Abstract
Background: Putrescine, spermidine, and spermine (i.e., polyamines) are small cationic amines synthesized by cells or acquired from the diet or gut bacteria. Polyamines are required for both normal and colorectal cancer (CRC) cell growth.
Objective: We investigated the association between dietary polyamines and risk of CRC incidence and mortality.
Design: The study was a prospective analysis in 87,602 postmenopausal women in the Women’s Health Initiative Observational Study. Multivariate Cox regression was used to calculate HRs and 95% CIs.
Results: Total dietary polyamine intake (mean ± SD: 289.2 ± 127.4 μmol/d) was not positively associated with CRC in fully adjusted models. Instead, intake ≥179.67 μmol/d was associated with reduced risk of CRC [HR (95% CI): 0.82 (0.68, 1.00), 0.81 (0.66, 0.99), 0.91 (0.74, 1.12), and 0.80 (0.62, 1.02) for quintiles 2–5, respectively, compared with quintile 1]. Reduced risk was not significant across all quintiles. Polyamines were not significantly associated with CRC-specific mortality in fully adjusted models. When stratified by risk factors for CRC, only body mass index (BMI) and fiber intake significantly modified the association between polyamine intake and CRC. In women with BMI (in kg/m2) ≤25 or fiber consumption above the median, polyamine intake was associated with significantly lower risk of CRC.
Conclusions: No positive association between dietary polyamines and CRC or CRC-specific mortality risk in women was observed. Instead, a protective effect of dietary polyamines was suggested in women with some CRC risk-lowering behaviors in particular. These results are consistent with emerging evidence that exogenous polyamines may be beneficial in colon health and warrant additional study.
Keywords: colon cancer, colorectal cancer, diet, dietary polyamines, dietary putrescine, polyamines, putrescine, Women's Health Initiative
INTRODUCTION
The cationic diamine putrescine and its triamine derivatives spermidine and spermine play an essential role in tumor growth, thereby serving as a rationale for targeting the inhibition of intracellular polyamine biosynthesis for the treatment and prevention of cancer (1–4). Relative to adjacent normal tissue and tissues undergoing normal rates of turnover, these polyamines are present at high concentrations in tumor cells (5–7). Pharmacologic targeting of intracellular polyamines via the inhibition of ornithine decarboxylase, which is the rate-limiting enzyme that converts ornithine to putrescine, has been extensively investigated as a drug-based strategy to suppress tumor growth (1). Although of limited efficacy for advanced cancers (8), an irreversible inhibitor of ornithine decarboxylase (oral difluoromethylornithine) has demonstrated growth supressive activities, on colorectal adenomatous polyps [i.e., premalignant precursor of colorectal cancer (CRC)] in animal (9) and human (10) studies. Thus, pharmacological studies have established evidence of a growth-promoting role of intracellular polyamines in early colorectal tumorigenesis and as a target for chemoprevention in high-risk populations.
Polyamines, from de novo synthesis, the diet, and products of gut microbiome metabolism, are made available to cells via active transport (11, 12). These extracellular sources of polyamines are essential for normal mammalian embryogenesis (13) and play a key role in the normal development and maintenance of the gastrointestinal tract (14). Polyamines are essential to normal cellular homeostasis (15). Inhibition studies showed that polyamines have important roles in the macromolecular synthesis of proteins and nucleic acids, regulation of gene and protein expression, and regulation of ion channels. More recent system-wide studies identified polyamines as immunomodulatory molecules and as important regulators in adult stem cell populations (16). The ability of probiotics to extend the life span in mice depends on bacterial-derived putrescine to inhibit colonic cell senescence (17). These findings are consistent with a progrowth effect on healthy colonic cells. Despite the recognized role of polyamines in both normal cellular homeostasis and intestinal tumor growth, there have been no studies, to our knowledge, that tested the association between dietary polyamines and human CRC.
The development of American (18) and Swedish (19, 20) dietary polyamine databases has facilitated the estimation of polyamine intake from foods for application to population studies. With these instruments, daily intake of polyamines (primarily from produce, fermented, and aged foods and beverages) has been estimated at ∼300 μmol in the United States (4, 18). With the use of the American database, we reported that dietary polyamine intake above the population median (>289 μmol/d) was associated with a significant 30% increase in odds of developing colorectal adenomatous polyps in patients with a history of forming colorectal adenomas (i.e., high-risk patient population for CRC) (4). Similarly, Raj et al. (21) reported that high polyamine intake reduced the efficacy of difluoromethylornithine when combined with sulindac for the prevention of colorectal adenomas in high-risk polyp formers enrolled in the difluoromethylornithine and sulindac trial previously mentioned (10). Despite these promising observations in patients at above-average risk of developing CRC, to our knowledge, no studies have investigated whether dietary polyamine intake influences carcinogenesis in a general population or in those with average risk of CRC.
