Synopsis
A finding of eosinophilia in the peripheral blood can be the manifestation of a large number of different medical conditions, including benign or malignant disorders. From a diagnostic standpoint eosinophilia can be divided into reactive (secondary) or clonal (primary). There are three main types of WHO-defined eosinophilia-associated myeloid neoplasms (MN-eos): 1) myeloid and lymphoid neoplasms associated with rearrangements of PDGFRA, PDGFRB or FGFR1; 2) chronic eosinophilic leukemia, not otherwise specified (CEL-NOS); and 3) idiopathic hypereosinophilic syndrome (HES). Imatinib mesylate, a PDGFRA and PDGFRB inhibitor, has revolutionized the treatment of molecularly defined MN-eos. Second generation molecules are available for patients who fail imatinib. Novel agents, such as the anti-IL5 antibody mepolizumab, have been successfully used for the treatment of HES. The discovery of new, recurrent molecular alterations in patients with MN-eos may improve the diagnosis and therapy of this group of patients. This review focuses on the hematologist’s approach to a patient with eosinophilia as well as treatment options for patients with eosinophilic myeloid neoplasms.
Keywords: eosinophilia, PDGFRA, PDGFRB, chronic eosinophilic leukemia, hypereosinophilic syndrome, imatinib
Introduction
The upper limit of normal for eosinophils in the peripheral blood is 3–5%, corresponding to an absolute eosinophil count (AEC) of 350–500/mm3.1 The severity of eosinophilia has been arbitrarily divided into mild (AEC 500–1,500/mm3), moderate (AEC 1,500–5,000/mm3), and severe (AEC >5,000/mm3),1,2 although the practical significance of this stratification is unclear.
Many different conditions can underlie a finding of eosinophilia. A first broad distinction should be made between reactive and clonal eosinophilia. The first condition is characterized by the proliferation of polyclonal, mature eosinophils and can be sustained by benign or malignant disorders. In the second, eosinophils represent the primary malignant clone and precursors can be found in the peripheral blood and/or bone marrow. As an additional category, idiopathic hypereosinophilic syndrome (HES) is a diagnosis of exclusion in patients with sustained eosinophilia and evidence of end-organ damage. It is important to identify the correct type of eosinophilia in a timely manner because a delay in referral and treatment can have profoundly detrimental consequences on patient outcomes. In the present review we will discuss the diagnostic approach to eosinophilia from the hematologist’s perspective, including elements of suspicion, diagnostic tests, and current treatment approaches for eosinophilia-associated myeloid neoplasms (MN-eos).
Reactive eosinophilia
Reactive eosinophilia is typically caused by increased levels of interleukin 5 (IL5). Concomitant elevation in IL4 and IL13 can lead to associated hypergammaglobulinemia (Ig)E.3 In Western countries, reactive eosinophilia is most commonly caused by allergic conditions, whereby increases in IL-5 are mediated by T helper 2 cells. A detailed clinical history and prick or radioallergosorbent tests usually allow prompt diagnosis and appropriate treatment.4 In developing countries, the main cause of eosinophilia is invasive parasitic infections (most commonly helminths). A thorough travel history is crucial to elicit clinical suspicion and subsequent testing.5 Other medical conditions that can present or associate with eosinophilia include a variety of pulmonary, dermatologic, or gastrointestinal disorders,6 adrenal insufficiency,7,8 and more rare entities such as HyperIgE syndrome9 or Wiscott-Aldrich syndrome.10 A systematic review of these disorders is offered elsewhere in this volume.
Reactive eosinophilia of hematologic/oncologic interest
Cancer cells are capable of secreting granulocyte/monocyte-colony stimulating factor, IL3 and IL5, which stimulate the proliferation of polyclonal eosinophils.11,12 Paraneoplastic eosinophilia occurs in a variety of solid malignancies including, but not limited to, head and neck, lung, gastrointestinal, ovarian, and cervical cancer. Its frequency is 0.5% to 7%.13 Eosinophilia is usually associated with advanced-stage disease and its prognostic value appears to vary (favorable, unfavorable or neutral) among tumor types. However, the available data on the clinical significance of tumor-associated tissue eosinophilia are limited and heterogeneous.14
Hodgkin’s lymphoma, especially the mixed cellularity or nodular sclerosis types, can present with peripheral blood or, less frequently, tissue or marrow eosinophilia. Eosinophils are recruited directly by Reed-Sternberg cells. Acute B-cell lymphoblastic leukemia (B-ALL) associated with t(5;14) can also present with eosinophilia. The t(5;14) juxtaposes the IL3 gene (on chromosome 5) and the Ig heavy chain (IgH) gene locus (on chromosome 14), resulting in enhanced IL3 transcription and consequent eosinophilia. Around 10% of cases of adult T-cell leukemia/lymphoma are associated with reactive, IL5-mediated peripheral blood eosinophilia, and 2–20% of patients with non-Hodgkin’s lymphoma (mostly of T-cell origin) present with elevated AEC (eosinophilia in lymphoroliferative disorders is reviewed in15)
Lymphocyte variant HES
In lymphocytic variant (LV) HES, peripheral blood eosinophilia is sustained by clonal T helper 2 cells,16 which may display different phenotypes, such as CD3-/CD4+, CD3+/CD4-/CD8- and CD3+/CD4+/CD8-. Increased serum IgE levels can also be present. Diagnosis of LV HES, which is not a WHO-defined entity, is not standardized. Demonstration of a clonally rearranged T-cell receptor, direct observation of cytokine production by cultured T cells or a finding of elevated TARC (a T-helper 2 cytokine) may be helpful in supporting the diagnosis. Up to a quarter of patients with LV HES ultimately develop an overt T-cell malignancy.17
