Abstract
Escherichia coli O157:H7 (EcO157) shed in cattle manure can survive for extended periods of time and intervention strategies to control this pathogen at the source are critical as produce crops are often grown in proximity to animal raising operations. This study evaluated whether neem (Azadirachta indica), known for its antimicrobial and insecticidal properties, can be used to amend manure to control EcO157. The influence of neem materials (leaf, bark, and oil) on the survival of an apple juice outbreak strain of EcO157 in dairy manure was monitored. Neem leaf and bark supplements eliminated the pathogen in less than 10 d with a D-value (days for 90% elimination) of 1.3 d. In contrast, nearly 4 log CFU EcO157/g remained after 10 d in neem-free manure control. The ethyl acetate extractable fraction of neem leaves was inhibitory to the growth of EcO157 in LB broth. Azadirachtin, a neem product with insect antifeedant properties, failed to inhibit EcO157. Application of inexpensive neem supplements to control pathogens in manure and possibly in produce fields may be an option for controlling the transfer of foodborne pathogens from farm to fork.
Keywords: neem, Azadirachta indica, E. coli O157:H7, bioscreen, survival, dairy manure, neem extracts, azadirachtin
1. Introduction
Concentrated animal feeding operations generate large amounts of manure waste [1], thus raising concerns about foodborne pathogen contamination of fruit and vegetable crops grown in the vicinity. A mid-sized dairy produces more than 12 million kilograms of manure per year [2] and the manure is usually stored on-site. This further increases the risk of pathogen contamination of produce grown nearby. Ruminants are primary reservoirs of many enteric pathogens including Escherichia coli O157:H7 (EcO157) [3,4]. EcO157 can cause life-threatening hemorrhagic colitis and in very severe cases causes hemolytic uremic syndrome [5]. Nineteen percent of all EcO157-associated outbreaks during 1998 to 2007 were due to the consumption of contaminated produce [6]. Pathogens attached to contaminated “ready to eat” produce are difficult to remove [7]. Therefore, prevention of pre-harvest contamination is critical. Thus, designing effective and inexpensive on-farm control strategies is essential.
Neem (Azardirachta indica) is a traditional and naturally available medicinal plant in India, South Africa, and Southeast Asia [8]. Almost every part of the neem tree has beneficial properties. Neem trees are grown extensively for their shade in India, for firewood in Ghana, and for reforestation in West Africa [9]. For centuries, neem twigs were used as teeth cleaning devices [9] as they are effective as antiplaque and anti-gingivitis agents [10] and thus some commercial herbal toothpastes contain neem as an active ingredient [11]. Water extracts of neem twigs inhibited growth of dental caries organisms Streptococcus mutans, S. salivarius, S. mitis, and S. sanguis [12]. Neem extracts have been reported to possess antibacterial, antifungal, antimalarial, and antiviral properties [10,13]. Neem leaves are used in India for curing diarrhea and cholera [14]. In addition, neem oil and leaves are used in popular medicine as antiparasitic, anti-inflammatory, antiulcer, antihyperglycemic, anticarcinogenic, and immunomodulatory agents [15,16]. Neem materials also affect more than 200 insect species as well as some mites and nematodes [9]. For example, an active ingredient from neem, azadirachtin, disrupts the metamorphosis of insect larvae and is thus used as a feeding deterrent [9]. Neem supplements and extracts inhibit many bacterial pathogens. Chloroform extracts of neem inhibited the growth of Listeria monocytogenes while ethanolic extracts showed higher inhibition for Staphylococcus aureus [17]. A water-soluble glycolipid, sulfonoquinovosyldiacylglyceride, isolated from the leaves of neem showed inhibitory activity against Salmonella typhi, Shigella dysenteriae, E. coli, and Vibrio cholerae [13]. Aquaneem, an emulsified product from neem kernels, inhibited pathogens of fish (Aeromonas hydrophila, Pseudomonas fluorescens, and E. coli) [18]. Extracts of neem cake, a waste byproduct of oil extraction, inhibited Campylobacter jejuni [19]. Extracts from neem leaves, seeds, and bark also act as nitrification inhibitors [20]. Thus, neem is proven to be effective against many bacterial pathogens including E. coli, but not against EcO157 according to an isolated study [17].
