Abstract
Background:
Titanium has been the most popular material of choice for dental implantology over the past few decades. Its properties have been found to be most suitable for the success of implant treatment. But recently, zirconia is slowly emerging as one of the materials which might replace the gold standard of dental implant, i.e., titanium.
Materials and Methods:
Literature was searched to retrieve information about zirconia dental implant and studies were critically analyzed. PubMed database was searched for information about zirconia dental implant regarding mechanical properties, osseointegration, surface roughness, biocompatibility, and soft tissue health around it. The literature search was limited to English language articles published from 1975 to 2015.
Results:
A total of 45 papers met the inclusion criteria for this review, among the relevant search in the database.
Conclusion:
Literature search showed that some of the properties of zirconia seem to be suitable for making it an ideal dental implant, such as biocompatibility, osseointegration, favourable soft tissue response and aesthetics due to light transmission and its color. At the same time, some studies also point out its drawbacks. It was also found that most of the studies on zirconia dental implants are short-term studies and there is a need for more long-term clinical trials to prove that zirconia is worth enough to replace titanium as a biomaterial in dental implantology.
Keywords: Biocompatibility, mechanical properties, osseointegration, surface roughening, titanium dental implant, zirconia, zirconia and biocompatibility, zirconia and implant surface coating, zirconia and osseointegration, zirconia dental implant
INTRODUCTION
Dental implants have improved the quality of life for many patients.[1] Currently titanium and titanium alloys are most widely used as dental implants due to their excellent biocompatibility, good mechanical properties, and long term follow-up in clinical success.[2,3] Even though titanium is a popular material, it has certain disadvantages such as greyish color, which is unaesthetic, especially in the anterior region where the gingival tissue is considerably thin.[4] Some studies have also reported of galvanic reaction that occurs after it comes in contact with saliva and fluoride.[5] Inflammatory response and bone resorption were also found to be induced due to titanium particles.[6]
In the last few years, zirconia dental implant has emerged as an alternative for titanium implant due to its potential to osseointegrate[7,8,9] and having other beneficial properties like its translucency and white color which mimics the natural teeth.[10,11] It is radiopaque similar to titanium and can be easily visualized on the radiograph.[12] Bacterial colonization around zirconia is found to be less as compared to that with titanium.[13] Some studies have reported that zirconia has more biocompatibility as compared to titanium, as the latter produces corrosion products at the implant site.[5]
This review of literature aims to discuss various properties of zirconia like osseointegration, biocompatibility, and less bacterial colonization, which make it a biomaterial suitable to be used as dental implant, and tries to find out whether the researches done till date authenticate its use.
MATERIALS AND METHODS
Literature search was done from 1975 to 2015 in PubMed database regarding mechanical properties, osseointegration, biocompatibility, soft tissue response, and antibacterial adhesion properties of zirconia. Literature search was only limited to English language articles. Keywords used in literature search were Zirconia Dental Implant, Zirconia AND Osseointegration, Zirconia AND soft tissue response, Zirconia AND Biocompatibility. Abstract were screened and full texts of potentially eligible articles were obtained. All articles on surface coating of zirconia on implant surfaces, are excluded from the review.
A total of 45 papers met the inclusion criteria for the review. All of these papers included in-vitro studies, in-vivo studies and case reports. Results of the literature search were discussed under different sections.
Mechanical properties of zirconia implants
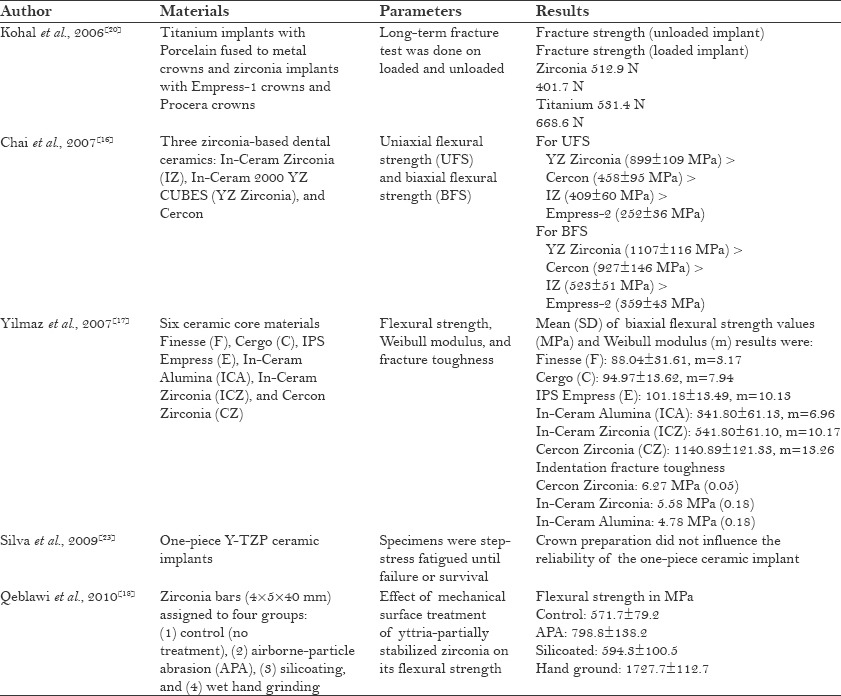
Yttria-stabilized tetragonal zirconia polycrystalline (Y-TZP) materials exhibit superior corrosion and wear resistance, as well as a high flexural strength (800–1000 MPa) compared to other dental ceramics[14,15,16,17] [Table 1]. It was also found that flexural strength of zirconia increases by mechanical modification of its surface.[18] When the compressive strength of blade type of zirconia implants was tested, it was found to be adequate in occlusion.[19] Fracture strength (512.9 N) of unloaded zirconia was found to be more than the fracture strength (401.7 N) of loaded zirconia[20] [Table 1]. A study performed by Kohal et al. found low fracture strength of two-piece zirconia implants in both loaded and unloaded conditions, due to which they were not recommended for clinical use[21] [Table 1]. It was also found that the implant preparation and cyclic loading decrease the fracture strength of one-piece zirconia implants, but these values were still within clinically acceptable limits to withstand average occlusal forces even after an extended interval of artificial loading.[22] Whereas Silva et al. reported in their study that crown preparation had no influence on the reliability of one-piece ceramic implant[23] [Table 1].
