Abstract
Mangrove wetlands serve as both a sink and source for arsenic (As), as mangrove plants are able to uptake and accumulate As. The present study used pot experiments to evaluate As accumulation and translocation in mangrove (Aegiceras corniculatum L.) seedlings grown in As contaminated soils. Results indicated that A. corniculatum seedlings grew normally under As stress with minute growth inhibition and biomass reduction at different As treatment concentrations in a range of 0–150 mg·kg−1. As concentrations in roots, stems and leaves were increased with increasing As treatment concentrations, but As accumulated mainly in roots, with accumulation rates of 74.54%–89.26% of the total As accumulation. In particular, relatively high bioconcentration factor (BCF) in root (2.12–1.79), low BCF in stem (0.44–0.14) and leaf (0.06–0.01), and thereby a low translocation factor (TF) in stem/root (0.21–0.08) and leaf/root (0.02–0.008) were observed. These results demonstrated that A. corniculatum is an As excluder with the innate capacity to tolerate As stress and root tissues may be employed as a bio-indicator of As in polluted sediments. Additionally, A. corniculatum is a potential candidate mangrove species for As phytostabilization in tropical and subtropical estuarine wetlands.
Keywords: arsenic, Aegiceras corniculatum L., accumulation, translocation, phytostabilization
1. Introduction
Arsenic (As) is a toxic environmental metalloid originating from geological and/or anthropogenic sources such as mining, burning of fossil fuels, use of fertilizers and agrochemicals, etc. [1,2]. As is a non-essential element for plants, also being highly phytotoxic and carcinogenic to humans through the food chain [3,4]. As is recognized as one of the most serious inorganic contaminants in natural water worldwide [5].
Mangroves are woody plants that grow on the coastal ecotone in tropical and subtropical latitudes, providing important ecological services including flood protection, prevention of shoreline erosion and salinity buffering [6,7]. Mangrove wetlands serve as both a sink and source for heavy metals (and also metalloids like As) in the coastline ecosystem, which prevents contaminant entry to adjacent waters but could increase the stress to mangrove plants [8,9]. High levels of As were found in mangrove sediments around the world, as 14.0 mg·kg−1 in Xiamen Bay (China) [10], 3.6–18.3 mg·kg−1 in the Sunderbans mangrove forest (India) [11], up to 70 mg·kg−1 in Espirito Santo (Brazil) [12], 0.52–35 mg·kg−1 in Sydney Estuary (Australia) [13]. Mangrove plants are able to survive under As stress, uptake and enrich As [14]. Recent field studies have shown that As is mainly accumulated in the roots and middle aerial part of Avicennia marina as compared to the upper part [15], As concentrations in fine nutritive roots of Avicennia marina were ~3-fold higher than those in sediments [13], while the uptake and bioaccumulation of As in Kandelia obovata are also significant [16]. However, field studies cannot provide more useful information on As accumulation and translocation in mangrove plants because of the frequent changes of As concentration and the lack of As concentration gradients in the natural environment. Thus, experimental study with more As concentration gradients, accessible for long-term observation and sampling, is urgently needed.
Aegiceras corniculatum L., an ecologically important tree species with a widespread distribution throughout the estuaries of southern China, was employed to evaluate As accumulation and translocation in mangrove plants. Data of plant growth and arsenic accumulation were collected through pot experiments with As addition to soils. The results of this study will provide useful information to improve our understanding of As tolerance in mangrove plants, and also its accumulation and translocation, and overall potential for As phytoremediation in tropical and subtropical estuarine wetlands.
2. Experimental Section
2.1. Soil Sampling
Surface soil samples (0–20 cm) were collected from mangrove sediments at Zhangjiang Estuary (23°55′ N, 117°25′ E, Fujian Province, China) for the pot experiments. Basic soil properties of the collected samples were analyzed based on standard methods [17], results are listed in Table 1.
Table 1.
