Abstract
BACKGROUND
Many alcoholics and heavy drinkers undergo repeated cycles of alcohol abstinence followed by relapse to alcohol drinking; a pattern that contributes to escalated alcohol intake over time. In rodents, alcohol drinking that is interspersed with periods of alcohol deprivation (imposed abstinence) increases alcohol intake during reaccess to alcohol. This is termed the “alcohol deprivation effect” or “ADE” and is a model of alcohol relapse in humans. We have previously reported that prazosin reduces alcohol drinking during both brief and prolonged treatment in rats selectively bred for alcohol preference (“P” rats). This study explores whether prazosin prevents alcohol “relapse” in P rats, as reflected by a reduced or abolished ADE.
METHODS
Adult male P rats were given 24-hour access to food and water and scheduled access to alcohol (15% and 30% v/v solutions presented concurrently) for 2 hrs/day. After 5 weeks rats underwent imposed alcohol deprivation for 2 weeks, followed by alcohol reaccess for 2 weeks, and this pattern was repeated for a total of 3 cycles. Rats were injected with prazosin (0, 0.5, 1.0, or 2.0 mg/kg BW, IP) once a day for the first 5 days of each alcohol reaccess cycle.
RESULTS
Alcohol intake increased on the first day of each alcohol reaccess cycle, demonstrating the formation of an ADE. The ADE was short-lived, lasting only 1 day, during each of the three cycles. Prazosin, in all doses tested, prevented the expression of an ADE in all three alcohol reaccess cycles.
CONCLUSIONS
Prazosin decreases alcohol intake in P rats even in a situation that would be expected to increase alcohol drinking, namely following periods of alcohol deprivation. This suggests that prazosin may be effective in reducing alcohol relapse that often occurs during attempts to achieve permanent alcohol abstinence in treatment-seeking alcoholics and heavy drinkers.
Keywords: alcohol preferring rats, prazosin, alcohol deprivation effect (ADE), alcohol relapse
Alcoholism is the most prevalent and widespread of all addictive diseases in the world and development of effective treatments is a high priority. Alcoholism is a chronic illness (Duka et al, 2002; Heinz et al, 2003) and alcoholics often find it difficult to maintain abstinence once it is achieved (Dawson et al, 2007; Hunt et al, 1971; Schuckit et al, 1997). Relapse to heavy drinking, after both short and prolonged periods of alcohol abstinence, is a common feature of alcoholism (American Psychiatric Association, 2000; Barrick and Connors, 2002). Depending on the definition of alcohol relapse used (Chiauzzi, 1991; Hunt et al, 1971), relapse rates over a 12-month period post-treatment can range from 50%, if relapse is defined as a return to pretreatment drinking levels (Armor et al, 1978), to as high as 90% if consumption of a single drink is used to define relapse (Orford and Edwards, 1977). Alcohol dependent individuals often repeatedly attempt to achieve abstinence without permanent success and hence undergo multiple alcohol withdrawal/detoxification episodes (Schuckit et al, 1997; Barrick and Connors, 2002). Multiple previous detoxifications are associated with heavier alcohol drinking during alcohol relapse (Malcolm et al, 2000).
Because relapse to alcohol drinking is a hallmark of alcoholism, animal models that exhibit this characteristic are useful for developing behavioral and pharmacological approaches that have the potential to attenuate or prevent alcohol relapse. Just like humans, rats also exhibit an increase in alcohol drinking when given reaccess to alcohol after a period of imposed “abstinence” (Sinclair and Senter, 1967; Heyser et al, 1997; Spanagel et al, 1996; Wolffgramm and Heyne, 1995). This increased alcohol intake that occurs when access to alcohol is reinstated following a period of alcohol deprivation in rodents is termed the “alcohol deprivation effect” or “ADE” (Sinclair and Senter, 1967; Heyser et al, 1997; Wolffgramm and Heyne, 1995). The ADE is thought to reflect an increase in the reinforcing value of alcohol (Heyser et al, 1997; McKinzie et al, 1998; Rodd-Henricks et al, 2000b; Spanagel et al, 1996; Wolffgramm and Heyne, 1995) and is not thought to be merely a consequence of physiological withdrawal since it is evident long after obvious withdrawal symptoms have disappeared which usually occurs within 1 week following termination of drinking in rats (Cicero et al., 1971). Instead, the ADE has been attributed to increased motivation to obtain alcohol due to increased alcohol craving during periods of alcohol withdrawal and abstinence (Heyser et al, 1997; Holter et al, 2000; Sinclair and Li, 1989). Because the ADE in rats is characterized by increased motivation to obtain alcohol (Sinclair and Li, 1989), is present even after prolonged periods of alcohol deprivation (McKinzie et al, 1998; Sinclair and Li, 1989; Sinclair et al, 1973) and is hardly modified by external stimuli such as taste adulterations (Spanagel et al, 1996), it is regarded by many as a good animal model of alcohol relapse in alcoholics (Froehlich and Li, 1991; Holter et al, 1998; 2000; McKinzie et al, 1998; Spanagel et al, 1996). Rats selectively bred for alcohol preference and high voluntary alcohol drinking (alcohol preferring or P rats) demonstrate a robust ADE in a variety of alcohol deprivation (imposed abstinence) conditions (McBride et al, 2002; McKinzie et al, 1998; Rodd-Henricks et al, 2000a; 2000b; 2001; Sinclair and Li, 1989) and are regarded by many as an appropriate animal analog of alcohol relapse in humans (Holter et al, 1998; 2000; McKinzie et al, 1998; Spanagel et al, 1996).
