Abstract
One new bicyclic lactam, cladosporilactam A (1), and six known 12-membered macrolides (2–7) were isolated from a gorgonian-derived Cladosporium sp. fungus collected from the South China Sea. Their complete structural assignments were elucidated by comprehensive spectroscopic investigation. Quantum chemistry calculations were used in support of the structural determination of 1. The absolute configuration of 1 was determined by calculation of its optical rotation. Cladosporilactam A (1) was the first example of 7-oxabicyclic[6.3.0]lactam obtained from a natural source. Compound 1 exhibited promising cytotoxic activity against cervical cancer HeLa cell line with an IC50 value of 0.76 μM.
Keywords: gorgonian-derived fungus, Cladosporium sp., bicyclic lactam, 12-membered macrolides, cytotoxic activity
1. Introduction
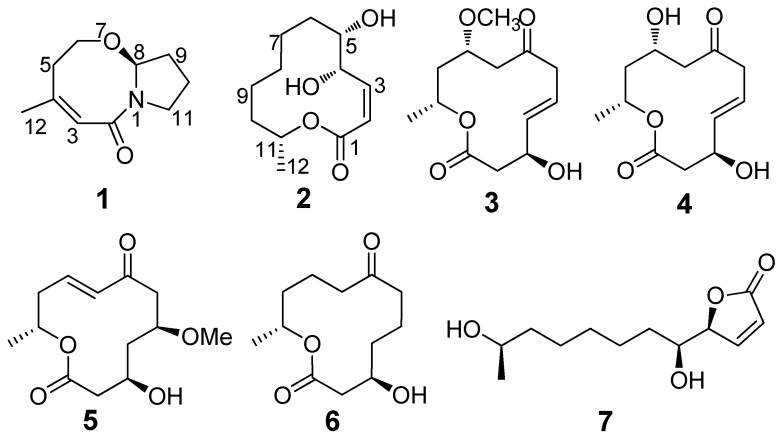
Marine fungi are known for their ability to sequester novel natural products with unique chemical scaffolds possessing a variety of potent biological activities [1]. The marine-derived Cladosporium fungi have been known to produce various secondary metabolites with novel structures and interesting biological activities such as cytotoxic sporiolides A and B [2], antibacterial Sumiki’s acid (5-hydroxymethylfuran-2-carboxylic acid) [3], and antifouling 3-phenyl-2-propenoic acid [4]. During our ongoing search for structurally novel and bioactive natural products from gorgonians and their symbiotic microorganisms in the South China Sea [5,6,7,8,9,10], we investigated the metabolites of 14 fungal strains cultured from the gorgonian Anthogorgia ochracea by integrated chemical and bioassay screening. Among them, the strain RA07-1 identified as Cladosporium sp. exhibited cytotoxicity and was selected for further chemical investigation. Bioassay-guided separation resulted in the isolation of a novel cytotoxic bicyclic lactam, cladosporilactam A (1), and six known 12-membered macrolides cladospolide B (2) [11], dendrodolides A, C, M, L (3, 4, 5, 6) [12], and iso-cladospolide B (7) [13] (Figure 1). Herein we report the isolation, structure elucidation, and bioactivities of these compounds.
Figure 1.
Structures of compounds 1–7.
2. Results and Discussion
Cladosporilactam A (1) was obtained as a colorless oil. The molecular formula was determined as C10H15O2N on the base of positive HRESIMS, indicating four degrees of unsaturation. The 1H and 13C NMR spectra (Figures S1 and S2) (Table 1) displayed signals for one methyl group, five methylenes including an oxygen-bearing methylene and a nitrogen-bearing methylene (δH 3.54; δC 44.9), two methines including an olefinic carbon (δH 5.84; δC 121.8) and an unusual oxygen-bearing carbon (δH 5.19; δC 88.4), and two quaternary carbons including a carbonyl carbon (δC 167.5) and an olefinic carbon (δC 145.4). Analysis of the 1H and 13C NMR spectra, with the aid of HMQC spectra (Figure S3), revealed the presence of a trisubstituted double bond. These functional groups accounted for two out of the four degrees of unsaturation, and the remaining two thus required a bicyclic nucleus of 1.