Properties of the soil used in the pot experiments. Values are mean ± SE (n = 5).
| Organic Matter mg·kg−1 | Available N mg·kg−1 | Available P mg·kg−1 | Available K mg·kg−1 | Total As mg·kg−1 | pH |
|---|---|---|---|---|---|
| 3.17 ± 0.11 | 97.85 ± 4.67 | 28.41 ± 1.07 | 612.59 ± 18.54 | 14.32 ± 0.38 | 6.63 ± 0.08 |
2.2. Pot Experiments
Hypocotyls of A. corniculatum collected from the same location were pre-cultured in plastic pots (35 cm in diameter, 18.5 cm in-depth) filled with sea sand. The sea sand used was prewashed with concentrated HCl and rinsed thoroughly with tap water as described by Du et al. [18]. The pre-cultivation in a half-strength Hoagland’s nutrient solution lasted four weeks, and the solution was replaced once a week [19]. The experiment was conducted in a natural light greenhouse, and the salinity of irrigated water was maintained at 12.
After pre-cultivation, uniformly-sized seedlings were selected. Five seedlings were transplanted to each pot with ~5 kg either non-contaminated or As contaminated soil (as Na2HAsO4•7H2O). As concentrations were set based on the baseline concentration of As in the experiment soil (14.32 mg·kg−1). As treatment concentrations were increased about one-fold or two-fold the baseline As concentration, respectively, resulting in a total of seven treatments (0, 15, 30, 60, 90, 120 or 150 mg·kg−1 As). Each treatment had five replicates. The pot experiments were conducted for 180 days in natural light greenhouses, with a day/night temperature of 33/25 °C, and relative humidity of 65%/85%. Plants were watered to maintain soil moisture at 70%–80% of the field water holding capacity by addition of tap water during the experiments.
2.3. Sampling and Analysis
At harvest, seedlings were thoroughly washed with distilled water, and the shoot lengths, stem heights and leaf sizes were measured. Seedlings were then separated into root, stem and leaf tissues, and oven dried at 105 °C for 15 min, then at 70 °C until the samples reached constant weight. Oven-dried plant tissues were weighed, then ground to powder and passed through 100-mesh sieves.
Precisely 100 mg of plant material was placed into clean digestion tubes for digestion with 1 mL HNO3 and H2O2 (8:2, v/v) on a heating block at 180 °C for 1 h, and subsequently at 200 °C for 45–60 min so as to evaporate the samples to dryness. The residue was taken up in 10 mL demineralized water. Arsenic concentrations were measured by atomic absorption spectrophotometry (AAS: model AA-6800, Shimadzu, Kyoto, Japan), and calculated by dry weight (DW). Each sample was analyzed three times, and the mean value was calculated. The variances of duplicate measurements were less than 5%. Results obtained from these analyses were in good agreement with the certified values (±5%). The variances of samples in the same pot or in different pots were less than 5% and 10%, respectively.
The bioconcentration factor (BCF) reflects the ability of plants to accumulate arsenic, and is defined BCF = Astissue/Assoil, where Astissues = concentration of As in plant tissues (roots, stems and leaves) and Assoil = concentration of As in soil [16,20,21]. The translocation factor (TF) reflects the ability of plants to translocate As, and is defined TF = Asaerial/Asroot, where Asaerial = concentration of As in plant’s aerial parts (stems and leaves) and Asroot = Concentration of As in roots [22,23].
2.4. Data Analysis
Data are presented mean ± SE of five replicates (n = 5). Two-way analysis of variance (ANOVA) was done on all the data to confirm the variability of data and validity of results using SPSS software (19.0, SPSS, Inc., Chicago, IL, USA). Duncan’s multiple range test (DMRT) was performed to determine the significant difference between treatments at 0.05 probability level.