We evaluated the survival and fate of an outbreak related strain of EcO157 in manure supplemented with neem materials (leaf, bark, and oil). Manure was from a medium-sized dairy in the central valley of California. The extractable fractions of neem leaves were also evaluated for their influence on the growth of EcO157 in 300-µL microcosms of nutrient medium. Microcosms were designed to determine the nature of extracts responsible for pathogen inhibition by neem.
2. Experimental Section
2.1. Neem Materials and Extracts
Neem oil, powdered leaf, and bark were obtained from Neem Tree Farms (Brandon, FL, USA). An aqueous extract of neem was prepared by extracting 50 g leaf powder with 200 mL RO pure deionized water. Extraction was carried out by shaking for 1 h on a gyratory shaker and the aqueous supernatant was separated by centrifugation at 10,000× g for 10 min. The aqueous fraction was concentrated to 40 mL by rotary flash evaporation at 40 °C. The residual leaf paste was extracted by mixing for 1 h with 250 mL of 1:1 ethanol-ethyl acetate on a gyratory shaker. The organic extract was concentrated by flash evaporation and reconstituted in 25 mL ethanol. This extract from hereon will be called “ethyl acetate extract”. Five milliliters of the ethanolic extract were evaporated to dryness and reconstituted in 50 mL ethyl acetate and extracted with 100 mL of 1% sodium bicarbonate to remove the acidic components. The bicarbonate-washed ethyl acetate fraction was concentrated to dryness, reconstituted in 5 mL ethanol, and used in assays to determine the influence of neem extracts on EcO157. Dilutions of both “ethyl acetate extracts” were made in ethanol.
2.2. Influence of Neem Materials on the Survival of EcO157 in Dairy Manure
Dairy manure used in this study was collected from a medium-sized dairy in Oakdale, CA, USA [21,22]. Survival of a green-fluorescent-protein (GFP) labeled EcO157 strain, MM123, was monitored in triplicate 10 g manure samples supplemented with 0%, 0.5%, and 5% levels (on a weight basis) of leaf, bark, or oil. MM123 is a spontaneous rifampicin- (100 µg/mL) and nalidixic acid- (50 µg/mL) resistant mutant of GFP-labeled apple juice outbreak strain RM2315 (plasmid-born GFP; wild type: FDA strain SEA13B88) [23,24]. Double antibiotic resistance aids in discriminating MM123 from native organisms in manure [22]. Manure mixed thoroughly with neem materials was spread evenly in the bottom of 300 mL Erlenmeyer flasks. The manure had a pH of 6.9 and was moist and fluffy. The manure was inoculated with 2 mL of MM123 in 0.01 M phosphate-buffered saline (PBS, pH 7.4) containing 8.3 × 108 CFU and thoroughly mixed prior to incubation at 37 °C for 10 days. Overnight growth of MM123 in LB broth supplemented with 50 µg/mL kanamycin (to select for GFP) was centrifuged and resuspended in PBS prior to inoculations. GFP-labeled EcO157 cells were monitored at various intervals from 100 mg manure samples. One hundred-microliter portions of 10-fold serial dilutions of manure in PBS were plated on LB agar supplemented with 100 µg/mL rifampicin, 50 µg/mL nalidixic acid, and 50 µg/mL kanamycin and incubated overnight at 37 °C. The fluorescent colonies of MM123 were counted on a UV Transilluminator (Fotodyne, Hartland, WI). Days for one log reduction (D-value) of the pathogen in manure were calculated from linear regressions of log cell number decline over time.
