Abstract
Imaging deep tissue can be extremely inefficient when the region of interest is non-planar and buried in a thick sample, yielding a severely limited effective field of view (FOV). Here we describe a novel technique, namely adaptive field microscopy, which improves the efficiency of 3D imaging by controlling the image plane. The plane of scanning laser focus is continuously reshaped in situ to match the conformation of the sample. The practicality is demonstrated for ophthalmic imaging, where a large area of the corneal epithelium of intact mouse eye is captured in a single frame with subcellular resolution.
Nonlinear optical microscopy is advantageous for imaging deep tissue by virtue of near-IR excitation and non-descanned signal detection [1, 2]. Deeper penetration of near-IR excitation has allowed live neurons in the mouse brain as deep as 1 mm to be imaged by two-photon excited fluorescence [2]. It also provides depth-resolved visualization via confined signal generation at a tight beam focus. While axial sectioning is beneficial for revealing three-dimensional spatial relationship of cells within thick tissue, undesirable loss of information could arise from it. One commonly finds that for a thick sample only a small fraction of the acquired image contains useable information because the image plane does not significantly overlap with the cellular layer of interest obliquely oriented or curved relative to the optic axis, leading to highly inefficient data acquisition. The effect is especially troublesome when studying stratified tissues, such as the epithelium and the retina. Under the circumstance the only way to visualize the entirety of a region of interest is to acquire a lot of z-scans encompassing the whole volume of tissue and then volumetrically reconstruct the layer of interest by post-processing. However, this is not only time consuming but also predisposes the tissue to excessive laser irradiation.
The problem could be circumvented with an ability to adjust the image plane to match the layer of interest. However, design of an optical imaging system with a desired field curvature and sharp foci across the field of view (FOV) is confounded because the field and aperture of an image-forming system are coupled through the Lagrange invariant. Minimization of the field and pupil aberrations can be simultaneously achieved by including additional elements, such as meniscus and field lenses, at the expense of the simplicity of optical system. Moreover, it is not a suitable approach for deep tissue imaging where it is desired that the field be reshaped in situ for arbitrary specimens with substantial variations in morphology.
Here we describe a novel control of the field for three-dimensional tissue imaging, namely adaptive field microscopy. The image plane of laser scanning microscopy is dynamically reconfigured, without compromising the quality of beam focus, to match a variety of surface geometry. As a result, the effective FOV is substantially increased, enhancing the efficiency and speed of three-dimensional imaging. The utility is demonstrated for topographic visualization of the intact mouse cornea.
The experimental configuration is depicted in Fig. 1. The basis of the setup was a custom-built two-photon microscope (TPM). Short pulses at around 800-nm wavelength from a mode-locked Ti:Sapphire laser (Chameleon, Coherent Inc.) were employed for excitation. A pair of galvo-scanning mirrors (6215H, Cambridge Technology) raster-scanned the focus of laser beam in the direction perpendicular to the optic axis and the emission from the sample is detected in a non-descanned mode using a photomultiplier tube (H10492-003, Hamamatsu). The frame and the line scan rates were 0.74 and 379 Hz, respectively. The setup for adaptive field microscopy included an additional path, i.e. the z-scanner, to which the beam was routed via a combination of a polarizing beam splitter and a quarter-wave plate. In the z-scanner the beam propagated through a telescope of unity magnification composed of an objective lens (UPlanSApo 20×/0.75NA, Olympus) and a mirror mounted on a voice coil transducer with the frequency response between 60 to 8000 Hz (W4-1720, Tang Band). The z-scanner was then followed by a relay telescope and an imaging objective lens (UPlanSApo 20×/0.75NA or XLUMPLFLN 20×/1.0NA, Olympus), which transferred the image of the mirror in the z-scanner onto the sample. Consequently, translation of the mirror in the z-scanner induced axial scanning of the laser focus in the sample. Since the focal plane in the z-scanner was telecentric, axial defocus does not cause alterations in magnification. The optical system of z-scanner has been previously demonstrated for remotely focusing the laser beam without introducing additional off-axis pupil aberrations, so that the sample does not have to move [3, 4]. Rapid axial refocusing using a high-speed scanner has been demonstrated for imaging fast calcium dynamics [5]. In our technique, the motion of the z-scanning mirror was synchronized with that of galvoscanners so that axial scanning of focus occurred in synchrony with lateral scanning. This allowed the shape of the image plane to be freely synthesized by modulating the electrical signal driving the transducer. An important consequence is that the curvature of field was no longer determined by a fixed Petzval sum but could be controlled by time-varying defocus.