3. Results and Discussion
3.1. Plant Growth
The effects of As treatments on A. corniculatum seedling growth after 180 days are shown in Table 2. Treatments of 15–120 mg·kg−1 As had no significant impact on plant growth (p > 0.05), but at 150 mg·kg−1 As, root length decreased by 6.04%, stem height by 6.48% and life size by 18.16% as compared to the control. Plant biomass also decreased significantly at 150 mg·kg−1 As (p < 0.05), with root biomass decreasing by 11.15%, stem biomass by 11.46%, leaf biomass by 20.56%, resulting in the total biomass being decreased by 13.60% as compared to the control. The toxicity of large doses of heavy metals (e.g., Cu, Pb or Zn, or multiple heavy metals) on mangrove plants can cause toxic effects such as reduction in seedling height, leaf area, biomass and root growth, leaf chlorosis or necrosis, to finally induce mortality [24,25,26]. However, mangrove plants are considered to operate as excluder species for non-essential elements [20,27]. In the present study, A. corniculatum seedlings survived and grew normally at soil treatments of 15–120 mg·kg−1 As without those typical symptoms. These results demonstrated that A. corniculatum is an As excluder with the innate capacity to tolerate As stress.
Table 2.
Effects of As treatments on A. corniculatum seedlings growth. Values are mean ± SE (n = 5). Different letters above same columns indicate significant differences at p < 0.05.
| As Treatment mg·kg−1 | Root Length cm | Stem Height cm | Leaf Size cm2 | Root Biomass g DW | Stem Biomass g DW | Leaf Biomass g DW |
|---|---|---|---|---|---|---|
| 0 | 17.05 ± 0.89 a | 14.67 ± 0.97 a | 12.39 ± 1.17 a | 2.03 ± 0.21 a | 3.09 ± 0.27 a | 1.67 ± 0.17 a |
| 15 | 16.98 ± 1.02 a | 14.50 ± 0.89 a | 11.97 ± 1.06 a | 2.05 ± 0.25 a | 3.13 ± 0.31 a | 1.68 ± 0.18 a |
| 30 | 17.10 ± 0.98 a | 14.70 ± 1.02 a | 11.74 ± 1.22 a | 2.08 ± 0.31 a | 3.14 ± 0.29 a | 1.71 ± 0.22 a |
| 60 | 17.07 ± 0.87 a | 14.76 ± 0.94 a | 12.09 ± 1.04 a | 2.07 ± 0.18 a | 3.12 ± 0.34 a | 1.68 ± 0.15 a |
| 90 | 16.96 ± 1.01 a | 14.59 ± 0.87 a | 11.95 ± 1.11 a | 2.04 ± 0.27 a | 3.10 ± 0.37 a | 1.64 ± 0.12 a |
| 120 | 16.85 ± 1.06 a | 14.43 ± 1.03 a | 11.84 ± 1.08 a | 1.97 ± 0.29 a | 3.06 ± 0.28 a | 1.61 ± 0.15 a |
| 150 | 16.02 ± 0.94 b | 13.72 ± 1.01 b | 10.14 ± 1.21 b | 1.81 ± 0.23 b | 2.73 ± 0.30 b | 1.32 ± 0.14 b |
3.2. As Accumulation
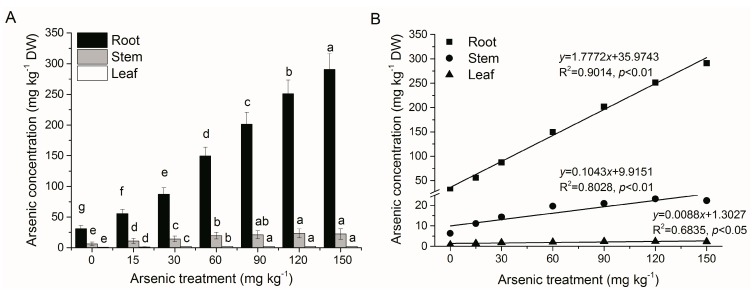
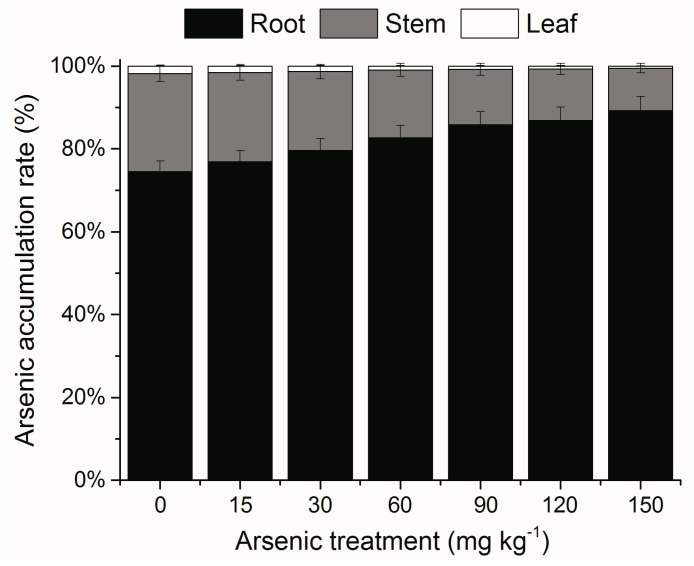
