Abstract
Introduction
Dyspnea is common among lung cancer patients. As most studies of dyspnea have reviewed patients with active cancer or immediately after treatment, its prevalence during the longer-term period once treatment has been completed is not well characterized. This study quantifies the prevalence of dyspnea among lung cancer survivors and identifies potential correlates that may be amenable to intervention.
Methods
Cross-sectional survey of 342 patients with disease-free, stage I, non-small cell lung cancer, assessed 1 to 6 years after surgical resection. Dyspnea was quantified using the Baseline Dyspnea Index. Any moderate/strenuous physical activity was measured using the Godin Leisure-Time Exercise Questionnaire. Mood disorder symptoms were assessed using the Hospital Anxiety and Depression Scale. Multiple regression analyses were used to examine demographic, medical, and health-related correlates of dyspnea.
Results
Mean age was 68.9 years. Average predicted preoperative forced expiratory volume in 1 second was 89.0%. Current dyspnea, defined by a Baseline Dyspnea Index score of 9 or less, existed among 205 (60%) individuals. For 133 (65%) of these patients, dyspnea was absent preoperatively. Multivariate correlates of current dyspnea included preoperative dyspnea (odds ratio [OR] = 5.31), preoperative diffusing capacity (OR = 0.98), lack of moderate/strenuous physical activity (OR = 0.41), and the presence of clinically significant depression symptoms (OR = 4.10).
Conclusions
Dyspnea is common 1 to 6 years after lung cancer resection, and is associated with the presence of preoperative dyspnea, reduced diffusing capacity, clinically significant depression symptoms, and lack of physical activity. Further research is needed to test whether strategies that identify and treat patients with these conditions attenuate dyspnea among lung cancer survivors.
Keywords: Dyspnea, Survivors, Carcinoma, Non-small cell lung
An estimated 215,000 individuals were diagnosed with lung cancer in the United States during 2008.1 Although lung cancer remains the leading cause of cancer mortality in men and women, the relatively high 5-year survival rate for stage I disease, now exceeding 55%,2 and the near doubling of the 5-year survival rate since the 1960s, provides some room for optimism. Even with the small 15 to 20% expected cure rate for all stages, there are about 40,000 newly diagnosed individuals annually who may become long-term survivors.
Understanding the prevalence and severity of pulmonary comorbidities is essential for targeting appropriate care to this growing number of survivors. To date, most studies have focused on the short-term (i.e., <1 year) morbidity associated with surgery, radiation, and chemotherapy.3–6 Not surprisingly, these studies have shown pulmonary symptoms to be highly prevalent. One study found that the most common symptoms 1 to 4 months after curative surgery were fatigue (57%) and dyspnea (49%).6 In another study, Dales et al.7 reported moderate to severe dyspnea among 31% of patients within 3 months of lung cancer resection.
Relatively little is known about the long-term prevalence, severity, and impact of respiratory symptoms. In a single study regarding dyspnea, Sarna et al.8 examined pulmonary disability 5 or more years after curative surgery. Among 142 patients, whose median time from surgery was 10 years, 63% reported chronic dyspnea, and 15% were so dyspneic that they could not leave their homes or dress themselves. These findings not only support the findings of other investigators regarding the high prevalence of dyspnea, but imply it can persist for a prolonged period of time.
This study builds on this earlier work implicating dyspnea as a chronic problem after lung-cancer surgery. The goals of this study are 2-fold: (1) to describe the prevalence and severity of current dyspnea among lung cancer survivors and (2) to identify specific correlates associated with dyspnea that may help clinicians target post-treatment rehabilitation strategies. In contrast to earlier work, this study includes a larger sample size and incorporates measures of depression and anxiety. Although dyspnea has been associated with reduced health-related quality of life among lung cancer survivors,8 there is a lack of information regarding its potential association with mood disorders in this population. Furthermore, the time period encompassed by this study, 1 to 6 years after resection, has not been extensively studied and is a critical period for the delivery of post-treatment interventions.
MATERIALS AND METHODS
Patient Selection
A random patient sample, stratified by time since diagnosis, was extracted from an institutional database containing demographic and medical information of all patients undergoing surgical resection for non-small cell lung cancer (NSCLC) at a comprehensive cancer center in New York City. At the time of patient accrual, the standard treatment for stage I NSCLC was surgical resection.