Table 1.
NMR spectroscopic data (500/125 MHz, CDCl3) for compound 1.
| Position | δC Type | δC-pred a Type | δH Mult. (J in Hz) |
|---|---|---|---|
| 2 | 167.5, C | 165.0, C | |
| 3 | 121.8, CH | 126.0, CH | 5.84, brs |
| 4 | 145.4, C | 146.9, C | |
| 5 | 36.3, CH2 | 36.8, CH2 | 2.56, dd (15.0, 10.0) |
| 2.17, dd (15.0, 7.5) | |||
| 6 | 66.3, CH2 | 61.2, CH2 | 4.00, dd (12.0, 7.5) |
| 3.43, dd (12.0, 10.0) | |||
| 8 | 88.4, CH | 87.4, CH | 5.19, d (4.5) |
| 9 | 34.2, CH2 | 33.7, CH2 | 1.93, m |
| 10 | 21.6, CH2 | 21.9, CH2 | 2.15, m |
| 1.91, m | |||
| 11 | 44.9, CH2 | 42.9, CH2 | 3.54, m |
| 12 | 25.6, CH3 | 30.1, CH3 | 1.87, s |
a Predicted δC for 8R-1.
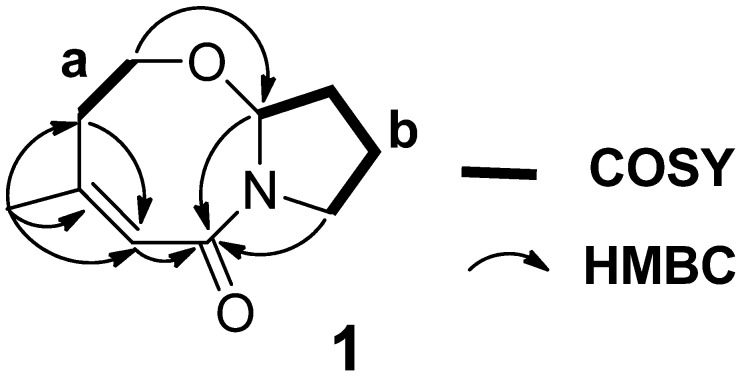
Extensive analysis of HMQC and COSY (Figure S4) gave two fragments (Figure 2, a and b). In order to determine the connectivity between the two partial structures, HMBC experiments (Figure S5) were carried out. The HMBC correlations (Figure 2) from H3-12 to C-3, C-4 and C-5, and from H2-5 to C-3 established the connections of C-4 with C-3, C-5 and C-12. And the HMBC correlation from H-6 to C-8 indicated the connection of C-6–O–C-8. While, both of H-8 and H2-11 showed HMBC cross-peaks with C-2 that suggested the amide carbonyl carbon C-2 was connected to C-8, the carbon bearing both oxygen and nitrogen, and C-11, the nitrogen-bearing carbon, via a nitrogen bridge. Thus, the planar structure of 1 was established.
Figure 2.
COSY and key HMBC correlations of 1.
NMR chemical shift calculation by quantum chemistry methods represents a powerful strategy for chemical structure interpretation [14]. To confirm the framework of 1, the gauge-independent atomic orbital (GIAO) based 13C NMR chemical shifts calculation [15] were carried out. Conformational searches were performed using MMFF94S force field for 8R-1. All geometries with relative energy from 0 to 5.0 kcal/mol were used in optimizations at the B3LYP/6-311+G(d) level first. The minimum geometries, 8R-1a (48.6%) and 8R-1b (51.4%) (Figure S6), with relative energy from 0 to 3.0 kcal/mol were further optimized by TDDFT at the B3LYP/6-311+G(d) level. Based on the optimized geometries, the NMR calculations were performed at the B3LYP/6-311G+(2d,p) level of theory. The relative chemical shifts of 8R-1a and 8R-1b were calculated based on the chemical shifts of TMS calculated by the same procedure. All the calculations were performed using the Gaussian 09 program [16]. The individual deviations, |Δδ|, between the predicted (δC-pred of 8R-1) and experimental 13C chemical shifts for 1 were less than 5.1 ppm (Table 1), suggesting the δC of 1 matched the calculated δC very well. These results demonstrate that the calculations may be sufficient to confirm the framework for 1.
