Abstract
Although scleroderma-associated interstitial lung disease (SSc-ILD) is a significant contributor to both morbidity and mortality, its pathogenesis is largely unclear. Pulmonary function tests (PFTs) and high resolution CT (HRCT) scanning continue to be the most effective tools to screen for lung involvement and to monitor for disease progression. More research and better biomarkers are needed to identify patients most at risk for developing SSc-ILD as well as to recognize which of these patients will progress to more severe disease. While immunosuppression remains the mainstay of treatment, anti-fibrotic agents may offer new avenues of treatment for patients with SSc-ILD in the future.
Keywords: Interstitial lung disease (ILD), Systemic Sclerosis (SSc), Cyclophosphamide, Rituximab, Stem Cell Transplantation
Introduction
In the past 35 years, since the introduction of angiotensin converting enzyme (ACE) inhibitor therapy as the first effective treatment for scleroderma renal crisis, scleroderma-associated interstitial lung disease (SSc-ILD) has become the leading SSc-related cause of death.1 In the largest study to date, SSc-ILD accounted for 35% of all disease-related deaths.2 Thus, the management of patients with SSc-ILD is of paramount importance. In this chapter, we present a brief discussion of the current knowledge of the pathogenesis of SSc-ILD, since such research may ultimately lead to targeted and more effective management of SSc-ILD. We then discuss the current state of management of SSc-ILD, beginning with early detection, followed by a discussion of disease staging and risk stratification, and finally a review of current and future treatment options.
Pathogenesis of SSc-ILD
Despite several decades of intense investigation, the pathogenesis of SSc-ILD remains unclear. It is likely that SSc-ILD represents a complex interplay between innate and acquired immunity, inflammation, and fibrosis, but the exact sequence of events remains uncertain. As lung biopsy is seldom required to establish a diagnosis of SSc-ILD, insight into the pathogenesis of SSc-ILD has been hampered by a relative lack of access to lung tissue, particularly early in the course of the disease when the greatest insight on disease initiation and mechanisms might be gained. When biopsy is performed, the SSc lung histopathology typically shows interstitial fibrosis with temporal homogeneity and with only a modest inflammatory cell infiltrate (i.e., fibrotic nonspecific interstitial pneumonia, NSIP).3 Cellular NSIP and UIP (usual interstitial pneumonia) are seen in a smaller proportion of cases.
Recent studies looking at gene expression profiles provide molecular insights into the pathogenesis of SSc-ILD (Table 1). Although not completely concordant, lung tissue gene expression and bronchoalveolar lavage (BAL) studies of early and late-stage SSc-ILD demonstrate abnormal expression of markers of macrophage migration and activation, as well as up-regulated expression of TGF-β and interferon-regulated genes.4–6 Genome wide association studies have found the gene for CXCL4 (chemokine [C-X-C motif] ligand 4), among others, to be highly and differentially expressed in certain SSc patients, and a recent proteome-wide analysis found serum levels of CXCL4 to be correlated with lung fibrosis.7 Polymorphisms at loci for additional genes have also been reported to be associated with the presence and/or severity of pulmonary fibrosis (see Table 1).3,8 Such genetic and molecular insights will likely lead to the future development of predictive serum biomarkers, as well as the development of safe and targeted therapies for patients who suffer from SSc-ILD.3,9
Table 1.
| Proposed Biologic Function | Associations with SSc-ILD |
|---|---|
| Alveolar epithelial homeostasis | SP-B, HGF |
| Immune regulation | IRAK-1, IRF-5, NLRP1, CXCL4, OAS1, IFI44, CCL18, CD163 |
| Fibroblast activation/matrix remodeling | COL1A, CTGF, MMP-12 |
SSc-ILD (systemic sclerosis-associated interstitial lung disease); SP-B (surfactant protein B); HGF (hepatocyte growth factor); IRAK-1 (interleukin-1 receptor-associated kinase 1); IRF5 (interferon regulatory factor 5); NLRP1 (NACHT, LRR and PYD domains-containing protein 1); CXCL4 (chemokine [C-X-C motif] ligand 4); OAS1 (2′-5′-oligoadenylate synthetase 1); IFI44 (interferon-induced protein 44); CCL18 (chemokine [C-C motif] ligand 18); CD163 (Cluster of Differentiation 163); COL1A (collagen, type I, alpha I); CTGF (CCN2, connective tissue growth factor); MMP-12 (matrix metalloproteinase 12).
Adapted from Herzog EL, Mathur A, Tager AM, et al. Interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis. How similar and distinct? Arthritis Rheumatol. 2014;66:1967–78; with permission.
Clinical Manifestations
Pulmonary involvement is common, occurring in over 80 percent of patients with SSc, and is often a significant source of morbidity and mortality.10 Lung involvement can occur in all subsets of the disease including limited cutaneous SSc, diffuse cutaneous SSc, and SSc sine scleroderma, and it can affect all aspects of the respiratory tract including the parenchyma, vasculature, airways, pleura, and musculature.11 Therefore, when a patient with SSc presents with symptoms of dyspnea, the differential diagnosis can be quite broad (Box 1).
Box 1. Differential diagnosis of dyspnea in SSc.
Interstitial lung disease
-
Pulmonary vascular disease
Pulmonary arterial hypertension
Thromboembolic disease
Pulmonary capillary hemangiomatosis
Pulmonary veno-occlusive disease
Pleural effusion
Spontaneous pneumothorax
Recurrent aspiration
-
Airways disease
Airflow limitation
Bronchiolitis obliterans
Follicular bronchiolitis
Bronchiectasis
Drug-associated pneumonitis
Lung cancer
Infection
Respiratory muscle weakness
Extrinsic chest wall restriction due to skin tightness
Anemia
Deconditioning
Inflammation or fibrosis of the pulmonary interstitium, ILD, is the most frequent pulmonary manifestation in SSc. Forty percent of patients have restrictive changes on pulmonary function tests (PFTs) while over 90 percent will have evidence of ILD at autopsy.12 The most common presenting symptom is dyspnea on exertion. Other indicators of ILD may include nonproductive cough, fatigue and chest pain. The most common finding on physical examination is the presence of dry (Velcro-like) crackles at the lung bases. However, some patients with SSc-ILD may not have any symptoms, and physical exam may be normal. Therefore, the clinician must remain ever vigilant, screening all patients initially and monitoring them frequently throughout the course of their disease.
