Abstract
Five new scalarane sesterterpenoids, felixins A–E (1–5), were isolated from the Formosan sponge Ircinia felix. The structures of scalaranes 1–5 were elucidated on the basis of spectroscopic analysis. Cytotoxicity of scalaranes 1–5 against the proliferation of a limited panel of tumor cell lines was evaluated.
Keywords: Ircinia felix, sponge, scalarane, sesterterpenoid, cytotoxicity
1. Introduction
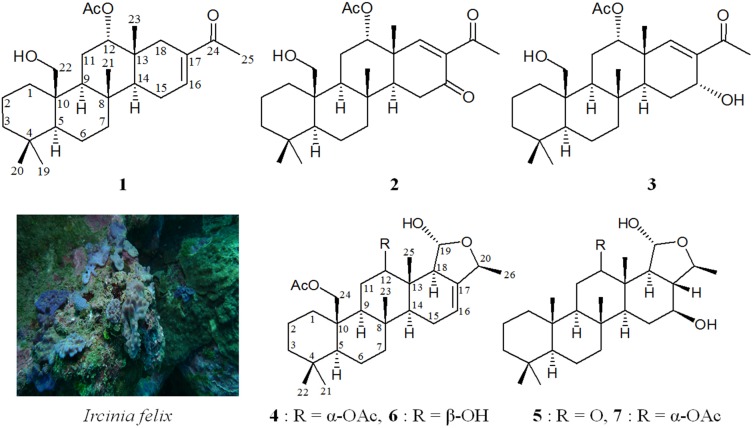
Marine sponges belonging to the genus Ircinia (family Irciniidae, order Dictyoceratida, class Demospongiae, phylum Porifera) have been proven to be not only an important source of various interesting natural substances [1,2,3,4,5], but have also played an interesting role in marine ecology [6,7,8,9,10] and medicinal use [11,12]. In continuing research aimed at the discovery of new bioactive substances from marine organisms, an organic extract of the sponge identified as Ircinia felix (Duchassaing and Michelotti, 1864) (Figure 1) exhibited cytotoxicity toward MOLT-4 (human acute lymphoblastic leukemia) tumor cells (IC50 < 6.25 μg/mL). We isolated five new scalarane sesterterpenoids, felixins A–E (1–5) from this organism (Figure 1). In this paper, we deal with the isolation, structure determination, and cytotoxicity of scalaranes 1–5.
Figure 1.
The sponge Ircinia felix and the structures of felixins A–E (1–5) and 12-deacetyl-23-acetoxy-20-methyl-12-epi-deoxyscalarin (6) and scalarane 7.
2. Results and Discussion
Felixin A (1) was isolated as a white powder and the molecular formula for this compound was determined to be C27H42O4 (seven unsaturations) using HRESIMS at m/z 453.29773 [M + Na]+ (calcd for C27H42O4 + Na, 453.29753). Comparison of the 13C NMR and DEPT data with the molecular formula indicated there must be an exchangeable proton, which required the presence of a hydroxy group. The IR spectrum of 1 showed strong bands at 3480, 1731 and 1662 cm−1, consistent with the presence of hydroxy, ester and α,β-unsaturated ketone groups. The 13C NMR and DEPT spectral data showed that this compound has 27 carbons (Table 1), including six methyls, nine sp3 methylenes (including an oxymethylene), four sp3 methines (including an oxymethine), four sp3 quaternary carbons, an sp2 methine and three sp2 quaternary carbons (including two carbonyls). Based on the 1H and 13C NMR spectra (Table 1), 1 was found to possess an acetoxy group (δH 2.08, 3H × s; δC 170.2, C; 21.5, CH3) and a ketonic carbonyl (δC 199.1, C-24). An additional unsaturated functionality was indicated by 13C resonances at δC 139.4 (CH-16) and 137.7 (C-17), suggesting the presence of a trisubstituted olefin. Thus, from the above data, three degrees of unsaturation were accounted for and 1 was identified as a tetracyclic sesterterpenoid analogue.
Table 1.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for scalarane 1.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.09 m; 0.52 ddd (12.8, 12.8, 3.2) | 34.4, CH2 | H2-2 | C-3, -10, -22 |
| 2 | 1.51 m; 1.39 m | 17.8, CH2 | H2-1, H2-3 | n. o. a |
| 3 | 1.43 ddd (12.8, 4.0, 4.0) | 41.7, CH2 | H2-2 | C-1, -4, -5, -19, -20 |
| 1.17 ddd (12.8, 12.8, 4.8) | ||||
| 4 | 33.0, C | |||
| 5 | 0.97 dd (12.0, 2.0) | 56.9, CH | H2-6 | C-3, -4, -6, -7, -9, -10, -20, -22 |
| 6 | 1.58 m; 1.45 m | 18.3, CH2 | H-5, H2-7 | C-5, -8 |
| 7 | 1.81 ddd (12, 8, 3.2, 3.2); 1.05 m | 41.9, CH2 | H2-6 | C-21 |
| 8 | 37.4, C | |||
| 9 | 1.35 br d (13.2) | 53.1, CH | H2-11 | C-5, -7, -8, -10, -11, -12, -14, -21, -22 |
| 10 | 41.8, C | |||
| 11 | 2.17 m; 1.96 m | 25.2, CH2 | H-9, H-12 | C-9, -13 |
| 12 | 4.72 dd (3.6, 2.0) | 77.1, CH | H2-11 | C-9, -14 |
| 13 | 35.8, C | |||
| 14 | 1.56 m | 48.0, CH | H2-15 | C-7, -8, -13, -15, -21, -23 |
| 15 | 2.34 m; 2.22 m | 24.0, CH2 | H-14, H-16 | C-16, -17 |
| 16 | 6.86 m | 139.4, CH | H2-15 | C-14, -24 |
| 17 | 137.7, C | |||
| 18 | 2.22 m; 1.92 m | 35.1, CH2 | C-13, -14, -16, -17, -23, -24 | |
| 19 | 0.87 s | 33.8, CH3 | C-3, -4, -5, -20 | |
| 20 | 0.77 s | 21.9, CH3 | C-3, -4, -5, -19 | |
| 21 | 1.10 s | 15.4, CH3 | C-7, -8, -9, -14 | |
| 22 | 4.03 d (11.6); 3.89 d (11.6) | 63.0, CH2 | C-1, -9, -10 | |
| 23 | 0.87 s | 19.6, CH3 | C-12, -13, -14 | |
| 24 | 199.1, C | |||
| 25 | 2.28 s | 25.2, CH3 | C-17, -24 | |
| 12-OAc | 170.2, C | |||
| 2.08 s | 21.5, CH3 | Acetate carbonyl |
a n. o. = not observed.