Table 1.
Mechanical properties of zirconia
ZrO2 is a polymorphic material and occurs in three forms: Monoclinic, tetragonal, and cubic. The monoclinic phase is stable at room temperatures up to 1170°C, the tetragonal at temperatures of 1170–2370°C, and the cubic form at over 2370°C.[24,25] Alloying pure zirconia with stabilizing oxides, such as CaO, MgO, Y2O3, or CeO2, allows the retention of the metastable tetragonal structure at room temperature. Dental procedures, such as grinding or sandblasting, can trigger a tetragonal to monoclinic transformation in the surface region.[15] Transformation from tetragonal phase to monoclinic phase is associated with volume expansion. This phase transformation results in compression of cracks, thereby retarding its growth and enhancing fracture toughness. This martensitic-like mechanism is known as transformation toughening.[26]
Due to severe environmental conditions of moisture and stress, the resulting zirconia may transform more aggressively to the monoclinic phase with catastrophic results. This type of high metastability is not good for dental implants. This mechanical property degradation in zirconia is known as “aging” of the material.[25] The transformation is enhanced in water or in vapor, while the most critical enhancing effects of temperature occur in the range of 200–300°C.[27,28] The transformation from tetragonal to monoclinic starts from surface and progresses to the core of the material. When the monoclinic phase dominates, it leads to reduction in strength, toughness, and density, which in turn leads to microcracking on the surface. This microcrack formation leads to penetration of water and causes corrosion.[27] Low temperature degradation of the material involves roughening, increased wear and microcracking, grain pull-out, generation of particle debris, and premature failure.[29] The aging process depends on various factors like porosity, residual stresses, grain size, and the content of stabilizer.[30] It was found that decrease in grain size and increase in stabilizing oxide content reduce the transformation rate.[28] Aging is accelerated due to changes in processing technique and can be avoided by more accurate processing.[27] Some in vitro studies have found that the aging reduces the mechanical properties of zirconia, even though within clinical acceptable limits, in simulated dental treatment conditions.[31,32]
Osseointegration of zirconia implants
One of the most important criteria for the success of implant treatment is osseointegration. Bone apposition takes place on different types of implant surfaces and depends on surface roughness of the implant.[33,34] Studies have shown that zirconia coating on the surface of titanium implants favours bone apposition,[35,36] which was found to be more than that of titanium implants with no coating.
Akagawa et al., in their study, found no significant difference in bone implant contact (BIC) between the loaded and unloaded zirconia implants. The BIC was 81.9% for the unloaded group and 69.8% for the loaded group[7] [Table 2].
Table 2.
Osseointegration of zirconia

Another study which examined the role of osseointegration around one-stage zirconia screw implant under various conditions for loading showed no difference in bone contact ratio among the single freestanding, connected freestanding, and implant-tooth supports of partially stabilized zirconia implants.[37] These findings were in agreement with another study which compared the BIC of submerged zirconia and non-submerged zirconia implants with submerged titanium as the control[38] [Table 2].
When BIC of zirconia implants was compared with that of titanium and alumina, there was no statistical difference between the BIC of all three types of implants.[39] Relatively bone healing around zirconia implants was found to be more than around titanium implants.[40] Some studies indicated that the zirconia implants might withstand occlusal loads over a longer period of time.[37,41]
Similar rate of bone apposition on zirconia and surface-modified titanium implant surfaces during early healing was found when a histological examination of early bone apposition around zirconia dental implants at 2 and 4 weeks after insertion was compared to that of surface-modified titanium implants.[42] There was no difference in osseointegration between acid-etched zirconia implants and acid-etched titanium implants.[43,44,45] This was true even when the implant surfaces were pharmacologically and chemically modified[46] [Table 2].
