Abstract
Background: Cardiovascular disease (CVD) is the leading cause of mortality and morbidity in the elderly and the ambient concentration of PM2.5 has been associated with several cardiovascular diseases. Methods: We describe the present state of planetary air pollution, analyze epidemiological studies linking PM2.5 and CVD, and discuss multiple pathophysiological mechanisms linking PM2.5 and CVD. Results: A few epidemiological studies show that the elderly appear specifically susceptible to adverse cardiovascular effects triggered by PM2.5 exposure. Plausible pathophysiological mechanisms include inflammatory dysfunction, oxidative stress, abnormal activation of the hemostatic system and disturbance of the autonomic nervous system. Conclusions: An in-depth knowledge of the chemical compounds, pathophysiological mechanisms, and epidemiological studies of PM2.5 are recommended to understand this important and modifiable factor contributing to geriatric CVD burden. We offer public health recommendations to reduce this preventable cause of disease and death.
Keywords: PM2.5, Cardiovascular disease (CVD), the elderly, susceptibility
1. Introduction
Cardiovascular diseases (CVD) include disorders of the heart (arrhythmias, coronary vessel and vascular disease, heart failure) and blood vessels (peripheral arterial diseases and venous thrombosis), particular those supplying the brain (ischemic and hemorrhagic stroke). Together, these disorders constitute the leading cause of death across the globe, with low- and middle-income countries most heavily affected. According to the World Health Organization (WHO), each year about 17.3 million people die of cardiovascular disease, accounting for 30% of all deaths [1]. In addition to genetic and behavioral risk factors (unhealthy diet, physical inactivity, tobacco, and alcohol use), the inhalation of air containing fine particulate matter (particle size less than or equal to 2.5 μm) is associated with CVD. Some researchers including Brook [2] have proven that PM2.5 is a modifiable exposure factor that contributes to cardiovascular morbidity and mortality. Elderly people have the highest rates of CVD and thus are the most susceptible population [3]. We discuss plausible PM2.5-related pathophysiological mechanisms of CVD and epidemiological studies linking PM2.5 and CVD, especially in susceptible people, to make recommendations for future public health and reduce this avoidable cause of disease and death.
2. Review of PM2.5
Atmospheric particulate matter (PM) is classified into four categories according to aerodynamic diameter: Total suspended particulate (TSP ≤ 100 μm); particulate matter (≤10 μm); fine particulate matter (≤2.5 μm), and ultrafine particles (≤0.1 μm). PM2.5 refers to atmospheric particles with diameters less than or equal to 2.5 micrometers. The influence of PM on humans depends on particle size, which is linked with its aerodynamic diameter (AD) (Table 1). The range of most PM10 particles is from 2.5 μm to 10 μm while PM2.5 and PM0.1 have ADs ≤ 2.5 μm and ≤0.1 μm. PM2.5 and PM0.1 particles may permeate the lung alveoli and enter into the bloodstream, and then cause adverse health effects [4,5,6]. Compared with PM10, PM2.5 has a small particle size, light quality and a relatively large specific surface area [7]. The smaller particle size may pose a higher risk for systemic cardiovascular effects [8].
Table 1.
Influences of particle size on human health.
| Particulate | Particle Size (≤μm) | Influences on Human Health |
|---|---|---|
| PM100 | 100 | Persist in the air and no evidence of adverse effects on human health |
| PM10 | 10 | Enter the respiratory system, deposit in the respiratory tract and cause respiratory diseases |
| PM2.5 | 2.5 | Get into the alveoli through the respiratory tract and then enter into the blood circulation, causing various diseases. |
| PM0.1 | 0.1 |
The major sources of particulate matter are broadly divided into two parts, human activities and natural phenomena, including wildfires, volcanoes, and land dust [9]. In addition, chemical reactions of primary emissions in the atmosphere cause the formation of secondary pollutants and the composition varies according to the pollution source [9,10,11].