From the 1H–1H COSY spectrum of 1 (Table 1), it was possible to establish the separate system that map out the proton sequences from H2-1/H2-2/H2-3, H-5/H2-6/H2-7, H-9/H2-11/H-12 and H-14/H2-15/H-16. These data, together with the key HMBC correlations between protons and quaternary carbons (Table 1), such as H2-3, H-5, H3-19, H3-20/C-4; H2-6, H-9, H-14, H3-21/C-8; H2-1, H-5, H-9, H2-22/C-10; H2-11, H-14, H2-18, H3-23/C-13; H2-15, H2-18, H3-25/C-17; and H-16, H2-18, H3-25/C-24, established the carbon skeleton of 1 as a 24-homo-25-norscalarane derivative [13]. The oxymethylene unit at δC 63.0 was correlated to the methylene protons at δH 4.03 and 3.89 in the HMQC spectrum. The methylene signals were 2J-correlated with C-10 (δC 41.8) and 3J-correlated with both C-1 (δC 34.4) and C-9 (δC 53.1), proving the attachment to a hydroxymethyl group at C-10 (Table 1). Thus, the remaining acetoxy group was positioned at C-12, an oxymethine (δH 4.72, δC 77.1) as indicated by analysis of the 1H–1H COSY correlations and characteristic NMR signals, although no HMBC correlation was observed between H-12 and the acetate carbonyl.
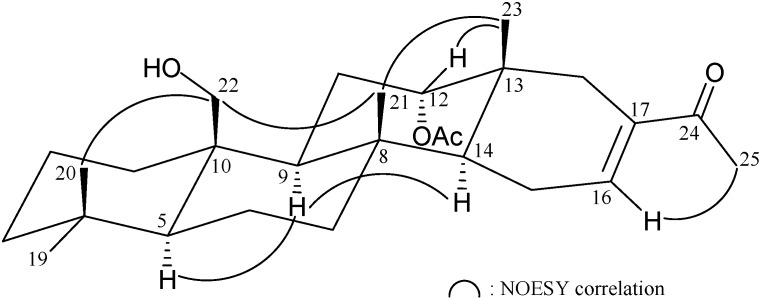
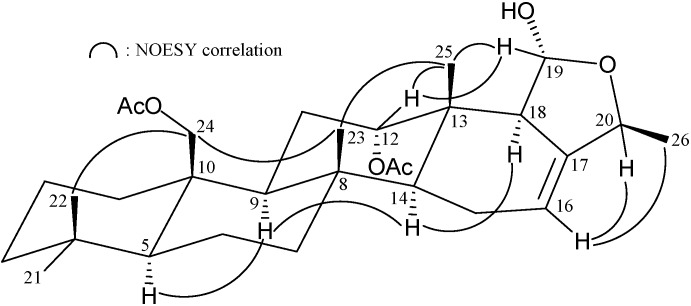
The relative stereochemistry of 1 was elucidated from the NOE interactions observed in an NOESY experiment (Figure 2). As per convention, when analyzing the stereochemistry of scalarane sesterterpenoids, H-5 and hydroxymethyl at C-10 were assigned to the α and β face, anchoring the stereochemical analysis because no correlation was found between H-5 and H2-22. In the NOESY experiment of 1, H-9 showed correlations with H-5 and H-14 but not with H3-21 and H2-22. Thus, both H-9 and H-14 must also be on α face whilst Me-21 and the hydroxymethyl at C-10 must be located on the β face. Moreover, the correlations of H3-23/H3-21 and H3-23/H-12, indicated the β-orientation of Me-23 and H-12 attaching at C-13 and C-12, respectively. The NOESY spectrum showed a correlation of H-16 with H3-25, revealing the E geometry of the C-16/17 double bond. Based on the above findings, the structure, including the relative configuration of 1 was established unambiguously.
Figure 2.
Selective NOESY correlations of 1.