As accumulation in A. corniculatum differed with As treatment levels and plant tissues (Figure 1A). As concentrations in roots, stems and leaves were increased by increasing of As treatment concentrations (0–150 mg·kg−1), with significantly linear expression (R2 > 0.68, p < 0.05) (Figure 1B). As mainly accumulated in roots (30.29–294.85 mg·kg−1 DW), at levels which were 4.77–13.20-fold higher than those in stems, and 34.23–127.64-fold higher than those in leaves at soil As concentrations of 0–150 mg·kg−1. As accumulation rates in roots were 74.54%–89.26% of the total As accumulation (average 82.23% ± 2.06%), while those in stems were 23.68%–10.23% (average 16.68% ± 1.88%) and in leaves only 1.78%–0.51% (average 1.09% ± 0.18%) (Figure 2). An increasing trend of As accumulation rates in roots was found in roots with increasing As treatment concentrations, while opposite trends were found in stems and leaves. High As accumulation in roots was observed in other species like rice [28], ferns [29] and the mangrove species Kandelia obovata [16]. Transport of non-essential elements was interpreted by cell wall immobilization and/or sequestering of the epidermal layers [20]. Thus mangrove plants absorb and store non-essential elements in the perennial tissues, especially roots [30]. A. corniculatum root tissues might be a bio-indicator of As in polluted sediments, as As concentrations in roots are reflective of environmental levels.
Figure 1.
Effects of As treatments on (A) As concentrations in root, stem and leaf, and (B) linear relationship between As treatment concentrations and As concentrations in root, stem and leaf in A. corniculatum seedlings. Values are mean ± SE (n = 5). Different letters above comparable columns in Figure 1A indicate significant differences at p < 0.05.
Figure 2.
Effects of As treatments on As accumulation rates in root, stem and leaf in A. corniculatum seedlings. Values are mean ± SE (n = 5).
3.3. Bioconcentration Factor and Translocation Factor
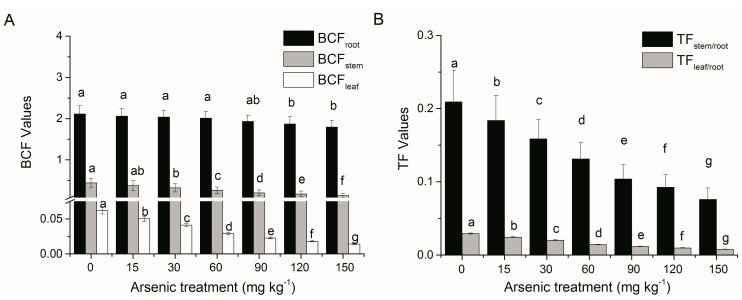
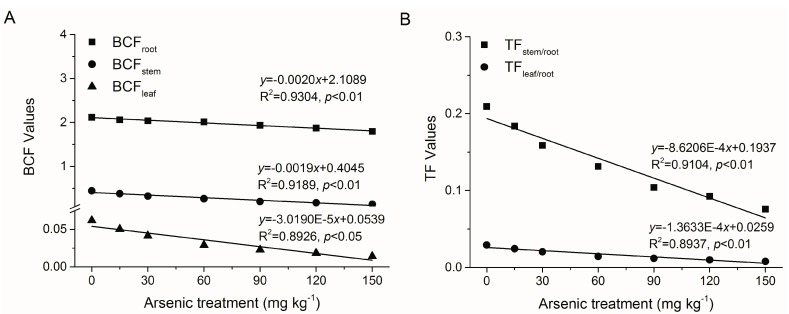
BCF and TF were further employed to evaluate the ability of A. corniculatum to accumulate and translocate As. At treatments of 15–120 mg·kg−1 As, BCFroot was reasonable-high (ranged of 2.12–1.79), but BCFstem (0.44–0.14) and BCFleaf (0.06–0.01) were low, with values at 150 mg·kg−1 As decreased by 15.18%, 69.29% and 77.25% as compared to the control, respectively (Figure 3A). Meanwhile, TFstem/root (0.21–0.08) and TFleaf/root (0.02–0.008) were also extremely low, and values at 150 mg·kg−1 As decreased by 63.80% and 73.18% as compared to the control, respectively (Figure 3B). Significant negative correlations were found between TF or BCF values and soil As concentrations (R2> 0.89, p < 0.05) (Figure 