The absolute configuration at C-8 of 1 was assigned by comparing the computed optical rotation (OR) with the experimental OR. The computed OR in the gas phase for 8R-1 was +70.4 at the B3LYP/6-311+G (2d,p) level. The recorded OR for 1 was −50.1 (c 0.80, MeOH). The absolute value of the recorded OR for 1 correspond to the computed value; however, the OR signs (negative vs. positive) contradict each other. These results of qualitative analysis allowed the assignment of the absolute configuration of 1 as 8S.
Although bicyclic lactams have been reported from marine-derived fungi, they were mainly obtained as 1,4-diazepine [17]. In present study, the isolated compound 1 is a 7-oxabicyclic[6.3.0]lactam. Compound 1 represents the first example of bicyclic lactam characterized with the bicyclic[6.3.0]lactam nucleus structure from a natural source. The carbon framework of 1 was similar to the linear chain amide product, fuscoatramide, isolated from the fungus Humicola fuscoatra [18]. This raises the intriguing possibility for that 1 might derive from a linear chain amide precursor by two biological acetal reaction steps.
The cytotoxic activities of 1–7 were evaluated against a panel of human tumor cell lines. Compound 1 exhibited significant cytotoxicity towards cervical cancer HeLa, mouse lymphocytic leukemia P388, human colon adenocarcinoma HT-29, and human lung carcinoma A549 cell lines with the IC50 values of 0.76, 1.35, 2.48, and 3.11 μM, respectively.
All the isolated compounds 1–7 were also evaluated for antibacterial activity against a panel of pathogenic bacteria, including Bacillus cereus, Tetragenococcus halophilus, Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Pseudomonas putida, Nocardia brasiliensis, and Vibrio parahaemolyticus (Table 2). Compounds 3–5 and 7 showed antibacterial activity againstall of the pathogenic bacteria, with MIC values ranging from 3.13 to 25.0 μM. Among the active compounds, 3 and 4 exhibited the same highest antibacterial activity against T. halophilus (MIC = 3.13 μM).
Table 2.
Tests of MIC (μM) for 1–7 against eight bacterial strains.
| Strains | Compounds | |||||
|---|---|---|---|---|---|---|
| 1, 2, 6 | 3 | 4 | 5 | 7 | Ciprofloxacin | |
| B. cereus | >25.0 | 12.5 | 25.0 | 6.25 | 6.25 | 1.56 |
| T. halophilus | >25.0 | 3.13 | 3.13 | 25.0 | 6.25 | 1.56 |
| S. epidermidis | >25.0 | 6.25 | 25.0 | 25.0 | 25.0 | 0.78 |
| S. aureus | >25.0 | 6.25 | 25.0 | 12.5 | 25.0 | 0.39 |
| E. coli | >25.0 | 12.5 | 12.5 | 25.0 | 25.0 | 1.56 |
| P. putida | >25.0 | 12.5 | 25.0 | 6.25 | 6.25 | 0.39 |
| N. brasiliensis | >25.0 | 6.25 | 12.5 | 25.0 | 12.5 | 0.78 |
| V. parahaemolyticus | >25.0 | 12.5 | 25.0 | 25.0 | 25.0 | 1.56 |
3. Experimental Section
3.1. General Experimental Procedures