Surface roughness of zirconia implants
While direct bone apposition can occur on different types of surfaces, it has been demonstrated that a certain degree of surface roughness is beneficial in accelerating bone apposition to the implant surface.[33,34] Since reduced treatment time is practiced more commonly in implant dentistry, the smooth surface of zirconia implants appears to be a disadvantage.[47] A study performed to investigate the osteoblastic response to Y-TZP with different surface topographies to increase the surface roughness by airborne particle abrasion and additionally acid etching showed cell proliferation with statistically significant higher values on day 3 for surface-treated zirconia as compared with machined zirconia. But no differences were found between the zirconia groups and sandblasted/acid-etched (SLA) titanium at 6 and 12 days.[48] It also found that roughening the zirconia implants enhances bone apposition and has a beneficial effect on the interfacial shear strength,[49] which was later contradicted by Hoffmann et al.[50]
High hardness of the zirconia implants makes the process of surface roughening very difficult. So, recently, laser has been used to engrave a pattern on the zirconia surface. A scanning electron microscopic (SEM) study done to find the influence of erbium-doped yttrium aluminium garnet (Er: YAG), carbon dioxide (CO2), and diode laser irradiation on the surface properties of polished zirconia implants demonstrated that diode and Er: YAG lasers did not cause any visible surface alterations. However, the CO2 laser produced distinct surface alterations to zirconia.[51]
Measurement of osseointegration
Torque removal forces have been used as a biomechanical measure of anchorage or osseointegration in which the greater forces required to remove implants may be interpreted as an increase in the strength of osseointegration.[52]
In the study of Sennerby et al., it was found that coated zirconia implants and titanium implants showed higher removal torque value than the machined zirconia implants. The findings suggested that surface-modified zirconia implants can reach firm stability in bone.[53] In another study wherein the removal torque values of machined zirconia implants, sandblasted zirconia implants, and acid-etched titanium implant were evaluated, machined zirconia had the least removal torque value. Acid-etched titanium implants had the highest removal torque value, followed by sandblasted zirconia implants. The findings suggest that sandblasted zirconia implants can achieve a higher stability in bone than machined zirconia implants.[49] Even when the zirconia was coated on titanium implants, it increased the removal torque value.[54] But in one of the studies that compared the biomechanical properties of six types of implant surfaces, it was found that removal torque value of zirconia implants was the least.[55]
It can be concluded that the removal of torque value of zirconia implants was improved after surface modification, but was not more than that of titanium implants.
Biocompatibility of zirconia implants
Various in vitro tests were conducted on osteoblasts, fibroblasts, lymphocytes, monocytes, and macrophages to test the biocompatibility of zirconia. It was observed that zirconia had no cytotoxic effect on osteoblasts and made the cells capable of elaborating the extracellular matrix by synthesizing various essential and structural proteins.[56] Zirconia does not induce pseudo-teratogenic effect, which makes it biocompatible.[57,58,59] Laser-modified zirconia showed better adhesion to osteoblasts due to the better wettability characteristics.[41] Zirconia does not provoke any inflammation pathway, as reported by Liagre et al.[60]
Wear products of zirconia could be cytotoxic as compared to titanium and other ceramics, when tested with fibroblasts.[61] But it was also noted that further studies are required to substantiate the evidence. Both powder and particles of zirconia tested in vitro on different cell lines (human and murine) of lymphocytes, monocytes, or macrophages did not induce high cytotoxicity or inflammation.[62]