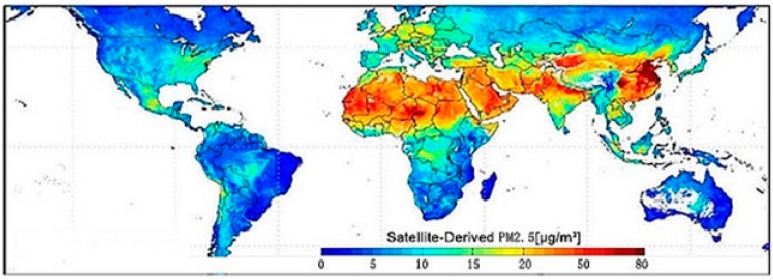
We may recall the Great Smog event in London in December 1952, when severe air pollution from domestic and industrial coal burning caused thousands of excess deaths, especially among the very young and elderly, and led to the passage in the UK of the 1956 Clean Air Act [12,13]. Nowadays, PM2.5 is considered the main culprit for the adverse effects of air pollution on human health [2]. PM2.5 pollution affects the whole planet, particularly densely populated metropolitan areas of eastern and southern China, northern India, and the emerging countries of South-East Asia. Parts of Europe and America are not spared as well (Figure 1). The concentration of PM2.5 in the area mentioned above may even exceed 100 μg/m3 [14]. By contrast, in 2012, the revised annual arithmetic mean in the USA was 12 μg/m3 for primary PM2.5 and 15 μg/m3 for secondary PM2.5, with a 24-hour 98th percentile value of 35 μg/m3 for both [15].
Figure 1.
The global distribution of PM2.5 averaged over 2001–2006 (Credit: Dalhousie University, Aaron van Donkelaar, http://www.nasa.gov/topics/earth/features/healthappoing.html).
3. Plausible Pathophysiological Mechanisms Linking PM2.5 Exposure and CVD
Several studies suggest that the elderly are especially susceptible to the harmful effects of PM exposure [2]. Older adults also represent a potentially susceptible population compared with children or younger adults because of the higher prevalence of preexisting cardiovascular and respiratory diseases. Epidemiological studies assessing the relationship between air pollution and CVD have appeared in the past 20 years [16]. High concentrations of PM2.5 have been associated with morbidity and mortality in both short- and long-term epidemiological studies [17].
Air particulate matter enters the human body mainly in two ways: (1) PM2.5 enters the respiratory system causing pulmonary and systemic inflammation, oxidative stress, affecting the coagulation system, changing autonomic nerve function, injuring the vascular endothelium and affecting vasomotor function; (2) Part of the particulate matter causes the above reaction through other routes, such as entering into the circulatory system via the digestive tract. Animal studies have shown that PM2.5 can be devoured by macrophages and endothelial cells, indicating that air pollution may have direct effects on the vascular system [4,5,6,18].
The relationship between air pollution and respiratory diseases, such as asthma and chronic obstructive pulmonary disease, is well established. Nevertheless, the relationship between air pollution and CVD remains unclear. The plausible pathophysiological mechanisms can be divided into the following several aspects [19].
3.1. Oxidative Stress and Inflammation
Both animal and human studies [20,21,22,23,24] have shown that inhaled particles may cause inflammation of the respiratory tract; in particular, PM2.5 inhalation can lead to the occurrence of systemic inflammation, increasing the risk of cardiovascular stress. PM2.5 can enter the alveolar epithelium, cause local inflammation and oxidative stress, resulting in the release from lung cells into bronchial fluid and the blood stream of several inflammatory mediators, such as IL-6, IL-8, TNF-α and interferon-γ [25]. These spread to the general circulation where they can plausibly modulate systemic effects. When mice are exposed to PM2.5 for six hours, there is increased expression of inflammation-related genes such as mRNA of TNF-α, TNF-β and IL-6, IL-8. A recent study finds that IL-6 can make a rapid response to air pollution and increase the production of C-reactive protein (CRP) [26]. CRP is the most important protein in acute phase reactants (APR), and it is also a sensitive indicator of inflammation associated with the risk of cardiovascular disease. Blood CRP increases in proportion to the concentration of PM2.5; for every increase of 100 μg/m3, blood CRP increases 8.1 mg/L [27]. Interestingly, coarse PM has been suggested to directly trigger inflammation by binding to toll-like receptor (TLR) 2 and 4 [28]. Higher TLR2 methylation may confer susceptibility to adverse cardiac autonomic effects of PM2.5 exposure in older individuals [29].