Participants were eligible if they met the following criteria: (1) previous diagnosis of stage IA or IB NSCLC, (2) had undergone surgical resection with curative intent, (3) were between one and 6 years post-treatment, (4) had no evidence of lung cancer at time of recruitment, (5) could be reached by phone, (6) had no psychiatric or cognitive impairment judged to interfere with participation (as defined by a score of <4 on the Brief Six-Item Screener),9 and (7) could give informed consent.
Procedure
Permission to contact survivors was obtained from each of their thoracic surgeons, who cosigned introductory letters explaining the nature of the study. Survivors received these letters along with consent forms and research authorizations. Within 2 weeks of sending the letters, a research assistant phoned survivors to determine eligibility and interest in the study. Interested participants provided verbal consent and could either complete a phone survey or a mailed paper and pencil survey. The survey required 45 to 60 minutes for completion and included several questions beyond the scope of this study. All participants were offered print educational resources for psychosocial and rehabilitation services. The study was approved by the Institutional Review Board.
Measures
Primary Outcome
Current dyspnea was measured using a self-report version of the Baseline Dyspnea Index (BDI),10 a scale that assesses dyspnea severity along three dimensions: (1) functional impairment in tasks of daily living caused by dyspnea, (2) intensity of everyday tasks a person can complete without experiencing dyspnea, and (3) general effort a person can expend before experiencing dyspnea. Dyspnea is graded on a five-point (0–4) scale from “very severe” to “no impairment” for each dimension. An additional qualifying item was added to indicate inability to function due to reasons other than dyspnea. Responses across the items are summed to achieve a final value of zero through 12 with lower scores indicating more severe dyspnea.
The BDI has been well validated in the literature11 and used to assess dyspnea in patients with emphysema undergoing lung volume reduction surgery,12 outpatient pleurodesis of malignant pleural effusions,13 and to evaluate the outcomes of such medical interventions as bronchodilator therapy14 and exercise training.15 Current dyspnea was defined as a score of 9 or less. This cutoff has been used in previous research16 and ensures that those categorized as having dyspnea experienced symptoms that were at least mild in severity (i.e., an average of 3 or less for each dimension). Examples in this cohort of the limitation experienced by patients with BDI scores of 9 include a 64-year-old woman who experienced breathlessness carrying heavy objects, and a 74-year-old woman who experienced dyspnea walking up a hill.
Demographics
Demographic variables collected via self-report were age, sex, marital/partnership status, employment status, race/ethnicity, and education (≤ high school diploma, at least some college).
Medical and Health-Related Correlates
Data regarding potential medical and health-related correlates of current dyspnea were prospectively identified by the investigators and obtained from the medical record. These included elapsed time since surgery, type of surgery (wedge resection, segmentectomy, lobectomy, bilobectomy, or pneumonectomy), preoperative dyspnea (yes/no), preoperative forced expiratory volume in 1 second (FEV1) expressed in percent predicted, preoperative diffusing capacity for carbon monoxide expressed in percent predicted, use of video-assisted thoracoscopic surgery only (yes/no), and preoperative body mass index (BMI). Pulmonary function tests are obtained for all patients as part of routine clinical care per recommendations of the American College of Chest Physicians.17 Therefore, the presence or absence of FEV1 and/or diffusing capacity measurements in the medical record did not influence patient selection. Also recorded was the presence of preoperative cardiac or pulmonary disease (yes/no). Cardiac disease was defined as atrial fibrillation, angina, previous coronary artery bypass graft surgery, hypertension, previous myocardial infarction, previous percutaneous transluminal coronary angioplasty, or valvular heart disease. Lung disease was defined as previous asthma, chronic obstructive pulmonary disease (COPD), interstitial lung disease, or tuberculosis (Table 2). Lung cancer was not considered a lung disease for the purpose of this analysis, as it was an inclusion criterion for all patients.
TABLE 2.
