Abstract
Background:
In daily practice of dentistry, we use same instruments on many patients. Before use, all instruments must be cleaned, disinfected, and sterilized to prevent any contamination. Pre-cleaning and sterilization of some devices can be difficult because of their small size and complex architecture. Dental burs and endodontic files are such instruments. Dental burs come in a variety of shapes and sizes, all with highly complex and detailed surface features.
Aim:
To determine the effectiveness of various disinfectants and sterilization techniques for disinfection and resterilization of dental burs and endodontic files.
Materials and Methods:
The materials used for the study were dental burs and endodontic files. Disinfectants used were Quitanet plus, glutaraldehyde, glass-bead sterilizer, and autoclave. The sterility of used dental burs and endodontic files was analyzed. Burs and files that had been used were pre-cleaned, resterilized, and then tested for various pathogens. Each item was transferred by sterile technique into Todd-Hewitt broth, incubated at 37°C for 72 h, and observed for bacterial growth.
Results:
The present study shows that the endodontic files and burs sterilized by autoclaving and glutaraldehyde showed complete sterilization. Burs and files immersed in glutaraldehyde (2.4%) for 12 h showed complete sterilization, whereas Quitanet plus solution and glass-bead sterilizer showed incomplete sterilization.
Conclusion:
The present study results indicate that autoclaving and glutaraldehyde (2.4%) showed complete sterilization. Other methods cannot be relied upon for sterilization.
Keywords: Disinfection, micro-organisms, sterilization, Todd-Hewitt broth
INTRODUCTION
Disinfection
It is the destruction or removal of all pathogenic micro-organisms which give rise to infection, but not necessarily their spore forms.
Sterilization
It is the process by which an article, surface, or medium is freed of all microorganisms either in the vegetative or spore state. With the whole world looking at the eradication of existing infectious diseases and preventing any new infections, sterilization of instruments is significant to ensure optimal patient care. In contemporary dental practice, the instruments directly come in contact with tissues, blood and tissue fluids, saliva, and gingival crevicular fluid which may seep through the rubber dam if not properly placed.
Dental health personnel are constantly exposed to the potential threat of developing an infection by occupational exposures to a variety of microbial pathogens; most common of all are hepatitis B virus (HBV), hepatitis C virus (HCV), tuberculosis (TB), and acquired immunodeficiency syndrome (AIDS).[1]
The prevention of cross-contamination of infectious diseases among dentists, dental staff, and patients is a major concern in dental practice. Also, aseptic technique is especially important in endodontics because microorganisms are the major cause of endodontic disease.
Diseases may be transmitted by indirect contact when dental instruments contaminated by one patient are reused for another patient without adequate disinfection or sterilization between uses.[2]
Apart from maxillofacial surgery, the potential for cross-infection is highest in the prosthodontic environment.[3] Dental burs have been identified as a source of cross-contamination between patient and dental personnel. They may become heavily contaminated with necrotic tissues, saliva, blood, and potential pathogens during use. However, it is difficult to do the pre-cleaning and sterilization of burs because of their complex architecture.[4]
Endodontic files are slender, tapered instruments, about 25 mm long, with intricate topography and spiral cutting edges, and are used for cleaning and shaping root canals during endodontic treatment. Because of their size and shape, it is difficult to remove all the biologic material during resterilization procedures.[5]
Hence, a strict sterilization protocol is essential as the risk of cross-infection is higher. Various methods have been proposed by many manufacturers for this purpose, namely, the autoclave, dry-heat sterilization, laser, chemical sterilization, etc.
Therefore, the present study was designed to investigate the pathological evaluation for sterilization of routinely used prosthodontic and endodontic instruments.
Aims and objectives
To investigate the effectiveness of various sterilization procedures commonly applied to used burs.
To investigate the effectiveness of various sterilization procedures commonly applied to used endodontic files.
MATERIALS AND METHODS
This is an in vitro study in which used burs and files were considered for sterilization. After sterilization, they were incubated for a period of 72 h. Ethical clearance was obtained from Sridevi Institute of Medical Sciences and Research Centre. Procedure and purpose of the study was explained to the participants from whom used burs and files were obtained, and their consent was taken. A total of 60 previously used No. 8 round-head carbon steel burs and 60 used endodontic No. 20 hand files were used in the present study.
Sampling
An unused bur was placed in a sterilized hand piece and used for cavity preparation. After use, the bur was removed from the hand piece. It was placed in a sterile tube and cleaned properly by scrubbing.
Similarly, an unused No. 20 file used for endodontic purpose was collected in a sterile tube and cleaned properly by scrubbing.