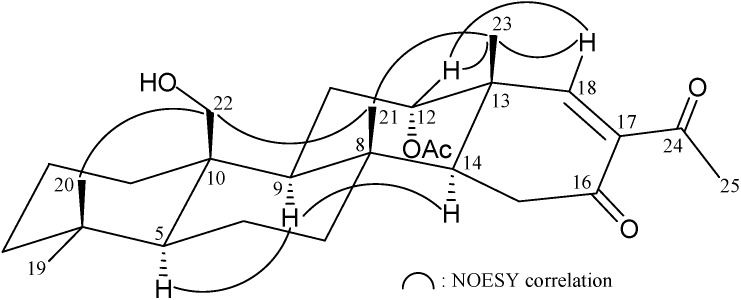
The HRESIMS of 2 (felixin B) exhibited a pseudomolecular ion peak at m/z 467.27707 [M + Na]+, with the molecular formula C27H40O5 (calcd C27H40O5 + Na, 467.27680), implying eight degrees of unsaturation. The IR absorptions of 2 showed the presence of hydroxy (3501 cm−1), ester carbonyl (1733 cm−1) and α,β-unsaturated ketone (1679 cm−1) functionalities. The 13C NMR and DEPT spectrum of 2 exhibited for all 27 carbons: two ketones (δC 197.9, C-24; 197.7, C-16), an ester carbonyl (δC 170.2, acetate carbonyl), a trisubstituted olefin (δC 163.9, CH-18; 136.6, C-17), an oxymethylene (δC 62.7, CH2-22), an oxymethine (δC 76.3, CH-12), six methyls, seven methylenes, three methines and four quaternary carbons. Both the 13C and 1H NMR data for the rings A–C portions were essentially same as those of 1. It also contained an acetoxy (δH 2.05), an acetyl (methyl ketone, δH 2.42) and a hydroxymethyl (δH 4.04 and 3.87) groups as in 1. Analysis of 1H–1H COSY and HMBC data (Table 2) revealed the planar structure. The same stereochemistry was shown by coupling constant and NOE data (Figure 3). The NOESY spectrum showed correlations of H-18 with H-12 and H3-23, revealing the Z geometry of the C-17/18 double bond.
Table 2.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for scalarane 2.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.11, m; 0.53 ddd (13.2, 13.2, 4.0) | 34.2, CH2 | H2-2 | C-2, -3, -10, -22 |
| 2 | 1.58–1.42 m | 18.3, CH2 | H2-1, H2-3 | n. o. a |
| 3 | 1.42 m; 1.19 m | 41.6, CH2 | H2-2 | C-4, -20 |
| 4 | 33.0, C | |||
| 5 | 0.96 m | 57.0, CH | H2-6 | C-3, -4, -6, -7, -9, -10, -20, -22 |
| 6 | 1.54 m; 1.42 m | 17.7, CH2 | H-5, H2-7 | n. o. |
| 7 | 1.78 ddd (12.8, 3.2, 3.2); 1.05 m | 41.0, CH2 | H2-6 | C-21 |
| 8 | 37.2, C | |||
| 9 | 1.31 br d (13.2) | 53.1, CH | H2-11 | C-5, -7, -8, -10, -11, -21, -22 |
| 10 | 41.7, C | |||
| 11 | 2.29 ddd (13.6, 13.6, 2.4); 2.05 m | 24.9, CH2 | H-9, H-12 | n. o. |
| 12 | 4.97 dd (2.8, 2.8) | 76.3, CH | H2-11 | n. o. |
| 13 | 41.4, C | |||
| 14 | 2.11 m | 48.9, CH | H2-15 | C-8, -13, -21, -23 |
| 15 | 2.57–2.40 m | 35.0, CH2 | H-14 | C-13, -14, -16 |
| 16 | 197.7, C | |||
| 17 | 136.6, C | |||
| 18 | 7.30 s | 163.9, CH | C-12, -14, -17, -24 | |
| 19 | 0.87 s | 33.8, CH3 | C-3, -4, -5, -20 | |
| 20 | 0.76 s | 21.8, CH3 | C-3, -4, -5, -19 | |
| 21 | 1.12 s | 15.7, CH3 | C-7, -8, -9, -14 | |
| 22 | 4.04 d (12.0); 3.87 d (12.0) | 62.7, CH2 | C-1, -9, -10 | |
| 23 | 1.17 s | 18.4, CH3 | C-12, -13, -14, -18 | |
| 24 | 197.9, C | |||
| 25 | 2.42 s | 30.6, CH3 | C-17, -24 | |
| 12-OAc | 170.2, C | |||
| 2.05 s | 21.2, CH3 | Acetate carbonyl |
a n. o. = not observed.
Figure 3.
Selective NOESY correlations of 2.
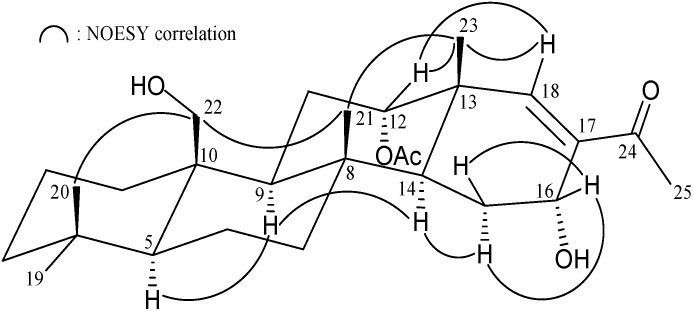
Felixin C (3) was isolated as a white solid. Its HRESIMS (m/z 469.29290 [M + Na]+) and NMR data (Table 3) established a molecular formula of C27H42O5 (calcd C27H42O5 + Na, 469.29245). The IR spectrum of 3 revealed the presence of hydroxy (νmax 3480 cm−1) ester (νmax 1731 cm−1) and α,β-unsaturated ketone (νmax 1662 cm−1) groups. By comparison of NMR data of 3 with those of 2 (Table 2 and Table 3), it was found that the ketone at C-16 in 2 (δC 197.7) was replaced by a hydroxy group (δC 63.3, δH 4.55, 1H, J = 3.6 Hz) in 3. Analyses of 1H–1H COSY and HMBC correlations established the planar structure of 3 (Table 3) as shown in Figure 1, which showed the C-16 positioning of the hydroxy group. Careful analysis of the NOESY spectrum of 3, in comparison with that of 2, allowed determination of the relative stereochemistry of A–C rings of felixin C (3) as shown in Figure 4. Moreover, the splitting pattern and J-value of proton at C-16 in 3, combined with the interactions observed between H-16 and both of the C-15 methylene protons revealed the α-orientation of the 16-OH. Furthermore, the correlations between the olefinic proton H-18/H3-23 and H-18/H-12 assigned the E-configuration of the double bond between C-17 and C-18.