Medical Characteristics of Sample (n = 342)
| Characteristic | n | % |
|---|---|---|
| Time since surgical resection (M = 3.5 y, SD = 1.2) | ||
| 1–<2 y | 37 | 10.8 |
| 2–<3 y | 99 | 28.9 |
| 3–<4 y | 88 | 25.7 |
| 4–<5 y | 75 | 21.9 |
| 5–<6 y | 43 | 12.6 |
| Missing (n) | (0) | |
| Type of surgical resection | ||
| Wedge | 40 | 11.7 |
| Segmentectomy | 30 | 8.8 |
| Lobectomy | 258 | 75.4 |
| Bilobectomy | 8 | 2.4 |
| Pneumonectomy | 6 | 1.7 |
| Missing (n) | (0) | |
| Preoperative dyspnea | ||
| No | 270 | 78.9 |
| Yes | 72 | 21.1 |
| Missing (n) | (0) | |
| Preoperative FEV1% (M = 89.0, SD = 20.3) | ||
| Severe (<50%) | 12 | 3.6 |
| Moderate (50–69%) | 48 | 14.4 |
| Mild (70%+) | 273 | 82.0 |
| Missing (n) | (9) | |
| Preoperative diffusing capacity % (M = 83.7, SD = 25.8) | ||
| Severe (<50%) | 17 | 5.5 |
| Moderate (50–69%) | 67 | 21.6 |
| Mild (70%+) | 226 | 72.9 |
| Missing (n) | (32) | |
| Video-assisted thoracic surgery (VATS) only | ||
| Yes | 61 | 18.0 |
| No | 280 | 82.0 |
| Missing (n) | (1) | |
| Preoperative body mass index (BMI) (M = 26.8, SD = 4.9) | ||
| <18.5 (underweight) | 6 | 1.7 |
| 18.5–24.9 (normal) | 133 | 38.9 |
| 25–29.9 (overweight) | 125 | 36.5 |
| ≥30 (obese) | 78 | 22.8 |
| Missing (n) | (0) | |
| Cardiac comorbidities | ||
| Atrial fibrillation | 9 | 3.4 |
| Angina | 2 | 0.8 |
| Coronary artery bypass graft | 10 | 3.8 |
| Hypertension | 74 | 28.0 |
| Myocardial infarction | 11 | 4.2 |
| Coronary angioplasty | 4 | 1.5 |
| Valvular condition | 2 | 0.8 |
| None | 159 | 57.6 |
| Missing (n) | (78) | |
| Pulmonary comorbidities | ||
| Asthma | 21 | 6.2 |
| COPD | 79 | 23.1 |
| Tuberculosis | 1 | 0.3 |
| None | 240 | 70.4 |
| Missing (n) | (1) | |
| Current smoking status | ||
| Current smoker | 19 | 5.5 |
| Former smoker | 268 | 78.4 |
| Never smoker | 55 | 16.1 |
| Missing (n) | (0) | |
| Moderate or strenuous physical activity | ||
| Any moderate/strenuous | 164 | 47.9 |
| None | 178 | 52.1 |
| Missing (n) | (0) | |
| Psychological distress | ||
| HADS depression (≥8) | 32 | 9.4 |
| HADS anxiety (≥8) | 69 | 20.2 |
| Missing (n) | (0) |
BDI, Baseline Dyspnea Index; HADS, Hospital Anxiety and Depression Scale.
The presence or absence of preoperative dyspnea was determined dichotomously by patient self-report on standard intake forms used by the Department of Thoracic Surgery as part of routine clinical practice. Typically, self-report occurs within 1 month of surgery. Preoperative BMI was calculated from the height and weight measured at the preoperative anesthesia visit.
Patients also reported current smoking status, physical activity, and the presence of depression and anxiety symptoms. Given recent Centers for Disease Control recommendations for older adults to get at least some moderate/strenuous physical activity,18 this variable was defined dichotomously as the presence or absence of any moderate or strenuous activity by self report using the Godin Leisure-Time Exercise Questionnaire.19 As respiratory symptoms have been associated with reduced health-related quality of life,8 we assessed the presence of clinically significant mood disorder symptoms using a standard cutoff of eight or more on each subscale of the Hospital Anxiety and Depression Scale (HADS).20
Statistical Plan
Variables listed earlier, prospectively identified as likely associated with current dyspnea (BDI ≤9), were examined via bivariate Pearson correlations. These were age, sex, employment status, education, elapsed time since surgical resection, type of surgery, preoperative dyspnea, preoperative FEV1, preoperative diffusing capacity, use of video-assisted thoracoscopic surgery only surgery, preoperative BMI, preoperative cardiac disease, preoperative lung disease excluding lung cancer, any history of tobacco use, any moderate/strenuous physical activity, clinically significant depression symptoms (HADS ≥8), and clinically significant anxiety symptoms (HADS ≥8). Multiple logistic regression was employed to examine the relative influence among the set of variables that had bivariate correlations with dyspnea at p < 0.05. Analyses were conducted using SAS v9.1, and a p value of <0.05 was used to determine statistical significance.