To date, only three medications have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of alcohol dependence and relapse: disulfiram (Antabuse) approved in 1949, naltrexone (Trexan) approved in 1994 and acamprosate (Campral) approved in 2004. Only about 20% of eligible patients receive these medications and, even when medications are given, not all alcohol dependent subjects respond well to them regardless of which medication is used. Clearly, a need exists for other agents to treat populations of alcohol dependent individuals who do not respond to the FDA approved medications that are currently available. One goal of our research program is to increase the number of pharmacotherapeutic agents available for the treatment of alcohol abuse, dependence and relapse. The physiologic processes and pathways that underlie alcohol relapse in humans and the alcohol deprivation effect in rats are not yet well understood but classic symptoms such as hyperactivity and anxiety have been noted during periods of alcohol deprivation in humans and rats and are thought to reflect central nervous system (CNS) hyperexcitability (Rasmussen et al, 2001). Alcohol, which is both anxiolytic and sympatho-suppressive (Koob and Le Moal, 1997; Kushner et al, 1990; Kushner et al, 1999), alleviates these symptoms and hence alcohol relapse in alcoholics is often viewed as an attempt to self-medicate (American Psychiatric Association, 2000; Cappell and LeBlanc, 1979; Koob, 2003).
One mechanism that underlies both anxiety and hyperexcitability in humans and rats is sympathetic activation and considerable evidence suggests that noradrenergic activation promotes and maintains alcohol drinking in rodents and humans. Prazosin is a postsynaptic α1-adrenergic receptor antagonist that blocks brain α1-adrenergic receptors that mediate central noradrenergic signaling (Menkes, 1981). Prazosin blocks noradrenergic excitation of the mesolimbic dopaminergic system (Sommermeyer et al, 1995) which plays a role in mediating the reinforcing properties of drugs of abuse, including alcohol (Weiss et al, 1992). Unlike other clinically available α1-adrenergic receptor antagonists, prazosin crosses the blood-brain barrier and is centrally active when administered systemically in clinically relevant doses. Prazosin was introduced in 1973 under the trade name “Minipress” as an antihypertensive drug, and has been used chronically by millions of people for hypertension (Lund-Johansen et al, 1993) and for urinary symptoms associated with benign prostatic hypertrophy (Hieble and Ruffolo, 1996). It has a long safety and clinical compliance record, and it is not associated with significant adverse side effects (Graham, 1984).
As part of our long-standing research program on the identification and characterization of potential pharmacotherapeutic agents for the treatment of alcoholism using a rodent model of alcoholism (Froehlich et al, 2003; Froehlich, 2010; Froehlich and Li, 1991), we have become interested in prazosin. Anxiolyic agents, such as prazosin, may substitute for the anxiolytic effects of alcohol and thereby reduce motivation to drink and reduce alcohol intake. In a series of preclinical studies we have found that prazosin decreased alcohol drinking/self-administration in many experimental conditions: in rats selectively bred for high voluntary alcohol drinking during both acute (Rasmussen et al, 2009) and prolonged (Froehlich et al, 2013a) treatment; when administered alone or in combination with naltrexone (Froehlich et al, 2013b); when administered during reaccess to alcohol following periods of alcohol deprivation (Rasmussen et al, 2009); and when administered during acute alcohol withdrawal (Walker et al, 2008). In humans, prazosin has been used to treat posttraumatic stress disorder (PTSD) where it reduces hyperarousal, overall PTSD severity (Raskind et al, 2003) and, parenthetically, alcohol drinking (Raskind et al, 2009). Prazosin also decreases relapse drinking in alcohol-dependent men without PTSD (Simpson et al, 2009) and a preliminary study reports that prazosin decreases stress- and cue-induced alcohol craving in alcohol-dependent individuals (Fox et al, 2012).
In the current study we examine the potential link between CNS hyperexcitability and alcohol relapse (“ADE” in rats) by determining whether a reduction of sympathetic activation, via prazosin treatment, might reduce the magnitude of, or even eliminate, the ADE in P rats. Such a finding would suggest that prazosin might be useful for reducing alcohol relapse in alcoholics and heavy drinkers.
MATERIALS AND METHODS
Subjects
Forty alcohol-naïve male rats from the 64th generation of selective breeding for alcohol preference (P line) were used in this study. The subjects were between 40–43 days of age and weighed 160 – 223 g at the start of the study. The rats were individually housed in stainless steel hanging cages located in an isolated vivarium with controlled temperature (21±1°C) and lighting conditions (a 12 hour light/dark cycle with lights off at 1000 hrs). Standard rodent chow (Laboratory Rodent Diet #7001, Harlan Teklad, Madison, WI) and water were available ad libitum throughout the study. All experimental procedures were approved by the Indiana University Institutional Animal Care and Use Committee and conducted in strict compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Alcohol Drinking Induction
All rats were provided with 24 hour (h) access to food, water, a 15% (v/v) alcohol solution and a 30 % (v/v) alcohol solution for 7 days a week for 5 weeks. In order to maximize alcohol intake in a 2-hour daily alcohol access period, all rats underwent a “step-down” procedure in which access to alcohol (15% v/v and 30% v/v) was reduced from 24 h/day to 2 h/day, 7 days a week, over the course of 14 weeks. Specifically, alcohol access was first reduced from 24 h to 6h/day, 7 days a week, for 4 weeks, then to 2 h/day, 7 days a week, for 5 weeks prior to initiation of alcohol deprivation and reaccess cycles. Rats were maintained with ad libitum access to food and water and scheduled access to a concurrent 3 bottle free-choice between water and 2 concentrations of alcohol (15% and 30% v/v) for 2 h/day (1000–1200 hrs), 7 days a week, throughout the experiment, except during periods of alcohol deprivation. After 5 weeks of daily 2h alcohol access, all rats were given imposed alcohol deprivation for 2 weeks followed by 2 weeks of reaccess to alcohol for 2 hrs a day and this cycle of alcohol access followed by alcohol deprivation was repeated three times. Food and water were continuously available during the alcohol deprivation periods. Rats in the control group were given access to water, but not alcohol, throughout the experiment. Alcohol intake (g/kg BW/2 hrs) and water intake (mls/kg BW/2 hrs and mls/kg BW/day) were recorded daily. Body weight was recorded daily during drug treatment and twice weekly at all other times. Multiple concentrations of alcohol were used to increase the magnitude of the ADE in P rats (Rodd-Hendricks, 2001).