Table 3.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for scalarane 3.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.08 m; 0.57 ddd (12.8, 12.8, 3.2) | 34.1, CH2 | H2-2 | n. o. a |
| 2 | 1.54 m; 1.39 m | 17.8, CH2 | H2-1, H2-3 | n. o. |
| 3 | 1.42 m; 1.16 m | 41.7, CH2 | H2-2 | C-20 |
| 4 | 33.0, C | |||
| 5 | 1.02 dd (12.8, 2.4) | 56.8, CH | H2-6 | C-4, -20, -22 |
| 6 | 1.54 m; 1.47 m | 18.4, CH2 | H-5, H2-7 | n. o. |
| 7 | 1.88 m; 1.11 m | 41.3, CH2 | H2-6 | C-8, -21 |
| 8 | 36.8, C | |||
| 9 | 1.45 m | 53.5, CH | H2-11 | C-10, -11, -21, -22 |
| 10 | 41.8, C | |||
| 11 | 1.96–1.81 m | 25.3, CH2 | H-9, H-12 | C-8, -10, -13 |
| 12 | 4.97 dd (2.8, 2.8) | 76.5, CH | H2-11 | n. o. |
| 13 | 41.4, C | |||
| 14 | 1.88 m | 44.0, CH | H2-15 | C-8, -13, -15, -16, -21, -23 |
| 15 | 1.88 m; 1.64 dd (14.0, 4.8) | 25.3, CH2 | H-14, H-16 | C-8, -13, -16, -17 |
| 16 | 4.55 d (3.6) | 63.3, CH | H2-15 | C-14, -17, -18 |
| 17 | 138.2, C | |||
| 18 | 6.59 s | 152.2, CH | C-12, -13, -14, -16, -24 | |
| 19 | 0.85 s | 33.8, CH3 | C-3, -4, -5, -20 | |
| 20 | 0.76 s | 21.8, CH3 | C-3, -4, -5, -19 | |
| 21 | 1.06 s | 16.4, CH3 | C-7, -8, -9, -14 | |
| 22 | 4.04 d (12.0); 3.90 d (12.0) | 62.8, CH2 | C-1, -9, -10 | |
| 23 | 1.06 s | 19.5, CH3 | C-12, -13, -14, -18 | |
| 24 | 201.4, C | |||
| 25 | 2.24 s | 25.4, CH3 | C-17, -24 | |
| 12-OAc | 170.9, C | |||
| 2.04 s | 21.4, CH3 | Acetate carbonyl |
a n. o. = not observed.
Figure 4.
Selective NOESY correlations of 3.
Moreover, two deoxoscalarin-like metabolites [13], felixins D (4) and E (5) were isolated from I. felix in this study. Felixin D (4) was isolated as white powder and its molecular formula was established as C30H46O6 from the HRESIMS at m/z 525.31849 (calcd C30H46O6 + Na, 525.31866). Eight degrees of unsaturation implied by the molecular formula were ascribed to five rings, a trisubstituted double bond (δC 141.2, C-17; 114.4, CH-16) and two ester carbonyl groups (δC 171.0, 170.9, 2 × C). The 1H NMR spectrum showed seven methyls (δH 2.10, 2.05, 2 × 3H, s, acetate methyls; 1.26, 3H, d, J = 6.0 Hz, H3-26; 0.98, 3H, s, H3-23; 0.89, 3H, s, H3-21; 0.83, 3H, s, H3-22; 0.78, 3H, s, H3-25); an acetoxymethylene (δH 4.59, 1H, d, J = 12.0 Hz; 4.16, 1H, d, J = 12.0 Hz, H2-24); three oxymethines (δH 5.21, 1H, d, J = 3.2 Hz, H-19; 4.91, 1H, dd, J = 3.2, 2.4 Hz, H-12; 4.62, 1H, br s, H-20); and an olefinic proton (δH 5.35, 1H, br s, H-16). The 13C NMR and DEPT spectra exhibited 30 signals, including seven methyls, eight sp3 methylenes (including an oxymethylene), seven sp3 methines (including three oxymethines), an sp2 methine, four sp3 quaternary carbons and three sp2 quaternary carbons (including two ester carbonyls). A typical sesterterpenoid carbons system bearing an acetoxymethylene and four methyl groups along rings A–D could be established by the HMBC correlations from the acetoxymethylene (CH2-24) and four methyl groups (Me-21, 22, 23 and 25) to the associated carbons and a deoxoscalarin skeleton could be obtained on the basis of further HMBC and 1H–1H COSY correlations (Table 4). The 1H–1H COSY correlations between H-18/H-19 and H-20/H3-26 and the HMBC correlations from H-19/C-20 and H3-26/C-17, -20, allowed the establishment of the hemiacetal ring E.