Eosinophilic myeloid disorders
Epidemiology
Analyses of the Surveillance, Epidemiology and End Results (SEER) database from 2001 to 2005 estimate the incidence rate of MN-eos at 0.036/100,000 people/year.18 The incidence of recurrent genetic abnormalities in patients with HES has been reported to range from 10% to 20% 19,20 HES is most commonly diagnosed between the ages of 20 and 50 with a male-to-female ratio of 1.47,18 although the vast majority of patients with MN-eos are male.19,20
Classification
There are three major types of MN-eos (Table 1). The 2008 WHO classification of myeloid neoplasms has recognized the pathogenetic, diagnostic and therapeutic importance of recurrent genetic abnormalities in patients with primary eosinophilia by creating the category “Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of platelet-derived growth factor receptor alpha (PDGFRA), platelet-derived growth factor receptor beta (PDGFRB), or fibroblast growth factor receptor 1 (FGFR1)”.21 A second WHO-defined MN-eos is chronic eosinophilic leukemia, not otherwise specified (CEL-NOS), included among the myeloproliferative neoplasms (MPN). This definition is operational and requires: 1) absence of the Philadelphia chromosome or rearrangements of PDGFRA, PDGFRB and FGFR1, and the exclusion of established myeloid neoplasms associated with eosinophilia; 2) demonstration of increased marrow blasts; 3) evidence of clonality of the eosinophil population.22 A diagnosis of idiopathic HES is one of exclusion and requires the exclusion of all the aforementioned primary and secondary causes of eosinophilia and the demonstration of an AEC >1,500/mm3 sustained for >6 months with concomitant tissue damage.22 Given the potential risk of end-organ damage when therapy is delayed, especially in patients with marked peripheral blood or tissue eosinophilia, a consensus definition of HE includes: AEC >1,500/mm3 on 2 occasions ≥4 weeks apart, and/or tissue HE (defined as >20% marrow eosinophils, extensive eosinophil infiltration in the pathologist’s opinion, or marked deposition of eosinophil granule proteins). A diagnosis of HES is made when there is concomitant end-organ damage that is attributable solely to eosinophilic infiltration.23
Table 1.
Classification and diagnostic criteria of primary hypereosinophilic disorders
| MYELOID AND LYMPHOID NEOPLASMS WITH EOSINOPHILIA AND ABNORMALITIES OF PDGFRA, PDGFRB, OR FGFR1 |
| PDGFRA rearrangements: |
| A Ph-negative MPN OR AML OR B/T-lymphoblastic leukemia/lymphoma |
| Prominent eosinophilia |
| Presence of FIP1L1-PDGFRA fusion gene |
| PDGFRB rearrangements: |
| Ph-negative MPN |
| Prominent eosinophilia§ |
| Presence of t(5;12)(q31-q33;p12) or variant OR ETV6-PDGFRB fusion gene OR other PDGFRB rearrangement |
| FGFR1 rearrangements: |
| A Ph-negative MPN OR AML OR B/T-lymphoblastic leukemia/lymphoma |
| Prominent eosinophilia§ |
| Presence of t(8;13)(p11;q12) or variant and presence of FGFR1 rearrangement in myeloid cells and/or lymphoblasts |
| CEL-NOS |
| AEC >1,500/µL |
| Blast cell count <20% and no other diagnostic criteria of AML |
| Blast cells >2% in peripheral blood or >5% in the bone marrow OR clonal cytogenetic or molecular abnormality |
| Absence of Ph- or BCR-ABL-positive or -negative MPN or MDS/MPN overlap disorder |
| Absence of PDFGRA, PDGFRB, or FGFR1 rearrangements |
| IDIOPATHIC HES |
| AEC >1,500/µL (sustained for >6 months) |
| Evidence of organ damage* |
| Exclusion of the following conditions: |
| 1. Reactive eosinophilia |
| 2. LV HES |
| 3. CEL-NOS |
| 4. WHO-defined MN-eos (ie, AML, MDS, MPN, MDS/MPN overlapping disorders) |
| 5. MN-eos with rearrangements of PDGFRA, PDGFRB, or FGFR1 |
neutrophilia or monocytosis can be present;
if no organ damage is present, a diagnosis of idiopathic hypereosinophilia is made.
Abbreviations: Ph, Philadelphia chromosome; MPN, myeloproliferative neoplasm; AML, acute myeloid leukemia; CEL-NOS, chronic eosinophilic leukemia, not otherwise specified; LV HES, lymphocyte variant hypereosinophilic syndrome; AEC, absolute eosinophil count; MDS, myelodysplastic syndrome; MN-eos, eosinophilia-associated myeloid neoplasm
Data from Bain BJ, Gilliland DG, Horny H-P, et al. Chronic eosinophilic leukaemia, not otherwise specified. In: Swerdlow S, Harris NL, Stein H, et al (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2008, pp 51–53; and Bain BJ, Gilliland DG, Horny H-P, et al. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, orFGFR1. In: Swerdlow S, Harris NL, Stein H, et al (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2008, pp 68–73.
Diagnostic work-up
Manifestations of eosinophilia are heterogeneous. Patients can be paucisymptomatic or experience a rapidly fatal course, mainly due to advanced cardiomyopathy or transformation into acute leukemia. Virtually any organ can be infiltrated by eosinophils. In addition to peripheral blood work and bone marrow examination (where indicated), the diagnostic work-up of patients presenting with eosinophilia should include at a minimum chest x-ray, pulmonary function tests, echocardiogram, and measurement of troponin levels. Further testing should be guided by the individual patient’s symptoms. Selected clinical features of eosinophilia, including “red flags” that should raise suspicion of eosinophilia related to a hematologic disorder are summarized in Table 2.
Table 2.