Experimental Design
After completion of the alcohol step-down procedure and 5 weeks of access to alcohol for 2 hrs a day, all rats were given multiple cycles of alcohol drinking and imposed alcohol deprivation and were treated with either prazosin or vehicle during the first 5 consecutive days of reaccess to alcohol in each cycle. Three cycles were given and each cycle involved alcohol deprivation for 2 weeks followed by 2 weeks of alcohol reaccess. This duration of alcohol access and deprivation have been found to be optimal for producing a large ADE in P rats (Rodd-Henricks et al. 2000a; 2000b; 2001).
The effects of prazosin on alcohol drinking was assessed during reaccess to alcohol following each of 3 alcohol deprivation periods. Four groups of rats received one of 4 doses of prazosin during reaccess to alcohol following each of 3 alcohol deprivation periods. Prazosin or saline was administered once a day at 15 min prior to onset of the daily 2 hour alcohol access period for the first 5 consecutive days of each of 3 alcohol reaccess periods. The remaining days of each alcohol reaccess period were not accompanied by drug treatment but alcohol access was maintained at 2 h/day. Alcohol intake, water intake, and body weight were recorded daily at the end of the 2 hour alcohol access period.
Alcohol Solutions
Alcohol solutions were prepared by diluting 95% alcohol (ethanol) with distilled, deionized water (D2H2O) to make a 15% (v/v) and a 30% (v/v) solution. The two alcohol solutions and water were presented concurrently in three separate calibrated glass drinking tubes. The positions of the tubes were rotated daily in order to prevent development of a side preference. Fluid intake was recorded to the nearest milliliter. Alcohol intake was converted from ml alcohol/kg BW to g alcohol/kg BW.
Drug Preparation and Delivery
Prazosin Hydrochloride (Fluka Chemical Corp., Milwaukee, WI) was dissolved in water and then diluted 1:1 with 0.9% (w/v) NaCl to achieve a 0.45% (w/v) saline solution. Prazosin (0.5 mg/ml/kg BW) was injected intraperitoneally (IP) in a dose of 0.5, 1.0, or 2.0 mg/kg BW. Due to limited solubility of prazosin in saline, increasing the dose of prazosin was achieved by increasing the volume injected (1.0 to 4.0 ml/kg BW). Rats in the control group were injected IP with vehicle (0.45% NaCl) in a volume of 3.0 ml/kg BW. All rats were handled, as if they were going to receive an IP injection, for 5 consecutive days prior to prazosin administration in order to reduce the stress associated with drug administration. This dose range was chosen because we have previously found that prazosin in doses of 1.0 – 2.0 mg/kg BW suppresses alcohol drinking (Rasmussen et al, 2009).
Assigning Rats to Treatment Groups
During the week prior to the first deprivation, baseline alcohol and water intake were calculated for each rat over 5 consecutive days and the rats were ranked in descending order in terms of average daily alcohol consumption. Rats were assigned to drug treatment groups in a manner that ensured that the groups did not differ in baseline alcohol intake prior to prazosin administration (Froehlich et al, 2013a; 2013b). Specifically, the top four drinkers were randomly assigned to one of the four prazosin treatment groups (0, 0.5, 1.0, or 2.0 mg/ml/kg BW, I.P.), followed by the next 4 highest alcohol drinkers, until all groups were complete.
Data Analyses
To test for the presence of an ADE, mean alcohol intake during the last 2 days prior to onset of alcohol deprivation was compared with alcohol intake on day 1 of alcohol reaccess using a two-way rm ANOVA (dose X day) with repeated measures on day in each of the 3 alcohol reaccess cycles. Fisher’s least significant difference (LSD) test was used to further analyze significant dose and day effects. To evaluate whether the ADE lasted for more than 1 day, we used this same approach, a two-way rm ANOVA (dose X day) with repeated measures on day, to compare alcohol intake during the last 2 days prior to onset of alcohol deprivation with alcohol intake on day 2 of alcohol reaccess in each of the 3 alcohol reaccess cycles. Fisher’s LSD was used to further analyze significant dose and day effects. To evaluate the effect of prazosin treatment on alcohol intake during the 5 days of prazosin treatment, a two-way rm ANOVA (dose X day) with repeated measures on day was used. Fisher’s LSD was used to further analyze significant dose and day effects. To evaluate alcohol intake following termination of prazosin treatment, mean alcohol intake during the last two days prior to onset of alcohol deprivation was compared to mean alcohol intake on both day 1 and day 2 following termination of prazosin treatment using a two-way rm ANOVAs (dose X day) with repeated measures on day. Fisher’s LSD test was used to further analyze significant dose and day effects. To compare the magnitude of prazosin-induced suppression of alcohol intake across the three alcohol reaccess cycles, percent suppression was calculated in each prazosin dose group on each day by taking the average alcohol intake in the vehicle group, subtracting each rat’s alcohol intake from the average intake in the vehicle group and dividing by average intake in the vehicle group. The mean percent suppression for each rat was averaged over the 5 days of prazosin treatment in each dose group and in each alcohol reaccess cycle. A two-way rm ANOVA (dose x cycle), with repeated measures on cycle, was performed and followed by Fisher’s LSD.
To evaluate the effects of prazosin on water intake in each of the three alcohol reaccess cycles, a one-way ANOVA was performed comparing mean water intake in each animal during the 5 days of prazosin treatment at each prazosin dose. Fisher’s LSD was used to further analyze significant dose effects.
Data are presented as mean ± SEM and significance was accepted at p<0.05, unless otherwise stated. Data were analyzed for extreme scores using the Dixon test (Dixon, 1950) with a conservative cutoff (p<0.01) which resulted in the deletion of 6 values: 4 in the vehicle group (day 1, cycle 1; day 1, cycle 2; day 4, cycle 3; day 5, cycle 3), 1 in the 1.0 mg/kg prazosin group (day 2, cycle 1), and 1 in the 2.0 mg/kg prazosin group (day 1, cycle 1).