RESULTS
A total of 1,017 patients with NSCLC were identified as potentially eligible from the institutional database. Of these, 507 did not meet eligibility criteria. The most common reasons for exclusion were current malignancy (n = 144 patients), more than 6 years since surgical resection (n = 95), deceased (n = 57), pathologic stage II–IV disease (n = 55), and the inability to speak English (n = 47). Among the 556 potentially eligible patients, 151 declined to participate. Reasons cited for declining participation included lack of interest (n = 23), wishing to avoid discussing cancer (n = 20) and feeling too poorly to participate (n = 10). Cumulatively, 359 eligible patients provided informed consent (65% participation rate) and 342 provided analyzable dyspnea data. Recruitment and data collection were conducted from September 2005 through July 2007. There were no statistically significant (p < 0.05) differences between those who completed the survey and those who declined participation with regard to mean age, sex, time since resection, pathologic stage, preoperative pulmonary function, type of resection, length of hospital stay, and number of surgical complications. However, individuals who completed the study were less likely to have reported preoperative dyspnea (21.1%) than those who declined participation (29.8%, p = 0.02).
Tables 1 and 2 show demographic and medical characteristics respectively. The sample was mostly female (63.5%), non-Hispanic White (93.0%), married or living with a partner (62.0%), and well educated (68.0% had some college education). The mean age was 68.9 years and the mean time since treatment completion was 3.5 years. Most (75.6%) patients had undergone lobectomy. In addition, 18 patients had received adjuvant chemotherapy, two of whom also received radiation. Nearly 60% percent of the sample was overweight or obese prior to surgery. Former smokers comprised 78.4% of the sample; 5.5% continued to smoke.
TABLE 1.
Demographic Characteristics of Sample (n = 342)
| Characteristic | n | % |
|---|---|---|
| Age (y) (M = 68.9, SD = 9.8) | ||
| 35–54 | 37 | 10.8 |
| 55–64 | 70 | 20.5 |
| 65–74 | 126 | 36.8 |
| 75+ | 109 | 31.9 |
| Missing (n) | (0) | |
| Sex | ||
| Male | 125 | 36.5 |
| Female | 217 | 63.5 |
| Missing (n) | (0) | |
| Married/partnered | ||
| No | 130 | 38.0 |