Selected clinical manifestations of sustained eosinophilia
| Organ/system | Manifestations | Findings |
|---|---|---|
| Non-hematologic | ||
| General | fatigue | |
| Fever | ||
| Dermatologic | pruritus | urticaria |
| angioedema | erythematous papules | |
| Cardiac | signs/symptoms of heart failure | inflammatory/infiltrative cardiomyopathy |
| endomyocardial fibrosis, including valvulopathy |
||
| signs/symptoms of systemic embolization |
mural platelet thrombi | |
| Pulmonary | cough, rhinitis | |
| shortness of breath | pleural effusion | |
| pulmonary infiltrates | ||
| Gastrointestinal | diarrhea, with or without blood | eosinophilic colitis |
| dysphagia/regurgitation | eosinophilic esophagitis/esophageal eosinophilia |
|
| vomiting/dyspepsia/malabsorption | eosinophilic gastroenteritis | |
| Neurologic | dysesthesia | polyneuropathy |
| loss of vision | optic neuritis | |
| Musculoskeletal | myalgias | eosinophilic myositis/fascitis |
| Hematologic | ||
| Peripheral blood | leukocytosis* | |
| eosinophilia* | ||
| neutrophilia | ||
| basophilia§ | ||
| left shift§ | ||
| circulating blasts§ | ||
| uni-or multilineage dysplasia§ | ||
| Pallor | anemia* | |
| bruisability/thrombosis | thrombocytopenia/thrombocytosis* | |
| Reticulo-endothelial | abdominal pain | hepato-/splenomegaly§ |
| hepatic/splenic infarct§ | ||
| lymph node swelling | superficial and/or deep adenopathy§ | |
if severe, upfront bone marrow examination should be performed;
Upfront bone marrow examination must be performed.
When clonal eosinophilia is suspected, peripheral blood smear and bone marrow sampling for morphology, conventional cytogenetics, and immunohistochemistry should be performed to ascertain whether an underlying WHO-defined myeloid disorder, such as systemic mastocytosis (SM), chronic myelogenous leukemia (CML), acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), or MDS/MPN overlap entities (i.e., chronic myelomonocytic leukemia, CMML), is present. Common marrow findings in patients with MN-eos include hypercellularity, prominent eosinophilia, with or without dysplasia, increased blasts, marrow fibrosis, and Charcot-Leyden crystals. Conventional cytogenetics can provide important diagnostic information. Indeed, rearrangements of genes commonly involved in the pathogenesis of MN-eos often have a cytogenetic counterpart (i.e., rearrangements of PDGFRA, PDGFRB and FGFR1 are associated with abnormalities of chromosomes 4q12, 5q31-33, and 8p11-13, respectively).21
For practical purposes, however, screening of primary eosinophilia is typically performed by reverse transcriptase-polymerase chain reaction (RT-PCR) of peripheral blood or interphase/metaphase fluorescent in-situ hybridization (FISH) to detect the FIP1L1-PDGFRA fusion gene. FISH probes are used to detect the cytogenetically occult 800-Kb deletion on chromosome 4q12 that generates FIP1L1-PDGFRA.24 Deletion of the CHIC2 gene, which is found in this region, is used as a surrogate marker for the FIP1L1-PDGFRA fusion gene in FISH.25 Finally, the FIP1L1-PDGFRA has been described in cases of eosinophilia-associated AML and T-cell lymphoblastic lymphoma.26
When FIP1L1-PDGFRA cannot be identified in a patient otherwise suspected to have primary eosinophilia, a search for other recurrent molecular abnormalities should be initiated. PDGFRB rearrangements have been identified in cases of CMML, atypical CML and juvenile myelomonocytic leukemia. Although rare, this molecular finding is of critical importance given the responsiveness of PDGFRB-driven disorders to imatinib mesylate (IM, see below). More than 20 fusion-gene partners of PDGFRB have been described.21,27 MN-eos sustained by fusion genes involving FGFR1 (formerly known as “8p11 myeloproliferative syndrome”) are very rare. Since the discovery of the ZNF198-FGFR1 fusion gene 17 years ago,28 more than 10 fusion partners of FGFR1 have been identified.27 These disorders can present as MPN, with or without peripheral or tissue eosinophilia, or as AML or T-cell lymphoblastic lymphoma. Currently, MN-eos that are “triple-negative” (i.e., lacking PDGFRA, PDGFRB and FGFR1 rearrangements) are diagnosed as CEL-NOS, idiopathic HES or idiopathic HE (if there is no organ damage).
Treatment
Patients with no symptoms or evidence of organ damage are generally observed without intervention. However, the clinical aggressiveness of CEL-NOS and HES and the availability of effective targeted therapy for molecularly defined entities have persuaded many clinicians to manage these patients proactively rather than conservatively. In patients with eosinophilia-associated WHO-defined myeloid or lymphoid malignancy, treatment should follow disease-specific guidelines.
Molecularly-defined MN-eos
IM is a multi-kinase inhibitor that blocks the activity of the BCR-ABL oncoprotein in CML, thereby inhibiting the proliferation and survival of the leukemic cells.29 Treatment of CML with IM has elicited unprecedented, high rates of deep cytogenetic and molecular responses and, ultimately, dramatically improved patient outcomes.30 On the basis of such tremendous success, IM was empirically tested in patients with MN-eos.
The first studies of IM (100–400 mg/day) in patients with HES were reported about a decade ago as case reports or small series. The majority of patients treated achieved early complete hematologic responses (CHR), usually defined as resolution of clinical symptoms and normalization of blood counts.31–33 The subsequent identification of FIP1L1-PDGFRA as a therapeutic target of IM24 enabled the selection of HES patients suitable for targeted therapy, leading to the re-classification of these MN-eos as WHO-defined entities.21 Moreover, the availability of a molecular marker improved the assessment and monitoring of response to IM. Several studies have shown that the majority of patients with FIP1L1-PDGFRA-positive disease treated with IM experience complete molecular remission (CMR), defined as no detectable fusion transcript by RT-PCR (Table 3). Results of these studies suggest that IM effectively suppresses the FIP1L1-PDGFRA clone. However, discontinuation of IM often results in disease re-appearance and clinical relapse. In one study, 5 patients with molecularly undetectable disease had molecular relapse upon IM dose de-escalation, but were able to re-gain molecular remission after resuming treatment.34 In another study, 6 of 11 patients who discontinued IM relapsed, while 5 maintained their molecular remission after 9–88 months.35 Although a few patients may maintain their remission after discontinuation, whether IM can eradicate the disease remains unclear at this time. Therefore, treatment discontinuation is currently considered experimental. Of note, because end-organ damage cannot be reversed with treatment in most cases, prompt initiation of IM is critical once a target molecular lesion is identified.