RESULTS
Alcohol Intake During Scheduled Access to Alcohol
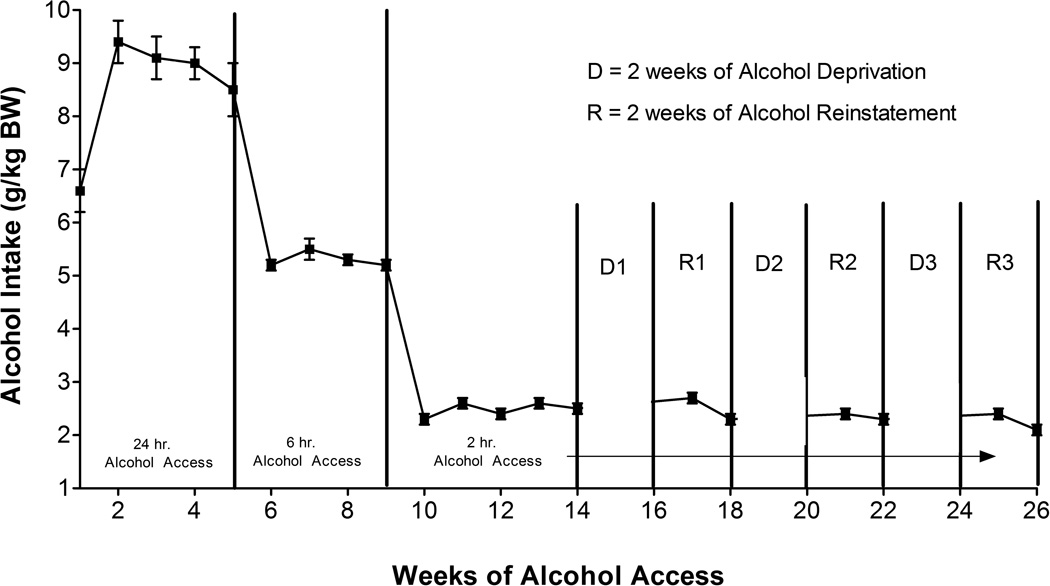
When given a 24-hour free-choice between two alcohol solutions (15% v/v and 30% v/v) and water for five weeks, rats of the P line consumed in excess of 9 g alcohol/kg BW/day by week 2 (Figure 1). When access to alcohol was reduced to 6 h/day for 4 weeks, P rats consumed in excess of 5 g alcohol/kg BW/day and when access to alcohol was further reduced to 2 h/day for 5 weeks (prior to initiation of alcohol deprivation), P rats consumed an average of 2.3 g alcohol/kg BW. These levels of alcohol consumption agree well with our prior findings in P rats (Froehlich et al, 2013a; Froehlich et al, 2013b; Rasmussen et al, 2009).
Fig. 1.
Alcohol intake (7 days/week) in P rats given scheduled access to alcohol (15% and 30% v/v) for 14 weeks prior to onset of 3 cycles of alcohol deprivation (D) for 2 weeks followed by 2 weeks of alcohol reaccess (R). Each point represents the mean ± SE.
Effects of Prazosin on alcohol and water intake following the first alcohol deprivation
With regard to alcohol intake, when alcohol intake prior to alcohol deprivation was compared with alcohol intake during reaccess to alcohol on day 1, there was an effect of dose, F (3, 34) = 2.7, p = 0.06, no effect of day, and a significant interaction, F (3, 34) = 4.68, p <0.01. Fisher’s LSD test revealed that alcohol intake increased significantly in the vehicle-treated group on the first day of alcohol reaccess compared with intake during predeprivation (p <0.05), indicating the presence of an alcohol deprivation effect (ADE) (Figure 2). Prazosin in doses of 0.5, 1.0 and 2.0 mg/kg BW eliminated the development of the ADE and a dose of 0.5 mg/kg BW prazosin actually suppressed alcohol drinking below predeprivation baseline levels (p =0.01)(Figure 2). When alcohol intake prior to alcohol deprivation was compared with alcohol intake during reaccess to alcohol on day 2, there was no effect of dose or day and no significant interaction, indicating that the ADE did not last for more than one day during the first alcohol reaccess cycle. When the effect of prazosin on alcohol intake during days 1–5 of alcohol reaccess was analyzed there was no effect of dose or day and no significant interaction. When the effect of terminating prazosin treatment on alcohol intake was examined, there was no effect of dose, an effect of day, F (2, 72) = 4.0, p <0.05, and no significant interaction. Fisher’s LSD test revealed that alcohol intake was lower on post-drug day 1 when compared with predeprivation alcohol intake (p<0.01).
Fig. 2.
Effect of daily prazosin treatment (0, 0.5, 1.0 or 2.0 mg/kg, IP) on 2-hour alcohol intake (g/kg BW) in P rats during the first alcohol reaccess cycle. Prazosin was injected prior to onset of alcohol access on each of the 5 days of alcohol reaccess. Each bar represents the mean ± SE. Predeprivation vs day 1 in the vehicle group = *p<0.05; predeprivation vs day 1 in the 0.5 mg/kg prazosin group = *p<0.05.
With regard to water intake during the first alcohol reaccess cycle, when mean water intake during prazosin treatment (days 1–5 of alcohol reaccess) was analyzed there was no significant effect of dose (Table 1) suggesting that prazosin did not alter water intake during the first alcohol reaccess cycle.
TABLE 1.