The optical rotations were measured on a JASCO P-1020 digital polarimeter (JASCO Corporation, Tokyo, Japan). UV spectra were recorded using a Milton-Roy spectrophotometer (Milton Roy, New York, NY, USA). IR spectra were recorded on a Nicolet-Nexus-470 spectrophotometer using KBr pellets (Thermo Electron, Waltham, MA, USA). NMR spectra were recorded on an Agilent DD2 500 MHz NMR spectrometer (500 MHz for 1H and 125 MHz for 13C) (JEOL, Tokyo, Japan), using TMS as internal standard. ESIMS and HRESIMS spectra were measured on a Micromass Q-TOF spectrophotometer (Waters Corp., Manchester, UK) and Thermo Scientific LTQ Orbitrap XL spectrometer (Thermo Fisher Scientific, Bremen, Germany). HPLC separation was performed using a Hitachi LA-2000 prep-HPLC system (Hitachi High Technologies, Tokyo, Japan) coupled with a Hitachi L-2455 photodiodearray detector (Hitachi High Technologies, Tokyo, Japan). A Kromasil C18 semi-preparative HPLC column (250 mm × 10 mm, 5 μm) (Eka Nobel, Bohus, Sweden) was used. Silica gel (200–300 mesh; Qingdao Marine Chemical Group Co., Qingdao, China) and Sephadex LH-20 (Amersham Biosciences Inc., Piscataway, NJ, USA) were used for column chromatography. Precoated silica gel GF254 plates (Yantai Zifu Chemical Group Co., Yantai, China) were used for analytical TLC.
3.2. Fungal Materials
The fungal strain Cladosporium sp. (RA07-1) was isolated from a piece of fresh tissue from the inner part of the gorgonian Anthogorgia ochracea (GXWZ-07), collected from Weizhou coral reef in the South China Sea in April 2011. The strain was deposited in the Key Laboratory of Marine Drugs, the Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao, China, with the GenBank (NCBI) accession number KP720581.
3.3. Extraction and Isolation
The fungus Cladosporium sp. RA07-1 was cultivated in a rice medium in 500 mL Erlenmeyer flasks (each containing rice 80 g, water 120 mL, sea salt 2.0 g) at 27 °C for four weeks. The fermented rice substrate (40 flasks) was extracted repeatedly with EtOAc (3 × 300 mL for each flask). The combined EtOAc layer was evaporated to dryness under reduced pressure to give an EtOAc extract. The EtOAc extract (10.0 g) was subjected to column chromatography (CC) on silica gel using a step gradient elution with EtOAc–petroleum ether (PE) (0%–100%) and then with MeOH–EtOAc (0%–100%) to afford six fractions (Fr.1–Fr.6). Fr.4 was subjected to Sephadex LH-20 CC eluting with mixtures of CH2Cl2/MeOH (1:1). Then purification by semi-preparative HPLC using a C18 column at a flow rate of 2.0 mL/min (MeOH/H2O, 35:65) yielded 1 (4.3 mg). Fr.5 was separated by silica gel CC (PE/EtOAC, 1:2) and semi-preparative HPLC (MeOH/H2O, 40:60) to offer 2 (3.5 mg), 3 (4.0 mg), 4 (2.0 mg), 5 (3.0 mg), 6 (4.0 mg), and 7 (4.0 mg).
Cladosporilactam A (1): Colorless oil; [α]20D −50.1° (c 0.80, MeOH); UV (MeOH) λmax (log ε): 213 (2.89) nm; IR (KBr) νmax 2931, 2364, 1658, 1611, 1428, 1114 cm–1; 1H and 13C NMR data, see Table 1; ESIMS m/z 385.4 [2M + Na]+, 220.2 [M + K]+, 204.2 [M + Na]+, 182.2 [M + H]+ (Figure S7); HRESIMS m/z 182.1174 [M + H]+ (calcd for C10H16O2N, 182.1176) (Figure S8).
Cladospolide B (2). White, amorphous solid; [α]20D +18.7° (c 0.10, MeOH) [lit. 11: +26.9° (c 0.40, MeOH)].