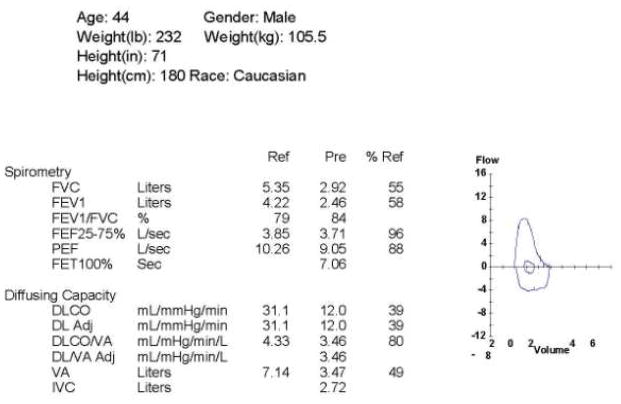
Pulmonary function tests (PFTs) play a major role in the investigation of lung involvement in SSc (Figure 1).13 Because changes in pulmonary function can occur before the onset of significant clinical symptoms, all patients should have screening PFTs at the time of presentation. These should include spirometry and single breath diffusion capacity for carbon monoxide (DLCO) at a minimum. Patients with SSc-ILD have a restrictive pattern on PFTs, marked by a decreased FVC. The FEV1/FVC ratio is typically normal, or sometimes even elevated, as the FEV1 decreases in proportion to the decline in FVC. Additionally, the parenchymal inflammation and fibrosis that occur in ILD lead to thickening of the interstitium, which results in a decreased DLCO.11 Thus, FVC and DLCO prove to be the most important and most commonly used diagnostic markers in SSc-ILD.13 In patients with SSc-ILD, progression of disease often varies and can be difficult to predict.11 Therefore, monitoring these patients with serial PFTs is a crucial aspect of the management of SSc-ILD as it can provide objective evidence of improvement or deterioration of lung function.13 In general with serial PFTs, changes of 10 percent in FVC and of 15 percent in DLCO are regarded as significant.13
Figure 1.
Pulmonary function tests from a patient with SSc-ILD demonstrating a restrictive pattern on the flow volume loop, decreased FVC, and decreased DLCO, but a preserved FEV1/FVC ratio.
High resolution CT (HRCT) scanning in which 3-mm or less sections of the lung are obtained is the most commonly used imaging modality for the evaluation of SSc-ILD (Figure 2), although CT with a limited number of slices to reduce radiation exposure and B-scale ultrasound imaging modalities are being explored. Compared to chest radiographs, advantages of HRCT include earlier detection of ILD as well as more accurate quantification of the extent of disease.11 The most common histopathological pattern seen in SSc-ILD is nonspecific interstitial pneumonia (NSIP). This appears on HRCT as ground glass opacities and pulmonary fibrosis, the distribution of which is typically peripheral, bilateral, and predominantly at the lung bases. Ground glass opacities are areas of increased lung attenuation thought to represent areas of active inflammation or early fibrosis; established pulmonary fibrosis is represented by reticular thickening of the interstitium with traction bronchiectasis.11 The extent of pulmonary fibrosis on HRCT correlates negatively with both FVC and DLCO.11 Therefore, HRCT imaging and PFTs, when used in combination, can be a powerful tool for predicting disease progression and mortality in SSc-ILD.
Figure 2.
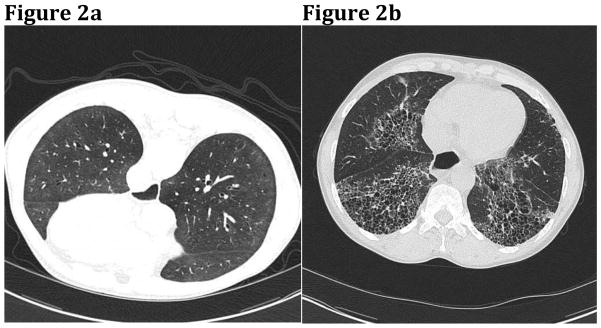
(A) HRCT with ground glass opacities in a patient with early SSc-ILD. (B) Fibrosis, honeycombing, and traction bronchiectasis in a patient with more advanced disease. I Courtesy of J. Ravenel, MD. Charleston, SC.
Risk Factors for Presence and Progression of SSc-ILD
Although sensitive screening techniques will identify lung disease in the majority of patients, many cases of SSc-ILD will be mild and not life threatening. Optimal management and prognosis for individual patients as well as risk stratification for future clinical trials will be dependent on the identification of certain clinical, serologic, radiographic, molecular, and genetic factors that individually or collectively impart a significantly increased risk for the development or progression of SSc-ILD. While we await the discovery and validation of useful SSc-ILD biomarkers, there are several known aspects of the disease that may provide prognostic and risk information to guide monitoring and treatment:
Gender and Race – Females are at higher overall risk for developing SSc, but males are more likely to develop severe SSc-ILD. African-American ethnicity is associated with earlier onset and greater severity of disease, especially SSc-ILD.15,16
Extent of Skin Involvement – The prevalence of SSc-ILD is higher in patients with diffuse cutaneous SSc (~50%) than in those with limited cutaneous SSc (~35%).17
Auto-Antibodies – Anti-topoisomerase I (anti-Scl-70) antibodies are strongly linked to the development SSc-ILD, with over 85% of Scl-70 antibody-positive SSc patients developing pulmonary fibrosis.18 Conversely, the presence of anti-centromere antibody (ACA) appears to be associated with a much lower likelihood of the development of SSc-ILD. U1-RNP, U3-RNP, Th/To and PM/Scl auto-antibodies are also associated with SSc-ILD.
Pulmonary Function Tests (PFTs)– The greatest risk of progression of SSc-ILD appears to be early in the course of disease (first 5 years). An abnormal forced vital capacity (FVC) early in the disease course has been shown to be an important predictor for eventual end-stage lung disease.19 Mortality is even more closely linked to lower initial PFTs (FVC and DLCO) than to lung histopathology (NSIP vs. UIP).14
High Resolution Computed Tomography (HRCT) Chest Imaging – Patients with more extensive fibrosis on HRCT chest scans (i.e., abnormalities involving >20% of the lung volume) are at significantly higher risk for rapid decline in lung function and death, compared with patients whose HRCT shows lesser involvement (<20% of the lung volume).20 In the Scleroderma Lung Study I (SLS I), the extent of fibrosis on baseline HRCT was a useful predictor of lung disease progression when untreated and of a favorable response to treatment with cyclophosphamide compared with placebo.21
Gastroesophageal Reflux Disease (GERD) – Although cause-and-effect remains to be proven, an association exists between gastroesophageal reflux and ILD. The extent of SSc-ILD as assessed by PFTs and by chest HRCT is correlated with the degree of esophageal reflux.22,23
Selected Biomarkers – Two lung-associated glycoproteins (KL-6, Krebs von den Lungen-6; SP-D, Surfactant Protein-D) and a chemokine secreted predominantly by alveolar macrophages (CCL18, C-C motif chemokine ligand 18) are reflective of active lung injury and may predict the progression of SSc-ILD.24–26 Other potential plasma biomarkers include: CXCL4, IL-6 (Interleukin-6), Chit 1 (Chitinase 1), TN-C (tenascin-C), LOX (lysly oxidase), and IL-33. 7,27–29
Development and validation of a composite index, comprised of two or more of these features, is needed and will help to optimize management of individual patients as well as to provide risk stratification for future clinical trials.