Fig. 1.
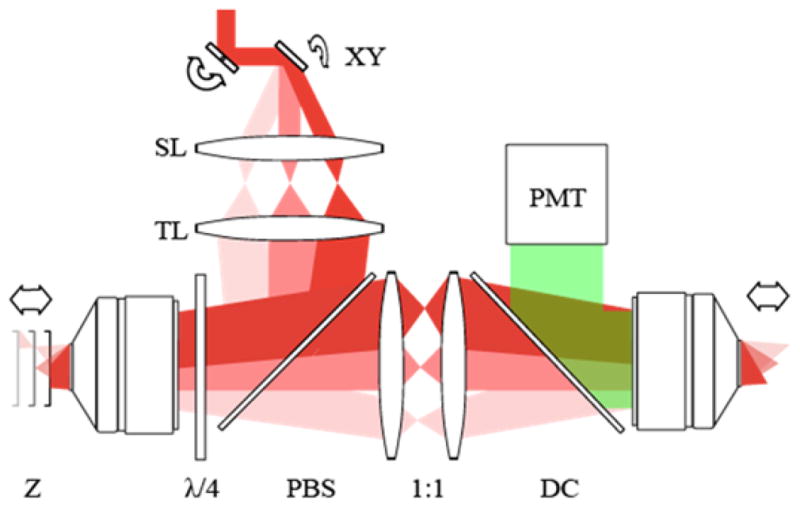
Experimental configuration; XY: galvo-scanning mirrors, SL: scan lens, TL: tube lens, Z: axial scanning mirror, λ/4: quarter-wave plate, PBS: polarizing beam splitter, DC: dichroic mirror, and PMT: photonmultiplier tube.
First, the capability of shaping the field was tested for the simplest adjustment, i.e. the tilting of the image plane. A slide of HeLa cells was placed under the microscopy at an inclination angle of 15 degrees with respect to the optic axis. The cells were imaged by autofluorescence excited at 800nm via two-photon absorption. The theoretical full-width at half-maximum (FWHM) resolution for 0.75NA objective lens in the lateral and axial dimensions was 0.4 and 2.1μm, respectively. Fig. 2 illustrates the merit of tilting the image plane. The images of TPM have a FOV of 400×400μm2. Under ordinary imaging condition, an insignificant fraction of the cell population was visualized (Fig. 2(a)) due to the narrow intersection of the plane of scanning laser focus, which is perpendicular to the optic axis, with the oblique sample. As a result, the effective FOV was significantly limited and visualizing the full extent of the sample required projection of z-stack of images. For a larger inclination angle, the useable view becomes even smaller while the sample extends further in the axial direction. Therefore the number of z-stack images necessary to reconstruct the whole sample could become impractically large, which is a situation frequently encountered during deep tissue imaging. Fig. 2(b) depicts the same area imaged with the z-scanning mirror at different positions. With an appropriate constant voltage bias applied to the transducer, identical image was obtained as Fig. 2(a) confirming that the translation of z-scanning mirror has the same effect as the movement of sample. This enabled tilting of the image plane to be implemented by synchronizing the motion of z-scanning mirror with the lateral scanning. A sawtooth waveform with the same frequency as the fast axis motion was supplied to the transducer. By increasing the amplitude, the tilt of the image plane was gradually increased until it matched the sample orientation. The entire FOV was filled with the image of cells (Fig. 2(c)) so that the population of cells could be imaged in a single shot without multiple scans over three-dimensional volume, allowing more rapid and less phototoxic image acquisition.
Fig. 2.
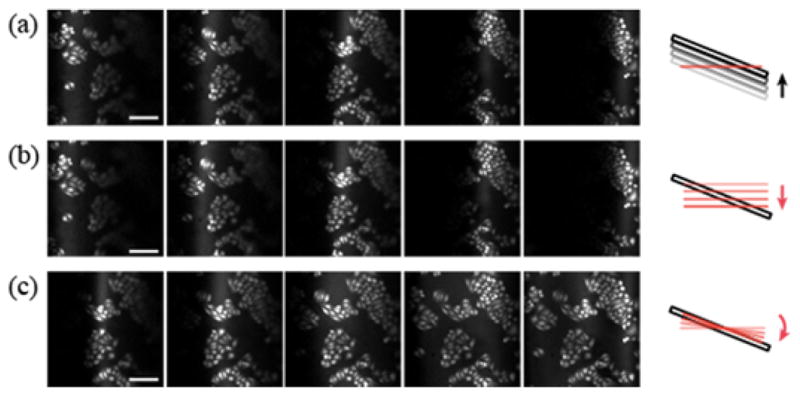
Full-field visualization of a slide of HeLa cells oriented at a 15° inclination. Z-stack images are acquired (a) by translating the sample in 20 μm steps along the optic axis and (b) by moving the z-scanning mirror. (c) The dynamic motion of the z-scanning mirror is synchronized with the fast axis of lateral scan and the tilt of image plane is gradually adjusted by increasing the amplitude. Scale bar, 100μm.