2.3. Growth of EcO157 with Neem Extracts
Growth of EcO157 strain MM149 in half-strength LB broth supplemented with aqueous or organic extracts of neem was monitored in a Bioscreen C microbial growth curve analysis system (Growth Curves USA, Piscataway, NJ). MM149 was previously isolated from dairy manure and was chosen for its superior survival characteristics in dairy wastewaters [21,22]. Wells of Bioscreen plate contained 270 µL of Murashige and Skoog basal salts (Fisher Scientific, Fairlawn, NJ) with 50% LB broth at pH 7.0, 10 µL of neem extract, and 20 µL of the inoculum. Overnight growth of MM149 resuspended in 0.01M PBS was adjusted to an optical density of 0.6 at 600 nm prior to inoculation. The inoculated plates were constantly shaken at medium speed during incubation at 37 °C, and growth was monitored at 1-h intervals by an onboard spectrophotometer equipped with a wide-band filter (420 to 580 nm). The effect of neem extracts at full, 1/10th, and 1/100th strengths were compared with treatments containing 1, 10, 100, and 1000 µg/mL of azadirachtin. Treatments were not replicated. Inoculated wells containing 10 µL of ethanol serve as controls for solvent effects on the growth of MM149.
3. Results
3.1. Survival of EcO157 in Dairy Manure Supplemented with Neem Materials
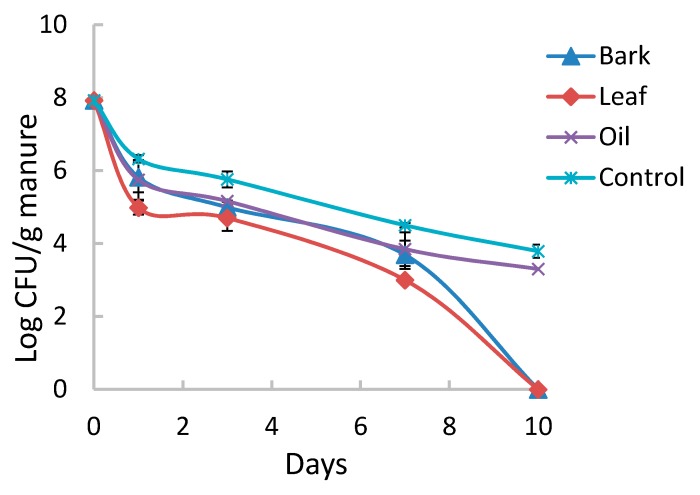
Mixing neem leaf at a concentration of 5% in dairy manure resulted in a 3 log reduction in numbers of MM123 within one day of incubation (Figure 1) and both leaf and bark supplements at this level eliminated the organism in <10 d. However, a 1.5 log reduction in EcO157 populations occurred in a day after inoculation of neem-free manure controls. D-value calculated based on a 10-day incubation with bark or leaf was 1.3 ± 0.0 d as compared to 2.4 ± 0.1 d for neem-free controls. Neem materials at a lower concentration of 0.5% were not effective in inhibiting EcO157. Neem oil did not cause any significant decreases in numbers of EcO157 compared to an untreated control.
Figure 1.
Growth of EcO157 in dairy manure supplemented with 5% neem materials.