Table 3.
Published studies of MN-eos with PDGFRA or PDGFRB rearrangements treated with imatinib mesylate
| Study | No. Patients | Gene rearrangement | Dose | Duration of Therapy | Response |
|---|---|---|---|---|---|
| Klion et al.56 | 6 | FIP1L1-PDGFRA | 100–400 mg/day | 1–12 months | CHR 100% CMR 5/6 (83%) |
| Metzgeroth et al.57 | 16 | FIP1L1-PDGFRA | 12 months | CHR 100% CMR 14/16 (87%) |
|
| Helbig G et al.58 | 6 | FIP1L1-PDGFRA | 100–400 mg/day * | Median, 30 months | CHR 100% CMR 5/6 (83%) |
| Legrand F et al.35,§ | 44 | FIP1L1-PDGFRA | Mean, 165 mg/day induction/58 mg/day maintenance |
Median, 52 months | CHR 100% CMR 43/44 (95%) |
| Baccarani et al.59 | 27 | FIP1L1-PDGFRA | 100 mg/day escalated to 400 mg/day |
Median, 1 month | CHR 100% CMR 100% |
| Jovanovic et al.60,§ | 11 | FIP1L1-PDGFRA | 100–400 mg/day | 12 months | CMR 9/11 (82%) |
| Arefi et al.61,§ | 8 | FIP1L1-PDGFRA | 100 mg/day | NR | CHR 100% MRg 100% |
| David et al.62,† | 20 | 10 ETV6-PDGFRB 7 various PDGFRB fusion partner 3 unknown PDGFRB fusion partner |
200–800 mg/day | 0.1–60 months | CHR 16/20 (80%) CCyR 15/20 (75%) CMR: 4/8 (50%) ETV6- PDGFRB, 1/8 (12%) other rearrangements |
| Cheah et al.63,‡ | 26 | 18 ETV6-PDGFRB 8 various PDGFRB fusion partners |
100–400 mg/day | Median, 10.2 years | OR 96% CCyR 52% CMR 32% |
Patients were transitioned to once-weekly dosing after achieving remission;
Multicenter retrospective study;
Multicenter, prospective study + review and update of data from the literature;
Multicenter retrospective study, including data from David M, Cross NC, Burgstaller S, et al. Durable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood. 2007;109(1):61–64.
Abbreviations: CHR, complete hematologic response, defined as resolution of symptoms and normalization of blood counts; CMR, complete molecular response defined, as no detectable transcript by RT-PCR; CCyR, complete cytogenetic remission, defined as normal karyptype; OR, objective response.
Primary or acquired resistance to IM has only occasionally been reported in patients with FIP1L1-PDGFRA-positive disease. The T674I point mutation within the ATP-binding domain of PDGFRA is the most common mechanism of acquired resistance to IM in PDGFRA-FIP1L1-positive disease. Its pharmacodynamic consequences are similar to those caused by T315I in CML, which renders BCR-ABL resistant to IM and other tyrosine kinase inhibitors (TKIs).24 T674I mutated clones are sensitive to nilotinib, sorafenib, and midostaurin in vitro. However, preliminary clinical experience with these agents has been disappointing.36 The D842V mutation has been found in patients whose disease progressed after treatment with nilotinib or sorafenib and is not sensitive in vitro to second generation TKIs. The low frequency of TKI-resistance in FIP1L1-PDGFRA-positive disease might be explained by the limited repertoire of mutations that can affect the PDGFRA kinase domain.37 Allogeneic hematopoietic stem cell transplantation has been performed successfully in patients with HES38 and should be considered a priority in cases of TKI-resistant PDGFRA-FIP1L1-positive disease.
IM has also been used successfully in MN-eos patients with a variety of other rearrangements involving PDGFRA or PDGFRB (more than half of whom harbor ETV6-PDGFRB fusion gene, see Table 3).
Eosinophilia-associated FGFR1-positive disease is the least common subtype of MN-eos. The clinical course is aggressive with frequent evolution into AML within 1–2 years. These disorder can present with or, more commonly, without peripheral blood or tissue eosinophilia. Eosinophilia-associated manifestations are also uncommon. Histopathology is usually consistent with T-cell lymphoblastic leukemia/lymphoma or a myeloid/T-cell phenotype.21 Treatment is directed at the lymphoma and usually involves intensive chemotherapy followed by allogeneic transplantation whenever possible. Data on the efficacy of multiple TKIs, including IM, ponatinib,39 dovitinib,40 and midostaurin41 in vitro are promising but experience in the clinical setting is limited.
IM and other TKIs have been generally well tolerated in patients with MN-eos, with a toxicity profile largely overlapping that observed in CML patients. However, because some patients have experienced cardiogenic shock after receiving IM,42 prophylactic steroids are recommended for the first 7–10 days of treatment in patients with known cardiac comorbidities and/or elevated baseline serum troponin levels attributable to eosinophil cardiac infiltration.
Recurrent rearrangements of genes other than PDGFRA, PDGFRB or FGFR1 have been identified in patients with MN-eos. The most important ones involve JAK2 and FLT3, each found to be fused with several different partners. Of 4 patients with JAK2-positive MN-eos treated with the JAK1/2 inhibitor ruxolitinib, all have been reported to achieve a hematologic and cytogenetic response.43,44 Another extremely rare condition, ETV6–FLT3-positive MN-eos, has been reported in 5 cases.45–48 Three patients received a FLT3 inhibitor46,48 and achieved clinical and cytogenetic responses. Because these 2 entities have a clinical-hematological phenotype similar to that of WHO-defined MN-eos and are driven by targetable molecular lesions, their recognition in the WHO framework appears appropriate.