Treating SSc-ILD
Whom and When to Treat
Identifying patients at risk for the development and progression of SSc-ILD should be the first step in management. As noted above, certain demographic, clinical, serologic, and radiographic elements may identify patients at high risk who, therefore, would warrant treatment. Biomarkers and genetic markers of lung fibrosis risk will surely be developed as the era of personalized medicine emerges and as we learn more about the pathogenesis of SSc-ILD. Since the initial PFT’s and the extent of fibrosis on HRCT scans seem to be important determinants of outcome, Goh and colleagues have proposed a simple staging system whereby extensive disease (>20% HRCT involvement) would warrant immunosuppressive treatment while limited disease (<20% HRCT involvement) would not.20 In situations where the extent of fibrosis on HRCT scan is indeterminate, the FVC (% predicted) would drive the decision to treat (<70% predicted) or not to treat (>70% predicted)(Figure 3).20 Another schema sets forth different criteria in determining whom to treat 30 (Box 2).
Figure 3.
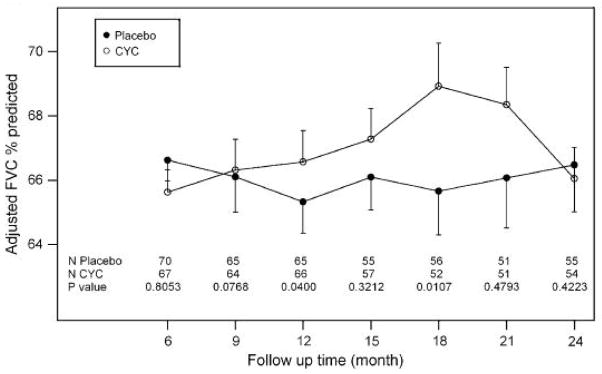
Time course from 6 to 24 months of mean values (+/−SE) for FVC % predicted of participants in the placebo and cyclophosphamide (CYC) SLS I treatment groups.
Box 2. When to Initiate Treatment.
-
Patients with limited or diffuse cutaneous SSc with dyspnea
AND
-
Within 5–7 years after onset of signs or symptoms attributable to SSc associated with:
-
Decline in their FVC% predicted by > 10% in the preceding 3–12 months
AND/OR
-
FVC% predicted of < 70% at time of presentation
AND/OR
Moderate extent of ILD on baseline HRCT (defined as >20% lung involvement)30
-
How to Treat
Immunosuppression
Cyclophosphamide
Over the past 25 years, immunosuppressive therapy has emerged as a treatment strategy in patients with SSc-ILD. Other forms of ILD, e.g., idiopathic pulmonary fibrosis (IPF), are not as responsive to immunosuppression. Cyclophosphamide is the only such therapy thus far tested in a randomized controlled trial (RCT) and shown to be effective in treating SSc-ILD. On the basis of a number of uncontrolled and retrospective studies, the Scleroderma Lung Study I (SLS I) was designed as a multicenter, double-blinded placebo-controlled RCT to evaluate the efficacy and safety of cyclophosphamide administered orally for one year in patients with symptomatic SSc-ILD and with evidence of disease activity by bronchoalveolar lavage findings and/or chest HRCT. SLS I was the first RCT to demonstrate efficacy of cyclophosphamide in improving lung function, relative to placebo, following one year of treatment.21 In addition to a modest improvement in the primary endpoint (2.53% adjusted FVC % predicted, p<0.03), one year of cyclophosphamide treatment also was associated with significant improvement in a number of secondary endpoints, e.g., total lung capacity (TLC % predicted), modified Rodnan skin score (mRSS), the patient-reported outcome of dyspnea (Transition Dyspnea Index), and several quality of life measures. After completion of 12 months of treatment with cyclophosphamide, the treatment effect on FVC % predicted increased further by 18 months, but was lost by 24 months.31 Follow-up HRCT scans at the end of the 12-month treatment period revealed that the change in extent of fibrosis from baseline was significantly worse in the placebo group than in the cyclophosphamide treatment group (p=0.012), and the difference in the 12-month change in fibrosis between the two treatment groups was significantly correlated with the favorable effect of cyclophosphamide on FVC, TLC, and dyspnea.32
A retrospective, multivariate regression analysis of SLS I found the maximal severity of reticular infiltrates on chest HRCT, the mRSS and the Mahler baseline dyspnea index at baseline to be independently correlated with treatment outcomes.33 When patients are stratified post hoc on the basis of whether 50% or more of any lung zone was involved by reticular infiltrates and/or whether patients had a mRSS of at least 23, a subgroup of patients emerges in whom the average treatment effect of cyclophosphamide on FVC was 9.81% at the 18 month assessment (i.e., 6 months after completing cyclophosphamide therapy). Conversely, there was no treatment effect in patients with less severe fibrosis on chest HRCT and a lower mRSS at baseline. This important retrospective analysis has implications for future clinical trial design in order to select patients likely to demonstrate responsiveness to the therapy to be tested.
In another trial, the Fibrosing Alveolitis in Scleroderma Trial (FAST), 45 patients with SSc-ILD were randomized to receive either intravenous cyclophosphamide (600 mg/m2 monthly) for 6 months followed by daily oral azathioprine 2.5 mg/kg/d [maximum 200 mg/d], or placebo infusions followed by oral placebo.34 At 12 months, a modest but non-statistically significant improvement in FVC was seen in the actively treated group (p = 0.08).
Based on expert consensus and evidence derived from both the SLS I and FAST trials, the EULAR Scleroderma Trials and Research (EUSTAR) group recommends cyclophosphamide for the treatment of SSc-ILD.35 A recent survey of SSc experts, however, revealed a lack of consensus on treatment decisions for both induction as well as maintenance therapy.36
Not all patients will respond to cyclophosphamide, with up to one-third of some cases showing continued decline in lung function.37 In view of cyclophosphamide’s limited efficacy, as well as short and long-term risk of toxicity, it is clear that alternate forms of immunosuppression are needed.
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is regarded as a safer, less toxic alternative to cyclophosphamide for the treatment of a number of immune-mediated conditions. Several uncontrolled, prospective or retrospective case series (summarized in Table 238–49) suggest that MMF may be effective in stabilizing or, in some cases, improving lung function in patients with SSc-ILD. Based on the results of such preliminary studies, SLS II was designed to evaluate the efficacy and safety of MMF (up to 1.5 g twice daily) for 2 years in comparison with oral cyclophosphamide (up to 2 mg/kg daily) for 1 year, followed by placebo for an additional year in symptomatic SSc-ILD patients with any evidence of ground-glass opacification on chest HRCT (www.clinicaltrials.gov). SLS II has completed enrollment and release of the first-year data is anticipated for 2015.
Table 2.