3.2. Growth of EcO157 with Neem Extracts
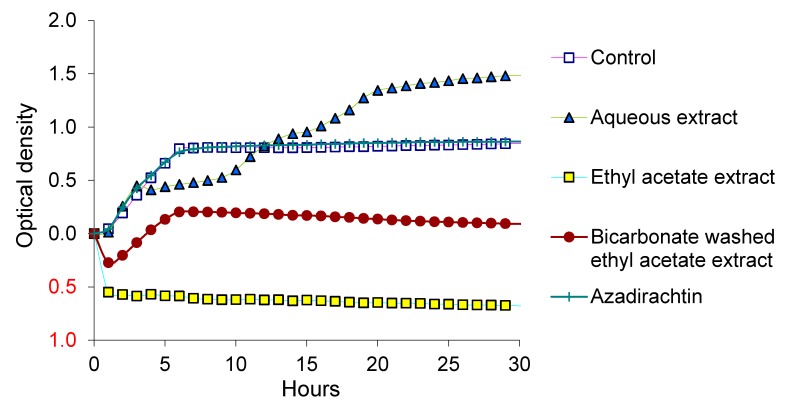
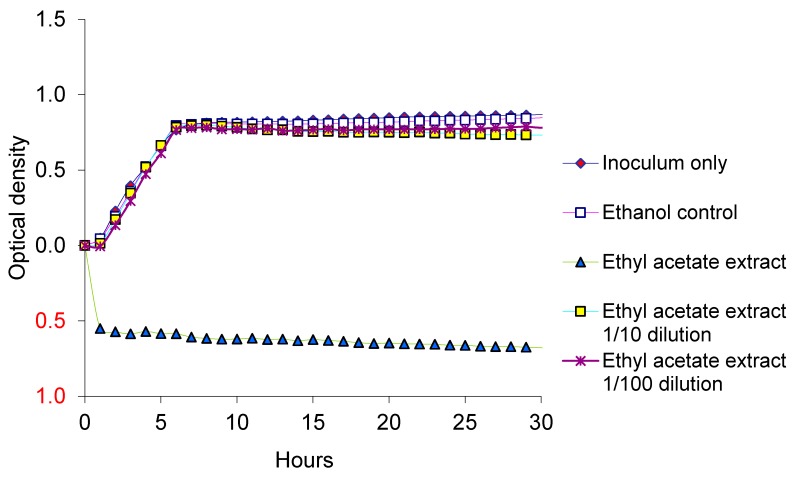
The inhibitory effects of neem leaf and bark encouraged us to determine the extractable component of neem responsible for the inhibition of the pathogen. Since both leaf and bark behaved similarly in decreasing the populations of EcO157, aqueous and ethyl acetate extracts of leaves (Table 1) were evaluated for the inhibition of dairy manure isolate MM149. Ethyl acetate extract applied at full strength (Table 1) inhibited the growth of MM149, whereas aqueous extract supported its growth (Figure 2). Some inhibition was observed also with full-strength bicarbonate-washed ethyl acetate extract. Extracts tested at 1/10th and 1/100th dilutions behaved similarly as controls (Figure 3). Ethanol at 10 µL per well did not enhance or inhibit the growth. Growth was not inhibited by azadirachtin even at the highest concentration of 1000 µg/mL (Figure 2).
Table 1.
Neem leaf extracts used in Bioscreen treatments.
| Extract | Leaf Equivalents/Well a, mg | Concentration/Well, % |
|---|---|---|
| Aqueous | 12.5 | 4.2 |
| Ethyl acetate | 20 | 6.7 |
| Bicarbonate washed ethyl acetate | 20 | 6.7 |
Notes: a Each Bioscreen C well received 10 µL of the extract. This concentration was considered as full-strength and two ten-fold dilutions were also tested.
Figure 2.
Growth of EcO157 in LB broth supplemented with full-strength aqueous or organic extracts of neem leaf. Optical densities at all sampling intervals were corrected for zero-time values. A control well contained 10 µL ethanol and 20 µL inoculum. Treatments of extracts at full strength (Table 1) were compared with azadirachtin at 1000 µg/mL.
Figure 3.
Growth of EcO157 in LB broth supplemented with ethyl acetate extracts of neem leaf. Optical densities at all sampling intervals were corrected for zero-time values.
Optical densities measured at various sampling intervals were corrected for zero time values ranging from 0.05 to 0.6 for different treatments. The highest optical density at zero time was obtained with the full strength ethyl acetate extract. Some precipitation was noticed in this treatment but not at lower concentrations or with water or bicarbonate-washed ethyl acetate extracts. Clumping was not observed in any treatments.
4. Discussion
EcO157 shed in feces of ruminants can survive for extended periods of time [25,26] and at times as long as 21 months in manure piles exposed to fluctuating environmental conditions [27]. In a recent study, we isolated a strain of EcO157 from dry produce field soil repeatedly during a 45-day period [26]. These observations indicate that some strains of EcO157 are very resilient and survive longer in austere environments and provide opportunities for the pathogen to be transported from farm to table. Intervention of pathogen transport from animal reservoirs to produce fields is essential. In this study, we explored the possibility of eliminating EcO157 from manure supplemented with neem materials known to have antimicrobial properties.