| Yes | 212 | 62.0 |
| Missing (n) | (0) | |
| Employment status | ||
| Employed/homemaker | 125 | 36.8 |
| Retired/unemployed | 215 | 63.2 |
| Missing (n) | (2) | |
| Race/ethnicity | ||
| Non-Hispanic white | 317 | 93.0 |
| Non-Hispanic black | 10 | 2.9 |
| Non-Hispanic Asian/Pacific | 4 | 1.2 |
| Islander | ||
| Non-Hispanic other | 2 | 0.6 |
| Hispanic | 8 | 2.3 |
| Missing (n) | (1) | |
| Education | ||
| ≤High school graduate | 109 | 32.0 |
| At least some college | 232 | 68.0 |
| Missing (n) | (1) |
Association between Preoperative and Current Dyspnea
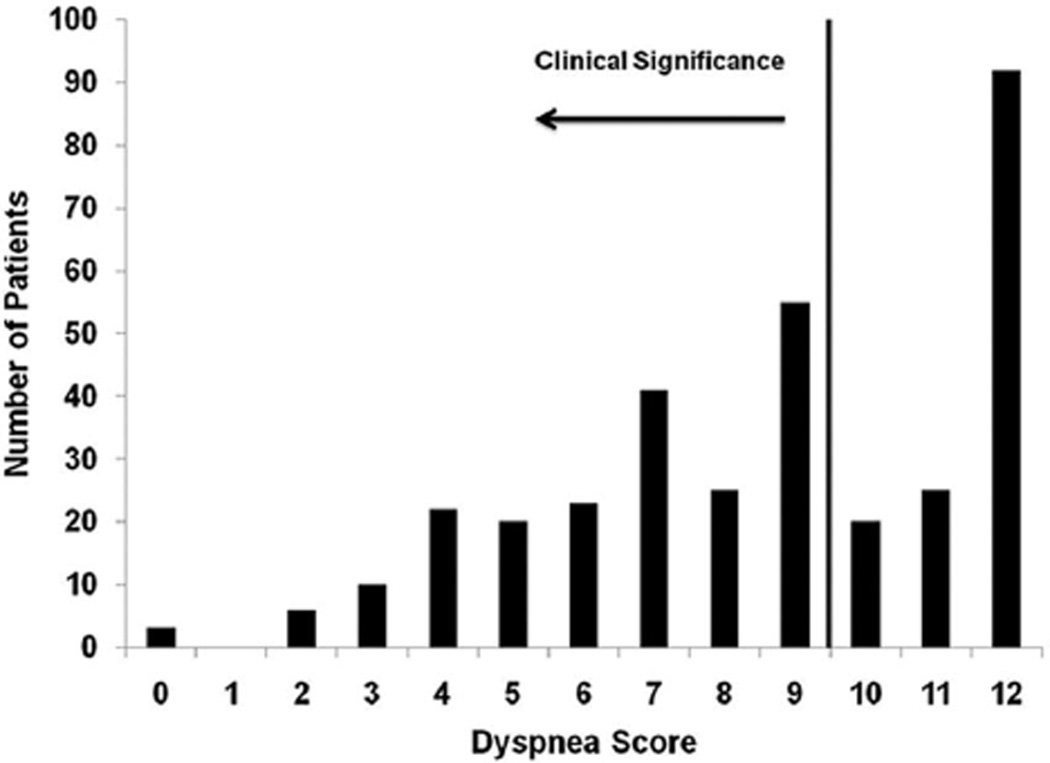
The distribution of current dyspnea, as reported by the BDI, is shown in Figure 1. The number of participants reporting current dyspnea was 205 (60%). Comparing rates of preoperative (21%) and current dyspnea (60%) revealed a nearly 3-fold increase (χ2 = 33.04, p < 0.001). Overall, 133 individuals (39% of total sample) reported current dyspnea who had not reported dyspnea before their surgery.
FIGURE 1.
Frequency of self-reported current dyspnea using the Baseline Dyspnea Index. Of 342 patients, 205 (60%) reported current dyspnea, defined as a score of 9 or less. Data are reported on a scale of zero through 12 with lower scores indicating more severe dyspnea.
Correlates of Dyspnea
Pearson correlations between current dyspnea and potential correlates described previously are shown in Table 3. The following were significantly associated (p < 0.05) with the presence of dyspnea: older age, being retired or unemployed, having less than a high school education, presence of preoperative dyspnea, reduced preoperative FEV1%, reduced preoperative diffusing capacity, presence of pulmonary disease, any history of tobacco use, any moderate or strenuous physical activity, and having clinically significant depression symptoms (HADS depression subscale ≥8). These variables were then included in a multivariate logistic regression. As shown in Table 4, preoperative dyspnea (odds ratio [OR] = 5.31), reduced preoperative diffusion capacity (OR = 0.98), any moderate or strenuous physical activity (OR = 0.41), and having clinically depression symptoms (OR = 4.10) were statistically significant multivariate correlates of current dyspnea. The selected indicators explained 32% of the variance in current dyspnea using an adjusted R2.
TABLE 3.