Dendrodolide A (3). White, amorphous solid; [α]20D +32.0° (c 0.20, MeOH) [lit. 12: +41.9° (c 0.37, CHCl3)].
Dendrodolide C (4). Colorless oil; [α]20D +98.4° (c 0.20, MeOH) [lit. 12: +122° (c 0.09, CHCl3)].
Dendrodolide M (5). Colorless oil; [α]20D +85.8° (c 0.20, MeOH) [lit. 12: +92.6° (c 0.105, CHCl3)].
Dendrodolide L (6). White, amorphous solid; [α]20D +23.6° (c 0.20, MeOH) [lit. 12: +10.7° (c 0.065, CHCl3)].
iso-Cladospolide B (7). White, amorphous solid; [α]20D −106.2° (c 0.20, MeOH) [lit. 13: −90° (c 0.23, MeOH)].
3.4. Biological Assays
The cytotoxic activities of 1–7 against mouse lymphocytic leukemia P388 and human lung carcinoma A549 cell lines were evaluated using the SRB method [19]. The cytotoxic activity against cervical cancer HeLa and human colon adenocarcinoma HT-29 cell lines were evaluated using the MTT method [20]. Adriamycin was used as a positive control.
Antibacterial activities were evaluated by the conventional broth dilution assay [21]. Eight pathogenic bacterial strains, Bacillus cereus, Tetragenococcus halophilus, Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Pseudomonas putida, Nocardia brasiliensis, and Vibrio parahaemolyticus were used, and ciprofloxacin was used as a positive control.
4. Conclusions
In summary, a bicyclic lactam, cytotoxic 7-oxabicyclic[6.3.0]lactam (1) has been isolated from a gorgonian-derived Cladosporium sp. fungus collected from the South China Sea. The structure of 1 was confirmed by quantum chemistry calculation methods. Compound 1 showed cytotoxic activity against HeLa, P388, HT-29 and A549 cell lines, suggesting that it might have potential to be developed as an antitumor agent.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 41176121; 41130858; 41322037; 41376145; 81172977), and Special Financial Fund of Innovative Development of Marine Economic Demonstration Project (No. GD2012-D01-001).
Supplementary Files
Author Contributions
F. Cao contribute to extraction, isolation and identification and manuscript preparation; Q. Yang contribute to quantum chemistry calculation; C.-L. Shao contribute to structure elucidation; C.-J. Kong contribute to NMR analysis; J.-J. Zheng and Y.-F. Liu contribute to bioactivities test; C.-Y. Wang was the project leader organizing and guiding the experiments and manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bugni T.S., Ireland C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004;21:143–163. doi: 10.1039/b301926h. [DOI] [PubMed] [Google Scholar]
- 2.Shigemori H., Kasai Y., Komatsu K., Tsuda M., Mikami Y., Kobayashi J. Sporiolides A and B, new cytotoxic twelve-membered macrolides from a marine-derived fungus Cladosporium species. Mar. Drugs. 2004;2:164–169. doi: 10.3390/md204164. [DOI] [Google Scholar]
- 3.Jadulco R., Proksch P., Wray V., Sudarsono, Berg A., Gräfe U. New macrolides and furan carboxylic acid derivative from the sponge-derived fungus Cladosporium herbarum. J. Nat. Prod. 2001;64:527–530. doi: 10.1021/np000401s. [DOI] [PubMed] [Google Scholar]
- 4.Qi S.H., Xu Y., Xiong H.R., Qian P.Y., Zhang S. Antifouling and antibacterial compounds from a marine fungus Cladosporium sp. F14. World J. Microbiol. Biotechnol. 2009;25:399–406. doi: 10.1007/s11274-008-9904-2. [DOI] [Google Scholar]