Out of the total 60 burs and 60 files, 12 burs and 12 files were taken as the control group (group E); the remaining 48 burs and 48 files were divided into four groups of 12 each and they were tested for the efficacy of sterilization with different methods such as autoclave (group A), glass bead (group B), glutaraldehyde (group C), and Quitanet plus solution (group D).
The 12 contaminated burs and files in group A were placed in an endodontic instrument box and subjected to autoclave at 121°C for 15 min at a pressure of 15 pounds.
The 12 contaminated burs and files in group B were wiped for 10 s with surgical spirit and placed in the periphery of the glass-bead sterilizer and sterilized for 45 s at 240°C.
The 12 contaminated burs and files in group C were placed in a sterile plastic container containing 2.4% glutaraldehyde solution and left in it for 12 h.
The 12 contaminated burs and files in group D were placed in a sterile plastic container containing Quitanet plus solution and left in it for 12 h.
The 12 contaminated burs and files in group E (control group) were put in separate tubes without doing any sterilization.
On completion of the procedure, the burs and files were transferred in a sterile fashion into test tubes containing a culture medium selected to grow oral bacteria (Todd-Hewitt broth). The control group comprised new unused instruments treated in an identical fashion before culturing. All samples (N = 120) were then placed in an incubator maintained at 37°C to mimic body temperature. The burs and files were examined daily over 72 h to check for evidence of bacterial growth. A color change, cloudy broth, and visible precipitate in the test tube were all considered as indicative of bacterial growth [Figure 1]. If the solution remained clear throughout the incubation period, the sample was considered sterile.
Figure 1.

Turbidity of test tubes for burs and files
RESULTS
In the study, the endodontic files sterilized by autoclaving in an instrument box at 121°C for 15 min at a pressure of 15 pounds (group A) showed total sterility.
Ten burs (83.3%) subjected to sterilization by glass-bead sterilizer and seven files (58.33%) after wiping for 10 s with surgical spirit and sterilized for 45 s at 240°C (group B) showed incomplete sterilization [Figures 2 and 3].
Figure 2.
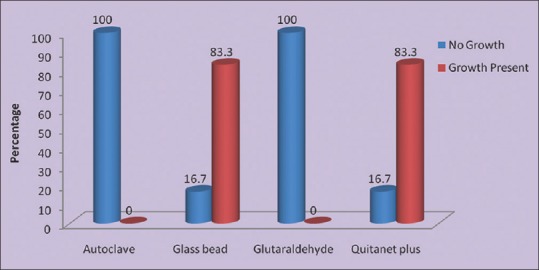
Sterilization efficiency of different methods for burs
Figure 3.

Sterilization efficiency of different methods for files
Burs and files sterilized by immersing in glutaraldehyde (2.4%) for 12 h (group C) showed complete sterilization.
Ten burs (83.3%) and nine files (75%) showed incomplete sterilization with Quitanet plus (group D) solution.
The control group (group E), for which the files after contamination were not sterilized by any method, showed growth in all samples.
Statistical analysis of the four sterilized groups using K analysis of variance (ANOVA) showed a statistically significant difference between groups with regard to their efficacies in sterilization of both with burs and files (P ≤ 0.05) [Tables 1 and 2].
Table 1.
Comparison of different methods of sterilization for burs

Table 2.
Comparison of different methods of sterilization for files

DISCUSSION
The risk of disease transmission is unknown in endodontic procedures; even if the risk of disease transmission is minimal during endodontic procedures, the high frequency of root canal treatments could increase the possibility of an adverse event.[5,6] This is one example of why it is so important to ensure that resterilization procedures are effective.
There are three principal methods currently available for sterilization of instruments: Steam under pressure (autoclave), dry heat, and chemiclave. Another method of sterilization, viz., laser, is also available but not widely used.
Many methods have been advocated for sterilization of endodontic instruments. Steam autoclaving and glass-bead sterilizers are among the commonly recommended methods of sterilization.
The goal of instrument sterilization in dentistry is to protect patients from cross-contamination via instruments.[7]
At present, only a few dental instruments cannot be sterilized and these are either disinfected or disposable.[8] The complex miniature architecture of dental burs and endodontic files makes precleaning and sterilization difficult.
For decades, clinicians have searched for the ideal chair-side sterilization method and several different mediums have been used.[9]
The present study showed that complete sterilization was possible by autoclaving the instruments in an endodontic box. This is significantly similar to the findings from studies of other researchers like Hurtt et al.,[9] Rajkumar et al.,[10] and Velez et al.[8] Damaging alterations of proteins by dry heat are a result of oxidation, desiccation, and changes in osmotic pressure owing to evaporation of moisture.