Bivariate Correlations Between Current Dyspnea and Selected Demographic and Medical Variables
| Characteristic | r | p |
|---|---|---|
| Age | 0.13 | 0.01 |
| Sex (male) | −0.04 | 0.50 |
| Employed (v. retired/unemployed) | −0.22 | <0.01 |
| Greater than high school education | −0.15 | 0.01 |
| Years since surgical resection | 0.05 | 0.35 |
| Type of resectiona | ||
| Lobectomy | −0.05 | 0.31 |
| Bilobectomy/pneumonectomy | 0.10 | 0.06 |
| Preoperative dyspnea | 0.31 | <0.01 |
| Preoperative FEV1% | −0.26 | <0.01 |
| Preoperative diffusing capacity % | −0.30 | <0.01 |
| Video-assisted thoracic surgery (VATS) only | 0.01 | 0.88 |
| Preoperative body mass index | 0.05 | 0.32 |
| Presence of cardiac disease | 0.05 | 0.40 |
| Presence of pulmonary disease | 0.12 | 0.02 |
| History of tobacco use | 0.14 | 0.01 |
| Any moderate/strenuous physical activity (currently) | −0.29 | <0.01 |
| Clinically significant symptoms of depression (HADS ≥8) | 0.18 | <0.01 |
| Clinically significant symptoms of anxiety (HADS ≥8) | 0.02 | 0.65 |
Type of lung resection as compared with segmentectomy/wedge resection.
HADS, Hospital Anxiety and Depression Scale.
TABLE 4.
Multivariate Correlates of Current Dyspnea (n = 304, adj R2 = 0.32)
| Characteristic | Odds Ratio (95% CI) | p |
|---|---|---|
| Age | 10.2 (0.99–1.05) | 0.24 |
| Employed (v. retired/unemployed) | 0.63 (0.34–1.16) | 0.14 |
| Greater than high school education | 0.93 (0.51–1.70) | 0.82 |
| Preoperative dyspnea | 5.31 (2.26–12.51) | <0.01 |
| Preoperative FEV1% | 0.99 (0.97–1.01) | 0.41 |
| Preoperative diffusing capacity % | 0.98 (0.97–0.99) | <0.01 |
| Presence of pulmonary disease | 0.76 (0.39–1.47) | 0.41 |
| History of tobacco use | 1.46 (0.67–3.18) | 0.34 |
| Any moderate/strenuous physical activity (currently) | 0.41 (0.24–0.71) | <0.01 |
| Clinically significant symptoms of depression (HADS ≥8) | 4.10 (1.01–16.55) | 0.05 |
CI, confidence interval; HADS, Hospital Anxiety and Depression Scale.
DISCUSSION
This study makes several notable contributions to our understanding of long-term lung cancer survivorship. We found dyspnea to be highly prevalent, occurring among 60% of the lung cancer survivors examined, and associated with the presence of preoperative dyspnea, lower preoperative diffusing capacity, lack of engagement in any moderate or strenuous physical activity, and the presence of clinically significant depression symptoms.
The identification of potentially modifiable risk factors associated with dyspnea is perhaps the most significant finding. This implies that strategies which improve physical activity or relieve depressive symptoms may result in improved breathlessness. An association between physical activity and improved clinical outcomes has been shown previously for a number of lung diseases, including COPD.21 Several studies also imply that improving physical activity is beneficial in the period immediately after lung cancer resection.22–23 In a study by Cesario et al.,22 mean 6-minute walk distance increased by nearly 100 m compared with the preoperative baseline among patients attending inpatient pulmonary rehabilitation after surgery. In a survey of patients surviving at least 5 years after lung cancer resection, those engaging in regular physical activity reported a better overall quality of life than those who did not.23
Data presented in this study suggest that the need for physical activity may extend beyond the immediate postoperative period. In addition, the high prevalence of dyspnea and the fact that fewer than half of patients reported pursuing any moderate/strenuous physical activity at all implies that a significant subset of lung cancer survivors exists who may potentially benefit from strategies that increase activity at suitable time points.
Depression symptoms were not very prevalent in this sample, occurring among about 10% of lung cancer survivors, but were nonetheless strongly associated with dyspnea. The question of whether treating depression relieves dyspnea among lung cancer patients has not been specifically addressed; however, there exists a limited literature regarding treating depression in other lung diseases. Patients with both depression and COPD who took nortriptyline reported less dyspnea performing low-demand physical activities, such as brushing teeth and combing hair, than those receiving placebo.24 Eiser et al.25 reported that patients with COPD and depression who took paroxetine, a serotonin reuptake inhibitor, for 3 months had improved depression and dyspnea scores, as well as exercise tolerance.