More complex shapes could be realized for the image plane with an appropriate waveform applied to the z-scanning mirror. The waveform was synthesized by summing sinusoidal waves with frequencies in the off-resonance range of the transducer. In principle it should be possible to synthesize a complex form of the image plane in 3D by a superposition of Zernike modes. We implemented three lowest modes of tilting and bending, as depicted in Fig. 3. The image plane was visualized by sweeping a thin film of fluorescein deposited on a glass coverslip across the optic axis. One-dimensional tilting (Fig. 3(a)) and bending (Fig. 3(b)) of the imaging field was obtained along a single axis. Two-dimensional manipulation of the field was achieved by tailoring a waveform as a function of coordinates of the slow and fast axes of lateral scan. 2D bending with spherical curvature is illustrated in Fig. 3(c). The actual range of the field depended on the mechanical response of transducer. The measured image planes allowed the calibration of the amplitudes of waveforms for each mode of field shaping. In order to achieve real-time matching of the image plane to the sample surface, we implemented continuous field shaping such that the conformational parameters of the image plane, such as the tilt angle and curvature, could be updated while monitoring acquired images.
Fig. 3.
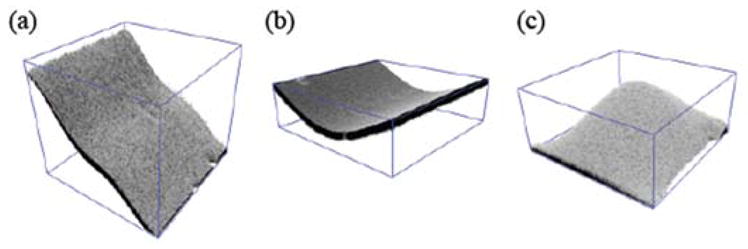
Various modes of field shaping. The size of FOV is 400×400μm2.
We investigated the limitations in the axial range of field that can be achieved with the z-scanner. The range of defocus was verified by measuring the axial resolution using sub-resolution fluorescent beads at various levels of defocus. It was possible to obtain axial resolution less than 3.0μm in the defocus range of approximately ±70μm. The spherical aberration that arises as an inevitable consequence of the Abbe condition [4] imposes the ultimate limit on the maximum range of defocus attainable with the z-scanner. Previously it was also noted that the lateral resolution of oblique plane imaging degrades with tilt angle because of coupling with the axial resolution [6, 7]. However, this is not necessarily a shortcoming of the technique. Since the imaged area increases in proportion to the loss of resolution, there is no net loss of information capacity of imaging, i.e. the number of resolvable points.
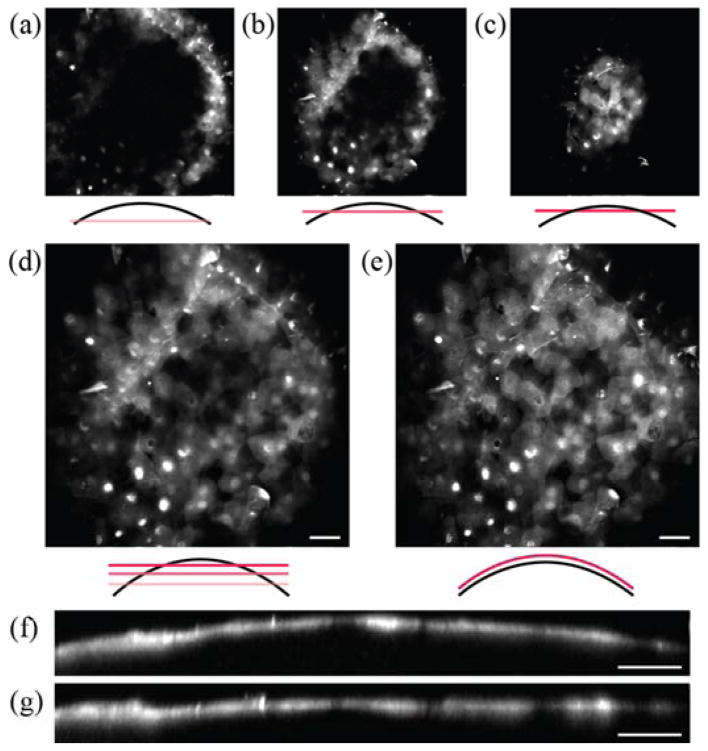
The ability to manipulate the plane of scanning laser focus is advantageous for imaging non-planar tissues, e.g. the cornea and retina in particular. To demonstrate the utility of adaptive field microscopy, we performed TPM imaging of the anterior segment of intact mouse eye. There have been previous imaging studies of the cornea by multiphoton microscopy [8, 9], but cellular-resolution imaging with a wide FOV is cumbersome due to the curved morphology of the corneal and retinal layers. We employed adaptive field TPM to improve the corneal imaging. Adult transgenic mice expressing GFP-tagged β-actin mRNA under the ubiquitin promoter [10] were euthanized as approved by Hunter College Institutional Animal Care and Use Committee. In this animal, the nuclei and cytoplasm of diverse cell types are fluorescently labeled. The intact eyes were enucleated and mounted under the objective lens. Adaptive field TPM was performed. The radius of curvature of the front surface of the eye was matched in real time by adjusting the amplitude of the waveform driving the z-scanning mirror, while concurrently monitoring the acquired images