Effect of daily prazosin treatment (0, 0.5, 1.0 or 2.0 mg/kg, IP) for 5 consecutive days on mean water intake (ml/kg BW± SEM/2 hr) in each of the three alcohol reaccess cycles. Prazosin was injected prior to onset of alcohol access on each of the 5 days of alcohol reaccess. Mean ± SE. Vehicle vs prazosin treatment
| ALCOHOL REACCESS 1 | |||
|---|---|---|---|
| Mean water intake during 5 days of Prazosin Treatment |
Post Drug Day 1 | Post Drug Day 2 | |
| VEH | 4.1 | 1.6 | 1.3 |
| ± 1.5 | ± 0.4 | ± 0.5 | |
| 0.5 mg | 5.2 | 1.4 | 0.9 |
| ± 2.3 | ± 0.4 | ± 0.4 | |
| 1.0 mg | 7.5 | 3.2 | 2.8 |
| ±4.9 | ± 0.6 | ± 1.2 | |
| 2.0 mg | 6.6 | 2.8 | 2.2 |
| ± 2.3 | ± 0.9 | ± 0.6 | |
| ALCOHOL REACCESS 2 | |||
|---|---|---|---|
| Mean water intake during 5 days of Prazosin Treatment |
Post Drug Day 1 | Post Drug Day 2 | |
| VEH | 2.6 | 2.3 | 0.7 |
| ± 1.0 | ± 0.8 | ± 0.4 | |
| 0.5 mg | 8.29** | 2.3 | 1.6 |
| ± 2.9 | ± 0.7 | ± 0.6 | |
| 1.0 mg | 9.32** | 3.4 | 2.4 |
| ± 3.9 | ± 1.0 | ± 0.6 | |
| 2.0 mg | 5.79* | 1.3 | 1.3 |
| ± 3.0 | ± 0.8 | ± 0.7 | |
| ALCOHOL REACCESS 3 | |||
|---|---|---|---|
| Mean water intake during 5 days of Prazosin Treatment |
Post Drug Day 1 | Post Drug Day 2 | |
| VEH | 1.8 | 1.3 | 1.6 |
| ± 0.8 | ± 0.6 | ± 0.5 | |
| 0.5 mg | 8.6** | 2.2 | 2.6 |
| ± 1.7 | ± 0.9 | ± 0.4 | |
| 1.0 mg | 9.2** | 2.5 | 2.5 |
| ± 3.8 | ± 0.7 | ± 0.8 | |
| 2.0 mg | 4.2* | 1.6 | 2.2 |
| ± 1.8 | ± 0.5 | ± 0.5 | |
p<0.05
p<0.001.
Effects of Prazosin on alcohol and water intake following the second alcohol deprivation
With regard to alcohol intake, when alcohol intake prior to alcohol deprivation was compared with alcohol intake during reaccess to alcohol on day 1, there was an effect of dose, F (3, 34) = 3.3, p = 0.05, an effect of day F (1, 34) = 8.4, p<0.01, and a significant interaction, F (3, 34) = 8.1, p <0.001. Fisher’s LSD test revealed that alcohol intake increased significantly in the vehicle-treated group on the first day of alcohol reaccess compared with intake during predeprivation (p <0.05), indicating the presence of an alcohol deprivation effect (ADE) (Figure 3). Prazosin in doses of 0.5, 1.0 and 2.0 mg/kg BW eliminated the development of the ADE and prazosin in doses of 0.5 mg/kg BW (p<0.05) and 2.0 mg/kg BW (p<0.001) suppressed alcohol drinking below predeprivation baseline levels (Figure 3). When alcohol intake prior to alcohol deprivation was compared with alcohol intake during reaccess to alcohol on day 2, there was no effect of dose, a significant effect of day F (1, 36) = 6.2, p< 0.05 and a significant interaction F (3, 36) = 3.3, p<0.05. Fisher’s LSD test revealed that alcohol intake in the vehicle-treated group was not increased on day 2 when compared with predeprivation alcohol intake indicating that the ADE did not last for more than one day during the second alcohol reaccess cycle. When the effect of prazosin on alcohol intake during days 1–5 of alcohol reaccess was analyzed, there was an effect of dose, F (3, 136), = 5.9, p<0.01, no effect of day and no significant interaction. Fisher’s LSD revealed that prazosin in doses of 0.5 , 1.0 and 2.0 mg/kg BW decreased alcohol intake compared with intake in the vehicle-treated group across the 5 days of prazosin treatment (p<0.01 for all doses). When the effect of terminating prazosin treatment on alcohol intake was examined, there was no effect of dose, an effect of day, F (2, 68) = 5.4, p <0.01, and no significant interaction. Fisher’s LSD test revealed that alcohol intake was lower on post-drug days 1 and 2 when compared with predeprivation alcohol intake (p<0.01 and p<0.05 respectively).
Fig. 3.
Effect of daily prazosin treatment (0, 0.5, 1.0 or 2.0 mg/kg IP) on 2-hour alcohol intake (g/kg BW) in P rats during the second alcohol reaccess cycle. Prazosin was injected prior to onset of alcohol access on each of the 5 days of alcohol reaccess. Each bar represents the mean ± SE. Predeprivation vs day 1 in the vehicle group = *p<0.05; predeprivation vs day 1 in the 0.5 mg/kg prazosin group = *p<0.05; predeprivation vs day 1 in the 2.0 mg/kg prazosin group = **p < 0.001. Vehicle vs prazosin treatment across all 5 days of alcohol reaccess for 0.5 mg/kg = † p < 0.01, for 1.0 mg/kg = † p < 0.01, for 2.0 mg.kg =† p < 0.01.
With regard to water intake during the second alcohol reaccess cycle, when mean water intake during prazosin treatment (days 1–5 of alcohol reaccess) was analyzed there was a significant effect of dose F (3, 34) = 10.6, p<0.001) (Table 1). Fisher’s LSD test revealed that prazosin, in doses of 0.5, 1.0 and 2.0 mg/kg BW, increased water intake when compared with intake in the vehicle-treated group (p< 0.001, p<0.001, p<0.05, respectively).