Table 4.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for scalarane 4.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 1.98 dd (13.2, 2.4) | 34.7, CH2 | H2-2 | C-5, -9, -24 |
| 0.57 ddd (13.2, 13.2, 2.4) | ||||
| 2 | 1.56 m; 1.43 m | 18.2, CH2 | H2-1, H2-3 | C-4 |
| 3 | 1.46 m; 1.15 m | 41.6, CH2 | H2-2 | C-2, -4, -21, -22 |
| 4 | 33.0, C | |||
| 5 | 1.04 dd (12.8, 2.0) | 56.8, CH | H2-6 | C-3, -4, -6, -7, -9, -10, -21, -22, -24 |
| 6 | 1.56 m; 1.38 dd (13.6, 3.2) | 17.9, CH2 | H-5, H2-7 | n. o. a |
| 7 | 1.79 ddd (12.8, 3.2, 3.2); 1.12 m | 41.7, CH2 | H2-6 | C-5, -8, -9, -14, -23 |
| 8 | 37.7, C | |||
| 9 | 1.46 m | 52.9, CH | H2-11 | C-1, -5, -8, -10, -11, -12, -14, -23, -24 |
| 10 | 40.1, C | |||
| 11 | 2.05–1.89 m | 25.1, CH2 | H-9, H-12 | C-8 |
| 12 | 4.91 dd (3.2, 2.4) | 74.6, CH | H2-11 | C-9, -14, acetate carbonyl |
| 13 | 36.9, C | |||
| 14 | 1.64 dd (11.2, 6.4) | 50.4, CH | H2-15 | C-7, -8, -9, -13, -15, -18, -25 |
| 15 | 2.16 m; 1.97 m | 22.9, CH2 | H-14, H-16 | C-8 |
| 16 | 5.35 br s | 114.4, CH | H2-15 | n. o. |
| 17 | 141.2, C | |||
| 18 | 2.82 br s | 54.7, CH | H-19 | n. o. |
| 19 | 5.21 d (3.2) | 96.7, CH | H-18 | C-20 |
| 20 | 4.62 m | 74.0, CH | H3-26 | n. o. |
| 21 | 0.89 s | 33.7, CH3 | C-3, -4, -5, -22 | |
| 22 | 0.83 s | 21.9, CH3 | C-3, -5, -21 | |
| 23 | 0.98 s | 15.4, CH3 | C-7, -9, -14 | |
| 24 | 4.59 d (12.0); 4.16 d (12.0) | 64.9, CH2 | C-1, -9, -10, acetate carbonyl | |
| 25 | 0.78 s | 14.7, CH3 | C-12, -14, -18 | |
| 26 | 1.26 d (6.0) | 17.6, CH3 | H-20 | C-17, -20 |
| 12-OAc | 170.9, C | |||
| 2.10 s | 21.5, CH3 | Acetate carbonyl | ||
| 23-OAc | 171.0, C | |||
| 2.05 s | 21.2, CH3 | Acetate carbonyl |
a n. o. = not observed.
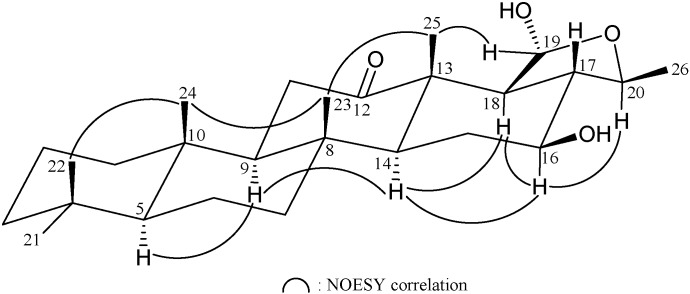
The relative stereochemistry of 4 was elucidated from the interactions observed in an NOESY experiment (Figure 5). In the NOESY experiment of 1, H-9 showed correlations with H-5 and H-14, but not with H3-23 and H2-24 at C-10. Thus, both H-5 and H-14 must be on α face whilst Me-23 and the acetoxymethylene at C-10 must be located on the β face. The correlations of H3-25 with H3-23 and H-12 indicated the β-orientation of Me-25 and H-12. H-18 correlated with H-14, but not with H-19, and H-19 correlated with H-12 and H3-25, assuming that H-18 and H-19 were α- and β-oriented, respectively. H-16 showed correlations with H-20 and H3-26, but not with H-18, revealing the E geometry of the C-16/17 double bond. It was found that the structure of 4 was similar with that of a known scalarane, 12-deacetyl-23-acetoxy-20-methyl-12-epi-deoxo- scalarin (6) [14], excepting the β-hydroxy group at C-12 in 6 was replaced by an α-acetoxy group in 4. The relative configuration of C-20 chiral carbon in 4 was elucidated by comparison the NMR data of CH-20 (δH 4.62, 1H, m; δC 74.0) of 4 with those of 6 (δH 4.67, 1H, m; δC 74.5), indicating H-20 in 4 was α-oriented.
Figure 5.
Selective NOESY correlations of 4.
The HRESIMS of 5 (felixin E) exhibited a pseudomolecular ion peak at m/z 441.29739 [M + Na]+, with the molecular formula C26H42O4 (calcd C26H42O4 + Na, 441.29753), implying six degrees of unsaturation. The IR absorptions of 5 showed the presence of hydroxy (3421 cm−1) and ketone (1701 cm−1) functionalities. The 13C NMR and DEPT spectrum of 5 exhibited for all 26 carbons: a ketone (δC 219.0, C-12), a hemiacetal (δC 97.1, CH-19), two oxymethines (δC 78.1, CH-20; 72.0, CH-16), six methyls, seven methylenes, five methines, and four quaternary carbons (Table 5). The NMR data of 5 were similar with those of 4, except for the acetoxymethylene group at C-10 and acetoxy group at C-12 in 4 were replaced by a methyl and a ketone group in 5, respectively. The C-16/17 trisubstituted olefin in 4 was replaced by a hydroxy group at C-16 in 5. The stereochemical configuration was identical to that of other scalarane sesterterpenes based on NOESY cross-peaks at H-5/H-9, H-9/H-14, H-14/H-16, H-14/H-18, H-16/H-18, H-16/H-20, H-19/H3-25, H3-22/H3-24, H3-23/H3-24 and H3-23/H3-25 (Figure 6). Furthermore, it was found that the structure of 5 was similar with that of known scalarane 7 [15], excepting the 12α-acetoxy group in 7 was replaced by a ketone group in 5.