to evaluate the extent of the overlap between the image plane and the sample. Fig. 4 presents images of the superficial endothelium of the cornea. Without field reshaping, the curved corneal layers could not be imaged in a single section but required z-stacks where a majority of each section contained dark pixels (Fig. 4(a)–(c)). When the z-scanner was turned on, a much larger corneal area could be instantly visualized in a single frame (Fig. 4(e)). Fig. 4(d) represents an average projection of 10 z-sections acquired over 20-μm depth without field shaping. An equivalent image of the same area was acquired in a single z-section by adaptive field microscopy (Fig. 4(e)). The difference was more apparent in the axial cross section in Fig. 4(f) and (g), where field shaping offsets the curvature of surface to effectively flatten the sample. Our results indicate that cellular-resolution images of the cornea can be acquired over a large surface area. This also suggests that measurement of the radii of curvature and quantitative analysis of other corneal metrics is feasible. Such a capability could be useful in conjunction with corneal topography.
Fig. 4.
The cornea of mouse eye imaged by adaptive field microscopy. The superficial epithelium is visualized. (a), (b), and (c), are z-sections that are 10 μm apart in depth with field shaping OFF. (d) The average z-projection image over 20-μm depth compared with (e) a single z-section with field shaping ON. (f) and (g) are the axial view of the corneal epithelium with and without field shaping. Scale bars, 40μm.
Information capacity of a lossless image-forming system is a conserved quantity that is determined by two imaging parameters – the resolution and the field of view. Methods of adaptive optics improve the former by manipulating the phase, or wavefront, in the pupil plane. Here we have described a homologous approach to enhance the latter by controlling the phase, or defocus, in the focal plane. We have demonstrated that the technique is capable of visualizing a large deformed area embedded within 3D at subcellular resolution. Field shapes conforming to a variety of surface morphology in 3D space is created by dynamic control of the image plane, substantially increasing the effective FOV for deep tissue imaging. Specifically, the tilt or bending of the image plane could be continuously adjusted in situ to match the oblique orientation or curvature of the sample plane within tissue. It is conceivable that the optimal field shaping can be implemented in a close-loop fashion by employing a field sensor similar to a wavefront sensor in adaptive optics. Although the technique is demonstrated for TPM here, it can be applied to other depth-resolving laser scanning microscopies, such as confocal microscopy and optical coherence tomography [11].
Our main objective with adaptive field microscopy has been to facilitate rapid surveillance of cellular features within thick tissue while avoiding excessive exposure to radiation during in vivo deep tissue imaging. The ability to make an efficient use of image pixels for the sample at hand is crucial for high-throughput, high-speed applications. Furthermore, it becomes even more important as the information capacity of an imaging system increases by means of wider image field and/or higher resolution. By imaging only the plane of interest, adaptive field microscopy enhances the speed of image acquisition and avoids photodamages. Various devices developed for fast modulation of defocus could allow even higher imaging speed [12, 13].