Effects of Prazosin on alcohol and water intake following the third alcohol deprivation
With regard to alcohol intake, when alcohol intake prior to alcohol deprivation was compared with alcohol intake during reaccess to alcohol on day 1, there was a significant effect of dose, F (3, 34) = 5.8, p <0.01, day F (1, 34) = 14.8, p<0.001, and a significant interaction, F (3, 34) = 8.8, p <0.001. Fisher’s LSD test revealed that alcohol intake increased significantly in the vehicle- treated group on the first day of alcohol reaccess compared with intake during predeprivation (p <0.05), indicating the presence of an ADE (Figure 4). Prazosin in doses of 0.5, 1.0 and 2.0 mg/kg BW eliminated the development of the ADE and prazosin in doses of 0.5 mg/kg BW (p<0.01) and 1.0 mg/kg BW (p<0.01) suppressed alcohol drinking below predeprivation baseline levels (Figure 4). When alcohol intake prior to alcohol deprivation was compared with alcohol intake during reaccess to alcohol on day 2, there was no effect of dose, a significant effect of day F (1, 34) = 21.2, p<0.001, and a significant interaction, F (3, 34) = 5.6, p <0.01. Fisher’s LSD revealed that alcohol intake in the vehicle-treated group was not increased on day 2 when compared with predeprivation intake, indicating that the ADE did not last for more than 1 day during the third alcohol reaccess cycle. When the effect of prazosin on alcohol intake during days 1–5 of alcohol reaccess was analyzed, there was an effect of dose, F (3, 34), = 9.1, p<0.001, no effect of day and a significant interaction, F (12, 134) = 2.2, p<0.05. Fisher’s LSD revealed that prazosin in doses of 0.5 and 1.0 mg/kg BW decreased alcohol intake compared with intake in the vehicle-treated group across the 5 days of prazosin treatment (p<0.001 for both doses) and that prazosin in a dose of 2.0 mg/kg BW decreased alcohol intake compared with intake in the vehicle-treated group on days 1, 2, and 3 of alcohol reaccess (p<0.01, p<0.05, and p<.01, respectively). When the effect of terminating prazosin treatment on alcohol intake was examined, there was no effect of dose, an effect of day, F (2, 67) = 15.7, p <0.001, and no significant interaction. Fisher’s LSD test revealed that alcohol intake was lower on post-drug days 1 and 2 when compared with predeprivation alcohol intake (p<0.001 and p<0.001, respectively).
Fig. 4.
Effect of daily prazosin treatment (0, 0.5, 1.0 or 2.0 mg/kg IP) on 2-hour alcohol intake (g/kg BW) in P rats during the third alcohol reaccess cycle. Prazosin was injected prior to onset of alcohol access on each of the 5 days of alcohol reaccess. Each bar represents the mean ± SE. Predeprivation vs. day 1 in the vehicle group = *p<0.05; predeprivation vs day 1 in the 0.5 mg/kg and 1.0 mg/kg BW prazosin groups = **p<0.01. Vehicle vs. prazosin in doses of 0.5mg/kg BW and 1.0 mg/kg BW across all 5 days of alcohol reaccess = †p < 0.001.
With regard to water intake during the third alcohol reaccess cycle, when mean water intake during prazosin treatment (days 1–5 of alcohol reaccess) was analyzed there was a significant effect of dose F (3, 34) = 23.1, p<0.001) (Table 1). Fisher’s LSD test revealed that prazosin, in doses of 0.5, 1.0, and 2.0 mg/kg BW, increased water intake when compared with intake in the vehicle-treated group (p< 0.001, p<0.001, p<0.05, respectively).
When the magnitude of prazosin-induced suppression of alcohol intake was compared across the three alcohol reaccess cycles, there was no effect of dose, a significant effect of cycle, F (2, 52) = 21.6 p<0.001, and no significant interaction. Fisher’s LSD tests revealed that the magnitude of suppression was significantly greater in cycles 2 and 3 compared to cycle 1 (p<0.001 and p<0.001, respectively) but the magnitude of suppression was not different in cycles 2 and 3.
DISCUSSION
We have previously shown that prazosin decreases both acute and chronic ongoing alcohol drinking in P rats (Froehlich et al, 2013a; 2013b; Rasmussen et al, 2009; 2014). We hypothesized that prazosin might also prevent the development of an ADE or “alcohol relapse”. To test this possibility, P rats were subjected to imposed cycles of alcohol deprivation followed by return of access to alcohol and reinstatement of alcohol drinking, a pattern that is similar to the one that is experienced by many alcohol dependent individuals who abstain from drinking then ‘fall off the wagon’ or relapse.
An increase in alcohol drinking (“relapse”) was seen in the current study during reaccess to alcohol following alcohol deprivation in all three alcohol reaccess cycles. This may be due, in part to several features of the experimental design that have previously been demonstrated to increase the reliability and robustness of the ADE in rats. These include single housing of rats which eliminates competition for access to alcohol, a free-choice between alcohol and water with food freely available, as opposed to operant self-administration of alcohol (McKinzie et al, 1998), establishing a stable baseline of chronic alcohol drinking prior to initiation of imposed abstinence, repeated 2-week cycles of alcohol drinking and imposed abstinence, and presenting rats with a concurrent free-choice between water and multiple alcohol concentrations rather than a single alcohol concentration (10 % v/v) during the alcohol reaccess period (Bell et al, 2003; McBride et al, 2002; Rodd-Henricks et al, 2000a; 2001), which more closely parallels the variety of alcohol choices available to alcoholics when they fail to abstain and relapse to drinking. However, the magnitude of the ADE in P rats is dependent in part on the duration of alcohol availability which may explain why the magnitude of the ADE in the current study and other studies with limited access to alcohol (McKinzie et al, 1998) is not as great as it is in paradigms where rats are given 24 hour access to alcohol (Rodd-Henricks et al, 2000a; 2000b).
The presence of an ADE in P rats in the current study supports a number of prior reports (McBride et al, 2002; McKinzie et al, 1998; Rodd-Henricks et al, 2000a; 2001; Sinclair and Li, 1989), but the fact that an ADE was seen even in the first alcohol reaccess cycle does not support the view that the expression of an ADE in selectively bred rat lines is dependent on repeated deprivations (Rodd-Henricks et al, 2000b). In the current study, the ADE was also relatively short-lived, lasting only 1 day during each of the three alcohol reaccess cycles, which contrasts with prior reports of a prolonged ADE in P rats (Rodd-Henricks et al, 2000a; 2001).
Prazosin, in all doses tested, prevented the development of an ADE in all three alcohol reaccess cycles. Prazosin also suppressed alcohol intake across the 5 days of prazosin treatment during alcohol reaccess cycles 2 and 3, and the magnitude of the prazosin-induced suppression of alcohol intake was greater, and persisted longer, during the second and third alcohol reaccess cycles when compared with the first. An increase in efficacy of prazosin over the course of repeated administration agrees well with prior reports of enhanced efficacy of prazosin for reducing PTSD symptomology (Raskind et al., 2013), cocaine-induced locomotor activity (Jiménez-Rivera et al., 2006), and cholesterol (Neusy and Lowenstein, 1986). However, increased efficacy of prazosin with repeated administration is not inevitable since prazosin does not become more effective at reducing blood pressure (Levy, 1989) or cocaine self-administration (Zhang and Kosten, 2005). The prazosin-induced increase in water intake, which accompanied the decrease in alcohol drinking, agrees well with our prior reports and those of others (Froehlich et al, 2013a; 2013b, Oryan et al, 2003; Rasmussen et al, 2009).