Table 5.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for scalarane 5.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 1.56 m; 0.76 m | 39.3 CH2 | H2-2 | C-5 |
| 2 | 1.64–1.34 m | 18.3, CH2 | H2-1, H2-3 | n. o. a |
| 3 | 1.80 m; 1.38 m | 41.6, CH2 | H2-2 | n. o. |
| 4 | 33.3, C | |||
| 5 | 0.94 m | 56.5, CH | H2-6 | C-4 |
| 6 | 1.64–1.34 m | 18.1, CH2 | H-5, H2-7 | n. o. |
| 7 | 1.81 m; 0.94 dd (13.2, 4.0) | 41.7, CH2 | H2-6 | C-5 |
| 8 | 37.8, C | |||
| 9 | 1.19 m | 61.4, CH | H2-11 | C-8, -12, -14, -23 |
| 10 | 38.2, C | |||
| 11 | 2.70 dd (14.0, 13.2); 2.32 dd (13.2, 2.4) | 35.3, CH2 | H-9 | C-8, -9, -12 |
| 12 | 219.0, C | |||
| 13 | 51.2, C | |||
| 14 | 1.21 m | 59.2, CH | H2-15 | C-12, -18 |
| 15 | 1.95 ddd (12.8, 4.4, 2.4); 1.41 m | 30.8, CH2 | H-14, H-16 | C-13 |
| 16 | 3.55 ddd (10.4, 10.4, 4.8) | 72.0, CH | H2-15, H-17 | n. o. |
| 17 | 1.62 m | 53.0, CH | H-16, H-18, H-20 | n. o. |
| 18 | 1.86 m | 59.2, CH | H-17, H-19 | C-13, -16, -19, -25 |
| 19 | 5.31 d (6.0) | 97.1, CH | H-18 | C-18, -20 |
| 20 | 4.10 qd (6.0, 3.2) | 78.1, CH | H-17, H3-26 | n. o. |
| 21 | 0.85 s | 33.2, CH3 | C-3, -4, -5, -22 | |
| 22 | 0.82 s | 21.3, CH3 | C-4, -21 | |
| 23 | 1.06 s | 16.9, CH3 | C-7, -8, -9, -14 | |
| 24 | 0.87 s | 15.6, CH3 | C-10 | |
| 25 | 1.24 s | 15.3, CH3 | C-12, -13, -14, -18 | |
| 26 | 1.38 d (6.0) | 20.5, CH3 | H-20 | C-17, -20 |
a n. o. = not observed.
Figure 6.
Selective NOESY correlations of 5.
The cytotoxicity of compounds 1–5 against MOLT-4 (human acute lymphoblastic leukemia), SUP-T1 (human T-cell lymphoblastic lymphoma), DLD-1 (human colorectal adenocarcinoma), LNCaP (human prostatic carcinoma), T-47D (human ductal carcinoma) and MCF7 (human breast adenocarcinoma) tumor cells are shown in Table 6. The results showed that compounds 1–5 were found to exhibit cytotoxicity against DLD-1 tumor cells. By comparison with the structures and cytotoxicity of scalaranes 2 and 3, implying that the presence of 16-ketone would enhance the activity.
Table 6.
Cytotoxic data of scalarane sesterterpenoids 1–5.
| Compounds | Cell Lines IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| MOLT-4 | SUP-T1 | DLD-1 | LNCaP | T-47D | MCF7 | |
| 1 | NA b | NA | 10.9 | 24.3 | NA | NA |
| 2 | 14.9 | 27.1 | 8.5 | NA | 32.2 | 23.0 |
| 3 | 18.5 | NA | 15.0 | NA | NA | NA |
| 4 | 12.8 | 31.6 | 7.9 | 21.5 | 20.2 | NA |
| 5 | 14.0 | 31.1 | 7.2 | NA | 22.7 | 24.3 |
| Doxorubicin a | 0.02 | 0.09 | 0.64 | 0.02 | 0.09 | 0.79 |
a Doxorubicin was used as a positive control; b NA = not active at 20 μg/mL for 72 h.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotation values were measured with a Jasco P-1010 digital polarimeter (Japan Spectroscopic Corporation, Tokyo, Japan). IR spectra were obtained on a Jasco FT-IR 4100 spectrophotometer (Japan Spectroscopic Corporation, Tokyo, Japan); absorptions are reported in cm−1. NMR spectra were recorded on a Varian Mercury Plus 400 NMR spectrometer (Varian Inc., Palo Alto, CA, USA) using the residual solvent (CDCl3, δH 7.26 ppm for 1H NMR and δC 77.1 ppm for 13C NMR) as the internal standard for 1H NMR and CDCl3 (δC 77.1 ppm) for 13C NMR. Coupling constants (J) are given in Hz. ESIMS and HRESIMS were recorded using a Bruker 7 Tesla solariX FTMS system (Bruker, Bremen, Germany). Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck, Darmstadt, Germany); spots were visualized by spraying with 10% H2SO4 solution followed by heating. Normal phase HPLC (NP-HPLC) was performed using a system comprised of a Hitachi L-7110 pump (Hitachi Ltd., Tokyo, Japan) and a Rheodyne 7725 injection port (Rheodyne LLC, Rohnert Park, CA, USA). Two normal phase columns (Supelco Ascentis® Si Cat #: 581515-U, 25.0 cm × 21.2 mm, 5.0 μm and 581514-U, 25.0 cm × 10.0 mm, 5.0 μm, Sigma-Aldrich. Com. St. Louis, MO, USA) was used for HPLC.
3.2. Animal Material
Specimens of the sponge Ircinia felix (Duchassaing and Michelotti, 1864) [16] were collected by hand using SCUBA equipment off the coast of the Southern Taiwan, in September 05, 2012 and stored in a freezer until extraction. A voucher specimen (NMMBA-TWSP-12005) was deposited in the National Museum of Marine Biology and Aquarium, Taiwan.