Although the association in this study between depression and dyspnea was independent of moderate/strenuous physical activity, evidence suggests that without treating depression symptoms, when present, lung cancer survivors will not adhere to advice and interventions designed to improve physical activity.26 Therefore, efforts to identify lung cancer survivors who have depressive symptomatology and to provide these patients additional psychosocial support could potentially mitigate depression as a barrier for post-treatment rehabilitation efforts.
Our results are similar to the only other study of long-term pulmonary impairment among lung cancer survivors receiving surgical resection. Sarna et al.8 examined respiratory symptoms among patients 5 or more years after surgery. Among 142 patients, they found dyspnea occurring among 63% of patients; 15% were so dyspneic that they could not leave their homes or dress themselves. Correlates of dyspnea included male gender, current smoking, bronchodilator use, exposure to second hand smoke, and more medical comorbidities. Depression was not examined. The lack of consistency in correlates of dyspnea between our sample and the sample of Sarna et al. may be explained by several demographic differences. Patients in the later study had longer duration time since surgery, poorer preoperative lung function as determined by FEV1, and were less likely to undergo pneumonectomy. Nevertheless, the similar finding that roughly 60% of long-term lung cancer survivors experience dyspnea underscores the clinical importance of this symptom for long-term management.
Strengths of our study include a good patient response rate (65%) and no indication of demographic differences between participants and decliners. Data on dyspnea and depression were assessed with well-validated measures. The size of the cohort, more than 340 patients, is larger than most reports of lung cancer survivorship.
Several limitations to the study should also be noted. Measurements of preoperative dyspnea were derived from routine clinical observations and documentation in the medical record, raising potential concerns about under-reporting of preoperative dyspnea. Further, study participants were less likely to have reported preoperative dyspnea than study decliners, raising the possibility that this study may underestimate the prevalence of current dyspnea among survivors of early-stage NSCLC. The study participants were early stage, relatively well educated, predominantly non-Hispanic white, and followed at a single institution; the findings may not generalize beyond these sample characteristics. Similarly, most patients had lobectomy, limiting inferences based on the amount of lung tissue resected. A small number of patients in this cohort received chemotherapy and radiation. It remains to be determined how adjuvant chemoradiation and targeted molecular-based chemotherapy may affect the spectrum of long-term complications experienced by lung cancer survivors. The cross-sectional design precludes making any causal inferences regarding direction of associations detected. It is possible, for instance, that some patients may engage in less moderate or strenuous activity because they experience more dyspnea. A prospective trial would be necessary to prove that increased activity actually attenuates dyspnea postoperatively.
Finally, any enthusiasm regarding the potential for improved physical activity to relieve dyspnea among lung cancer patients may be tempered by the historical low adherence rates to exercise after pulmonary and cardiac rehabilitation programs. Consequently, future research in this area should not only address whether improved physical activity is helpful, but also to what degree any perceived benefit can be sustained.
CONCLUSION
Previous reports of NSCLC survivors have generally been limited to the short-term period after surgery. This study is among the first to quantify the presence and correlates of dyspnea among the growing number of long-term lung cancer survivors. Dyspnea is indeed highly prevalent and may persist long after the immediate treatment period. These findings also suggest that modifiable risk factors for dyspnea may exist. Future research is needed to test whether screening and intervening for depression and physical inactivity among lung cancer survivors has beneficial effects with regards to dyspnea.
ACKNOWLEDGMENTS
The authors also thank Melissa Ozim and Syncia Sabain for their assistance with data collection and management and the study participants for their valued contribution.