4A,B). However, BCFroot values decreased slightly with increasing As treatment concentrations (0–150 mg·kg−1), while BCFstem and BCFleaf decreased sharply, resulting in great reductions in TFstem/root and TFleaf/root values. These results demonstrated that most of the As absorbed from the soil was retained in the roots and only small amounts were transported to the stems and leaves. Mangroves with high BCFroot (e.g., Kandelia obovata [16], Avicennia marina [13,31], Phragmites australis [32]) are appropriate candidates for phytostabilisation, retaining metallic inputs and thereby reducing transport to adjacent estuarine and marine systems. Besides, it was suggested that plant species with high BCFroot (>1) and low TFs (<1) could be considered as a potential candidate for the phytostabilization [33,34]. In the present study, high BCFroot (>1) and low TFs (<1) were observed at high soil As concentrations, suggesting that A. corniculatum is a potential candidate mangrove species for As phytostabilization in tropical and subtropical estuarine wetlands.
Figure 3.
Effects of As treatments on (A) bioconcentration factor (BCF) of root, stem and leaf, and (B) translocation factor (TF) from root to stem and leaf in A. corniculatum seedlings. Values are mean ± SE (n = 5). Different letters above comparable columns indicate significant differences at p < 0.05.
Figure 4.
Linear relationship between As treatment concentrations and (A) bioconcentration factor (BCF) of root, stem and leaf, and (B) translocation factor (TF) from root to stem and root to leaf in A. corniculatum seedlings. Values are mean ± SE (n = 5).
4. Conclusions
In the present study, A. corniculatum seedlings survived and grew normally without typical symptoms of As toxicity, demonstrating that A. corniculatum is an As excluder with an innate capacity to tolerate As stress. As was mainly accumulated in roots, with As concentrations increasing with increasing As treatment concentration, indicating that A. corniculatum root tissues may be employed as a bio-indicator of As in polluted sediments. Additionally, relatively high BCFroot (>1) and low TFs (<1) suggested that A. corniculatum is a potential candidate mangrove species for As phyto-stabilization in tropical and subtropical estuarine wetlands.
Acknowledgments
This work was jointly supported by the National Natural Important Scientific Research Program of China (2013CB956504) and National Natural Science Foundation of China (No. 31370516, 31170471). The authors would like to thank Dou Y.Y. for assistance in sample culture and John Merefield for English grammar.