Therefore, we sought to test the hypothesis that dietary polyamines increase risk of CRC and CRC-specific mortality in an average-risk cohort. In addition, because dietary polyamines may act as tumor promoters only in a high-risk background (4), we explored whether risk factors that increase the likelihood of having initiated colorectal epithelial cells (e.g., family history of CRC and older age) modify the effect of dietary polyamines on CRC. With the use of data from the WHI (Women’s Health Initiative), which is a longitudinal cohort of postmenopausal women with dietary data, adjudicated outcomes, and extended follow-up, we provide the first study to our knowledge of dietary polyamine exposure and CRC risk in average-risk women.
METHODS
Study population
A total of 93,676 postmenopausal women aged 50–79 y were enrolled in the WHI Observational Study from 1993 to 1998 (22). Briefly, women throughout the United States who were either not eligible for or not interested in WHI clinical trials were invited to participate in the Observational Study. Women were ineligible if they had a life expectancy <3 y. Our study excluded participants without follow-up data (n = 473), with reported daily energy intakes <600 or >5000 kcal/d (n = 3534), or with an incomplete or missing food-frequency questionnaire (n = 94) at baseline. Participants who used a Hawaii-specific food-frequency questionnaire (n = 1973) that was not compatible with the dietary polyamine database were also excluded. A total of 87,602 women were available for the analysis, with a mean follow-up of 12.4 y; 1.4% of them experienced a CRC event while on study.
Dietary data
Average daily dietary intake over the preceding 3 mo was estimated at baseline by using the 122 line-item WHI food-frequency questionnaire (23). Dietary polyamine (putrescine, spermidine, and spermine) estimates were calculated by the Fred Hutchinson Cancer Research Center Nutrition Assessment Shared Resource by adapting the Zoumas-Morse (18) dietary polyamine database for use with the WHI food-frequency questionnaire in conjunction with the Nutrient Data System for Research (software version 2005) developed by the Nutrition Coordinating Center, University of Minnesota Food and Nutrient Database. Before ranking the top contributors to total dietary polyamines, food items were grouped into distinct categories (e.g., all poultry together and all cheeses together).
Nondietary exposure data
At baseline, multiple questionnaires that detailed medical, demographic, dietary supplement, and family histories (first-degree relative) were administered (22). Exposure variables used in analyses from these questionnaires are defined in Statistical methods.
Outcome measures
CRC and CRC-specific mortality outcomes were reported to the WHI via mail or telephone annually, and cases were adjudicated on the basis of medical records, pathology reports, and an additional blinded review as previously described (24). Bowel screening for CRC in the study was wholly at the discretion of the participant and her physician. Our analysis includes outcome data through 17 September 2012. There were 1245 adjudicated cases of CRC (1133 cases with a known stage at diagnosis) during follow-up, of which 1047 cases were documented to be of colonic origin, and 157 were documented to be of rectal origin. Of 11,607 deaths during the observational period, 335 deaths were attributed to CRC.
Statistical methods
Associations between dietary nutrients related to CRC or polyamines were calculated by using Pearson’s correlation. The percentage of contribution of distinct food categories to total dietary polyamine exposure was calculated at the person level and then ranked by the mean percentage. Cox regression was used to calculate HRs and 95% CIs for the association between quintiles of dietary polyamines and CRC. Only participants with CRC were at risk of CRC-specific death, and non-CRC-specific death is a competing risk of CRC-specific death. Thus, risk of CRC death was analyzed only in participants who had invasive, Surveillance, Epidemiology, and End Results-staged (24) CRC (local, regional, or distant but not in situ) by using a competing risk model to calculate a sub-HR, 95% CI, and P value as outlined by Fine and Gray (25) and adjusted for the stage at diagnosis and all model 3 covariates.