Idiopathic HES and CEL-NOS
Systemic corticosteroids exert quick and effective eosinophil-lytic activity and remain the first-line treatment for patients with primary eosinophilia without a defined molecular lesion. However, treatment duration is limited by numerous side effects. Among patients treated with 30–40 mg of prednisone daily, with subsequent tapering to a maintenance dose, objective response rates range from 65%–85%.49,50 Disease progression while on prednisone doses >10 mg daily warrants the addition of a second agent. Hydroxyurea has been used alone or together with corticosteroids in previously untreated or steroid-refractory HES patients with response rates around 70%.51 For patients who fail to respond to corticosteroids and hydroxyurea, interferon (IFN)-α represents a viable option.52 Reported response rates are around 50% and increase to 75% with the addition of prednisone. The optimal induction and maintenance doses are not defined.49 IFN-α therapy is burdened with side effects (e.g., flu-like syndrome, fatigue, cytopenia, mood disorders, hypothyroidism) in a significant proportion of patients. The pegylated formulation of IFN-α may decrease the incidence and severity of these complications while preserving efficacy.53 Other treatment options for patients who did not respond to or were intolerant of the above agents, include vincristine, cyclophosphamide, etoposide or cladribine, alone or in combination with cytarabine, and cyclosporin-A.36 The “molecularly blind” use of IM in this patient population has limited efficacy and the few responses observed are conceivably explained by the presence of occult molecular targets.
Novel monoclonal antibodies (MoAb) that target the pathophysiology of eosinophilia have been used in patients whose disease cannot be controlled with conventional approaches. Mepolizumab, a MoAb against IL5, was compared as a steroid-sparing agent with placebo in a randomized trial.54 Significantly more patients in the mepolizumab arm achieved doses of prednisone <10 mg daily for >8 weeks. Some patients were able to avoid prednisone for at least 3 months. Alemtuzumab, an anti-CD52 MoAb, induced CHR in the majority of patients.55 However, the response duration is short and patients typically require a maintenance regimen. Moreover, because alemtuzumab is profoundly immune suppressive, close monitoring and anti-infectious prophylaxis are recommended during and after treatment. A more extensive review of MoAbs for the treatment of HES is offered elsewhere in this volume.
Conclusion
A finding of eosinophilia, isolated or in conjunction with other clinical manifestations, opens a broad differential diagnosis for clinicians, as it can subtend many different disorders, acute or chronic, benign or malignant. In the context of myeloid neoplasms that present or are associated with eosinophilia, an equally large number of different conditions must be considered in the diagnostic approach of patients. For molecularly defined MN-eos, targeted agents such as IM represent definitive therapy for most patients. In contrast, the relatively vast armamentarium used to treat CEL-NOS or HES has yielded insufficient results. As our knowledge of the pathogenesis of MN-eos expands and new targeted molecules become available, more cases currently labeled as CEL-NOS/HES will likely be re-classified as WHO-defined entities. Thus, in the future it will be important to devise MN-eos-specific molecular diagnostic panels that encompass core genetic driving lesions, thus providing clinicians with reliable and timely guidance for patient management.
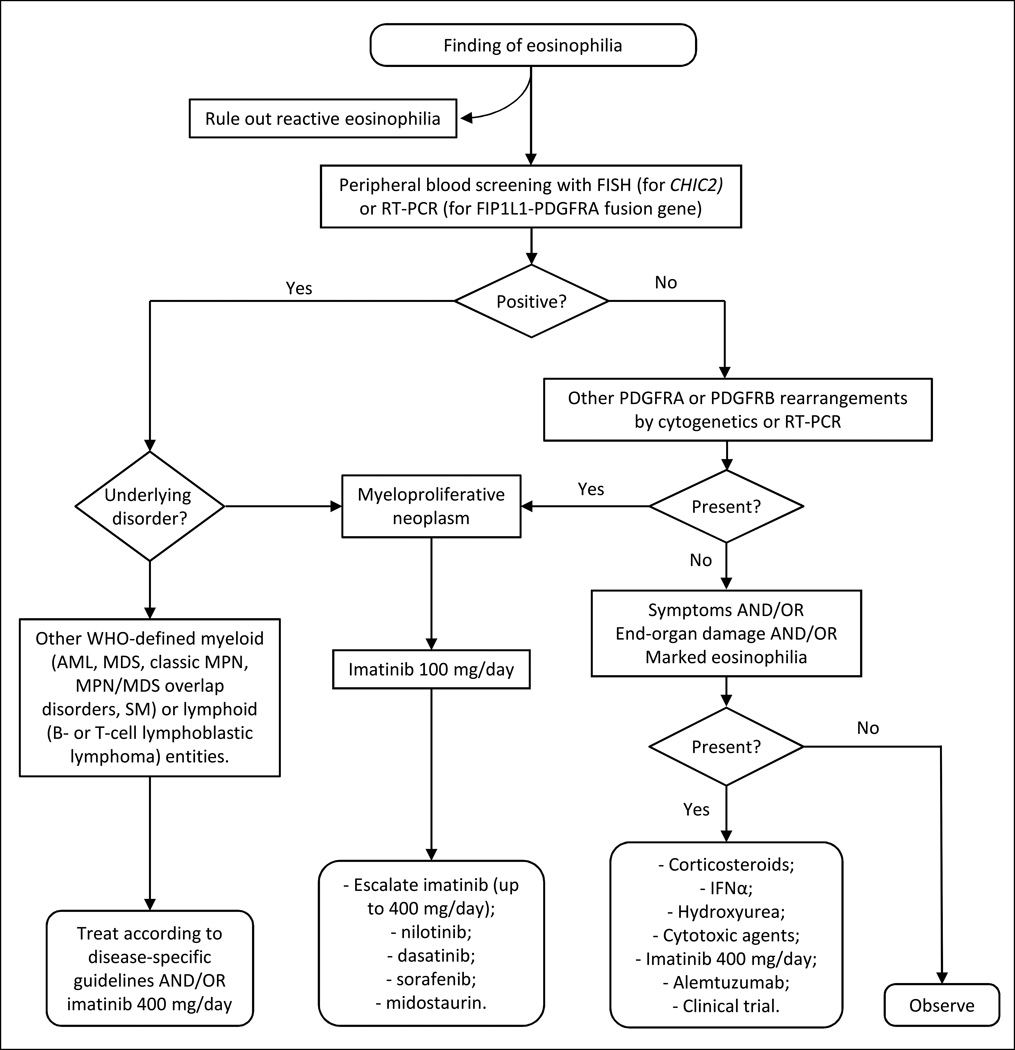
Figure 1.