Neem leaf or bark eliminated the apple juice outbreak strain MM123 from dairy manure (Figure 1) in less than 10 days, whereas nearly 4 log CFU of the pathogen/g survived in manure without neem. A large effect of neem treatments did not become noticeable until after day 7 of the experiment. To our knowledge, this is the first report of neem supplements inhibiting the growth of EcO157 in manure. Although neem oil is used in popular medicines [16] and found to be bactericidal to E. coli [28], the oil is not effective against EcO157 in manure. However, extracts of waste byproducts (neem cake) after oil extraction inhibited C. jejuni, a foodborne pathogen associated with contaminated meat and poultry, in a broth model meat system [19]. Generic non-pathogenic E. coli were also inhibited in this model system, but it is not certain if the data can be extrapolated to pathogenic EcO157 or other shiga-toxigenic E. coli. Nonetheless, EcO157 in dairy manure was controlled by neem supplements.
Since neem supplements controlled EcO157 in manure, we explored the nature of the active ingredient. The solvent extractable organic fraction from neem leaves is effective in totally inhibiting the growth of EcO157 in LB broth. The removal of acidic organic components with bicarbonate also resulted in some inhibition of the pathogen. Thus, it appears that the inhibitory activity of neem leaves is inherent to the organic fraction extracted by ethyl acetate containing both basic and acidic components. In contrast, chloroform and ethanol extracts of neem were previously found to be not inhibitory to EcO157 but inhibited the growth of two other foodborne pathogens, L. monocytogenes and S. aureus [17]. Even though the organic extractable components are inhibitory to foodborne pathogens in this and a previous study, azadirachtin, a potent insect antifeedant [9] extracted from neem, was not found to be inhibitory to EcO157. In addition, we found that an aqueous extract of neem leaves enhanced the growth of EcO157 although water extracts of neem chewing sticks were found inhibitory to supra-gingival plaque organisms including generic E. coli [10]. However, in a different study, water extracts were not found to be inhibitory to multi-drug-resistant E. coli [29]. Thus, results from the current study are distinct in that pathogenic EcO157 are inhibited by supplements of neem leaf or bark and the active ingredient for inhibition is extractable by ethyl acetate.
Foodborne pathogens in manure can be controlled with inexpensive treatments such as composting and/or supplementation of neem materials. Furthermore, pathogen transfer from point sources such as dairies and feedlots can be minimized by maintaining a safe setback distance for produce cultivation [30]. Produce fields can also be treated with neem supplements to control the pathogen transfer from soil to plants, although field tests are warranted to determine the efficacy of neem. Neem supplements could be a viable option for foodborne pathogen control wherever neem is grown.
5. Conclusions
Foodborne pathogen contamination from “ready to eat” produce is difficult to remove. Treating with neem supplements could be an inexpensive way to prevent pre-harvest contamination via manure from nearby animal raising operations. Supplementation of neem leaf and bark to manure resulted in elimination of pathogenic EcO157 in less than 10 days. The active principle for inhibition of neem leaves is localized in the organic extractable fraction. Neem supplementation to manure piles on dairies and feedlots, and also to produce fields, could be a novel strategy for on-site pathogen control.
Acknowledgments
We thank Chester Sarreal for technical assistance. The work was funded by the U.S. Department of Agriculture, Agricultural Research Service CRIS project 5325-42000-046.