Statistical models were adjusted for potential confounders of the polyamine-CRC risk association or strong predictors of CRC on the basis of previous literature. Model 1 was adjusted only for age (continuous) (4, 26). Model 2 was adjusted as for model 1 and for total estimated dietary energy, household income (<$10,000–$19,999, $20,0000–$49,999, $50,000–$99,999, or >$100,000), smoking pack-years (continuous) (27, 28), recreational physical activity (continuous metabolic equivalent task hour per week) (29), race-ethnicity (non-Hispanic white, black or African American, Hispanic or Latino, or other) (30), hormone-replacement therapy use (ever or never) (31), history of colonoscopy or sigmoidoscopy (yes or no) (32), and BMI (in kg/m2; continuous) (33). Model 3 was adjusted as for model 2 and for continuous dietary exposures including total fiber and calcium because they are present in foods with a high polyamine content (4, 18, 19) and are related to CRC risk (34, 35). Arginine (36–38), dietary fat (37, 39), and folate equivalents (40) were also added to model 3 because of their putative effects on polyamine metabolism, presence in high-polyamine foods (4, 18–20), and association with CRC risk (37, 41–44). The association between CRC and dietary putrescine alone and, separately, spermidine plus spermine was evaluated under model 3 conditions. Schoenfeld’s residuals were tested, and there was no evidence that proportional hazards assumptions were violated. Likelihood ratio tests were conducted to analyze the overall effect of adding dietary polyamines (quintiles) to each Cox model.
Potential effect modifiers of the dietary polyamine–CRC association were tested by using a likelihood ratio test that compared model 3 to a model that included an interaction term. The following potential modifiers were evaluated because they are indicative of an initiated colorectal epithelium where polyamines may have a different effect than in a noninitiated colorectum (4, 13, 21, 45, 46): personal history of colorectal polyp removed, family history of CRC, older age, overweight or obese status, not using aspirin, and low fiber intake. In addition, arginine (21, 36, 47) and folate and folic acid (4, 40, 48) are involved in endogenous polyamine metabolism and, thus, have the potential to modify exogenous polyamine uptake and use. Further, the gut microbiome is responsive to BMI (49, 50) and fiber (51) and is believed to produce and use polyamines (17, 52, 53). Fiber, arginine, folate, and folic acid amounts were categorized into above (i.e., high intake) compared with below (i.e., low intake) median intakes to test for an effect modification. A sensitivity analysis was conducted to compare Cox-regression results with and without events that occurred within the first year of follow-up and with and without participants with a personal history of CRC. Participants were dropped from analyses when covariate data were missing (numbers of missing data by covariate were as follows: alcohol use, 29; adjusted energy intake, 8370; BMI, 1029; race-ethnicity, 242; education, 701; annual household income, 3823; previous colonoscopy or sigmoidoscopy, 1306; polyp or adenoma removed in subjects who received a colonoscopy or sigmoidoscopy, 1386; previous CRC, 7556; family history of CRC, 834; any hormone-replacement therapy use, 85; estrogen use, 64; estrogen plus progesterone use, 33; aspirin use, one; NSAID use, one; smoking, 3856; and physical activity, 992). Analyses were conducted with STATA SE 12.0 software (StataCorp LP).
RESULTS
Baseline participant and dietary characteristics
For the analyses of CRC endpoints, 87,602 participants were included with an mean ± SD follow-up time of 12.4 ± 4.0 y. The mean ± SD age of participants at enrollment was 63 ±7 y, and BMI was 27.3 ± 5.9. Mean daily energy intake was 1574.2 ± 599.4 kcal/d, and total estimated polyamine intake (sum of putrescine, spermidine, and spermine) was 289.2 ±127.4 μmol/d. Mean daily intakes of specific dietary polyamines were 189.5, 64.7, and 34.9 μmol/d for putrescine, spermidine, and spermine, respectively. Participants who were not white, with no more than a high school education, or with household income <$20,000, were more often in the lowest quintile of total dietary polyamine intake (Table 1). In addition, participants in the lowest quintile had the highest mean smoking pack-years and the lowest mean physical activity.
TABLE 1.