Outcomes of Clinical Trials of Myophenolate Mofetil for SSc
| Author, Year, Ref | Patients (n) | Baseline PFTs* | Regimen | Pulmonary Results |
|---|---|---|---|---|
| Swigris et al., 2006 (38) | 28 CTD* patients, 11 with SSc-ILD | FVC 65% (56–76%) | MMF 2 g/d for median 371 days | FVC, TLC, and DLCO improved by 2.3%, 4.0%, and 2.6%, respectively, which approached statistical significance |
| Liossis et al., 2006 (39) | 6 SSc patients with ILD and alveolitis | FVC 71% (32–80%) | MMF 2 g/d plus low-dose prednisolone for up to 12 months | Improvement in FVC from 65.6% to 76.2% (p = 0.057) and in DLCO from 64.2% to 75.4% (p = 0.033) |
| Nihtyanova et al., 2007(40) | 172 early SSc patients, 109 MMF-treated | Progressive ILD in 27.5% of MMF group prior to treatment | In MMF group, MMF for 1 year (79%) and for 12–36 months (59%) | 12% (MMF) versus 19% (control) developed progressive ILD (p < 0.04); 5-year survival 95.4% vs. 85.7% (p = 0.027) |
| Zamora et al., 2008 (41) | 17 SSc-ILD patients | FVC 72% DLCO 52% |
MMF 2 g/d for 12–24 months | At 12 month, FVC improved by 2.6% and DLCO by 1.4%. At 24 month, FVC improved on average 2.4% |
| Gerbino et al., 2008 (42) | 13 SSc-ILD, patients with early disease | FVC 70%, DLCO 51% | MMF median dose 2 g/d for median 21 months | FVC improved by mean of 4% in contrast to a decrease of 5% during a median of 14 months prior to MMF Rx |
| Derk et al., 2010 (43) | 15 dcSSc patients with disease duration < or = 48 months | FVC 99%, DLCO 71.2% | MMF maximum dose 3 g/d for 13 months | Non-significant trend for improvement in PFTs |
| Koutroumpas et al., 2010 (44) | 10 dc SSc patients with ILD | FVC 79.5%, DLCO 80.67% | MMF 2 g/d for median 12 months | Significant increase in FVC and non-significant increase in DLCO at 12 months (p=0.04 and 0.66, respectively) |
| Le et al., 2011 (45) | 98 dcSSc patients with active skin disease and mean disease duration 21.9 +/− 27.6 months | FVC 79.4%, DLCO 77.4% | MMF maximum dose 3 g/d for 12 months | Significant decrease in mRSS at 12 months, but no significant difference in the FVC and DLCO |
| Simeon-Aznar et al., 2011 (46) | 14 SSc-ILD patients with median duration of lung symptoms of 32 months; 10 with prior immunosuppression | FVC 64%, DLCO 40% | MS (mycophenolate sodium), 720 mg twice daily for 12 months | Non-significant change in FVC or DLCO. 6 patients showed >10% improvement in FVC, 5 remained stable, 3 declined >10% in FVC |
| Mendoza et al., 2012 (47) | 25 dcSSc patients with disease duration <24 months, 15 with evaluable PFT’s | TLC 89.5% DLCO 69.0% |
MMF mean dose 2 g/d, average duration therapy 18.2 +/− 8.7 months | Significant improvement in skin and non-significant change in TLC and DLCO. Only 3/15 patients showed >10% decline in TLC |
| Henes et al., 2013 (48) | 8 evaluable SSc patients with ILD and median disease duration 26 months | Median FVC 78% Median DLCO 75.1% |
MS (mycophenolate sodium) up to 720 mg twice daily for up to 6 months | Stabilization of PFT’s with non-significant changes in FVC and DLCO; non-significant increase in lung density by HRCT histography |
| Panopoulos et al., 2013 (49) | 10 SSc-ILD patients with mean disease duration 5.8 +/1 6.8 years | FVC 79.0%, TLC 71.5% DLCO 56.8% |
MMF mean daily dose 1.5 g/d, up to > 2 g/d in 8 patients, for up to 24 months | No significant change in FVC, TLC or DLCO. Significant worsening of 2-year HRCT scores in MMF patients compared with matched cyclophosphamide-treated patients |
PFTs, Pulmonary Function Tests; CTD, Connective Tissue Disease; FVC, Forced Vital Capacity (mean % predicted); TLC, Total Lung Capacity (mean % predicted); DLCO, Diffusing Capacity for Carbon Monoxide (mean % percent predicted); MMF, mycophenolate mofetil; ILD, interstitial lung disease; dcSSc, diffuse cutaneous systemic sclerosis; SSc-ILD, systemic sclerosis-associated interstitial lung disease; mRSS, modified Rodnan skin score; HRCT, high resolution computed tomography.
Azathioprine
Published experience on the use of azathioprine for patients with SSc-ILD has been less robust and less enthusiastic than for cyclophosphamide or MMF. In a non-blinded trial comparing azathioprine to cyclophosphamide in patients with diffuse cutaneous SSc, azathioprine did not appear to halt the deterioration in lung function.50 Azathioprine was used as maintenance therapy following monthly pulse intravenous cyclophosphamide in the FAST study, but the effect of azathioprine on the outcome of the trial is impossible to discern.34
Rituximab
Rituximab, a monoclonal antibody directed against the B cell CD20 antigen, has been proposed as a potential therapy for patients with SSc-ILD. In one study rituximab therapy (n=8) was associated with statistically significant improvement in FVC and DLCO and stabilization of HRCT chest imaging compared to a matched control group (n=6) on a variety of different background medications.51 The EUSTAR group recently evaluated rituximab treatment in a nested case-control designed study.52 Among the 63 patients treated with rituximab, there were 9 who had SSc-ILD (defined by an FVC <70% predicted and evidence of ILD on chest HRCT). At a median follow-up of 6 months (range, 4–12 months), FVC remained stable and DLCO improved compared with baseline. In comparison, matched control SSc-ILD patients showed a decline in FVC resulting in significant differences between rituximab-treated and matched controls. There was no significant difference in change in DLCO between rituximab-treated and matched control patients. These authors also observed a statistically significant improvement in skin thickness (mRSS) for the rituximab-treated patients (n=63).52 The side-effect profile of the drug appeared acceptable and no serious adverse events were reported. Such studies support the need for a prospective, double-blinded RCT to assess the efficacy and safety of rituximab in patients with SSc-ILD, either as induction or maintenance therapy.
Autologous Hematopoietic Stem Cell Transplantation
Cell based therapies, usually autologous hematopoietic stem cell transplantation (HSCT), have been designed to “reset” an auto-reactive immune system and ameliorate SSc. Following high-dose cyclophosphamide and ATG (anti-thymocyte globulin) with/without total body irradiation (TBI), autologous CD34+ stem cells are re-infused to rescue the ablated immune system. Treatment–related mortality can be quite high due to infection and other complications, so autologous HSCT remains experimental and is offered mainly to selected patients considered to be at high risk for disease-related morbidity and mortality. Proper patient selection continues to evolve as more is learned about risk factors for treatment-related mortality.