We have demonstrated the utility of adaptive field microscopy in ophthalmic imaging where the ability to account for the geometrical variations of sample is extremely useful. In optometry and ophthalmic imaging there exists a technique analogous to the z-scanner in our method, i.e. Badal optometer [14]. When incorporated in an ophthalmic imaging system with Maxwellian viewing, Badal optometer could alter defocus while preserving the magnification. The apparatus has been widely used for studying accommodation in optometry or compensating the refractive power of ocular lens during in vivo retinal imaging. The distinguishing feature of our technique is that it is capable of controlling the curvature of the field as well as defocus. Adaptive field ophthalmoscopy would allow one to use the same instrument for imaging eyes of various sizes stemming from the age and species. It is also conceivable that the cornea and retina of small animals could be imaged while compensating for not only ocular refraction and but also accommodation. Furthermore, it could be incorporated into clinical ophthalmoscopy.
Adaptive field microscopy provides a new opportunity in optical design of an image-forming system. Traditional optical design achieves a flat field, i.e. the near-zero Petzval sum, by compromising other characteristics. In adaptive field technique, the image-forming system is not stationary but changes over time. Due to the dynamics nature, an arbitrary shape of field can be synthesized without affecting other imaging characteristics. The Petzval sum is no longer the determinant of the field. Conversely, the freedom from optimizing the field aberrations may allow designing a simpler optical system.
Acknowledgments
This work is supported by NIH EB013571.
Footnotes
OCIS Codes: (170.0170) Medical optics and biotechnology, (180.0180) Microscopy, (330.7327) Visual optics, ophthalmic instrumentation http://dx.doi.org/10.1364/OL.99.099999
References
- 1.Denk W, Strickler J, Webb W. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 2.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nature Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 3.Botcherby E, Juskaitis R, Booth M, Wilson T. Aberration-free optical refocusing in high numerical aperture microscopy. Optics Letters. 2007;32:2007–2009. doi: 10.1364/ol.32.002007. [DOI] [PubMed] [Google Scholar]
- 4.Botcherby EJ, Juskaitis R, Booth MJ, Wilson T. An optical technique for remote focusing in microscopy. Optics Communications. 2008;281:880–887. [Google Scholar]
- 5.Botcherby EJ, Smith CW, Kohl MM, Debarre D, Booth MJ, Juskaitis R, Paulsen O, Wilson T. Aberration-free three-dimensional multiphoton imaging of neuronal activity at kHz rates. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2919–2924. doi: 10.1073/pnas.1111662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CW, Botcherby EJ, Wilson T. Resolution of oblique-plane images in sectioning microscopy. Optics Express. 2011;19:2662–2669. doi: 10.1364/OE.19.002662. [DOI] [PubMed] [Google Scholar]
- 7.Smith CW, Botcherby EJ, Booth MJ, Juskaitis R, Wilson T. Agitation-free multiphoton microscopy of oblique planes. Optics Letters. 2011;36:663–665. doi: 10.1364/OL.36.000663. [DOI] [PubMed] [Google Scholar]
- 8.Aptel F, Olivier N, Deniset-Besseau A, Legeais JM, Plamann K, Schanne-Klein MC, Beaurepaire E. Multimodal nonlinear imaging of the human cornea. Investigative Ophthalmology & Visual Science. 2010;51:2459–2465. doi: 10.1167/iovs.09-4586. [DOI] [PubMed] [Google Scholar]
- 9.Tan H, Sun Y, Lo W, Lin S, Hsiao C, Chen Y, Huang S, Lin W, Jee S, Yu H, Dong C. Multiphoton fluorescence and second harmonic generation imaging of the structural alterations in keratoconus ex vivo. Investigative Ophthalmology & Visual Science. 2006;47:5251–5259. doi: 10.1167/iovs.06-0386. [DOI] [PubMed] [Google Scholar]
- 10.Park HY, Lim H, Yoon YJ, Follenzi A, Nwokafor C, Lopez-Jones M, Meng XH, Singer RH. Visualization of dynamics of single endogenous mRNA labeled in live mouse. Science. 2014;343:422–424. doi: 10.1126/science.1239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, Fujimoto JG. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grewe BF, Voigt FF, Van’t Hoff M, Helmchen F. Fast two-layer two-photon imaging of neuronal cell populations using an electrically tunable lens. Biomedical Optics Express. 2011;2:2035–2046. doi: 10.1364/BOE.2.002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivier N, Mermillod-Blondin A, Arnold CB, Beaurepaire E. Two-photon microscopy with simultaneous standard and extended depth of field using a tunable acoustic gradient-index lens. Optics Letters. 2009;34:1684–1686. doi: 10.1364/ol.34.001684. [DOI] [PubMed] [Google Scholar]
- 14.Atchison DA, Bradley A, Thibos LN, Smith G. Useful variations of the Badal optometer. Optometry and Vision Science. 1995;72:279–284. doi: 10.1097/00006324-199504000-00010. [DOI] [PubMed] [Google Scholar]