Author Contributions
Gui-Rong Wu conceived the study idea, designed the experiments, analyzed the data, and wrote the manuscript. Hua-Long Hong finished a part of the experiments, analyzed the data. Chong-Ling Yan co-conceived the study idea and critically revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mandal B.K., Suzuki K.T. Arsenic round the world: A review. Talanta. 2002;58:201–235. doi: 10.1016/S0039-9140(02)00268-0. [DOI] [PubMed] [Google Scholar]
- 2.Bissen M., Frimmel F.H. Arsenic—A review. Part I: Occurrence, toxicity, speciation, mobility. Acta Hydrochim. Hydrobiol. 2003;31:9–18. doi: 10.1002/aheh.200390025. [DOI] [Google Scholar]
- 3.Naujokas M.F., Anderson B., Ahsan H., Aposhian H.V., Graziano J., Thompson C., Suk W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao F.J., McGrath S.P., Meharg A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Ann. Rev. Plant Biol. 2010;61:535–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- 5.Tuzen M., Citak D., Mendil D., Soylak M. Arsenic speciation in natural water samples by coprecipitation-hydride generation atomic absorption spectrometry combination. Talanta. 2009;78:52–56. doi: 10.1016/j.talanta.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Giri C., Ochieng E., Tieszen L.L., Zhu Z., Singh A., Loveland T., Masek J., Duke N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011;20:154–159. doi: 10.1111/j.1466-8238.2010.00584.x. [DOI] [Google Scholar]
- 7.Lee S.Y., Primavera J.H., Dahdouh-Guebas F., McKee K., Bosire J.O., Cannicci S., Diele K., Fromard F., Koedam N., Marchand C. Ecological role and services of tropical mangrove ecosystems: A reassessment. Glob. Ecol. Biogeogr. 2014;23:726–743. doi: 10.1111/geb.12155. [DOI] [Google Scholar]
- 8.Lewis M., Pryor R., Wilking L. Fate and effects of anthropogenic chemicals in mangrove ecosystems: A review. Environ. Pollut. 2011;159:2328–2346. doi: 10.1016/j.envpol.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 9.He B., Li R.L., Chai M.W., Qiu G.Y. Threat of heavy metal contamination in eight mangrove plants from the Futian mangrove forest, China. Environ. Geochem. Health. 2014;36:467–476. doi: 10.1007/s10653-013-9574-3. [DOI] [PubMed] [Google Scholar]
- 10.Vane C.H., Harrison I., Kim A., Moss-Hayes V., Vickers B., Hong K. Organic and metal contamination in surface mangrove sediments of South China. Mar. Pollut. Bull. 2009;58:134–144. doi: 10.1016/j.marpolbul.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee M., Massolo S., Sarkar S.K., Bhattacharya A.K., Bhattacharya B.D., Satpathy K.K., Saha S. An assessment of trace element contamination in intertidal sediment cores of Sunderban mangrove wetland, India for evaluating sediment quality guidelines. Environ. Monit. Assess. 2009;150:307–322. doi: 10.1007/s10661-008-0232-7. [DOI] [PubMed] [Google Scholar]
- 12.Mirlean N., Medeanic S., Garcia F., Travassos M.P., Baisch P. Arsenic enrichment in shelf and coastal sediment of the Brazilian subtropics. Cont. Shelf Res. 2012;35:129–136. doi: 10.1016/j.csr.2012.01.006. [DOI] [Google Scholar]
- 13.Chaudhuri P., Nath B., Birch G. Accumulation of trace metals in grey mangrove Avicennia marina fine nutritive roots: The role of rhizosphere processes. Mar. Pollut. Bull. 2014;79:284–292. doi: 10.1016/j.marpolbul.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Kirby J., Maher W., Chariton A., Krikowa F. Arsenic concentrations and speciation in a temperate mangrove ecosystem, NSW, Australia. Appl. Organomet. Chem. 2002;16:192–201. doi: 10.1002/aoc.283. [DOI] [Google Scholar]
- 15.Parveen R., Zahir E., Siddiqui A.F. Arsenic enrichment in mangroves, and sediments along Karachi coast. J. Coast. Life Med. 2013;1:59–64. [Google Scholar]
- 16.Liu C.W., Chen Y.Y., Kao Y.H., Maji S.K. Bioaccumulation and translocation of arsenic in the ecosystem of the Guandu Wetland, Taiwan. Wetlands. 2014;34:129–140. doi: 10.1007/s13157-013-0491-0. [DOI] [Google Scholar]