Early success of small phase I and phase II clinical trials of autologous HSCT for SSc patients, together with improvement in treatment-related mortality as centers gain more experience with patient selection, conditioning regimens and supportive care, led to the conception of two phase II/III multi-center trials comparing autologous HSCT to monthly (x12) intravenous cyclophosphamide, one in Europe (ASTIS, Autologous Stem cell Transplantation International Scleroderma) and one in the USA (SCOT, Scleroderma: Cyclophosphamide Or Transplantation)(Table 3). Each trial has competed enrollment and patients are being followed to compare the safety and efficacy of HSCT with the control arm of monthly pulse intravenous cyclophosphamide. Initial results of the ASTIS trial were recently published.53 Among the 156 patients in the ASTIS trial, lung involvement was frequent (86.5% overall): chest HRCT scans were abnormal in 83.3% and PFT’s were consistent with mild to moderate restrictive lung disease (FVC 81.4% [18.4], mean [SD]; TLC 80.7% [16.6], mean [SD]; DLCO 58.5% [14.1], mean [SD]). Low DLCO may have been due to SSc-PAH in some cases (diagnosed in 6.6%) of the overall ASTIS study population. Follow-up of event-free survival of the intention to treat (ITT) populations was 5.8 years at the time of the initial report. Overall results indicate that patients treated with autologous HSCT experienced more events in the first year but had improved long-term event-free survival compared with patients treated with cyclophosphamide. During year one, there were 11 deaths (13.9%, including 8 treatment-related deaths) in the HSCT group vs. 7 deaths (9.1%, none treatment-related) in the cyclophosphamide group (RR 1.53 [95% CI, 0.4–5.4]). After year two of follow-up, there were 12 deaths (15.2%) in the HSCT group vs. 13 (16.9%) in the cyclophosphamide group; after four years of follow-up, there were 13 deaths (16.5%) in the HSCT group vs. 20 (20.6%) in the cyclophosphamide group. As expected, the mRSS decreased in both treatment groups, with a significantly greater reduction in the group receiving HSCT (mean difference -11.1, range −7.3 to −15.0, p<0.001). There was also a statistically significant difference favoring HSCT in lung function: mean change in FVC (6.3% predicted vs. −2.8% predicted) and TLC (5.1% predicted vs. −1.3% predicted). No statistically significant difference in DLCO was observed. It is noteworthy that 7 of 8 treatment-related deaths occurred in current or former smokers; this is an important observation that should be taken into account for future trial designs of HSCT. The SCOT trial completed enrollment and results are not expected until 2016. The STAT trial, which is still enrolling subjects, is a multi-center, non-comparative study that will look at event-free survival when maintenance MMF therapy for up to 2 years is used following autologous HSCT.
Table 3.
Comparison of 3 Randomized Trials of Autologous HSCT for Systemic Sclerosis
| ASTIS | SCOT | ASSIST | |
|---|---|---|---|
| Study Design | Phase II, Non-Myeloablative, multicenter, event-free survival study | Phase II/III, Myeloablative, multicenter, event-free survival study | Phase II, Non-Myeloablative, singlecenter, treatment failure study |
| Inclusion Criteria | 18–65 years old Disease duration ≤4 years, skin score ≥15, at least one predefined major organ involved or Disease duration ≤2 years, skin score ≥20, elevated acute phase reactants and/or proteinuria |
18–69 years old Disease duration ≤ 5 years Diffuse cutaneous SSc, skin score ≥16 plus either pulmonary disease or prior renal crisis |
18–60 years old Disease duration ≤ 4 years mRSS ≥15 and internal organ involvement or restricted skin involvement (mRSS ≤15) but coexistent pulmonary involvement |
| Exclusion Criteria | Predefined severe organ damage Prior cyclophosphamide total >5 g iv or >2 mg/kg po for > 3 months |
Predefined severe organ damage Prior cyclophosphamide >6 months or >3 g/m2 |
Predefined severe organ damage Prior cyclophosphamide >6 month |
| Mobilizing Regimen | Cyclophosphamide and G-CSF | G-CSF | Cyclophosphamide and G-CSF |
| Conditioning Regimen | Cyclophosphamide 200 mg/kg Rabbit ATG |
Cyclophosphamide 120 mg/kg Equine ATG TBI 800 cGy (with lung and renal shielding) |
Cyclophosphamide 200 mg/kg Rabbit ATG |
| Graft Manipulation | CD34+ cell selection | CD34+ cell selection | No CD34+ cell selection |
| Primary End Point | Survival without organ failure at 3 years | Event-free survival without organ failure at 54 months | Improvement at 12 months defined as a decrease in mRSS (>25% for those with initial mRSS >14) or an increase in FVC > 10% |
| Control Arm | Cyclophosphamide 750 mg/m2 iv monthly x 12; cross over to HSCT not allowed | Cyclophosphamide 750 mg/m2 iv monthly x 12; cross over to HSCT not allowed | Cyclophosphamide 1000 mg/m2 iv monthly x 6 |
| Current Status | Completed 156 patients enrolled and randomized. Primary analysis reported in 2014 (van Laar et al, JAMA) |
Completed enrollment 2011 Results pending follow-up |
Completed 19 patients enrolled and randomized (10 HSCT, 9 cyclophosphamide of whom 7 crossed over to HSCT after cyclophosphamide failure). Primary analysis reported in 2011 (Burt et al, 2011). ASSIST IIb currently underway to test less intensive conditioning regimen (clinicaltrials.gov/ct2/show/NCT01445821) |
A single-center, open-label phase II trial of autologous HSCT without CD34+ cell selection (ASSIST, Autologous Stem Cell Systemic Sclerosis Immune Suppression Trial) showed short-term superiority of HSCT in 10 patients who had significant regression of skin thickness (mRSS) and improvement in lung function, as well as reduction in the extent of lung disease on chest HRCT.54 Eight of nine control patients (cyclophosphamide-treated) showed disease progression, and seven of these patients then crossed over to HSCT therapy. After 2 years of follow-up, 11/18 patients showed persistent improvement in skin score, chest HRCT and FVC (but not TLC or DLCO).
Another clinical trial, STAT (Scleroderma Treatment with Autologous Transplant), is currently enrolling patients (clinicaltrials.gov/ct2/show/NCT01413100). In this clinical trial, selected patients with active SSc-ILD who have failed conventional immunosuppressive therapy will receive autologous CD34+ stem cells followed by maintenance therapy with MMF for up to two years.
Other Investigational Immunosuppressive Agents
In addition to the aforementioned immunosuppressive treatments (Table 4), a number of other drugs with immunosuppressive properties are currently under investigation to treat SSc, including an IL-6 receptor blocker (tocilizumab), a T-cell co-stimulatory blocker (abatacept), and a monoclonal antibody directed against B-cell activating factor (belimumab). It remains to be seen if any of such agents will prove to be an effective therapy for SSc-ILD.
Table 4.