Biocompatibility tests were also conducted in vivo for zirconia, and it was found that when it was implanted in the soft tissue, it became encapsulated by a thin layer of fibrous tissue similar to that seen in the case of alumina.[63,64] Also, there was no cytotoxicity in the soft tissue in relation to wear products of zirconia.[65] Zirconia was also found to be biocompatible to hard tissue when tested in vivo according to the findings of a study which inserted pellets of stabilized zirconia with 6% Y2O3 into the femur of monkeys.[66] When compared with alumina, zirconia showed no difference in bone reaction.[67,68] In the study by Kohal et al., it was found that cell proliferation around zirconia was comparable to titanium, but surface modification of zirconia did not show improvement in osseointegration.[69] Biocompatibility of zirconia was also found to be good in another study conducted by Gredes et al., in which they tested a newly created zirconia implant.[70]
Soft tissue response to zirconia implants
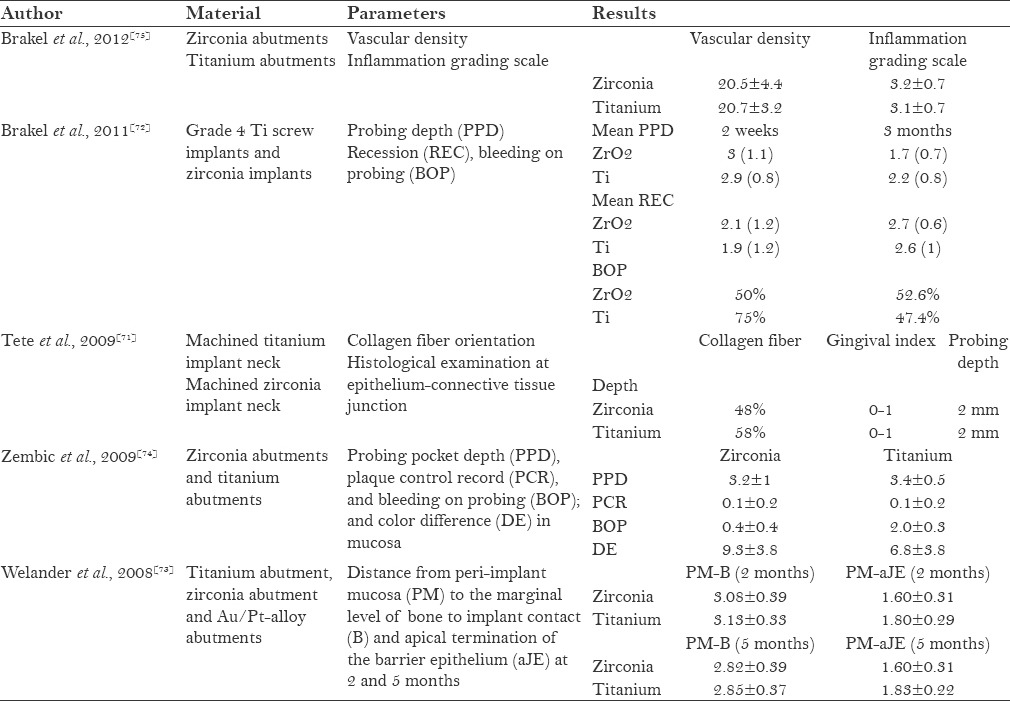
Studies conducted on the soft tissue response of zirconia implants [Table 3] have reported comparable findings for both zirconia and titanium. Tete et al. found that the collagen fiber orientation around zirconia implants was parallel to the implant surface, similar to that of titanium.[71] Brakel et al. reported that zirconia had similar probing depth as titanium.[72] Regarding the healing of soft tissue around the zirconia abutment and titanium abutment, it was reported by Wellander et al. that titanium had better soft tissue healing as compared to zirconia. The distance from the peri-implant mucosa to the apical termination of the barrier epithelium for zirconia was found to be less than that of titanium. The same study also found that zirconia had less mucosal color change as compared to titanium,[73] which was contradicted by Zembic et al.[74] Brakel et al. found no significant difference in the soft tissue response around zirconia and titanium abutments.[75] This finding was similar to the study finding of Kohal et al., wherein zirconia and titanium implants were inserted in the extraction sites of monkeys and both implants showed same peri-implant soft tissue dimensions.[9]
Table 3.
Soft tissue response to zirconia implants
Bacterial colonization around zirconia implants
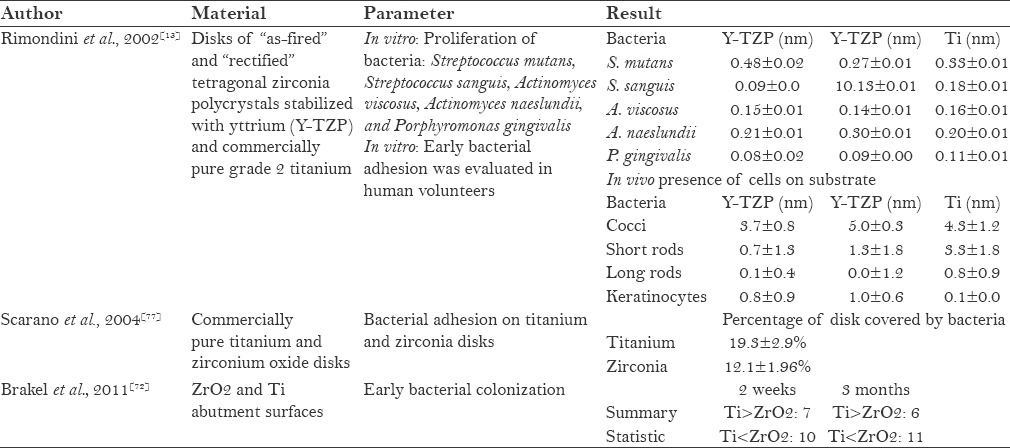
Bacterial colonisation is commonly found around the natural tooth due to humid environment and constant temperature inside the oral cavity.[76] Since the microflora around implants is similar to that of natural teeth, microbial pathogens (i.e. Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, or Prevotella intermedia) associated with periodontitis may contribute to implant failure.[76] When zirconia was introduced in orthopedics, some studies evaluated the adhesion of oral bacteria in vitro.[62] In a study which compared the inhibition of growth and adhesion of selected oral bacteria on titanium and zirconia, difference was found in the adhesion of some selected oral bacteria [Table 4]. But in an in vivo study, zirconia showed significantly lesser adhesion of bacteria than titanium,[13,77] which was contradicted by Brakel et al. and Egawa et al., who reported that the bacterial adhesion of zirconia was similar to that of titanium.[72,78]
Table 4.
Bacterial colonization around zirconia implants
With fewer studies on bacterial adhesion on zirconia surface, it can be concluded that plaque formation on this surface might be less.[72,79]
CONCLUSION
Limited amount of research on zirconia proves that zirconia is biocompatible with the surrounding tissues. Compared to titanium, its osseointegration is inferior and shows improvement after surface modification. Strength of zirconia is good, but comparatively lesser than that of titanium. Zirconia is osseoconductive as reported in some studies and has also shown favourable interaction with the soft tissue. It has been found that zirconia reduces plaque formation on the implant surface, which leads to good healing and successful implant treatment.