3.2. Abnormal Activation of the Haemostatic System
Another probably detrimental effect of PM2.5 exposure is an abnormal activation of the hemostatic system. Epidemiological studies have associated PM2.5 inhalation with venous thrombosis and a shorter prothrombin plasma-clotting time. The diffuse pulmonary inflammation caused by PM2.5 may spread to the circulatory system, causing abnormal activation of the hemostatic system [30]. Animal models and in vitro cell studies show that PM2.5 increases fibrinogen and tissue factors [31], and fine particulate matter in blood vessels can directly activate platelets. Platelets play an important role in thrombus formation and can make the blood hypercoagulable states. PM2.5 exposure may increase the risk of acute thrombosis, as in myocardial infarction and ischemic stroke. At the same time, fibrinogen is also an important risk factor for stroke [32].
3.3. Disturbance of Autonomic Nervous System
PM particles disturb the autonomic nervous system (ANS) [33]. Under normal circumstances, the rhythmic activity of the heart is controlled by the activity of autorhythmic cells in the sinoatrial node, which is regulated by the vagus nerve. Acute exposure to PM2.5 can stimulate the ANS and increase the risk of arrhythmia and acute cardiovascular events, with serious impact on the elderly [34]. A number of studies in a recent meta-analysis support an inverse association between PM exposure and heart rate variability [35]. Heart rate variability (HRV) refers to the cyclical changes of sinus rhythm, and it is an important index of tonicity and sympathetic-parasympathetic balance. Some research finds that exposure of PM2.5 is linked with HRV change in the elderly. Compared with exposure to clean air for two hours, HRV drops 35.7% after inhalation of 21.2~80.3 μg/m³ PM2.5 [36]. Moreover, in a 48-hour moving average, PM2.5 was found to have a strong effect on the decrement in HRV [37]. Additionally, the effects of PM2.5 on subjects with hypertension were larger than on the subjects without hypertension [38]. The decrease of HRV reflects direct perturbation of the cardiac autonomic nervous system, and may serve as a prelude to heart disease.
3.4. Injury of Endothelial Cells
Because vascular endothelial injury is an important pathological basis of cardiovascular disease, the damage of vascular endothelial cells by PM2.5 is one possible CVD mechanism. Cardiovascular endothelial cells treated in vitro with PM2.5 for 24 hours display adhesion molecules and apoptosis [39]. Cell mortality rates increase with PM2.5, suggesting that PM2.5 may damage the vascular endothelium, thereby causing CVDs. Recently, some studies suggest that specific metals may be important components responsible for PM2.5-induced cardiovascular effects and that the reduced capacity of endothelial repair may play a critical role [40].
4. Epidemiological Studies Linking PM2.5 and CVD in the Elderly
Though several studies have shown exposure to PM2.5 can increase risk of CVD [2,19,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57], few studies focused on the elderly, one of susceptible subpopulations, Kan et al. found that risk of CVD exposed to PM10 increased 0.26%, compared with people 5–44 years of age or 45–64 years of age[58,59]. Another Chinese studies in Beijing showed that ambient PM2.5 adversely affected cardiac autonomic function of the elderly people with heart diseases [38]. Dominici and colleagues analyzed acute effect of fine particulate air pollution on elderly people (age > 65 years) and found that the largest association between PM10 and congestive heart failure, a 0.72% increase in risk per 10 μg/m3 elevation in same-day PM10 concentration [49]. This is more than the results reported by Brook with an increasing of 0.18% in the general population [2]. However, annual average exposure to higher levels of black carbon (per 0.26 μg/m3 elevation), a marker of traffic-related PM, was associated with a 1.1% increase in carotid intima media thickness (CIMT) in a cohort of elderly men living in the Boston area [60]. Short-term PM exposure has been more strongly associated with cardiac mortality among older individuals [61]. In an elderly population (aged 65 and older) across Eastern USA, researchers found that both short-term and long-term exposure were significantly associated with risk of deep vein thrombosis (DVT) [62]. While the majority of studies and expert-consensus opinions consistently agree that PM2.5 can increase the risk of CVD, it is noteworthy that some studies had not find relationships between PM2.5 and risk of CVD [63,64,65,66].
5. Conclusions
From a point of pathophysiology, the elderly is a susceptible population to cardiovascular injury by PM2.5 and a few epidemiological studies showed more risk of CVD increased in the elderly exposed to the same levels of PM2.5. Due to increasing of PM2.5 level and aged populations, more attention should be paid to CVD risk and PM2.5 exposure in the elderly. In addition, the relationship between PM2.5 concentration and CVD risk appears not to have a “safe” threshold. The “alarm” threshold periodically claimed by regulatory agencies during certain seasons should therefore be considered advisory, and efforts should be made to keep level of pollutants as low as possible [2]. Thus, besides of reducing air pollution, avoidance of exposure to PM2.5 pollution and societal and personal measures to reduce other risk factors should be recommended for the elderly [67,68]:
Walking or cycling in parks or country roads instead of busy streets during rush hours.
