similarly to FAS-deficient mice. The regulation of RIP1–RIP3 activity was attributed to proteolytic activity of the caspase 8–cFLIPL complex.
- 24.Zhang H, et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that mice lacking both Fadd and Rip1 complete embryonic development. Thus, dysregulation of RIP1 underlies the embryonic lethality, as well as the defects in T cell function, in FADD-deficient mice.
- 25.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nature Rev. Mol. Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 26.Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem. Sci. 2010;35:434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; A virus-encoded RHIM-containing suppressor of programmed necrosis was identified in this study, unveiling a natural RIP3-dependent, RIP1-independent death pathway in mammalian host defence.
- 28.Kalai M, et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ. 2002;9:981–994. doi: 10.1038/sj.cdd.4401051. [DOI] [PubMed] [Google Scholar]
- 29.Feoktistova M, et al. cIAPs block ripoptosome formation, a RIP1/Caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that TLR3 signalling engages a cytosolic caspase 8-activating complex (the ripoptosome) that requires RIP1 kinase activity, is repressed by cIAP and is differentially controlled by cFLIPL and cFLIPs.
- 30.Tenev T, et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]; This study identified that cellular responses to genotoxic stress involve the formation of a cytosolic caspase 8-activating complex (the ripoptosome), with a requirement for RIP1 kinase activity and repression by cIAP.
- 31.Wallach D, Kovalenko A, Kang TB. ‘Necrosome’-induced inflammation: must cells die for it? Trends Immunol. 2011;32:505–509. doi: 10.1016/j.it.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Bonnet MC, et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Welz PS, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 34.Gunther C, et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerlach B, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 36.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nature Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 37.Walczak H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol. Rev. 2011;244:9–28. doi: 10.1111/j.1600-065X.2011.01066.x. [DOI] [PubMed] [Google Scholar]
- 38.Silke J. The regulation of TNF signalling: what a tangled web we weave. Curr. Opin. Immunol. 2011;23:620–626. doi: 10.1016/j.coi.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Wajant H, Scheurich P. TNFR1-induced activation of the classical NF-κB pathway. FEBS J. 2011;278:862–876. doi: 10.1111/j.1742-4658.2011.08015.x. [DOI] [PubMed] [Google Scholar]
- 40.Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nature Immunol. 2002;3:1013–1018. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 41.Kischkel FC, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muzio M, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 43.Sprick MR, et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 44.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 45.Irmler M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 46.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 48.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 49.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 50.Bertrand MJ, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Vince JE, et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (TNF) to efficiently activate NF-κB and to prevent TNF-induced apoptosis. J. Biol. Chem. 2009;284:35906–35915. doi: 10.1074/jbc.M109.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varfolomeev E, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]; This report revealed that TNFR1 pro-death signalling is blocked by the E3 ligase cIAP in part through inhibition of the RIP1-dependent assembly of a caspase 8-activating complex, but that this inhibition is reversed by the deubiquitylating enzyme CYLD.
- 54.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 55.Vanlangenakker N, et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;18:656–665. doi: 10.1038/cdd.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-α feedforward signaling. Cell. 2011;145:92–103. doi: 10.1016/j.cell.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 57.Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nature Rev. Mol. Cell Biol. 2011;12:439–452. doi: 10.1038/nrm3143. [DOI] [PubMed] [Google Scholar]
- 58.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 59.Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIPL. Biochem. J. 2004;382:651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Micheau O, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 61.Oberst A, et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J. Biol. Chem. 2010;285:16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pop C, et al. FLIPL induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem. J. 2011;433:447–457. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan FK, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]; This study presented initial evidence that some mammalian viruses encode suppressors of programmed necrosis.
- 64.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu JV, et al. Complementary roles of FADD and RIPK3 in T cell homeostasis and antiviral immunity. Proc. Natl Acad. Sci. USA. 2011;108:15312–15317. doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell. Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 67.O’Donnell MA, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nature Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified the RIP1 deubiquitylating enzyme CYLD as a key target of caspase 8 in suppressing necrosis following death receptor signalling.
- 68.Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J. Exp. Med. 2011;208:633–641. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hedrick SM, Ch’en IL, Alves BN. Intertwined pathways of programmed cell death in immunity. Immunol. Rev. 2010;236:41–53. doi: 10.1111/j.1600-065X.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ch’en IL, et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc. Natl Acad. Sci. USA. 2008;105:17463–17468. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that caspase 8-deficient T cells fail to proliferate in response to antigens owing to RIP1-dependent necroptosis.