Suggested treatment algorithm for patients with primary eosinophilia (MN-eos).
Key points.
Eosinophilia can subtend a broad differential diagnosis of acute or chronic, benign or malignant disorders.
Suspecting an eosinophilia-associated myeloid neoplasm is important for prompt initiation of effective therapy.
Molecular characterization of eosinophilia-associated myeloid neoplasms is critical for selecting the most appropriate targeted therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- 1.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338(22):1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. British journal of haematology. 2006;133(5):468–492. doi: 10.1111/j.1365-2141.2006.06038.x. [DOI] [PubMed] [Google Scholar]
- 3.Valent P, Gleich GJ, Reiter A, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol. 2012;5(2):157–176. doi: 10.1586/ehm.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lombardi C, Passalacqua G. Eosinophilia and Diseases: Clinical Review of 1862 Cases. Arch Intern Med. 2003;163:1371–1373. doi: 10.1001/archinte.163.11.1371-b. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis Infection. CID. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 6.Simon D, Wardlaw A, Rothenberg ME. Organ-specific eosinophilic disorders of the skin, lung, and gastrointestinal tract. The Journal of allergy and clinical immunology. 2010;126(1):3–13. doi: 10.1016/j.jaci.2010.01.055. quiz 14–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spry C. Eosinophilia in Addison's Disease. Yale J Biol Med. 1976;49(4):411–413. [PMC free article] [PubMed] [Google Scholar]
- 8.Beishuizen A, Vermes I, Hylkema BS, Haanen C. Relative eosinophilla and functional adrenal insufficiency in critically ill patients. The Lancet. 1999;353(9165):1675–1676. doi: 10.1016/s0140-6736(99)01346-x. [DOI] [PubMed] [Google Scholar]
- 9.Sowerwine KJ, Holland SM, Freeman AF. Hyper-IgE syndrome update. Annals of the New York Academy of Sciences. 2012;1250:25–32. doi: 10.1111/j.1749-6632.2011.06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. The Journal of allergy and clinical immunology. 2006;117(4):725–738. doi: 10.1016/j.jaci.2006.02.005. quiz 739. [DOI] [PubMed] [Google Scholar]
- 11.Pandit R, Scholnik A, Wulfekuhler L, Dimitrov N. Non-small-cell lung cancer associated with excessive eosinophilia and secretion of interleukin-5 as a paraneoplastic syndrome. American journal of hematology. 2007;82(3):234–237. doi: 10.1002/ajh.20789. [DOI] [PubMed] [Google Scholar]
- 12.Stefanini M, Claustro JC, Motos RA, Bendigo LL. Blood and bone marrow eosinophilia in malignant tumors. Role and nature of blood and tissue eosinophil colony-stimulating factoRs) in two patients. Cancer. 1991;68(3):543–548. doi: 10.1002/1097-0142(19910801)68:3<543::aid-cncr2820680317>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Isaacson NH, Rapoport P. EOSINOPHILIA IN MALIGNANT TUMORS: ITS SIGNIFICANCE*. Annals of Internal Medicine. 1946;25(6):893–902. doi: 10.7326/0003-4819-25-6-893. [DOI] [PubMed] [Google Scholar]
- 14.Jain M, Kasetty S, Khan S, Jain NK. Tissue Eosinophilia in Head and Neck Squamous Neoplasia: an Update. Exp Oncol. 2014;36(3):157–161. [PubMed] [Google Scholar]
- 15.Roufosse F, Garaud S, de Leval L. Lymphoproliferative disorders associated with hypereosinophilia. Seminars in hematology. 2012;49(2):138–148. doi: 10.1053/j.seminhematol.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Simon HU, Plotz SG, Dummer R, Laser K. Abnormal Clones of T Cells Producing Interleukin-5 in Idiopathic Eosinophilia. N Engl J Med. 1999;341:1112–1120. doi: 10.1056/NEJM199910073411503. [DOI] [PubMed] [Google Scholar]
- 17.Roufosse F, Cogan E, Goldman M. Lymphocytic variant hypereosinophilic syndromes. Immunology and allergy clinics of North America. 2007;27(3):389–413. doi: 10.1016/j.iac.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Crane MM, Chang CM, Kobayashi MG, Weller PF. Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence. The Journal of allergy and clinical immunology. 2010;126(1):179–181. doi: 10.1016/j.jaci.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardanani A, Brockman SR, Paternoster SF, et al. FIP1L1-PDGFRA fusion: prevalence and clinicopathologic correlates in 89 consecutive patients with moderate to severe eosinophilia. Blood. 2004;104(10):3038–3045. doi: 10.1182/blood-2004-03-0787. [DOI] [PubMed] [Google Scholar]
- 20.Pardanani A, Ketterling RP, Li CY, et al. FIP1L1-PDGFRA in eosinophilic disorders: prevalence in routine clinical practice, long-term experience with imatinib therapy, and a critical review of the literature. Leukemia research. 2006;30(8):965–970. doi: 10.1016/j.leukres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Bain BJ, Gilliland DG, Horny H-P, Vardiman JW. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, orFGFR1. In: Swerdlow S, Harris NL, Stein H, Jaffe ES, Theile J, Vardiman JW, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp. 68–73. [Google Scholar]
- 22.Bain BJ, Gilliland DG, Horny HP, Vardiman JW. Chronic Eosinophilic Leukemia, not Otherwise Specified. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp. 51–53. [Google Scholar]