Disclosure: Supported by grants T32CA009461-25 and R03CA115212-02 from the National Cancer Institute and the Byrne Foundation. The authors thank P30 CA08748 which provides partial support for the Behavioral Research Methods Core.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 3.Charpidou AG, Gkiozos I, Tsimpoukis S, et al. Therapy-induced toxicity of the lungs: an overview. Anticancer Res. 2009;29:631–639. [PubMed] [Google Scholar]
- 4.De Ruysscher D, Dehing C, Yu S, et al. Dyspnea evolution after high-dose radiotherapy in patients with non-small cell lung cancer. Radiother Oncol. 2009;91:353–359. doi: 10.1016/j.radonc.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Foroulis CN, Lioulias AG, Papakonstantinou C. Simple laboratory parameters which can determine the clinical state of patients after pneumonectomy for lung cancer. J Thorac Oncol. 2009;4:55–61. doi: 10.1097/JTO.0b013e3181914d6a. [DOI] [PubMed] [Google Scholar]
- 6.Sarna L, Cooley ME, Brown JK, et al. Symptom severity 1 to 4 months after thoracotomy for lung cancer. Am J Crit Care. 2008;17:455–467. quiz 468. [PMC free article] [PubMed] [Google Scholar]
- 7.Dales RE, Belanger R, Shamji FM, et al. Quality-of-life following thoracotomy for lung cancer. J Clin Epidemiol. 1994;47:1443–1449. doi: 10.1016/0895-4356(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 8.Sarna L, Evangelista L, Tashkin D, et al. Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest. 2004;125:439–445. doi: 10.1378/chest.125.2.439. [DOI] [PubMed] [Google Scholar]
- 9.Callahan CM, Unverzagt FW, Hui SL, et al. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Mahler DA, Weinberg DH, Wells CK, et al. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 11.Eakin EG, Sassi-Dambron DE, Ries AL, et al. Reliability and validity of dyspnea measures in patients with obstructive lung disease. Int J Behav Med. 1995;2:118–134. doi: 10.1207/s15327558ijbm0202_3. [DOI] [PubMed] [Google Scholar]
- 12.Yusen RD, Trulock EP, Pohl MS, et al. Results of lung volume reduction surgery in patients with emphysema. The Washington University Emphysema Surgery Group. Semin Thorac Cardiovasc Surg. 1996;8:99–109. [PubMed] [Google Scholar]
- 13.Saffran L, Ost DE, Fein AM, et al. Outpatient pleurodesis of malignant pleural effusions using a small-bore pigtail catheter. Chest. 2000;118:417–421. doi: 10.1378/chest.118.2.417. [DOI] [PubMed] [Google Scholar]
- 14.Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957–965. doi: 10.1378/chest.115.4.957. [DOI] [PubMed] [Google Scholar]
- 15.Sassi-Dambron DE, Eakin EG, Ries AL, et al. Treatment of dyspnea in COPD. A controlled clinical trial of dyspnea management strategies. Chest. 1995;107:724–729. doi: 10.1378/chest.107.3.724. [DOI] [PubMed] [Google Scholar]
- 16.Napolis LM, Sette A, Bagatin E, et al. Chronic dyspnea and altered respiratory function in former workers with asbestosis evaluated to determine pension benefits. J Bras Pneumol. 2004;30:528–534. [Google Scholar]
- 17.Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132:161S–177S. doi: 10.1378/chest.07-1359. [DOI] [PubMed] [Google Scholar]
- 18.Physical Activity for Everyone: Centers for Disease Control and Prevention, 2009 [Google Scholar]
- 19.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 20.Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J (Clin Res Ed) 1986;292:344. doi: 10.1136/bmj.292.6516.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ries AL, Kaplan RM, Limberg TM, et al. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823–832. doi: 10.7326/0003-4819-122-11-199506010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Cesario A, Ferri L, Galetta D, et al. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung Cancer. 2007;57:175–180. doi: 10.1016/j.lungcan.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Clark MM, Novotny PJ, Patten CA, et al. Motivational readiness for physical activity and quality of life in long-term lung cancer survivors. Lung Cancer. 2008;61:117–122. doi: 10.1016/j.lungcan.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33:190–201. doi: 10.1016/S0033-3182(92)71995-1. [DOI] [PubMed] [Google Scholar]
- 25.Eiser N, Harte R, Spiros K, et al. Effect of treating depression on quality-of-life and exercise tolerance in severe COPD. COPD. 2005;2:233–241. [PubMed] [Google Scholar]
- 26.Doorenbos A, Given B, Given C, et al. Physical functioning: effect of behavioral intervention for symptoms among individuals with cancer. Nurs Res. 2006;55:161–171. doi: 10.1097/00006199-200605000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]