3.3. Extraction and Isolation
Sliced bodies of Ircinia felix (wet weight 1210 g) were extracted with ethyl acetate (EtOAc). The EtOAc layer (5.09 g) was separated on silica gel and eluted using a mixture of n-hexane and EtOAc (stepwise, 100:1–pure EtOAc) to yield 11 fractions A–K. Fraction F was separated by NP-HPLC using a mixture of n-hexane and EtOAc (3:1) as the mobile phase to yield 16 fractions F1–F16. Fraction F4 was purified by NP-HPLC using a mixture of n-hexane and acetone (3:1, flow rate: 1.0 mL/min) to afford 1 (1.3 mg, tR = 50 min). Fraction G was chromatographed on silica gel and eluted using n-hexane/acetone (6:1–2:1) to afford four fractions G1–G4. Fraction G2 was separated by NP-HPLC using a mixture of dichloromethane (DCM) and EtOAc (5:1, flow rate: 2.0 mL/min) to afford 2 (5.8 mg, tR = 210 min), 3 (5.3 mg, tR = 324 min) and twelve subfractions G2A–G2L. Fraction G2L was further separated by NP-HPLC using a mixture of DCM and acetone (8:1) as the mobile phase to afford 4 (4.4 mg, tR = 45 min). Fraction I was separated by NP-HPLC using a mixture of DCM and acetone (4:1) as the mobile phase to afford 5 (3.7 mg, tR = 126 min).
Felixin A (1): white solid; mp 191–193 °C; −84 (c 0.4, CHCl3); IR (neat) νmax 3480, 1731, 1662 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS: m/z 453 [M + Na]+; HRESIMS: m/z 453.29773 (calcd for C27H42O4 + Na, 453.29753).
Felixin B (2): white solid; mp 92–94 °C; +34 (c 0.3, CHCl3); IR (neat) νmax 3501, 1733, 1679 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2; ESIMS: m/z 467 [M + Na]+; HRESIMS: m/z 467.27707 (calcd for C27H40O5 + Na, 467.27680).
Felixin C (3): white solid; mp 194–196 °C; +35 (c 0.3, CHCl3); IR (neat) νmax 3480, 1731, 1662 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 3; ESIMS: m/z 469 [M + Na]+; HRESIMS: m/z 469.29270 (calcd for C27H42O5 + Na, 469.29245).
Felixin D (4): white solid; mp 94–97 °C; +22 (c 0.2, CHCl3); IR (neat) νmax 3441, 1738 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 4; ESIMS: m/z 525 [M + Na]+; HRESIMS: m/z 525.31849 (calcd for C30H46O6 + Na, 525.31866).
Felixin E (5): white solid; mp 151–153 °C; −5 (c 1.2, CHCl3); IR (neat) νmax 3421, 1701 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 5; ESIMS: m/z 441 [M + Na]+; HRESIMS: m/z 441.29739 (calcd for C26H42O4 + Na, 441.29753).
3.4. MTT Antiproliferative Assay
MOLT-4, SUP-T1, DLD-1, LNCaP, T-47D and MCF7 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM glutamine and antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin) at 37 °C in a humidified atmosphere of 5% CO2. Cells were seeded at 4 × 104 per well in 96-well culture plates before treatment with different concentrations of the tested compounds. The compounds were dissolved in dimethyl sulfoxide (less than 0.02%) and made concentrations of 1.25, 2.5, 5, 10 and 20 μg/μL prior to the experiments. After treatment for 72 h, the cytotoxicity of the tested compounds was determined using a MTT cell proliferation assay (thiazolyl blue tetrazolium bromide, Sigma-M2128). The MTT is reduced by the mitochondrial dehydrogenases of viable cells to a purple formazan product. The MTT-formazan product was dissolved in DMSO. Light absorbance values (OD = OD570 − OD620) were recorded at wavelengths of 570 and 620 nm using an ELISA reader (Anthos labtec Instrument, Salzburg, Austria) to calculate the concentration that caused 50% inhibition (IC50), i.e., the cell concentration at which the light absorbance value of the experiment group was half that of the control group. These results were expressed as a percentage of the control ± SD established from n = 4 wells per one experiment from three separate experiments [17,18,19].
4. Conclusions
Sponges have been well-recognized as an important source of potential bioactive marine natural products. Our studies on Ircinia felix for the extraction of natural substances, have led to the isolation of five new scalaranes, felixins A–E (1–5) and compounds 1–5 are potentially cytotoxic toward DLD-1 tumor cells. These results suggest that continuing investigation of novel secondary metabolites together with the potentially useful bioactivities from this marine organism are worthwhile for future drug development.
Acknowledgments
This research was supported by grants from the National Dong Hwa University; the National Museum of Marine Biology and Aquarium; the Asia-Pacific Ocean Research Center, National Sun Yat-sen University; the Ministry of Science and Technology (Grant No. NSC 103-2911-I-002-303; MOST 104-2911-I-002-302; MOST 103-2325-B-039-008; MOST 103-2325-B-039-007-CC1; MOST 103-2325-B-291-001; MOST 104-2325-B-291-001; MOST 104-2320-B-291-001-MY3 and NSC 101-2320-B-291-001-MY3); the National Health Research Institutes (NHRI-EX103-10241BI), and in part from the grant from Chinese Medicine Research Center, China Medical University (the Ministry of Education, the Aim for the Top University Plan), Taiwan, awarded to Yang-Chang Wu and Ping-Jyun Sung.