Summary of Immunosuppressive Therapies in SSc-ILD
| Drug | Proposed Mechanism of Action | Status of Investigation |
|---|---|---|
| Cyclophosphamide | Alkylating agent that prevents cell division by cross-linking DNA strands and decreasing DNA synthesis | SLS I: multicenter, double-blinded placebo-controlled RCT FAST : multicenter, double-blinded placebo-controlled RCT |
| Mycophenolate mofetil | Exhibits a cytostatic effect on T and B lymphocytes through the inhibition of de novo guanosine nucleotide synthesis | SLS II: multicenter, double-blinded placebo-controlled RCT (ongoing) |
| Azathioprine | Imidazolyl derivative of mercaptopurine that incorporates its metabolites into replicating DNA and halts replication | Single unblinded RCT of 60 patients |
| Rituximab | Monoclonal antibody directed against the CD20 antigen on B lymphocytes | Small RCT of 14 patients Nested case-control designed study |
| Autologous | HSCT Administration of hematopoietic progenitor cells derived from the individual with the disorder to “reset” an autoreactive immune system | ASTIS: phase II, multicenter, event-free survival study SCOT: phase II/III, multi-center, eventfree survival study (ongoing) ASSIST: single-center, open-label phase II trial |
Anti-Fibrotic Therapy
Given the magnitude of the impact of fibrosis - estimated to contribute to as much as 45% of the mortality in Western developed countries55 – the pace of development of effective anti-fibrotic drugs has been disappointing. The bleak picture for anti-fibrotic therapy to treat SSc may be improving, as new animal models that more faithfully replicate the human disease are emerging, more promising biomarkers are being developed, and greater knowledge on the mechanisms and pathways of fibrosis is being gained.27 In 2014, the Food and Drug Administration (FDA) approved two new drugs – perfinidone and nintedanib – for the treatment of patients with IPF. Both drugs, which act by down-regulating the expression or signaling by TGF-β, are now undergoing preliminary safety and efficacy studies in SSc-ILD patients. Many other new therapeutics that target specific growth factors, cytokines or pathways (e.g., monoclonal CTGF antibodies, tocilizumab, endostatin 1-derived peptide, caveolin scaffolding domain), as well as multiple existing drugs that might be repurposed to treat fibrosis (e.g., PPAR-γ agonists [e.g., rosiglitazone]56–58, statins [rosuvastatin]59, fluoroquinolone antibiotics [e.g., ciprofloxacin]60, and thrombin inhibitors [e.g., dabigatran]61) loom on the horizon. Given that the pathogenic mechanisms of fibrotic diseases in general and SSc in particular consist of complex networks of multiple and often redundant pathways, blocking a single molecule or pathway will likely not be sufficient and, like cancer, it may be necessary to treat patients with multiple drugs that affect different pathways.62
Adjunctive Therapy
General Measures
In addition to immunosuppressive therapies, patients with SSc-ILD should receive the same supportive care measures used in other types of ILD. These should include supplemental oxygen (if indicated), appropriate vaccinations, and pulmonary rehabilitation therapy. Patients with SSc-ILD should receive yearly influenza vaccination and periodic vaccination against pneumococcal pneumonia. Furthermore, those patients on treatment with cyclophosphamide should receive prophylaxis against Pneumocystis jirovecii (formerly Pneumocystis carinii or PCP). Additionally, the association between GERD and ILD demands aggressive management of reflux symptoms in patients with SSc. This can be accomplished through the use of pharmacologic agents, such as proton pump inhibitors (PPIs) and H2-blockers, and augmented by non-pharmacologic methods, such as elevating the head of the bed and avoiding lying down for several hours after a meal. These general measures are not to be overlooked as they can have a significant impact on easing the symptom burden and improving the quality of life in patients with SSc-ILD (Box 3).
Box 3. Supportive Care Measures.
-
Supplemental oxygen
If indicated
-
Appropriate vaccinations
Influenza
Streptococcus pneumoniae
-
Prophylaxis against Pneumocystis jirovecii
If indicated
Pulmonary rehabilitation
Treatment of GERD
Medications (PPI, H2-antagonists)
Reflux precautions
Intravenous Immunoglobulin
Intravenous immunoglobulin (iv Ig) therapy has been shown to be useful as adjunctive therapy in some myositis-associated ILD patients not responding to steroids and immunosuppressive drugs.63 When SSc is complicated by an inflammatory myositis, ILD occurs not infrequently (e.g., anti-PM/Scl antibody positive patients). In such cases, iv Ig therapy might be a useful adjunctive measure when conventional treatment proves inadequate.
Lung Transplantation
Given the lack of highly effective medical therapy, some SSc patients will progress to end-stage lung disease. Lung transplantation should be considered for selected patients who progress despite medical therapy, but transplant centers have been reluctant to consider SSc-ILD patients given the high prevalence of gastroesophageal reflux and its attendant risks for aspiration, bronchiolitis obliterans and allograft rejection. Querying the United Network for Organ Sharing database revealed that less than 1% (196 of 25,260) of all lung transplants performed in the United States from January 1988 to January 2013 for end-stage lung disease were done in patients with SSc.64 Nevertheless, reviews of transplant outcomes comparing SSc patients with IPF patients and with PAH patients have shown similar 2-year and 5-year outcomes for SSc patients (72% and 55%, respectively).65,66 A recent review of the medical literature reporting outcomes of lung transplantation in SSc patients found no reports of recurrence of SSc in the lung allograft.67 In a recent report of 10 SSc patients, severity of GERD was shown to impact the 1-year survival rate.68 Esophageal pH monitoring should be considered in patients with SSc-ILD, as this test could identify those patients in whom laparoscopic anti-reflux surgery should be performed to minimize GERD and its detrimental effects while awaiting lung transplantation.
Key Points.
ILD is the leading cause of mortality for SSc patients.
Early diagnosis is critical for managing patients with SSc-ILD.
PFT’s and HRCT chest imaging are used for screening and management.
Immunosuppression can be beneficial, but better therapies are needed.