- 5.Zheng J.J., Shao C.L., Chen M., Gan L.S., Fang Y.C., Wang X.H., Wang C.Y. Ochracenoids A and B, guaiazulene-based analogues from gorgonian Anthogorgia ochracea collected from the South China Sea. Mar. Drugs. 2014;12:1569–1579. doi: 10.3390/md12031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao F., Shao C.L., Chen M., Zhang M.Q., Xu K.X., Meng H., Wang C.Y. Antiviral C-25 epimers of 26-acetoxy steroids from the South China Sea gorgonian Echinogorgia rebekka. J. Nat. Prod. 2014;77:1488–1493. doi: 10.1021/np500252q. [DOI] [PubMed] [Google Scholar]
- 7.Sun X.P., Cao F., Shao C.L., Chen M., Liu H.J., Zheng C.J., Wang C.Y. Subergorgiaols A–L, 9,10-secosteroids from the South China Sea gorgonian Subergorgia rubra. Steroids. 2015;94:7–14. doi: 10.1016/j.steroids.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Cao F., Shao C.L., Wang Y., Xu K.X., Qi X., Wang C.Y. Polyhydroxylated sterols from the South China Sea gorgonian Verrucella umbraculum. Helv. Chim. Acta. 2014;97:900–908. doi: 10.1002/hlca.201300397. [DOI] [Google Scholar]
- 9.Shao C.L., Xu R.F., Wei M.Y., She Z.G., Wang C.Y. Structure and absolute configuration of fumiquinazoline L, an alkaloid from a gorgonian-derived Scopulariopsis sp. fungus. J. Nat. Prod. 2013;76:779–782. doi: 10.1021/np4002042. [DOI] [PubMed] [Google Scholar]
- 10.Wei M.Y., Wang C.Y., Liu Q.A., Shao C.L., She Z.G., Lin Y.C. Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a gorgonian Dichotella gemmacea. Mar. Drugs. 2010;8:941–949. doi: 10.3390/md8040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirota A., Sakai H., Isogai A. New plant growth regulators, cladospolide A and B, macrolides produced by Cladosporium cladosporioides. Agric. Biol. Chem. 1985;49:731–735. doi: 10.1271/bbb1961.49.731. [DOI] [Google Scholar]
- 12.Sun P., Xu D.X., Mándi A., Kurtán T., Li T.J., Schulz B., Zhang W. Absolute configuration, and conformational study of 12-membered macrolides from the fungus Dendrodochium sp. associated with the Sea Cucumber Holothuria nobilis Selenka. J. Org. Chem. 2013;78:7030–7047. doi: 10.1021/jo400861j. [DOI] [PubMed] [Google Scholar]
- 13.Smith C.J., Abbanat D., Bernan V.S., Maiese W.M., Greenstein M., Jompa J., Tahir A., Ireland C.M. Novel polyketide metabolites from a species of marine fungi. J. Nat. Prod. 2000;63:142–145. doi: 10.1021/np990361w. [DOI] [PubMed] [Google Scholar]
- 14.Bifulco G., Dambruoso P., Gomez-Paloma L., Riccio R. Determination of relative configuration in organic compounds by NMR spectroscopy and computational methods. Chem. Rev. 2007;107:3744–3779. doi: 10.1021/cr030733c. [DOI] [PubMed] [Google Scholar]
- 15.Wolinski K., Hilton J.F., Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990;112:8251–8260. doi: 10.1021/ja00179a005. [DOI] [Google Scholar]
- 16.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09. Gaussian, Inc.; Wallingford, CT, USA: 2009. [Google Scholar]
- 17.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2015;32:116–211. doi: 10.1039/C4NP00144C. [DOI] [PubMed] [Google Scholar]
- 18.Joshi B.K., Gloer J.B., Wicklow D.T. Bioactive natural products from a sclerotium-colonizing isolate of Humicola fuscoatra. J. Nat. Prod. 2002;65:1734–1737. doi: 10.1021/np020295p. [DOI] [PubMed] [Google Scholar]
- 19.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Appendio G., Gibbons S., Giana A., Pagani A., Grassi G., Stavri M., Smith E., Rahman M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008;71:1427–1430. doi: 10.1021/np8002673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.