Relapse is one of the most significant problems facing recovering alcoholics (Miller, 1996) and the discovery of an effective treatment for preventing alcohol relapse is a high priority. In the current study rats underwent three cycles of alcohol deprivation and reaccess to alcohol, which is analogous to alcoholics who repeatedly attempt to remain abstinent but are not successful and relapse to alcohol drinking (“fall off the wagon”). The results suggest that prazosin may be useful in decreasing alcohol relapse in alcoholics and heavy drinkers who are trying to abstain. The fact that prazosin was effective when administered immediately prior to alcohol reaccess suggests that its utility may not require continuous treatment. Prazosin may be effective if used on an “as needed” basis: immediately prior to encountering situations known to represent a risk for relapse. The fact that the magnitude of the prazosin effect increased with repeated treatments also suggests that this drug may be uniquely valuable when used intermittently (“as needed”) rather than continuously.
Abstinence is not viewed by everyone as the only acceptable goal for alcoholics and heavy drinkers. Data suggest that a goal of reduced drinking or “controlled drinking” can be as valuable as abstinence for some drinkers. (Sobell and Sobell, 1973; Jellinek, 1960) and reduced drinking and behavioral self-control training is seen by some as a good first step toward abstinence (Miller, et al, 1991). Marlatt and colleagues have pointed out that, if drinking in moderation were not an option, many people would not seek treatment, even if the treatment ultimately leads to abstinence (Owen and Marlatt, 2001). Recently, Jaffe and colleagues reported that a program called “moderation management”, that employs a variety of self-help tools and is designed to achieve moderate drinking and harm reduction, rather than abstinence, is useful for increasing number of days abstinent per month and for reducing blood alcohol concentrations by 50% even on drinking days in heavy drinkers (Jaffe, 2011; Kosok, 2006). The results of the present study suggest that addition of prazosin to an array of self-help tools may further facilitate a reduction in drinking and movement toward abstinence. Because prazosin is orally active, safe, well-tolerated, and inexpensive, it holds promise as a potential pharmacotherapeutic agent for the treatment of alcohol relapse.
ACKNOWLEDGEMENTS
We thank Dr. Ting-Kai Li and the Indiana Alcohol Research Center for supplying the selectively bred rats used in this study. This work was supported by NIH Grants AA018604 (JCF and DDR), AA07611 (JCF) and AA13881 (DDR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Armor D, Polich J, Stambul H. Alcoholism and treatment. New York: Wiley; 1978. [Google Scholar]
- Barrick C, Connors GJ. Relapse prevention and maintaining abstinence in older adults with alcohol-use disorders. Drug Aging. 2002;19:583–594. doi: 10.2165/00002512-200219080-00004. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Kuc KA, Lumeng L, Li TK, Murphy JM, McBride WJ. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on the intake of ethanol by male and female periadolescent alcohol-preferring (P) rats. Alcohol. 2003;29:137–148. doi: 10.1016/s0741-8329(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE. Tolerance to, and physical dependence on, ethanol: why do we study them? Drug Alcohol Depend. 1979;4:15–31. doi: 10.1016/0376-8716(79)90038-3. [DOI] [PubMed] [Google Scholar]
- Chiauzzi EJ. Preventing relapse in the addictions: A biopsychosocial approach. New York: Pergamon Press; 1991. [Google Scholar]
- Cicero TJ, Snider SR, Perez VJ, Swanson LW. Physical dependence on and tolerance to alcohol in the rat. Physiol Behav. 1971;6:191–198. doi: 10.1016/0031-9384(71)90088-6. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: A 3-year follow-up. Alcohol Clin Exp Res. 2007;31(12):2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Analysis of extreme values. Annals of Mathematical Statistics. 1950;21:488–506. [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Kindling of withdrawal: A study of craving and anxiety after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2002;26:785–795. [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC. What aspects of human alcohol use disorders can be modeled using selectively bred rat lines? Substance Use and Misuse. 2010;45:1727–1741. doi: 10.3109/10826084.2010.482424. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Li TK. Animal models for the study of alcoholism: Utility of selected lines. J Addictive Diseases. 1991;10:61–71. doi: 10.1300/J069v10n01_05. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, O'Malley S, Hyytia P, Davidson D, Faren C. Preclinical and clinical studies on naltrexone: What have they taught each other? Alcoholism: Clinical and Experimental Research. 2003;27:533–539. doi: 10.1097/01.ALC.0000057943.57330.AB. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, Rasmussen DD. Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcoholism: Clinical and Experimental Research. 2013a;37:1552–1560. doi: 10.1111/acer.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer B, Rasmussen DD. Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcoholism: Clinical and Experimental Research. 2013b;37:1763–1770. doi: 10.1111/acer.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RM. Selective alpha 1-adrenergic antagonists: Therapeutically relevant antihypertensives. Am J Cardiol. 1984;53:16A–20A. doi: 10.1016/0002-9149(84)90829-4. [DOI] [PubMed] [Google Scholar]
- Heinz A, Schafer M, Higley JD, Krystal JH, Goldman D. Neurobiological correlates of the disposition and maintenance of alcoholism. Pharmacopsychiatry. 2003;36(Suppl 3):255–258. doi: 10.1055/s-2003-45139. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- Hieble JP, Ruffolo RRJ. The use of alpha-adrenoceptor antagonists in the pharmacological management of benign prostatic hypertrophy: An overview. Pharmacol Res. 1996;33:145–160. doi: 10.1006/phrs.1996.0022. [DOI] [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety related behavior during ethanol deprivation in rats. Behavioral Pharmacology. 1998;9:41–48. [PubMed] [Google Scholar]
- Holter SM, Linthorst AC, Reul JM, Spanagel R. Withdrawal symptoms in a long-term model of voluntary alcohol drinking in Wistar rats. Pharmacology, Biochemistry and Behavior. 2000;66:143–151. doi: 10.1016/s0091-3057(00)00196-9. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Jaffe A. Abstinence is not the only option: Learning to drink responsibly with help. 2011 Psychology Today ( http://www.psychologytoday.com/node/56573.