Most of the studies on zirconia implants are short-term studies and evidence of success in long-term clinical trials is lacking. More research is needed on zirconia dental implants before we could use it for frequent treatment needs, as compared to titanium implants.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kuboki T, Okamoto S, Suzuki H, Kanyama M, Arakawa H, Sonoyama W, et al. Quality of life assessment of bone-anchored fixed partial denture patients with unilateral mandibular distal-extension edentulism. J Prosthet Dent. 1999;82:182–7. doi: 10.1016/s0022-3913(99)70154-x. [DOI] [PubMed] [Google Scholar]
- 2.Depprich R, Zipprich H, Ommerborn M, Naujoks C, Wiesmann HP, Kiattavorncharoen S, et al. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008;4:30. doi: 10.1186/1746-160X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinemann SG. Titanium-the material of choice? Periodontol 2000. 1998;17:7–21. doi: 10.1111/j.1600-0757.1998.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 4.Heydecke G, Kohal R, Gläser R. Optimal esthetics in single-tooth replacement with the Re-Implant system: A case report. Int J Prosthodont. 1999;12:184–9. [PubMed] [Google Scholar]
- 5.Tschernitschek H, Borchers L, Geurtsen W. Nonalloyed titanium as a bioinert metal-A review. Quintessence Int. 2005;36:523–30. [PubMed] [Google Scholar]
- 6.Sterner T, Schütze N, Saxler G, Jakob F, Rader CP. Effects of clinically relevant alumina ceramic, zirconia ceramic and titanium particles of different sizes and concentrations on TNF-alpha release in a human macrophage cell line. Biomed Tech (Berl) 2004;49:340–4. doi: 10.1515/BMT.2004.063. [DOI] [PubMed] [Google Scholar]
- 7.Akagawa Y, Ichikawa Y, Nikai H, Tsuru H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993;69:599–604. doi: 10.1016/0022-3913(93)90289-z. [DOI] [PubMed] [Google Scholar]
- 8.Scarano A, Di Carlo F, Quaranta M, Piattelli A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J Oral Implantol. 2003;29:8–12. doi: 10.1563/1548-1336(2003)029<0008:BRTZCI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Kohal RJ, Weng D, Bächle M, Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: An animal experiment. J Periodontol. 2004;75:1262–8. doi: 10.1902/jop.2004.75.9.1262. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad I. Yttrium-partially stabilized zirconium dioxide posts: An approach to restoring coronally compromised nonvital teeth. Int J Periodontics Restorative Dent. 1998;18:455–65. [PubMed] [Google Scholar]
- 11.Jackson MC. Restoration of posterior implants using a new ceramic material. J Dent Technol. 1999;16:19–22. [PubMed] [Google Scholar]
- 12.Pekkan G, Pekkan K, Hatipoglu MG, Tuna SH. Comparative radiopacity of ceramics and metals with human and bovine dental tissues. J Prosthet Dent. 2011;106:109–17. doi: 10.1016/S0022-3913(11)60104-2. [DOI] [PubMed] [Google Scholar]
- 13.Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002;17:793–8. [PubMed] [Google Scholar]
- 14.Denry I, Kelly JR. State of the art of zirconia for dental applications. Dent Mater. 2008;24:299–307. doi: 10.1016/j.dental.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20:1–25. doi: 10.1016/s0142-9612(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 16.Chai J, Chu FC, Chow TW, Liang BM. Chemical solubility and flexural strength of Zirconia- based ceramics. Int J Proshodont. 2007;20:587–95. [PubMed] [Google Scholar]
- 17.Yilmaz H, Aydin C, Gul BE. Flexural strength and fracture toughness of dental core ceramics. J Prosthet Dent. 2007;98:120–8. doi: 10.1016/S0022-3913(07)60045-6. [DOI] [PubMed] [Google Scholar]
- 18.Qeblawi DM, Muñoz CA, Brewer JD, Monaco EA., Jr The effect of zirconia surface treatment on flexural strength and shear bond strength to a resin cement. J Prosthet Dent. 2010;103:210–20. doi: 10.1016/S0022-3913(10)60033-9. [DOI] [PubMed] [Google Scholar]
- 19.Minamizato T. Slip-cast zirconia dental roots with tunnels drilled by laser process. J Prosthet Dent. 1990;63:677–84. doi: 10.1016/0022-3913(90)90326-8. [DOI] [PubMed] [Google Scholar]
- 20.Kohal RJ, Klaus G, Strub JR. Zirconia-implant supported all-ceramic crowns withstand long-term load: A pilot investigation. Clin Oral Implants Res. 2006;17:565–71. doi: 10.1111/j.1600-0501.2006.01252.x. [DOI] [PubMed] [Google Scholar]
- 21.Kohal RJ, Finke HC, Klaus G. Stability of prototype two-piece zirconia and titanium implants after artificial aging: An in vitro pilot study. Clin Implant Dent Relat Res. 2009;11:323–9. doi: 10.1111/j.1708-8208.2008.00116.x. [DOI] [PubMed] [Google Scholar]