- 17.Lu R. Chemistry Analysis Methods of Soil and Agriculture. Agricultural Science Publishing House; Beijing, China: 1999. [Google Scholar]
- 18.Du J.N., Yan C.L., Li Z.D. Formation of iron plaque on mangrove Kandalar. Obovata (SL) root surfaces and its role in cadmium uptake and translocation. Mar. Pollut. Bull. 2013;74:105–109. doi: 10.1016/j.marpolbul.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Hoagland D.R., Arnon D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950;347:32. [Google Scholar]
- 20.MacFarlane G.R., Koller C.E., Blomberg S.P. Accumulation and partitioning of heavy metals in mangroves: A synthesis of field-based studies. Chemosphere. 2007;69:1454–1464. doi: 10.1016/j.chemosphere.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 21.Baker A., Brooks R. Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1:81–126. [Google Scholar]
- 22.Marchiol L., Assolari S., Sacco P., Zerbi G. Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ. Pollut. 2004;132:21–27. doi: 10.1016/j.envpol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Ma L.Q., Rathinasabapathi B., Liu Y., Zeng G. Uptake and translocation of arsenite and arsenate by Pteris vittata L.: Effects of silicon, boron and mercury. Environ. Exp. Bot. 2010;68:222–229. doi: 10.1016/j.envexpbot.2009.11.006. [DOI] [Google Scholar]
- 24.MacFarlane G.R., Burchett M.D. Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove Avicennia marina (Forsk.) Vierh. Mar. Environ. Res. 2002;54:65–84. doi: 10.1016/S0141-1136(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 25.Huang G.Y., Wang Y.S. Physiological and biochemical responses in the leaves of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) exposed to multiple heavy metals. J. Hazard. Mat. 2010;182:848–854. doi: 10.1016/j.jhazmat.2010.06.121. [DOI] [PubMed] [Google Scholar]
- 26.MacFarlane G., Burchett M. Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar. Pollut. Bull. 2001;42:233–240. doi: 10.1016/S0025-326X(00)00147-8. [DOI] [PubMed] [Google Scholar]
- 27.Bayen S. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ. Int. 2012;48:84–101. doi: 10.1016/j.envint.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Lei M., Tie B.Q., Zeng M., Qing P.F., Song Z.G., Williams P.N., Huang Y.Z. An arsenic-contaminated field trial to assess the uptake and translocation of arsenic by genotypes of rice. Environ. Geochem. Health. 2013;35:379–390. doi: 10.1007/s10653-012-9501-z. [DOI] [PubMed] [Google Scholar]
- 29.Feng R.W., Wang X.L., Wei C.Y., Tu S.X. The accumulation and subcellular distribution of arsenic and antimony in four fern plants. Int. J. Phytoremediation. 2015;17:348–354. doi: 10.1080/15226514.2013.773281. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y.W., Peng Y.S., Li X.L., Chen G.Z. Accumulation and partitioning of heavy metals in mangrove rhizosphere sediments. Environ. Earth Sci. 2011;64:799–807. doi: 10.1007/s12665-011-0904-4. [DOI] [Google Scholar]
- 31.MacFarlane G.R., Pulkownik A., Burchett M.D. Accumulation and distribution of heavy metals in the grey mangrove, Avicennia marina (Forsk.) Vierh.: Biological indication potential. Environ. Pollut. 2003;123:139–151. doi: 10.1016/S0269-7491(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 32.Weis J.S., Weis P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004;30:685–700. doi: 10.1016/j.envint.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Yoon J., Cao X.D., Zhou Q.X., Ma L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006;368:456–464. doi: 10.1016/j.scitotenv.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Meng L., Guo Q., Mao P., Tian X. Accumulation and tolerance characteristics of zinc in Agropyron cristatum plants exposed to zinc-contaminated soil. Bull. Environ. Contam. Toxicol. 2013;91:298–301. doi: 10.1007/s00128-013-1039-y. [DOI] [PubMed] [Google Scholar]