Dry heat sterilization is slower and requires temperatures higher than those used in moist heat sterilization. This study showed that sterilization by a glass-bead sterilizer was not able to completely eliminate microorganisms and that total sterility was not found. The present study result was contradictory to that of previous research done by Rajkumar et al.,[10] but it was the same as that of Hurtt et al.[9] who performed their study with salt instead of glass bead.
In the present study, immersing the files in glutaraldehyde solution for 12 h resulted in complete sterilization, which is similar to the results of the study done by Hurtt et al.[9] But glutaraldehyde solution cannot be relied upon completely to sterilize endodontic instruments as it is used in a limited number of applications, rather than as a general disinfectant, as it is a toxic chemical. Contact with glutaraldehyde liquid and vapor can severely irritate the eyes, and at higher concentrations, it burns the skin. Breathing glutaraldehyde can irritate the nose, throat, and respiratory tract, causing coughing and wheezing, nausea, headaches, drowsiness, nosebleeds, and dizziness.[11]
Quitanet plus is commonly used nowadays in the dental office for sterilization of burs and files. It can be concluded that it is not an effective means of sterilizing burs and files.
Routine sterilization procedures were not effective for previously used burs and files, and further research is warranted to devise an effective sterilization protocol. Future studies should focus on determining the best method of pre-cleaning these devices. If such procedures cannot be devised, perhaps the instruments should be considered single-use devices. This would reduce the risk of transmission of all infectious agents, including prions.[12]
CONCLUSION
In the present study, autoclaving and glutaraldehyde resulted in complete sterilization. Glass-bead sterilizer can be used as an alternative when these two methods are not available, but the problem with glutaraldehyde sterilization is tissue toxicity.
Though autoclave is an effective method for sterilizing endodontic files, the time taken by it to sterilize is more. Hence there is a need for an alternative method which has a good sterilization capacity and involves less time.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Farias AF, Rai S, Rao T. Life on the edge for the Indian dentist: A look at infection control and its application for Indian dental offices. Int J Infect Control. 2014;10:1–8. [Google Scholar]
- 2.Woods R, Amerena V, David P, Fan PL, Heydt H, Marianos D. Sterilization: Part 1. Instrument preparation. FDI World. 1996;5:7–10. [PubMed] [Google Scholar]
- 3.Whitworth CL, Martin MV, Gallagher M, Worthington HV. A comparison of decontamination methods used for dental burs. Br Dent J. 2004;197:635–40. doi: 10.1038/sj.bdj.4811832. discussion 623. [DOI] [PubMed] [Google Scholar]
- 4.Gordon BL, Burke FJ, Bagg J, Marlborough HS, McHugh ES. Systematic review of adherence to infection control guidelines in dentistry. J Dent. 2001;29:509–16. doi: 10.1016/s0300-5712(01)00043-4. [DOI] [PubMed] [Google Scholar]
- 5.Smith A, Dickson M, Aitken J, Bagg J. Contaminated dental instruments. J Hosp Infect. 2002;51:233–5. doi: 10.1053/jhin.2002.1213. [DOI] [PubMed] [Google Scholar]
- 6.Ingrosso L, Pisani F, Pocchiari M. Transmission of the 263K scrapie strain by the dental route. J Gen Virol. 1999;80:3043–7. doi: 10.1099/0022-1317-80-11-3043. [DOI] [PubMed] [Google Scholar]
- 7.Miller CH. Sterilization. Disciplined microbial control. Dent Clin North Am. 1991;35:339–55. [PubMed] [Google Scholar]
- 8.Vélez AE, Thomas DD, del Río CE. An evaluation of sterilization of endodontic instruments in artificial sponges. J Endod. 1998;24:51–3. doi: 10.1016/S0099-2399(98)80215-X. [DOI] [PubMed] [Google Scholar]
- 9.Hurtt CA, Rossman LE. The sterilization of endodontic hand files. J Endod. 1996;22:321–2. doi: 10.1016/S0099-2399(96)80268-8. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar K, Lakshminarayanan L. The effectiveness of two commonly used methods of sterilizing endodontics. J Indian Dent Assoc. 2001;72:245–8. [Google Scholar]
- 11.World Health Organization (WHO) Hyderabad, Andhra Pradesh: Environment Protection Training and Research Institute; 2004. Bio-Medical Waste Management Self Learning Document for Nurses and Paramedical; pp. 1–74. [Google Scholar]
- 12.Morrison A, Conrod S. Dental burs and endodontic files: Are routine sterilization procedures effective? J Can Dent Assoc. 2009;75:39. [PubMed] [Google Scholar]