- 71.Gaide O, et al. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nature Immunol. 2002;3:836–843. doi: 10.1038/ni830. [DOI] [PubMed] [Google Scholar]
- 72.Ruefli-Brasse AA, French DM, Dixit VM. Regulation of NF-κB-dependent lymphocyte activation and development by paracaspase. Science. 2003;302:1581–1584. doi: 10.1126/science.1090769. [DOI] [PubMed] [Google Scholar]
- 73.Ruland J, et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 74.Kawadler H, Gantz MA, Riley JL, Yang X. The paracaspase MALT1 controls caspase-8 activation during lymphocyte proliferation. Mol. Cell. 2008;31:415–421. doi: 10.1016/j.molcel.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Misra RS, et al. Caspase-8 and c-FLIPL associate in lipid rafts with NF-κB adaptors during T cell activation. J. Biol. Chem. 2007;282:19365–19374. doi: 10.1074/jbc.M610610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eimon PM, et al. Delineation of the cell-extrinsic apoptosis pathway in the zebrafish. Cell Death Differ. 2006;13:1619–1630. doi: 10.1038/sj.cdd.4402015. [DOI] [PubMed] [Google Scholar]
- 77.Naitza S, et al. The Drosophila immune defense against Gram-negative infection requires the death protein dFADD. Immunity. 2002;17:575–581. doi: 10.1016/s1074-7613(02)00454-5. [DOI] [PubMed] [Google Scholar]
- 78.Leulier F, Rodriguez A, Khush RS, Abrams JM, Lemaitre B. The Drosophila caspase Dredd is required to resist Gram-negative bacterial infection. EMBO Rep. 2000;1:353–358. doi: 10.1093/embo-reports/kvd073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-κB signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Challa S, Chan FK. Going up in flames: necrotic cell injury and inflammatory diseases. Cell. Mol. Life Sci. 2010;67:3241–3253. doi: 10.1007/s00018-010-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 82.Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-κB activation via the DNA-dependent activator of IFN regulatory factors. J. Immunol. 2008;181:6427–6434. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rebsamen M, et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-κB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Fruh K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J. Virol. 2010;84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 86.Galluzzi L, et al. Viral strategies for the evasion of immunogenic cell death. J. Intern. Med. 2010;267:526–542. doi: 10.1111/j.1365-2796.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 87.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 88.Roy CR, Mocarski ES. Pathogen subversion of cell-intrinsic innate immunity. Nature Immunol. 2007;8:1179–1187. doi: 10.1038/ni1528. [DOI] [PubMed] [Google Scholar]
- 89.Vercammen D, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clem RJ, Fechheimer M, Miller LK. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 91.Chen P, Tian J, Kovesdi I, Bruder JT. Interaction of the adenovirus 14.7-kDa protein with FLICE inhibits Fas ligand-induced apoptosis. J. Biol. Chem. 1998;273:5815–5820. doi: 10.1074/jbc.273.10.5815. [DOI] [PubMed] [Google Scholar]
- 92.Taylor JM, Barry M. Near death experiences: poxvirus regulation of apoptotic death. Virology. 2006;344:139–150. doi: 10.1016/j.virol.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 93.Lagunoff M, Carroll PA. Inhibition of apoptosis by the γ-herpesviruses. Int. Rev. Immunol. 2003;22:373–399. doi: 10.1080/08830180305218. [DOI] [PubMed] [Google Scholar]
- 94.McCormick AL. Control of apoptosis by human cytomegalovirus. Curr. Top. Microbiol. Immunol. 2008;325:281–295. doi: 10.1007/978-3-540-77349-8_16. [DOI] [PubMed] [Google Scholar]
- 95.Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor α and caspase inhibitors: novel mechanism for killing virus-infected cells. J. Virol. 2000;74:7470–7477. doi: 10.1128/jvi.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mocarski ES. In: Human Herpesviruses: Biology, Therapy and Immunoprophylaxis. Arvin A, et al., editors. Cambridge Univ. Press; Cambridge, UK: 2007. pp. 204–230. [PubMed] [Google Scholar]
- 97.Cicin-Sain L, et al. Dominant-negative FADD rescues the in vivo fitness of a cytomegalovirus lacking an antiapoptotic viral gene. J. Virol. 2008;82:2056–2064. doi: 10.1128/JVI.01803-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Skaletskaya A, et al. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl Acad. Sci. USA. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brune W. Inhibition of programmed cell death by cytomegaloviruses. Virus Res. 2011;157:144–150. doi: 10.1016/j.virusres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 100.Manzur M, Fleming P, Huang DC, Degli-Esposti MA, Andoniou CE. Virally mediated inhibition of Bax in leukocytes promotes dissemination of murine cytomegalovirus. Cell Death Differ. 2009;16:312–320. doi: 10.1038/cdd.2008.152. [DOI] [PubMed] [Google Scholar]
- 101.Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J. Biol. Chem. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was the first report of a virus-encoded RHIM-containing inhibitor of RHIM-dependent signalling pathways.
- 102.Mack C, Sickmann A, Lembo D, Brune W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl Acad. Sci. USA. 2008;105:3094–3099. doi: 10.1073/pnas.0800168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lembo D, Brune W. Tinkering with a viral ribonucleotide reductase. Trends Biochem. Sci. 2009;34:25–32. doi: 10.1016/j.tibs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 104.McCormick AL, Skaletskaya A, Barry PA, Mocarski ES, Goldmacher VS. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology. 2003;316:221–233. doi: 10.1016/j.virol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 105.Paterson S, et al. Antagonistic coevolution accelerates molecular evolution. Nature. 2010;464:275–278. doi: 10.1038/nature08798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferguson TA, Choi J, Green DR. Armed response: how dying cells influence T-cell functions. Immunol. Rev. 2011;241:77–88. doi: 10.1111/j.1600-065X.2011.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 108.Krysko DV, D’Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- 109.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 111.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol. Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 112.Salmena L, Hakem R. Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J. Exp. Med. 2005;202:727–732. doi: 10.1084/jem.20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chun HJ, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 114.Wang J, et al. Inherited human caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 115.Rosenberg S, Zhang H, Zhang J. FADD deficiency impairs early hematopoiesis in the bone marrow. J. Immunol. 2011;186:203–213. doi: 10.4049/jimmunol.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beisner DR, Ch’en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J. Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- 117.Imtiyaz HZ, et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J. Immunol. 2006;176:6852–6861. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lemmers B, et al. Essential role for caspase-8 in Toll-like receptors and NFκB signaling. J. Biol. Chem. 2007;282:7416–7423. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- 119.Kovalenko A, et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J. Exp. Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee P, et al. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–523. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li C, et al. Development of atopic dermatitis-like skin disease from the chronic loss of epidermal caspase-8. Proc. Natl Acad. Sci. USA. 2010;107:22249–22254. doi: 10.1073/pnas.1009751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ben Moshe T, et al. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology. 2007;45:1014–1024. doi: 10.1002/hep.21495. [DOI] [PubMed] [Google Scholar]