Author Contributions
Subbarao Ravva conceived and designed the experiments. Anna Korn performed the experiments. Both analyzed, wrote, edited, and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cole D.J., Hill V.R., Humenik F.J., Sobsey M.D. Health, safety, and environmental concerns of farm animal waste. Occup. Med. 1999;14:423–448. [PubMed] [Google Scholar]
- 2.McGarvey J.A., Miller W.G., Sanchez S., Stanker L. Identification of bacterial populations in dairy wastewaters by use of 16S rRNA gene sequences and other genetic markers. Appl. Environ. Microbiol. 2004;70:4267–4275. doi: 10.1128/AEM.70.7.4267-4275.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elder R.O., Keen J.E., Siragusa G.R., Barkocy-Gallagher G.A., Koohmaraie M., Laegreid W.W. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA. 2000;97:2999–3003. doi: 10.1073/pnas.97.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pell A.N. Manure and microbes: Public and animal health problem? J. Dairy Sci. 1997;80:2673–2681. doi: 10.3168/jds.S0022-0302(97)76227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen M.A., Casey T.A. Environmental and food safety aspects of Escherichia coli O157:H7 infections in cattle. Crit. Rev. Microbiol. 2001;27:57–73. doi: 10.1080/20014091096701. [DOI] [PubMed] [Google Scholar]
- 6.DeWaal C.S., Tian X.A., Plunkett D. Outbreak Alert! 2009. Center for Science in the Public Interest. [(accessed on 3 March 2015)]. Available online: http://cspinet.org/new/pdf/outbreakalertreport09.pdf.
- 7.Lynch M.F., Tauxe R.V., Hedberg C.W. The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiol. Infect. 2009;137:307–315. doi: 10.1017/S0950268808001969. [DOI] [PubMed] [Google Scholar]
- 8.Vimala K., Kanny K., Varaprasad K., Kumar N.M., Reddy G.S. Novel-porous-Ag(0) nanocomposite hydrogels via green process for advanced antibacterial applications. J. Biomed. Mater. Res. A. 2014;102:4616–4624. doi: 10.1002/jbm.a.35136. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council . Neem: A Tree for Solving Global Problems. National Academy Press; Washington, DC, USA: 1992. [PubMed] [Google Scholar]
- 10.Rao D.S., Penmatsa T., Kumar A.K., Reddy M.N., Gautam N.S., Gautam N.R. Antibacterial activity of aqueous extracts of Indian chewing sticks on dental plaque: An in vitro study. J. Pharm. Bioallied Sci. 2014;6:S140–S145. doi: 10.4103/0975-7406.137426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenner F., Jaleel V.A., Kulshrestha R., Maheswar G., Rao P.K., Kranthi J. Evaluating the antimicrobial activity of commercially available herbal toothpastes on microorganisms associated with diabetes mellitus. J. Contemp. Dent. Pract. 2013;14:924–929. doi: 10.5005/jp-journals-10024-1427. [DOI] [PubMed] [Google Scholar]
- 12.Chava V.R., Manjunath S.M., Rajanikanth A.V., Sridevi N. The efficacy of neem extract on four microorganisms responsible for causing dental caries viz Streptococcus mutans, Streptococcus salivarius, Streptococcus mitis and Streptococcus sanguis: An in vitro study. J. Contemp. Dent. Pract. 2012;13:769–772. doi: 10.5005/jp-journals-10024-1227. [DOI] [PubMed] [Google Scholar]
- 13.Bharitkar Y.P., Bathini S., Ojha D., Ghosh S., Mukherjee H., Kuotsu K., Chattopadhyay D., Mondal N.B. Antibacterial and antiviral evaluation of sulfonoquinovosyldiacylglyceride: A glycolipid isolated from Azadirachta indica leaves. Lett. Appl. Microbiol. 2014;58:184–189. doi: 10.1111/lam.12174. [DOI] [PubMed] [Google Scholar]
- 14.Thakurta P., Bhowmik P., Mukherjee S., Hajra T.K., Patra A., Bag P.K. Antibacterial, antisecretory and antihemorrhagic activity of Azadirachta indica used to treat cholera and diarrhea in India. J. Ethnopharmacol. 2007;111:607–612. doi: 10.1016/j.jep.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y.X., Cao M., Shi D.X., Yin Z.Q., Jia R.Y., Xu J., Wang C., Lv C., Liang X.X., He C.L., et al. Toxicological evaluation of neem (Azadirachta indica) oil: Acute and subacute toxicity. Environ. Toxicol. Pharmacol. 2013;35:240–246. doi: 10.1016/j.etap.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Subapriya R., Nagini S. Medicinal properties of neem leaves: A review. Curr. Med. Chem. Anticancer Agents. 2005;5:149–146. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- 17.Mahfuzul Hoque M.D., Bari M.L., Inatsu Y., Juneja V.K., Kawamoto S. Antibacterial activity of guava (Psidium guajava L.) and neem (Azadirachta indica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodborne Pathog. Dis. 2007;4:481–488. doi: 10.1089/fpd.2007.0040. [DOI] [PubMed] [Google Scholar]