Using more public transport rather than private cars and motorbikes.
Avoiding outdoor exercise at times of high PM2.5 (especially those with cardiorespiratory disorders).
Using an air purifier to optimize indoor air quality.
Choosing appropriate respirators that fit snugly on the face if air pollution is severe.
Reducing other known cardiovascular risk factors, such as smoking and alcohol.
Warranting more aggressive use of primary and secondary preventive therapies, including antiplatelet agents, lipid-lowering agents, and treatments for hypertension or diabetes.
Acknowledgments
The authors gratefully acknowledge Peter Spencer at Global Health Center, Oregon Health & Science University and James J. Collins at Saginaw Valley State University for their helpful discussion and critical comments.
Author Contributions
Chenchen Wang and Yifan Tu drafted the manuscript. Chenchen Wang and Yifan Tu provided key input in the literature search and contributed equally to the article. As the mentor, Rongzhu Lu oversaw idea formation, editing and coordination. Zongliang Yu reviewed and edited the manuscript focusing on assessment of clinical data. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.World Health Organization Cardiovascular Diseases (CVDs). Fact Sheet No. 317, March 2013. [(accessed on 29 November 2014)]. Available online: http://www.heart.org/idc/groups/heartpublic/@wcm/@sop/@smd/documents/downloadable/ucm_319574.pdf.
- 2.Brook R.D., Rajagopalan S., Pope C.A., 3rd, Brook J.R., Bhatnagar A., Diez-Roux A.V., Holguin F., Hong Y., Luepker R.V., Mittleman M.A., et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 3.Sacks J.D., Stanek L.W., Luben T.J., Johns D.O., Buckley B.J., Brown J.S., Ross M. Particulate matter-induced health effects: Who is susceptible? Environ. Health Perspect. 2011;119:446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franck U., Odeh S., Wiedensohler A., Wehner B., Herbarth O. The effect of particle size on cardiovascular disorders—The smaller the worse. Sci. Total Environ. 2011;409:4217–4221. doi: 10.1016/j.scitotenv.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 5.Valavanidis A., Fiotakis K., Vlachogianni T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 6.Mills N.L., Amin N., Robinson S.D., Anand A., Davies J., Patel D., de la Fuente J.M., Cassee F.R., Boon N.A., Macnee W., et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am. J. Respir. Crit. Care Med. 2006;173:426–431. doi: 10.1164/rccm.200506-865OC. [DOI] [PubMed] [Google Scholar]
- 7.Gilar M., Belenky A., Wang B.H. High-throughput biopolymer desalting bysolid-phase extraction prior to mass spectrometric analysis. J. Chromatogr. A. 2001;921:3–13. doi: 10.1016/S0021-9673(01)00833-0. [DOI] [PubMed] [Google Scholar]
- 8.Araujo J.A., Nel A.E. Particulate matter and atherosclerosis: Role of particle size, composition and oxidative stress. Par. Fibre Toxicol. 2009;6 doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee B.J., Kim B., Lee K. Air pollution exposure and cardiovascular disease. Toxicol. Res. 2014;30:71–75. doi: 10.5487/TR.2014.30.2.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamble J.F. PM2.5 and mortality in long-term prospective cohort studies: Cause-effect or statistical associations? Environ. Health Perspect. 1998;106:535–549. doi: 10.1289/ehp.98106535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunekreef B., Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur. Respir. J. 2005;26:309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- 12.Wilkins E.T. Air pollution and the London fog of December, 1952. J. R. Sanit. Inst. 1954;74:1–21. [PubMed] [Google Scholar]
- 13.Logan W.P. Mortality from fog in London. BMJ. 1956;1:722–725. doi: 10.1136/bmj.1.4969.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W., Tang D.G., Liu O.J., Yue X., Pan Z., Ding Y. Research on current pollution status and pollution characteristics of PM2.5 in China. Res. Environ. Sci. 2000;13:1–5. (Huan Jing Ke Xue Yan Jiu) (In Chinese) [Google Scholar]
- 15.EPA National Ambient Air Quality Standards (NAAQS) [(accessed on 16 February 2014)];2012 Available online: http://www.epa.gov/air/criteria.html.