Author Contributions
Yang-Chang Wu and Ping-Jyun Sung designed the whole experiment and contributed to manuscript preparation. Ya-Yuan Lai researched data. Mei-Chin Lu, Li-Hsueh Wang, Jih-Hung Chen and Lee-Shing Fang analyzed the data and performed data acquisition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Martinez A., Duque C., Sato N., Tanaka R., Fujimoto Y. (18R)-Variabilin from the sponge Ircinia felix. Nat. Prod. Lett. 1995;6:1–6. doi: 10.1080/10575639508044080. [DOI] [Google Scholar]
- 2.Martinez A., Duque C., Hara N., Fujimoto Y. Variabilin 11-methyloctadecanoate, a branched-chain acid ester of furanosesterterpene tetronic acid, from the sponge Ircinia felix. Nat. Prod. Lett. 1995;6:281–284. doi: 10.1080/10575639508043171. [DOI] [Google Scholar]
- 3.Martinez A., Duque C., Sato N., Fujimoto Y. (8Z,13Z,20Z)-Strobilinin and (7Z,13Z,20Z)-felixinin: New furanosesterterpene tetronic acids from marine sponges of the genus Ircinia. Chem. Pharm. Bull. 1997;45:181–184. doi: 10.1248/cpb.45.181. [DOI] [Google Scholar]
- 4.Martínez A., Duque C., Fujimoto Y. Novel fatty acid esters of (7E, 12E, 18R, 20Z)-variabilin from the marine sponge Ircinia felix. Lipids. 1997;32:565–569. doi: 10.1007/s11745-997-0072-6. [DOI] [PubMed] [Google Scholar]
- 5.Granato A.C., de Oliveira J.H.H.L., Seleghim M.H.R., Berlinck R.G.S., Macedo M.L., Ferreira A.G., da Rocha R.M., Hajdu E., Peixinho S., Pessoa C.O., et al. Produtos naturais da ascídia Botrylloides giganteum, das esponjas Verongula gigantea, Ircinia felix, Cliona delitrix e do nudibrânquio Tambja eliora, da costa do Brasil. Quim. Nova. 2005;28:192–198. doi: 10.1590/S0100-40422005000200005. [DOI] [Google Scholar]
- 6.Waddell B., Pawlik J.R. Defenses of Caribbean sponges against invertebrate predators. II. Assays with sea stars. Mar. Ecol. Prog. Ser. 2000;195:133–144. doi: 10.3354/meps195133. [DOI] [Google Scholar]
- 7.Duque C., Bonilla A., Bautista E., Zea S. Exudation of low molecular weight compounds (thiobismethane, methyl isocyanide, and methyl isothiocyanate) as a possible chemical defense mechanism in the marine sponge Ircinia felix. Biochem. Syst. Ecol. 2001;29:459–467. doi: 10.1016/S0305-1978(00)00081-8. [DOI] [PubMed] [Google Scholar]
- 8.Pawlik J.R., McFall G., Zea S. Does the odor from sponges of the genus Ircinia protect them from fish predators? J. Chem. Ecol. 2002;28:1103–1115. doi: 10.1023/A:1016221415028. [DOI] [PubMed] [Google Scholar]
- 9.Freeman C.J., Gleason D.F. Chemical defenses, nutritional quality, and structural components in three sponges: Ircinia felix, I. campana, and Aplysina fulva. Mar. Biol. 2010;157:1083–1093. doi: 10.1007/s00227-010-1389-5. [DOI] [Google Scholar]
- 10.Freeman C.J., Gleason D.F. Does concentrating chemical defenses within specific regions of marine sponges results in enhanced protection from predators? Hydrobiologia. 2012;687:289–297. doi: 10.1007/s10750-011-0792-3. [DOI] [Google Scholar]
- 11.Gómez-Guiñán Y., Hidalgo J., Jiménez M., Salcedo J. Actividad antibacteriana de extractos orgánicos de Penicillium sp. (Moniliales) aislados de la esponja Ircinia felix (Demospongiae) Rev. Biol. Trop. 2003;51:141–147. [PubMed] [Google Scholar]
- 12.Sepčić K., Kauferstein S., Mebs D., Turk T. Biological activities of aqueous and organic extracts from tropical marine sponges. Mar. Drugs. 2010;8:1550–1566. doi: 10.3390/md8051550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González M.A. Scalarane sesterterpenoids. Curr. Bioact. Comp. 2010;6:178–206. doi: 10.2174/157340710793237362. [DOI] [Google Scholar]
- 14.Fontana A., Mollo E., Ortea J., Gavagnin M., Cimino G. Scalarane and homoscalarane compounds from the nudibranchs Glossodoris sedan and Glossodoris dalli: Chemical and biological properties. J. Nat. Prod. 2000;63:527–530. doi: 10.1021/np990506z. [DOI] [PubMed] [Google Scholar]
- 15.Roy M.C., Tanaka J., de Voogd N., Higa T. New scalarane class sesterterpenes from an Indonesian sponge, Phyllospongia sp. J. Nat. Prod. 2002;65:1838–1842. doi: 10.1021/np020311i. [DOI] [PubMed] [Google Scholar]
- 16.Pronzato R., Malva R., Manconi R. The taxonomic status of Ircinia fasciculata, Ircinia felix, and Ircinia variabilis (Dictyoceratida, Irciniidae) Boll. Mus. Ist. Biol. Univ. Genova. 2004;68:553–563. [Google Scholar]
- 17.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 18.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 19.Lu M.-C., Hwang S.-L., Chang F.-R., Chen Y.-H., Chang T.-T., Hung C.-S., Wang C.-L., Chu Y.-H., Pan S.-H., Wu Y.-C. Immunostimulatory effect of Antrodia camphorata extract on functional maturation of dendritic cells. Food Chem. 2009;113:1049–1057. doi: 10.1016/j.foodchem.2008.08.089. [DOI] [Google Scholar]