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katherine Culp Silver, Email: silverk@musc.edu, Fellow, Adult & Pediatric Rheumatology, Medical University of South Carolina, Suite 816, Clinical Sciences Building, 96 Jonathan Lucas Street, Charleston, SC 29425, 843-792-3484
Richard M. Silver, Email: silverr@musc.edu, Distinguished University Professor, Director, Division of Rheumatology & Immunology, Medical University of South Carolina, Suite 816, Clinical Sciences Building, 96 Jonathan Lucas Street, Charleston, SC 29425, 843-792-3484
References
- 1.Steen VD, Medsger TA., Jr Changes in causes of death in systemic sclerosis. Ann Rheum Dis. 2007;66:940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyndal AJ, Banert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–15. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 3.Herzog EL, Mathur A, Tager AM, Feghali-Bostwick C, Schneider F, Varga J. Interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis. How similar and distinct? Arthritis Rheum. 2014;66:1967–78. doi: 10.1002/art.38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu E, Shi H, Jordan RM, Lyons-Weiler J, Pilewski JM, Feghali-Bostwick CA. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 2011;63:783–94. doi: 10.1002/art.30159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christmann RB, Sampaio-Barros P, Stifano G, Borges CL, de Carvalho CR, Kairalla R, et al. Association of interferon- and transforming growth factor-β–regulated genes and macrophage activation with systemic sclerosis–related progressive lung fibrosis. Arthritis Rheum. 2014;66:714–25. doi: 10.1002/art.38288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindahl GE, Stock CJ, Shi-Wen X, Leoni P, Sestini P, Howat SL, et al. Microarray profiling reveals suppressed interferon stimulated gene program in fibroblasts from scleroderma-associated interstitial lung disease. Respir Res. 2013;14:80. doi: 10.1186/1465-9921-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Bon L, Affandi AJ, Broen J, et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Eng J Med. 2014;370:433–443. doi: 10.1056/NEJMoa1114576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assassi S, Radstake TR, Mayes MD, Martin J. Genetics of scleroderma: implications for personalized medicine? BMC Med. 2013;11:9. doi: 10.1186/1741-7015-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver RM, Feghali-Bostwick C. Molecular insights into systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 2014;66:485–7. doi: 10.1002/art.38287. [DOI] [PubMed] [Google Scholar]
- 10.Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;81(2):139–53. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hant FN, Herpel LB, Silver RM. Pulmonary Manifestations of Scleroderma and Mixed Connective Tissue Disease. Clin Chest Med. 2010;31(3):433–49. doi: 10.1016/j.ccm.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Varga J. Systemic sclerosis: an update. Bull NYU Hosp Jt Dis. 2008;66(3):198–202. [PubMed] [Google Scholar]
- 13.Behr J, Furst DE. Pulmonary function tests. Rheumatology (Oxford) 2008 Oct;47(Suppl 5):v65–7. doi: 10.1093/rheumatology/ken313. [DOI] [PubMed] [Google Scholar]
- 14.Bouros D, Wells AU, Nicholson AG, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165:1581–6. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 15.Silver RM, Bogatkevich G, Tourkina E, Nietert PJ, Hoffman S. Racial differences between blacks and whites with systemic sclerosis. Curr Opin Rheumatol. 2012;24(6):642–648. doi: 10.1097/BOR.0b013e328356d9dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steen V, Domsic RT, Lucas M, Fertig N, Medsger TA., Jr A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum. 2012;64:2986–2994. doi: 10.1002/art.34482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker UA, Tyndall A, Czirjak L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR scleroderma trials and research group database. Ann Rheum Dis. 2007;66:754–63. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs DC, Vaughan RW, Welsh KI, Myers A, duBois RM, Black CM. Immunogenetic prediction of pulmonary fibrosis in systemic sclerosis. Lancet. 1991 Sep 14;338(8768):661–2. doi: 10.1016/0140-6736(91)91235-m. [DOI] [PubMed] [Google Scholar]
- 19.Morgan C, Knight C, Lunt M, Black CM, Silman AJ. Predictors of end stage lung disease in a cohort of patients with scleroderma. Ann Rheum Dis. 2003;62:146–50. doi: 10.1136/ard.62.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh NS, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–54. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 21.Tashkin D, Elashoff R, Clements P, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;25(354):2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 22.Savarino E, Bazzica M, Zentilin P, et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med. 2009;179:408–13. doi: 10.1164/rccm.200808-1359OC. [DOI] [PubMed] [Google Scholar]
- 23.Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum. 2010;40(3):241–9. doi: 10.1016/j.semarthrit.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Hant FN, Ludwicka-Bradley A, Wang HJ, et al. Scleroderma Lung Study Research Group. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol. 2009;36:773–80. doi: 10.3899/jrheum.080633. [DOI] [PubMed] [Google Scholar]
- 25.Yanaba K, Hasegawa M, Hamaguchi Y, et al. Longitudinal analysis of serum KL-6 levels in patients with systemic sclerosis: association with the activity of pulmonary fibrosis. Clin Exp Rheumatol. 2003;21:429–36. [PubMed] [Google Scholar]
- 26.Kodera M, Hasegawa M, Komura K, et al. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis. Arthrtis Rheum. 2005;52:2889–96. doi: 10.1002/art.21257. [DOI] [PubMed] [Google Scholar]
- 27.Fan MH, Feghali-Bostwick CA, Silver RM. Update on scleroderma-associated interstitial lung disease. Curr Opin Rheumatol. 2014;26:630–636. doi: 10.1097/BOR.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Laurentis A, Sestini P, Pantelidis P, Hoyles R, et al. Serum interleukin-6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumtol. 2013;40:435–446. doi: 10.3899/jrheum.120725. [DOI] [PubMed] [Google Scholar]
- 29.Lee CG, Herzog EL, Ahangari F, Zhou Y, Gulati M, Lee CM, Peng X, Feghali-Bostwick C, Jimenez SA, Varga J, Elias JA. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-β signaling. J Immunol. 2012;189:2635–2644. doi: 10.4049/jimmunol.1201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Au K, Khanna D, Clements PJ, Furst DE, Tashkin DP. Current concepts in disease-modifying therapy for systemic sclerosis-associated interstitial lung disease: lessons from clinical trials. Curr Rheumatol Rep. 2009;11:111–9. doi: 10.1007/s11926-009-0016-2. [DOI] [PubMed] [Google Scholar]
- 31.Tashkin DP, Elashoff R, Clements PJ, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldin JG, Lynch DA, Strollo DC, et al. Follow-Up HRCT after Treatment of scleroderma-interstitial lung disease with cyclophosphamide demonstrates evidence for treatment effect. Am J Respir Crit Care Med. 2008;177(A768):91. doi: 10.1164/rccm.200705-655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth MD, Tseng CH, Clements PJ, et al. Predicting treatment outcomes and responder subsets in scleroderma-related interstitial lung disease. Arthritis Rheum. 2011;63(9):2797–808. doi: 10.1002/art.30438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 35.Kowal-Bielecka O, Landewe R, Avouac J, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR) Ann Rheum Dis. 2009;60:620–8. doi: 10.1136/ard.2008.096677. [DOI] [PubMed] [Google Scholar]
- 36.Walker KM, Pope J on behalf of the participating members of the Scleroderma Clinical Trials Consortium (SCTC) and Canadian Scleroderma Research Group (CSRG) Semin Arthritis Rheum. 2012;42:42–55. [Google Scholar]