- 22.Kohal RJ, Wolkewitz M, Tsakona A. The effects of cyclic loading and preparation on the fracture strength of zirconium-dioxide implants: An in vitro investigation. Clin Oral Implants Res. 2011;22:808–14. doi: 10.1111/j.1600-0501.2010.02067.x. [DOI] [PubMed] [Google Scholar]
- 23.Silva NR, Coelho PG, Fernandes CA, Navarro JM, Dias RA, Thompson VP. Reliability of one-piece ceramic implant. J Biomed Mater Res B Appl Biomater. 2009;88:419–26. doi: 10.1002/jbm.b.31113. [DOI] [PubMed] [Google Scholar]
- 24.Chevalier J, Gremillard L, Virkar AV, Clarke DR. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J Am Ceram Soc. 2009;92:1901–20. [Google Scholar]
- 25.Suresh A, Mayo MJ, Porter WD, Rawn CJ. Crystallite and grain-size-dependent phase transformations in Yttria-Doped zirconia. J Am Ceram Soc. 2003;86:360–2. [Google Scholar]
- 26.Garvie RC, Hannink RH, Pascoe RT. Ceramic steel? Nature. 1975;258:703–4. [Google Scholar]
- 27.Sato T, Shimada M. Transformation of Yttria-Doped tetragonal ZrO2 polycrystals by annealing in water. J Am Ceram Soc. 1985;68:356–9. [Google Scholar]
- 28.Swab JJ. Low temperature degradation of Y-TZP materials. J Mater Sci. 1991;26:6706–14. [Google Scholar]
- 29.Chevalier J. What future for zirconia as a biomaterial? Biomaterials. 2006;27:535–43. doi: 10.1016/j.biomaterials.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 30.Deville S, Chevalier J, Gremillard L. Influence of surface finish and residual stresses on the ageing sensitivity of biomedical grade zirconia. Biomaterials. 2000;27:2186–92. doi: 10.1016/j.biomaterials.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe M, Iio S, Fukuura I. Ageing behaviour of Y-TZP. In: Claussen N, Ruhle M, Heuer AH, editors. Science and Technology of Zirconia II (Advances in Ceramics) Vol. 12. Columbus, OH, USA: The American Ceramic Society; 1984. pp. 391–8. [Google Scholar]
- 32.Att W, Grigoriadou M, Strub JR. ZrO2 three-unit fixed partial dentures: Comparison of failure load before and after exposure to a mastication simulator. J Oral Rehabil. 2007;34:282–90. doi: 10.1111/j.1365-2842.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- 33.Zechner W, Tangl S, Fürst G, Tepper G, Thams U, Mailath G, et al. Osseous healing characteristics of three different implant types. Clin Oral Implants Res. 2003;14:150–7. doi: 10.1034/j.1600-0501.2003.140203.x. [DOI] [PubMed] [Google Scholar]
- 34.Albrektsson T, Wennerberg A. Oral implant surfaces: Part 2--Review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 2004;17:544–64. [PubMed] [Google Scholar]
- 35.Sollazzo V, Pezzetti F, Scarano A, Piattelli A, Bignozzi CA, Massari L, et al. Zirconium oxide coating improves implant osseointegration in vivo. Dent Mater. 2008;24:357–61. doi: 10.1016/j.dental.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Franchi M, Bacchelli B, Martini D, Pasquale VD, Orsini E, Ottani V, et al. Early detachment of titanium particles from various different surfaces of endosseous dental implants. Biomaterials. 2004;25:2239–46. doi: 10.1016/j.biomaterials.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Akagawa Y, Hosokawa R, Sato Y, Kamayama K. Comparison between freestanding and tooth-connected partially stabilized zirconia implants after two years’ function in monkeys: A clinical and histologic study. J Prosthet Dent. 1998;80:551–8. doi: 10.1016/s0022-3913(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 38.Stadlinger B, Hennig M, Eckelt U, Kuhlisch E, Mai R. Comparison of zirconia and titanium implants after a short healing period. A pilot study in minipigs. Int J Oral Maxillofac Surg. 2010;39:585–92. doi: 10.1016/j.ijom.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Dubruille JH, Viguier E, Le Naour G, Dubruille MT, Auriol M, Le Charpentier Y. Evaluation of combinations of titanium, zirconia, and alumina implants with 2 bone fillers in the dog. Int J Oral Maxillofac Implants. 1999;14:271–7. [PubMed] [Google Scholar]
- 40.Schultze-Mosgau S, Schliephake H, Radespiel-Tröger M, Neukam FW. Osseointegration of endodontic endosseous cones: Zirconium oxide vs titanium. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:91–8. doi: 10.1016/s1079-2104(00)80022-0. [DOI] [PubMed] [Google Scholar]
- 41.Kohal RJ, Papavasiliou G, Kamposiora P, Tripodakis A, Strub JR. Three-dimensional computerized stress analysis of commercially pure titanium and yttrium-partially stabilized zirconia implants. Int J Prosthodont. 2002;15:189–94. [PubMed] [Google Scholar]
- 42.Hoffmann O, Angelov N, Gallez F, Jung RE, Weber FE. The zirconia implant-bone interface: A preliminary histologic evaluation in rabbits. Int J Oral Maxillofac Implants. 2008;23:691–5. [PubMed] [Google Scholar]