- 16.Franchini M., Mannucci P.M. Air pollution and cardiovascular disease. Thromb. Res. 2012;129:230–234. doi: 10.1016/j.thromres.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Franchini M., Mannucci P.M. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood. 2011;118:2405–2412. doi: 10.1182/blood-2011-04-343111. [DOI] [PubMed] [Google Scholar]
- 18.Nemmar A., Vanbilloen H., Hoylaerts M.F., Hoet P.H., Verbruggen A., Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001;164:1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- 19.Brook R.D., Franklin B., Cascio W., Hong Y., Howard G., Lipsett M., Luepker R., Mittleman M., Samet J., Smith S.C., Jr., Tager I. Expert Panel on Population and Prevention Science of the American Heart Association. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 20.Quay J.L., Reed W., Samet J., Devlin R.B. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-kappa B activation. Am. J. Respir. Cell Mol. Biol. 1998;19:98–106. doi: 10.1165/ajrcmb.19.1.3132. [DOI] [PubMed] [Google Scholar]
- 21.Veronesi B., Oortgiesen M., Carter J.D., Devlin R.B. Particulate matter initiates inflammatory cytokine release by activation of capsaicin and acid receptors in a human bronchial epithelial cell line. Toxicol. Appl. Pharmacol. 1999;154:106–115. doi: 10.1006/taap.1998.8567. [DOI] [PubMed] [Google Scholar]
- 22.van Eeden S.F., Tan W.C., Suwa T., Mukae H., Terashima T., Fujii T., Qui D., Vincent R., Hogg J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10) Am. J. Respir. Crit. Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 23.Hartz A.M., Bauer B., Block M.L., Hong J.S., Miller D.S. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008;22:2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Törnqvist H., Mills N.L., Gonzalez M., Miller M.R., Robinson S.D., Megson I.L., Macnee W., Donaldson K., Söderberg S., Newby D.E., et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am. J. Respir. Crit. Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- 25.Martinelli N., Olivieri O., Girelli D. Air particulate matter and cardiovascular disease: A narrative review. Eur. J. Intern. Med. 2013;24:295–302. doi: 10.1016/j.ejim.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Rückerl R., Greven S., Ljungman P., Aalto P., Antoniades C., Bellander T., Berglind N., Chrysohoou C., Forastiere F., Jacquemin B., et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ. Health Perspect. 2007;115:1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pope C.A., 3rd, Hansen M.L., Long R.W., Nielsen K.R., Eatough N.L., Wilson W.E., Eatough D.J. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ. Health Perspect. 2004;112:339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker S., Dailey L., Soukup J.M., Silbajoris R., Devlin R.B. TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol. Appl. Pharmacol. 2005;203:45–52. doi: 10.1016/j.taap.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Zhong J., Colicino E., Lin X., Mehta A., Kloog I., Zanobetti A., Byun H.M., Bind M.A., Cantone L., Prada D., et al. Cardiac autonomic dysfunction: Particulate air pollution effects are modulated by epigenetic immunoregulation of Toll-like Receptor 2 and dietary flavonoid Intake. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seaton A., MacNee W., Donaldson K., Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345:176–178. doi: 10.1016/S0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 31.Bonzini M., Tripodi A., Artoni A., Tarantini L., Marinelli B., Bertazzi P.A., Apostoli P., Baccarelli A. Effects of inhalable particulate matter on blood coagulation. J. ThrombHaemost. 2010;8:662–668. doi: 10.1111/j.1538-7836.2009.03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters A., Fröhlich M., Döring A., Immervoll T., Wichmann H.E., Hutchinson W.L., Pepys M.B., Koenig W. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur. Heart J. 2001;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- 33.Rhoden C.R., Wellenius G.A., Ghelfi E., Lawrence J., González-Flecha B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochim. Biophys. Acta. 2005;1725:305–313. doi: 10.1016/j.bbagen.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Liao D., Duan Y., Whitsel E.A., Zheng Z.J., Heiss G., Chinchilli V.M., Lin H.M. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: A population-based study. Am. J. Epidemiol. 2004;159:768–777. doi: 10.1093/aje/kwh109. [DOI] [PubMed] [Google Scholar]