- Jellinek EM. The disease concept of alcoholism. New Haven: Hillhouse; 1960. [Google Scholar]
- Jiménez-Rivera CA, Feliu-Mojer M, Vázquez-Torres Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Ann NY Acad Sci. 2006;1074:390–402. doi: 10.1196/annals.1369.039. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kosok A. The moderation management programme in 2004: What type of drinker seeks controlled drinking? International Journal of Drug Policy. 2006;17:295–303. [Google Scholar]
- Kushner MG, Sher KJ, Beitman BD. The relation between alcohol problems and the anxiety disorders. Am J Psychiatry. 1990;147:685–695. doi: 10.1176/ajp.147.6.685. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Erickson DJ. Prospective analysis of the relation between DSM-III anxiety disorders and alcohol use disorders. Am J Psychiatry. 1999;156:723–732. doi: 10.1176/ajp.156.5.723. [DOI] [PubMed] [Google Scholar]
- Levy P. Effects of prazosin on blood pressure and diabetic control in patients with type II diabetes mellitus and mild essential hypertension. Am J Med. 1989;86:59–86. doi: 10.1016/0002-9343(89)90132-0. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen P, Hjermann I, Iversen BM, Thaulow E. Selective alpha-1 inhibitors: First- or second-line antihypertensive agents? Cardiology. 1993;83:150–159. doi: 10.1159/000175963. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts JS, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000;22:159–164. doi: 10.1016/s0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Le AD, Noronha A. Central nervous system mechanisms in alcohol relapse. Alcohol Clin Exp Res. 2002;26:280–286. [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li T-K. The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcoholism: Clinical and Experimental Research. 1998;22:1170–1176. [PubMed] [Google Scholar]
- Menkes DB, Baraban JM, Aghajanian GK. Prazosin selectively antagonizes neuronal responses mediated by α1-adrenoceptors in brain. Naunyn-Schmiedeberg's Arc Pharmacol. 1981;317:273–275. doi: 10.1007/BF00503830. [DOI] [PubMed] [Google Scholar]
- Miller WR. What is a relapse? Fifty ways to leave the wagon. Addiction. 1996;91:15–27. [PubMed] [Google Scholar]
- Miller WR, Page AC. Warm turkey: Other routes to abstinence. J Substance Abuse Treat. 1991;8:227–232. doi: 10.1016/0740-5472(91)90043-a. [DOI] [PubMed] [Google Scholar]
- Neusy AJ, Lowenstein J. Effects of prazosin, atenolol, and thiazide diuretic on plasma lipids in patients with essential hypertension. Am J Med. 1986;80:94–99. doi: 10.1016/0002-9343(86)90166-x. [DOI] [PubMed] [Google Scholar]
- Orford J, Edwards G. Alcoholism: A comparison of treatment and advice, with a study of influence of marriage. New York: Maudsley Monographs No 26, Oxford University Press; 1977. [Google Scholar]
- Oryan S, Eidi M, Eidi A, Kohanrooz B. Effect of α1-adrenoceptors and muscarinic cholinoceptors on water intake in rats. European Journal of Pharmacology. 2003;477:123–127. doi: 10.1016/j.ejphar.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Owen P, Marlatt GA. Should abstinence be the goal for alcohol treatment? The American Journal on Addictions. 2001;10:289–295. [PubMed] [Google Scholar]
- Raskind MA. Sustained recovery from chronic alcohol dependence with prazosin treatment of PTSD. Alcohol Clin Exp Res. 2009;33(Suppl):311A. [Google Scholar]
- Raskind MA, Peskind ER, Kanter KD, Petrie EC, Radant A, Thompson CE, Dobie DJ, Rein RJ, Straits-Tröster K, Thomas RG, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, Homas D, Hill J, Daniels C, Calohan J, Millard JP, Rohde K, O’Connell J, Pritzl D, Feiszli K, Petrie EC, Gross C, Mayer CL, Freed MC, Engel C, Peskind ER. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003–1010. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The α1 adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcoholism: Clinical and Experimental Research. 2009;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen D, Beckwith LE, Kincaid CL, Froehlich JC. Combining the α1-adrenergic receptor antagonist, prazosin, with the β-adrenergic receptor antagonist, propranolol, reduces alcohol drinking more effectively than either drug alone. Alcoholism: Clinical and Experimental Research. 2014 doi: 10.1111/acer.12441. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li T-K. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcoholism: Clinical and Experimental Research. 2000a;24:8–16. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li T-K. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000b;24:747–753. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcoholism: Clinical and Experimental Research. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Bucholz KK. Periods of abstinence following the onset of alcohol dependence in 1,853 men and women. J Stud Alcohol. 1997;58:581–589. doi: 10.15288/jsa.1997.58.581. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Increased preference for ethanol in rats following alcohol deprivation. Psychonomic Science. 1967;8:11–12. [Google Scholar]
- Sinclair JD, Li T-K. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC. Alcoholics treated by individualized behavior therapy: One year treatment outcome. Behaviour Research and Therapy. 1973;11:599–618. doi: 10.1016/0005-7967(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Sommermeyer H, Frielingsdorf J, Knorr A. Effects of prazosin on the dopaminergic neurotrtansmission in rat brain. Eur J Pharmacol. 1995;276:267–270. doi: 10.1016/0014-2999(95)00062-p. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Hölter SM, Allingham K, Landgraf R, Zieglgansberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. The effects of α1-noradrenergic receptor antagonism on dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Hurd YL, Ungerstedt U, Markou A, Plotsky PM, Koob GF. Neurochemical correlates of cocaine and ethanol self-administration. Annals N Y Acad Sci. 1992;654:220–241. doi: 10.1111/j.1749-6632.1992.tb25970.x. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behavioural Brain Research. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an α-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]