- 43.Depprich R, Zipprich H, Ommerborn M, Mahn E, Lammers L, Handschel J, et al. Osseointegration of zirconia implants: An SEM observation of the bone-implant interface. Head Face Med. 2008;4:25. doi: 10.1186/1746-160X-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gahlert M, Röhling S, Wieland M, Sprecher CM, Kniha H, Milz S. Osseointegration of zirconia and titanium dental implants: A histological andhistomorphometrical study in the maxilla of pigs. Clin Oral Implants Res. 2009;20:1247–53. doi: 10.1111/j.1600-0501.2009.01734.x. [DOI] [PubMed] [Google Scholar]
- 45.Gahlert M, Roehling S, Sprecher CM, Kniha H, Milz S, Bormann K. In vivo performance of zirconia and titanium implants: A histomorphometric study in mini pig maxillae. Clin Oral Implants Res. 2012;23:281–6. doi: 10.1111/j.1600-0501.2011.02157.x. [DOI] [PubMed] [Google Scholar]
- 46.Langhoff JD, Voelter K, Scharnweber D, Schnabelrauch M, Schlottig F, Hefti T, et al. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: A study in sheep. Int J Oral Maxillofac Surg. 2008;37:1125–32. doi: 10.1016/j.ijom.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Özkurt Z, Kazazoğlu E. Zirconia dental implants: A literature review. J Oral Implantol. 2011;37:367–76. doi: 10.1563/AAID-JOI-D-09-00079. [DOI] [PubMed] [Google Scholar]
- 48.Bächle M, Butz F, Hübner U, Bakalinis E, Kohal RJ. Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin Oral Implants Res. 2007;18:53–9. doi: 10.1111/j.1600-0501.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 49.Gahlert M, Gudehus T, Eichhorn S, Steinhauser E, Kniha H, Erhardt W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin Oral Implants Res. 2007;18:662–8. doi: 10.1111/j.1600-0501.2007.01401.x. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann O, Angelov N, Zafiropoulos GG, Andreana S. Osseointegration of zirconia implants with different surface characteristics: An evaluation in rabbits. Int J Oral Maxillofac Implants. 2012;27:352–8. [PubMed] [Google Scholar]
- 51.Stübinger S, Homann F, Etter C, Miskiewicz M, Wieland M, Sader R. Effect of Er: YAG, CO (2) and diode laser irradiation on surface properties of zirconia endosseous dental implants. Lasers Surg Med. 2008;40:223–8. doi: 10.1002/lsm.20614. [DOI] [PubMed] [Google Scholar]
- 52.Klokkevold PR, Nishimura RD, Adachi M, Caputo A. Osseointegration enhanced by chemical etching of the titanium surface. A torque removal study in the rabbit. Clin Oral Implants Res. 1997;8:442–7. doi: 10.1034/j.1600-0501.1997.080601.x. [DOI] [PubMed] [Google Scholar]
- 53.Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005;7(Suppl 1):S13–20. doi: 10.1111/j.1708-8208.2005.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 54.Alzubaydi TL, Alameer SS, Ismaeel T, Alhijazi AY, Geetha M. In vivo studies of the ceramic coated titanium alloy for enhanced osseointegration in dental applications. J Mater Sci Mater Med. 2009;20(Suppl 1):S35–42. doi: 10.1007/s10856-008-3479-1. [DOI] [PubMed] [Google Scholar]
- 55.Ferguson SJ, Langhoff JD, Voelter K, von Rechenberg B, Scharnweber D, Bierbaum S, et al. Biomechanical comparison of different surface modifications for dental implants. Int J Oral Maxillofac Implants. 2008;23:1037–46. [PubMed] [Google Scholar]
- 56.Josset Y, Oum’Hamed Z, Zarrinpour A, Lorenzato M, Adnet JJ, Laurent-Maquin D. In vitro reactions of human osteoblasts in culture with zirconia and alumina ceramics. J Biomed Mater Res. 1999;47:481–93. doi: 10.1002/(sici)1097-4636(19991215)47:4<481::aid-jbm4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 57.Torricelli P, Verné E, Brovarone CV, Appendino P, Rustichelli F, Krajewski A, et al. Biological glass coating on ceramic materials: In vitro evaluation using primary osteoblast cultures from healthy and osteopenic rat bone. Biomaterials. 2001;22:2535–43. doi: 10.1016/s0142-9612(00)00444-0. [DOI] [PubMed] [Google Scholar]
- 58.Lohmann CH, Dean DD, Köster G, Casasola D, Buchhorn GH, Fink U, et al. Ceramic and PMMA particles differentially affect osteoblast phenotype. Biomaterials. 2002;23:1855–63. doi: 10.1016/s0142-9612(01)00312-x. [DOI] [PubMed] [Google Scholar]
- 59.Bächle M, Butz F, Hübner U, Bakalinis E, Kohal RJ. Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin Oral Implants Res. 2007;18:53–9. doi: 10.1111/j.1600-0501.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 60.Liagre B, Moalic S, Vergne P, Charissoux JL, Bernache-Assollant D, Beneytout JL. Effects of alumina and zirconium dioxide particles on arachidonic acid metabolism and proinflammatory interleukin production in osteoarthritis and rheumatoid synovial cells. J Bone Joint Surg Br. 2002;84:920–30. doi: 10.1302/0301-620x.84b6.12457. [DOI] [PubMed] [Google Scholar]