- 37.Mittoo S, Wigley FM, Wise RA, Woods A, Xian H, Hummers LK. Long term effects of cyclophosphamide treatment on lung function and survival in scleroderma patients with interstitial lung disease. Open Rheumatol J. 2011;5:1–6. doi: 10.2174/1874312901105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swigris JJ, Olson AL, Fischer A, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006;130:30–6. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 39.Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology (Oxford) 2006;45:1005–8. doi: 10.1093/rheumatology/kei211. [DOI] [PubMed] [Google Scholar]
- 40.Nihtyanova SI, Brough GM, Black CM, Denton CP. Mycophenolate mofetil in diffuse cutaneous systemic sclerosis – a retrospective analysis. Rheumatology (Oxford) 2007;46:442–5. doi: 10.1093/rheumatology/kel244. [DOI] [PubMed] [Google Scholar]
- 41.Zamora AC, Wolters PJ, Collard HR, et al. Use of mycophenolate mofetil to treat scleroderma-associated interstitial lung disease. Respir Med. 2008;102:150–5. doi: 10.1016/j.rmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Gerbino AJ, Goss CH, Molitor JA. Effect of mycophenolate mofetil on pulmonary function in scleroderma-associated interstitial lung disease. Chest. 2008;133:455–60. doi: 10.1378/chest.06-2861. [DOI] [PubMed] [Google Scholar]
- 43.Derk CT, Grace E, Shenin M, Naik M, Schulz S, Xiong W. A prospective open-label study of mycophenolate mofetil for the treatment of diffuse systemic sclerosis. Rheumatology. 2009;48:1595–9. doi: 10.1093/rheumatology/kep295. [DOI] [PubMed] [Google Scholar]
- 44.Koutroumpas A, Ziogas A, Alexiou I, Barouta G, Sakkas LI. Mycophenolate mofetil in systemic sclerosis-associated interstitial lung disease. Clin Rheumatol. 2010;29:1167–8. doi: 10.1007/s10067-010-1498-z. [DOI] [PubMed] [Google Scholar]
- 45.Le EN, Wigley FM, Shah AA, Boin F, Hummers LK. Long-term experience of mycophenolate mofetil for treatment of diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2011;70:1104–7. doi: 10.1136/ard.2010.142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simeon-Aznar CP, Fonollosa-Pia V, Tolosa-Vilella C, Selva-O’Callaghan A, Solans-Laque R, Vilardeli-Tarres M. Effect of mycophenolate sodium in scleroderma-related interstitial lung disease. Clin Rheumatol. 2011;30:1393–8. doi: 10.1007/s10067-011-1823-1. [DOI] [PubMed] [Google Scholar]
- 47.Mendoza FA, Nagle SJ, Lee JB, Jimenez SA. A prospective observational study of mycophenolate mofetil treatment in progressive diffuse cutaneous systemic sclerosis of recent onset. J Rheumatol. 2012;39:1241–7. doi: 10.3899/jrheum.111229. [DOI] [PubMed] [Google Scholar]
- 48.Henes JC, Horger M, Amberger C, et al. Enteric-coated mycophenolate sodium for progressive systemic sclerosis – a prospective open-label study with CT histography for monitoring pulmonary fibrosis. Clin Rheumatol. 2013;32:673–8. doi: 10.1007/s10067-012-2155-5. [DOI] [PubMed] [Google Scholar]
- 49.Panopoulos ST, Bournia VK, Trakada G, Giavri I, Kostopoulos C, Sfikakis PP. Mycophenolate versus cyclophosphamide for progressive interstitial lung disease associated with systemic sclerosis: a 2-year case control study. Lung. 2013;191:483–9. doi: 10.1007/s00408-013-9499-8. [DOI] [PubMed] [Google Scholar]
- 50.Nadashkevich O, Davis P, Fritzler M, Kovalenko W. A randomized unblinded trial of cyclophosphamide versus azathioprine in the treatment of systemic sclerosis. Clin Rheumatol. 2006;25:205–12. doi: 10.1007/s10067-005-1157-y. [DOI] [PubMed] [Google Scholar]
- 51.Daoussis D, Liossis SN, Tsamandas AC, et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) 2010;49:271–80. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordan S, Distler JHW, Maurer B, Huscher D, van Laar JM, Allanore Y, Distler O on behalf of the EUSTAR Rituximab study group. doi: 10.1136/annrheumdis-2013-204522. [DOI] [Google Scholar]
- 53.Van Laar JM, Farge D, Sont JK, et al. for the EBMT/EULAR Scleroderma Study Group. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis. A randomized clinical trial. JAMA. 2014;311:2490–8. doi: 10.1001/jama.2014.6368. [DOI] [PubMed] [Google Scholar]
- 54.Burt RK, Shah SJ, Dill K, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomized phase 2 trial. Lancet. 2011;378:498–506. doi: 10.1016/S0140-6736(11)60982-3. [DOI] [PubMed] [Google Scholar]
- 55.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antonelli A, Ferri C, Ferrari SM, et al. Peroxisome proliferator-activated receptor γ agonists reduce cell proliferation and viability and increase apoptosis in systemic sclerosis fibroblasts. Br J Dermatol. 2013;168(1):129–35. doi: 10.1111/j.1365-2133.2012.11199.x. [DOI] [PubMed] [Google Scholar]
- 57.Bogatkevich GS, Highland KB, Akter T, Silver RM. The PPARγ Agonist Rosiglitazone Is Antifibrotic for Scleroderma Lung Fibroblasts: Mechanisms of Action and Differential Racial Effects. Pulm Med. 2012:545172. doi: 10.1155/2012/545172. Epub 2011 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei J, Ghosh AK, Sargent JL, et al. PPARγ downregulation by TGFβ in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS One. 2010;5(11):e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timar O, Szekanecz Z, Kerekes G, et al. Rosuvastatin improves impaired endothelial function, lowers high sensitivity CRP, complement and immuncomplex production in patients with systemic sclerosis--a prospective case-series study. Arthritis Res Ther. 2013;15:R105. doi: 10.1186/ar4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruben EC, Manuel VR, Agustin OR, et al. Ciprofloxacin utility as antifibrotic in the skin of patients with scleroderma. J Dermatol. 2010;37:323–9. doi: 10.1111/j.1346-8138.2010.00826.x. [DOI] [PubMed] [Google Scholar]
- 61.Atanelishvili I, Liang J, Akter T, et al. Thrombin increases lung fibroblast survival while promoting alveolar epithelial cell apoptosis via the endoplasmic reticulum stress marker, CCAAT enhancer-binding homologous protein. Am J Respir Cell Mol Biol. 2014;50:893–902. doi: 10.1165/rcmb.2013-0317OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenbloom J, Mendoza FA, Jimenez SA. Strategies for anti-fibrotic therapies. Biochimica et Biophysica Acta. 2013;1832:1088–1103. doi: 10.1016/j.bbadis.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki Y, Hayakawa H, Miwa S, et al. Intravenous immunoglobulin therapy for refractory interstitial lung disease associated with polymyositis/dermatomyositis. Lung. 2009;187(3):201–6. doi: 10.1007/s00408-009-9146-6. [DOI] [PubMed] [Google Scholar]
- 64.De Cruz S, Ross D. Lung transplantation in patients with scleroderma. Curr Opin Rheumatol. 2013;25:714–8. doi: 10.1097/01.bor.0000434670.39773.a8. [DOI] [PubMed] [Google Scholar]
- 65.Shitrit D, Amitai A, Peled N, et al. Lung transplantation in patients with scleroderma: case series, review of the literature, and criteria for transplantation. Clin Transplant. 2009;23:178–83. doi: 10.1111/j.1399-0012.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 66.Schachna L, Medsger TA, Jr, Dauber JH, et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54:3954–61. doi: 10.1002/art.22264. [DOI] [PubMed] [Google Scholar]
- 67.Khan IY, Singer LG, de Perrot M, et al. Survival after lung transplantation in systemic sclerosis. A systematic review. Respir Med. 2013;107:2081–7. doi: 10.1016/j.rmed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 68.Fisichella PM, Reder NP, Gagermeier J, Kovacs EJ. Usefulness of pH monitoring in predicting the survival status of patients with scleroderma awaiting lung transplantation. J Surg Res. 2014;189:232–7. doi: 10.1016/j.jss.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]