- 18.Das B.K., Mukherjee S.C., Sahu B.B., Murjani G. Neem (Azadirachta indica) extract as an antibacterial agent against fish pathogenic bacteria. Indian J. Exp. Biol. 1999;37:1097–1100. [PubMed] [Google Scholar]
- 19.Del Serrone P., Nicoletti M. Antimicrobial activity of a neem cake extract in a broth model meat system. Int. J. Environ. Res. Public Health. 2013;10:3282–3295. doi: 10.3390/ijerph10083282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbasi M.K., Hina M., Tahir M.M. Effect of Azadirachta indica (neem), sodium thiosulphate and calcium chloride on changes in nitrogen transformations and inhibition of nitrification in soil incubated under laboratory conditions. Chemosphere. 2011;82:1629–1635. doi: 10.1016/j.chemosphere.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Ravva S.V., Korn A. Extractable organic components and nutrients in wastewater from dairy lagoons influence the growth and survival of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007;73:2191–2198. doi: 10.1128/AEM.02213-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravva S.V., Sarreal C.Z., Duffy B., Stanker L.H. Survival of Escherichia coli O157:H7 in wastewater from dairy lagoons. J. Appl. Microbiol. 2006;101:891–902. doi: 10.1111/j.1365-2672.2006.02956.x. [DOI] [PubMed] [Google Scholar]
- 23.Ravva S.V., Sarreal C.Z., Mandrell R.E. Altered protozoan and bacterial communities and survival of Escherichia coli O157:H7 in monensin-treated wastewater from a dairy lagoon. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0054782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravva S.V., Sarreal C.Z., Mandrell R.E. Identification of protozoa in dairy lagoon wastewater that consume Escherichia coli O157:H7 preferentially. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0015671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandrell R.E. Enteric human pathogens associated with fresh produce: Sources, transport and ecology. In: Fan X., Niemira B.A., Doona C.J., Feeherry F., Gravani R.B., editors. Microbial Safety of Fresh Produce: Challenges, Perspectives and Strategies. IFT/Blackwell Publishing; Oxford, UK: 2009. pp. 3–42. [Google Scholar]
- 26.Ravva S.V., Cooley M.B., Sarreal C.Z., Mandrell R.E. Fitness of outbreak and environmental strains of Escherichia coli O157:H7 in aerosolizable soil and association of clonal variation in stress gene regulation. Pathogens. 2014;3:528–548. doi: 10.3390/pathogens3030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudva I.T., Blanch K., Hovde C.J. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 1998;64:3166–3174. doi: 10.1128/aem.64.9.3166-3174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain D., Jayaram L., Prabhu V.M., Bhat G.K. Antibacterial effect of neem (Azadirachta indica) oil on multidrug resistant bacteria isolated from human infections. Int. J. Biol. Med. Res. 2013;4:3544–3546. [Google Scholar]
- 29.Dahiya P., Purkayastha S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J. Pharm. Sci. 2012;74:443–450. doi: 10.4103/0250-474X.108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry E.D., Wells J.E., Bono J.L., Woodbury B.L., Kalchayanand N., Norman K.N., Suslow T.V., Lopez-Velasco G., Millner P.D. Effect of proximity to a cattle feedlot on Escherichia coli O157:H7 contamination of leafy greens and evaluation of the potential for airborne transmission. Appl. Environ. Microbiol. 2015;81:1101–1110. doi: 10.1128/AEM.02998-14. [DOI] [PMC free article] [PubMed] [Google Scholar]