- 61.Ito A, Tateishi T, Niwa S, Tange S. In vitro evaluation of the cytocompatibility of wear particles generated by UHMWPE/zirconia friction. Clin Mater. 1993;12:203–9. doi: 10.1016/0267-6605(93)90074-h. [DOI] [PubMed] [Google Scholar]
- 62.Hisbergues M, Vendeville S, Vendeville P. Zirconia: Established facts and perspectives for a biomaterial in dental implantology. J Biomed Mater Res B Appl Biomater. 2009;88:519–29. doi: 10.1002/jbm.b.31147. [DOI] [PubMed] [Google Scholar]
- 63.Christel P, Meunier A, Heller M, Torre JP, Peille CN. Mechanical properties and short-term in-vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J Biomed Mater Res. 1989;23:45–61. doi: 10.1002/jbm.820230105. [DOI] [PubMed] [Google Scholar]
- 64.Ichikawa Y, Akagawa Y, Nikai H, Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo. J Prosthet Dent. 1992;68:322–6. doi: 10.1016/0022-3913(92)90338-b. [DOI] [PubMed] [Google Scholar]
- 65.Styles JA, Wilson J. Comparison between in vitro toxicity of two novel fibrous mineral dusts and their tissue reaction in vivo. Ann Occup Hyg. 1976;19:63–8. doi: 10.1093/annhyg/19.1.63. [DOI] [PubMed] [Google Scholar]
- 66.Helmer JC, Driskell TD. South Carolina: Clemson University; 1969. Research on Bioceramics: Symposium on Use of Ceramics as Surgical Implants. [Google Scholar]
- 67.Wagner W, Rixecker H, Wahlmann UW. Morphometric comparison of histologic bone reactions after implantation of mono- and polycrystalline aluminium oxide pins. In: Christel P, Meunier A, Lee AJ, editors. Biological and Biomechanical Performance of Biomaterials. Amsterdam: Elsevier; 1986. pp. 129–34. [Google Scholar]
- 68.Christel PS. Zirconia: The second generation of ceramics for total hip replacement. Bull Hosp Jt Dis Orthop Inst. 1989;49:170–7. [PubMed] [Google Scholar]
- 69.Kohal RJ, Bächle M, Att W, Chaar S, Altmann B, Renz A, et al. Osteoblast and bone tissue response to surface modified zirconia and titanium implant materials. Dent Mater. 2013;29:763–76. doi: 10.1016/j.dental.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 70.Gredes T, Kubasiewicz-Ross P, Gedrange T, Dominiak M, Kunert-Keil C. Comparison of surface modified zirconia implants with commercially available zirconium and titanium implants: A histological study in pigs. Implant Dent. 2014;23:502–7. doi: 10.1097/ID.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 71.Tetè S, Mastrangelo F, Bianchi A, Zizzari V, Scarano A. Collagen fiber orientation around machined titanium and zirconia dental implant necks: An animal study. Int J Oral Maxillofac Implants. 2009;24:52–8. [PubMed] [Google Scholar]
- 72.van Brakel R, Cune MS, van Winkelhoff AJ, de Putter C, Verhoeven JW, van der Reijden W. Early bacterial colonization and soft tissue health around zirconia and titanium abutments: An in vivo study in man. Clin Oral Implants Res. 2011;22:571–7. doi: 10.1111/j.1600-0501.2010.02005.x. [DOI] [PubMed] [Google Scholar]
- 73.Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res. 2008;19:635–41. doi: 10.1111/j.1600-0501.2008.01543.x. [DOI] [PubMed] [Google Scholar]
- 74.Zembic A, Sailer I, Jung RE, Hämmerle CH. Randomized-controlled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin Oral Implants Res. 2009;20:802–8. doi: 10.1111/j.1600-0501.2009.01717.x. [DOI] [PubMed] [Google Scholar]
- 75.van Brakel R, Meijer GJ, Verhoeven JW, Jansen J, de Putter C, Cune MS. Soft tissue response to zirconia and titanium implant abutments: An in vivo within-subject comparison. J Clin Periodontol. 2012;39:995–1001. doi: 10.1111/j.1600-051X.2012.01931.x. [DOI] [PubMed] [Google Scholar]
- 76.Ong ES, Newman HN, Wilson M, Bulman JS. The occurrence of periodontitis-related microorganisms in relation to titanium implants. J Periodontol. 1992;63:200–5. doi: 10.1902/jop.1992.63.3.200. [DOI] [PubMed] [Google Scholar]
- 77.Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J Periodontol. 2004;75:292–6. doi: 10.1902/jop.2004.75.2.292. [DOI] [PubMed] [Google Scholar]
- 78.Egawa M, Miura T, Kato T, Saito A, Yoshinari M. In vitro adherence of periodontopathic bacteria to zirconia and titanium surfaces. Dent Mater J. 2013;32:101–6. doi: 10.4012/dmj.2012-156. [DOI] [PubMed] [Google Scholar]
- 79.Re D, Pellegrini G, Francinetti P, Augusti D, Rasperini G. In vivo early plaque formation on zirconia and feldspathic ceramic. Minerva Stomatol. 2011;60:339–48. [PubMed] [Google Scholar]