- 23.Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. The Journal of allergy and clinical immunology. 2012;130(3):607–612. e609. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cools J, Deangelo DJ, Gotlib J, et al. A Tyrosine Kinase Created by Fusion of the PDGFRA and FIP1L1 Genes as a Therapeutic Target of Imatinib in Idiopathic Hypereosinophilic Syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 25.Pardanani A, Ketterling RP, Brockman SR, et al. CHIC2 deletion, a surrogate for FIP1L1-PDGFRA fusion, occurs in systemic mastocytosis associated with eosinophilia and predicts response to imatinib mesylate therapy. Blood. 2003;102(9):3093–3096. doi: 10.1182/blood-2003-05-1627. [DOI] [PubMed] [Google Scholar]
- 26.Metzgeroth G, Walz C, Score J, et al. Recurrent finding of the FIP1L1-PDGFRA fusion gene in eosinophilia-associated acute myeloid leukemia and lymphoblastic T-cell lymphoma. Leukemia. 2007;21(6):1183–1188. doi: 10.1038/sj.leu.2404662. [DOI] [PubMed] [Google Scholar]
- 27.Gotlib J, Cools J. Five years since the discovery of FIP1L1-PDGFRA: what we have learned about the fusion and other molecularly defined eosinophilias. Leukemia. 2008;22(11):1999–2010. doi: 10.1038/leu.2008.287. [DOI] [PubMed] [Google Scholar]
- 28.Xiao S, Nalabolu SR, Aster JC, et al. FGFR1 is fused with a novel zinc-finger gene, ZNF198, in the t(8;13 leukaemia/lymphoma syndrome. Nat Genetics. 1998;18:84–87. doi: 10.1038/ng0198-84. [DOI] [PubMed] [Google Scholar]
- 29.Druker BJ, Tamura S, Fau - Buchdunger E, Buchdunger E Fau, Ohno S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 30.Druker BJ, Guilhot F, O’Brien SG, et al. Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 31.Schaller JL, Burkland GA. Case report: rapid and complete control of idiopathic hypereosinophilia with imatinib mesylate. MedGenMed. 2001;3(5):9. [PubMed] [Google Scholar]
- 32.Gleich GJ, Leiferman KM, Pardanani A, Tefferi A, Butterfield JH. Treatment of hypereosinophilic syndrome with imatinib mesilate. The Lancet. 2002;359(9317):1577–1578. doi: 10.1016/S0140-6736(02)08505-7. [DOI] [PubMed] [Google Scholar]
- 33.Cortes J, Ault P, Koller C, et al. Efficacy of imatinib mesylate in the treatment of idiopathic hypereosinophilic syndrome. Blood. 2003;101(12):4714–4716. doi: 10.1182/blood-2003-01-0081. [DOI] [PubMed] [Google Scholar]
- 34.Klion AD, Robyn J, Maric I, et al. Relapse following discontinuation of imatinib mesylate therapy for FIP1L1/PDGFRA-positive chronic eosinophilic leukemia: implications for optimal dosing. Blood. 2007;110(10):3552–3556. doi: 10.1182/blood-2007-07-100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legrand F, Renneville A, Macintyre E, et al. The Spectrum of FIP1L1-PDGFRA-Associated Chronic Eosinophilic Leukemia: New Insights Based on a Survey of 44 Cases. Medicine. 2013 doi: 10.1097/MD.0b013e3182a71eba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. American journal of hematology. 2014;89(3):325–337. doi: 10.1002/ajh.23664. [DOI] [PubMed] [Google Scholar]
- 37.von Bubnoff N, Gorantla SP, Engh RA, et al. The low frequency of clinical resistance to PDGFR inhibitors in myeloid neoplasms with abnormalities of PDGFRA might be related to the limited repertoire of possible PDGFRA kinase domain mutations in vitro. Oncogene. 2011;30(8):933–943. doi: 10.1038/onc.2010.476. [DOI] [PubMed] [Google Scholar]
- 38.Halaburda K, Prejzner W, Szatkowski D, Limon J, Hellmann A. Allogeneic bone marrow transplantation for hypereosinophilic syndrome: long-term follow-up with eradication of FIP1L1- PDGFRA fusion transcript. Bone marrow transplantation. 2006;38(4):319–320. doi: 10.1038/sj.bmt.1705437. [DOI] [PubMed] [Google Scholar]
- 39.Lierman E, Smits S, Cools J, Dewaele B, Debiec-Rychter M, Vandenberghe P. Ponatinib is active against imatinib-resistant mutants of FIP1L1-PDGFRA and KIT, against FGFR1-derived fusion kinases. Leukemia. 2012;26(7):1693–1695. doi: 10.1038/leu.2012.8. [DOI] [PubMed] [Google Scholar]
- 40.Wasag B, Lierman E, Meeus P, Cools J, Vandenberghe P. The kinase inhibitor TKI258 is active against the novel CUX1-FGFR1 fusion detected in a patient with T-lymphoblastic leukemia/lymphoma and t(7;8)(q22;p11) Haematologica. 2011;96(6):922–926. doi: 10.3324/haematol.2010.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Deangelo DJ, Kutok JL, et al. PKC412 inhibits the zinc finger 198-fibroblast growth factor receptor 1 fusion tyrosine kinase and is active in treatment of stem cell myeloproliferative disorder. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(40):14479–14484. doi: 10.1073/pnas.0404438101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitini V, Arrigo C, Azzarello D, et al. Serum concentration of cardiac Troponin T in patients with hypereosinophilic syndrome treated with imatinib is predictive of adverse outcomes. Blood. 2003;102(9):3456–3457. doi: 10.1182/blood-2003-07-2393. [DOI] [PubMed] [Google Scholar]