- 35.Pieters N., Plusquin M., Cox B., Kicinski M., Vangronsveld J., Nawrot T.S. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: A meta-analysis. Heart. 2012;98:1127–1135. doi: 10.1136/heartjnl-2011-301505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devlin R.B., Ghio A.J., Kehrl H., Sanders G., Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur. Respir. J. 2003;40:76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- 37.Montiel-Dávalos A., Ibarra-Sánchez Mde J., Ventura-Gallegos J.L., Alfaro-Moreno E., López-Marure R. Oxidative stress and apoptosis are induced in human endothelial cells exposed to urban particulate matter. Toxicol. In Vitro. 2010;24:135–141. doi: 10.1016/j.tiv.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Xu M.M., Jia Y.P., Li G.X., Liu L.Q., Mo Y.Z., Jin X.B., Pan X.C. Relationship between ambient fine particles and ventricular repolarization changes and heart rate variability of elderly people with heart disease in Beijing, China. Biomed. Environ. Sci. 2013;26:629–637. doi: 10.3967/0895-3988.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Park S.K., O’Neill M.S., Vokonas P.S., Sparrow D., Schwartz J. Effects of air pollution on heart rate variability: The VA normative aging study. Environ. Health Perspect. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niu J., Liberda E.N., Qu S., Guo X., Li X., Zhang J., Meng J., Yan B., Li N., Zhong M., et al. The role of metal components in the cardiovascular effects of PM2.5. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lippmann M., Ito K., Hwang J.S., Maciejczyk P., Chen L.C. Cardiovascular effects of nickel in ambient air. Environ. Health Perspect. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell M.L., Ebisu K., Peng R.D., Samet J.M., Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am. J. Respir. Crit. Care Med. 2009;179:1115–1120. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dominici F., Peng R.D., Barr C.D., Bell M.L. Protecting human health from air pollution: Shifting from a single-pollutant to a multipollutant approach. Epidemiology. 2010;21:187–194. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brook R.D. Cardiovascular effects of air pollution. Clin. Sci. (Lond) 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- 45.Lifetips Who’s at Risk for Heart Disease? Heart Disease Tips. 2015. [(accessed on 16 February 2015)]. Available online: http://heartdisease.lifetips.com//cat/63924/who-s-at-risk-for-heart-disease/index.html.
- 46.Johnston F.H., Hanigan I.C., Henderson S.B., Morgan G.G. Evaluation of interventions to reduce air pollution from biomass smoke on mortality in Launceston, Australia: Retrospective analysis of daily mortality, 1994–2007. BMJ. 2013;346 doi: 10.1136/bmj.e8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kunzli N., Jerrett M., Garcia-Esteban R., Basagana X., Beckermann B., Gilliland F., Medina M., Peters J., Hodis H.N., Mack W.J. Ambient air pollution and the progression of atherosclerosis in adults. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann B., Moebus S., Mohlenkamp S., Stang A., Lehmann N., Dragano N., Schmermund A., Memmesheimer M., Mann K., Erbel R., Jockel K.H., Heinz Nixdorf Recall Study Investigative Group Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- 49.Dominici F., Peng R.D., Bell M.L., Pham L., McDermott A., Zeger S.L., Samet J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dockery D.W., Pope C.A., 3rd, Xu X., Spengler J.D., Ware J.H., Fay M.E., Ferris B.G., Jr., Speizer F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 51.Qiu H., Yu I.T., Wang X., Tian L., Tse L.A., Wong T.W. Differential effects of fine and coarse particles on daily emergency cardiovascular hospitalizations in Hong Kong. Atmos. Environ. 2013;64:296–302. doi: 10.1016/j.atmosenv.2012.09.060. [DOI] [Google Scholar]
- 52.Cruz A.M., Sarmento S., Almeida S.M., Silva A.V., Alves C., Freitas M.C., Wolterbeek H. Association between atmospheric pollutants and hospital admissions in Lisbon. Environ. Sci. Pollut. Res. Int. 2015;22:5500–5510. doi: 10.1007/s11356-014-3838-z. [DOI] [PubMed] [Google Scholar]