- 43.Schwaab J, Knut M, Haferlach C, et al. Limited duration of complete remission on ruxolitinib in myeloid neoplasms with PCM1-JAK2 and BCR-JAK2 fusion genes. Annals of hematology. 2015;94(2):233–238. doi: 10.1007/s00277-014-2221-y. [DOI] [PubMed] [Google Scholar]
- 44.Bain BJ, Ahmad S. Should myeloid and lymphoid neoplasms with PCM1-JAK2 and other rearrangements of JAK2 be recognized as specific entities? British journal of haematology. 2014;166(6):809–817. doi: 10.1111/bjh.12963. [DOI] [PubMed] [Google Scholar]
- 45.Vu HA, Xinh PT, Masuda M, et al. FLT3 is fused to ETV6 in a myeloproliferative disorder with hypereosinophilia and a t(12;13)(p13;q12) translocation. Leukemia. 2006;20(8):1414–1421. doi: 10.1038/sj.leu.2404266. [DOI] [PubMed] [Google Scholar]
- 46.Walz C, Erben P, Ritter M, et al. Response of ETV6-FLT3-positive myeloid/lymphoid neoplasm with eosinophilia to inhibitors of FMS-like tyrosine kinase 3. Blood. 2011;118(8):2239–2242. doi: 10.1182/blood-2011-03-343426. [DOI] [PubMed] [Google Scholar]
- 47.Chonabayashi K, Hishizawa M, Matsui M, et al. Successful allogeneic stem cell transplantation with long-term remission of ETV6/FLT3-positive myeloid/lymphoid neoplasm with eosinophilia. Annals of hematology. 2014;93(3):535–537. doi: 10.1007/s00277-013-1843-9. [DOI] [PubMed] [Google Scholar]
- 48.Falchi L, Mehrotra M, Newberry KJ, et al. ETV6-FLT3 fusion gene-positive, eosinophilia-associated myeloproliferative neoplasm successfully treated with sorafenib and allogeneic stem cell transplant. Leukemia. 2014;28(10):2090–2092. doi: 10.1038/leu.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. The Journal of allergy and clinical immunology. 2009;124(6):1319–1325. e1313. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helbig G, Wisniewska-Piaty K, Francuz T, Dziaczkowska-Suszek J, Kyrcz-Krzemien S. Diversity of clinical manifestations and response to corticosteroids for idiopathic hypereosinophilic syndrome: retrospective study in 33 patients. Leukemia & lymphoma. 2013;54(4):807–811. doi: 10.3109/10428194.2012.731602. [DOI] [PubMed] [Google Scholar]
- 51.Fauci AS, Harley JB, Roberts WC, Ferrans VJ, Gralnick HR, Bjornson BH. The Idiopathic Hypereosinophilic Syndrome: Clinical, Pathophysiologic, and Therapeutic Considerations. Ann Intern Med. 1982;97:78–92. doi: 10.7326/0003-4819-97-1-78. [DOI] [PubMed] [Google Scholar]
- 52.Butterfield JH. Interferon treatment for hypereosinophilic syndromes and systemic mastocytosis. Acta haematologica. 2005;114(1):26–40. doi: 10.1159/000085560. [DOI] [PubMed] [Google Scholar]
- 53.Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer. 2007;110(9):2012–2018. doi: 10.1002/cncr.23018. [DOI] [PubMed] [Google Scholar]
- 54.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358(12):1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 55.Verstovsek S, Tefferi A, Kantarjian H, et al. Alemtuzumab therapy for hypereosinophilic syndrome and chronic eosinophilic leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(1):368–373. doi: 10.1158/1078-0432.CCR-08-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klion AD, Robyn J, Akin C, et al. Molecular remission and reversal of myelofibrosis in response to imatinib mesylate treatment in patients with the myeloproliferative variant of hypereosinophilic syndrome. Blood. 2004;103(2):473–478. doi: 10.1182/blood-2003-08-2798. [DOI] [PubMed] [Google Scholar]
- 57.Metzgeroth G, Walz C, Erben P, et al. Safety and efficacy of imatinib in chronic eosinophilic leukaemia and hypereosinophilic syndrome: a phase-II study. British journal of haematology. 2008;143(5):707–715. doi: 10.1111/j.1365-2141.2008.07294.x. [DOI] [PubMed] [Google Scholar]
- 58.Helbig G, Stella-Holowiecka B, Majewski M, et al. A single weekly dose of imatinib is sufficient to induce and maintain remission of chronic eosinophilic leukaemia in FIP1L1-PDGFRA-expressing patients. British journal of haematology. 2008;141(2):200–204. doi: 10.1111/j.1365-2141.2008.07033.x. [DOI] [PubMed] [Google Scholar]
- 59.Baccarani M, Cilloni D, Rondoni M, et al. The efficacy of imatinib mesylate in patients with FIP1L1-PDGFR -positive hypereosinophilic syndrome. Results of a multicenter prospective study. Haematologica. 2007;92(9):1173–1179. doi: 10.3324/haematol.11420. [DOI] [PubMed] [Google Scholar]
- 60.Jovanovic JV, Score J, Waghorn K, et al. Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1- PDGFRA–positive chronic eosinophilic leukemia. Blood. 2007;109:4635–4640. doi: 10.1182/blood-2006-10-050054. [DOI] [PubMed] [Google Scholar]
- 61.Arefi M, Garcia JL, Briz MM, et al. Response to imatinib mesylate in patients with hypereosinophilic syndrome. International journal of hematology. 2012;96(3):320–326. doi: 10.1007/s12185-012-1141-7. [DOI] [PubMed] [Google Scholar]
- 62.David M, Cross NC, Burgstaller S, et al. Durable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood. 2007;109(1):61–64. doi: 10.1182/blood-2006-05-024828. [DOI] [PubMed] [Google Scholar]
- 63.Cheah CY, Burbury K, Apperley JF, et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood. 2014;123(23):3574–3577. doi: 10.1182/blood-2014-02-555607. [DOI] [PMC free article] [PubMed] [Google Scholar]