- 53.Zhou M., He G., Fan M., Wang Z., Liu Y., Ma J., Ma Z., Liu J., Liu Y., Wang L., Liu Y. Smog episodes, fine particulate pollution and mortality in China. Environ. Res. 2015;136:396–404. doi: 10.1016/j.envres.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 54.Siponen T., Yli-Tuomi T., Aurela M., Dufva H., Hillamo R., Hirvonen M.R., Huttunen K., Pekkanen J., Pennanen A., Salonen I., et al. Source-specific fine particulate air pollution and systemic inflammation in ischaemic heart disease patients. Occup. Environ. Med. 2015;72:277–283. doi: 10.1136/oemed-2014-102240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cesaroni G., Forastiere F., Stafoggia M., Andersen Z.J., Badaloni C., Beelen R., Caracciolo B., de Faire U., Erbel R., Eriksen K.T., et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348 doi: 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai S.S., Chang C.C., Liou S.H., Yang C.Y. The effects of fine particulate air pollution on daily mortality: A case-crossover study in a subtropical city, Taipei, Taiwan. Int. J. Environ. Res. Public Health. 2014;11:5081–5093. doi: 10.3390/ijerph110505081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wellenius G.A., Schwartz J., Mittleman M.A. Particulate air pollution and hospital admissions for congestive heart failure in seven United States cities. Am. J. Cardiol. 2006;97:404–408. doi: 10.1016/j.amjcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 58.Kan H., Chen B., Zhao N., London S.J., Song G., Chen G., Zhang Y., Jiang L., HEI Health Review Committee Part 1 A time-series study of ambient air pollution and daily mortality in Shanghai, China. Res. Rep. Health Eff. Inst. 2010;154:17–78. [PubMed] [Google Scholar]
- 59.Kan H., London S.J., Chen G., Zhang Y., Song G., Zhao N., Jiang L., Chen B. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: The Public Health and Air Pollution in Asia (PAPA) Study. Environ. Health Perspect. 2008;116:1183–1188. doi: 10.1289/ehp.10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilker E.H., Mittleman M.A., Coull B.A., Gryparis A., Bots M.L., Schwartz J., Sparrow D. Long-term exposure to black carbon and carotid intima-media thickness: The normative aging study. Environ. Health Perspect. 2013;121:1061–1067. doi: 10.1289/ehp.1104845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan J., Ishikawa N., Sheppard L., Siscovick D., Checkoway H., Kaufman J. Exposure to ambient fine particulate matter and primary cardiac arrest among persons with and without clinically recognized heart disease. Am. J. Epidemiol. 2003;157:501–509. doi: 10.1093/aje/kwg015. [DOI] [PubMed] [Google Scholar]
- 62.Kloog I., Zanobetti A., Nordio F., Coull B.A., Baccarelli A.A., Schwartz J. Effects of airborne fine particles (PM 2.5) on deep vein thrombosis admissions in the northeastern United States. J. Thromb. Haemost. 2015;13:768–774. doi: 10.1111/jth.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barclay J.L., Miller B.G., Dick S., Dennekamp M., Ford I., Hillis G.S., Ayres J.G., Seaton A. A panel study of air pollution in subjects with heart failure: Negative results in treated patients. Occup. Environ. Med. 2009;66:325–334. doi: 10.1136/oem.2008.039032. [DOI] [PubMed] [Google Scholar]
- 64.O’Donnell M.J., Fang J., Mittleman M.A., Kapral M.K., Wellenius G.A. Investigators of the Registry of Canadian Stroke Network. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology. 2011;22:422–431. doi: 10.1097/EDE.0b013e3182126580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willocks L.J., Bhaskar A., Ramsay C.N., Lee D., Brewster D.H., Fischbacher C.M., Chalmers J., Morris G., Scott E.M. Cardiovascular disease and air pollution in Scotland: No association or insufficient data and study design. BMC Public Health. 2012;12 doi: 10.1186/1471-2458-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang M., Beelen R., Stafoggia M., Raaschou-Nielsen O., Andersen Z.J., Hoffmann B., Fischer P., Houthuijs D., Nieuwenhuijsen M., Weinmayr G., et al. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: Results from the ESCAPE and TRANSPHORM projects. Environ. Int. 2014;66:97–106. doi: 10.1016/j.envint.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 67.Newby D.E., Mannucci P.M., Tell G.S., Baccarelli A.A., Brook R.D., Donaldson K., Forastiere F., Franchini M., Franco O.H., Graham I., et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015;36:83–93. doi: 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartog J.J., Boogaard H., Nijland H., Hoek G. Do the health benefits of cycling outweigh the risks? Cien. Saude Colet. 2011;16:4731–4744. doi: 10.1590/S1413-81232011001300022. [DOI] [PubMed] [Google Scholar]