Participant characteristics at baseline across quintiles of total dietary polyamine intake in the WHI Observational Study (n = 87,602)1
| Total dietary polyamine intake,2 μmol/d |
|||||
| Participant characteristics at baseline | Quintile 1 (21.8–179.6) | Quintile 2 (179.7–243.0) | Quintile 3 (243.1–304.9) | Quintile 4 (305.0–384.7) | Quintile 5 (384.8–1293.7) |
| Participants, n (%) | 17,521 (20.0)3 | 17,520 (20.0) | 17,520 (20.0) | 17,520 (20.0) | 17,520 (20.0) |
| Age, y | 63.3 ± 7.44 | 63.5 ± 7.4 | 63.7 ± 7.3 | 63.9 ± 7.3 | 63.7 ± 7.4 |
| BMI, kg/m2 | 27.5 ± 6.0 | 27.3 ± 5.9 | 27.2 ± 5.7 | 27.1 ± 5.8 | 27.3 ± 6.0 |
| Race-ethnicity, n (%) | |||||
| Non-Hispanic white | 14,173 (81.2) | 15,004 (85.9) | 15,224 (87.1) | 15,492 (88.7) | 15,257 (87.3) |
| Black or African American | 1807 (10.4) | 1344 (7.7) | 1266 (7.2) | 1053 (6.0) | 1210 (6.9) |
| Hispanic or Latino | 884 (5.1) | 636 (3.6) | 542 (3.1) | 509 (2.9) | 587 (3.4) |
| Other | 598 (3.4) | 479 (2.7) | 453 (2.6) | 411 (2.4) | 431 (2.5) |
| Education, n (%) | |||||
| High school diploma or less | 5072 (29.2) | 3928 (22.6) | 3415 (19.6) | 2969 (17.1) | 2660 (15.3) |
| Some college | 8467 (48.7) | 8510 (49.0) | 8482 (48.8) | 8368 (48.1) | 8021 (46.2) |
| At least a college graduate | 3838 (22.1) | 4935 (28.4) | 5492 (31.6) | 6049 (34.8) | 6695 (38.5) |
| Annual household income, n (%) | |||||
| <$10,000–$19,999 | 3349 (20.7) | 2542 (15.7) | 2380 (14.6) | 2181 (13.4) | 2168 (13.3) |
| $20,000–$49,999 | 7319 (45.2) | 7107 (43.8) | 7099 (43.5) | 7107 (43.6) | 6877 (42.3) |
| $50,000–$99,999 | 4112 (25.4) | 4805 (29.6) | 4953 (30.4) | 5065 (31.1) | 5255 (32.3) |
| ≥$100,000 | 1406 (8.7) | 1782 (11.0) | 1876 (11.5) | 1953 (12.0) | 1961 (12.1) |
| Previous bowel screening,5 n (%) | 8967 (52.2) | 9263 (53.8) | 9423 (54.7) | 9727 (56.4) | 9819 (56.9) |
| Previous polyp or adenoma removed,6 n (%) | 1705 (19.6) | 1695 (18.8) | 1673 (18.3) | 1702 (18.0) | 1728 (18.1) |
| Previous colorectal cancer, n (%) | 185 (1.1) | 145 (0.8) | 132 (0.8) | 172 (1.0) | 176 (1.0) |
| Family history of colorectal cancer, n (%) | 2624 (16.6) | 2704 (16.9) | 2592 (16.2) | 2750 (17.1) | 2751 (17.1) |
| Any hormone replacement therapy use, n (%) | |||||
| Never | 7292 (41.7) | 6791 (39.8) | 7042 (40.2) | 7004 (40.0) | 7124 (40.7) |
| Ever (past or current) | 10,211 (58.3) | 10,527 (60.2) | 10,466 (59.8) | 10,500 (60.0) | 10,380 (59.3) |
| Unopposed estrogen use, n (%) | |||||
| Never | 10,826 (61.8) | 10,937 (62.5) | 11,067 (63.2) | 10,994 (62.8) | 11,167 (63.8) |
| Ever (past or current) | 6681 (38.2) | 6597 (37.5) | 6444 (36.8) | 6513 (37.2) | 6342 (36.2) |
| Estrogen plus progesterone use, n (%) | |||||
| Never | 13,038 (74.4) | 12,476 (71.3) | 12,395 (70.8) | 12,414 (70.9) | 12,420 (70.9) |
| Ever (past or current) | 4477 (25.6) | 5034 (28.8) | 5122 (29.2) | 5103 (29.1) | 5090 (29.1) |
| Aspirin use, n (%) | |||||
| ≤1 y | 15,234 (87.0) | 15,103 (86.2) | 14,831 (84.7) | 14,771 (84.3) | 14,699 (83.9) |
| >1 y | 2287 (13.1) | 2417 (13.8) | 2690 (15.4) | 2749 (15.7) | 2820 (16.1) |
| NSAID use,7 n (%) | |||||
| ≤1 y | 15,575 (88.9) | 15,476 (88.3) | 15,491 (88.4) | 15,552 (88.8) | 15,419 (88.0) |
| >1 y | 1946 (11.1) | 2044 (11.7) | 2030 (11.6) | 1968 (11.2) | 2100 (12.0) |
| Smoking, pack-years | 11.7 ± 20.3 | 10.8 ± 19.4 | 9.9 ± 18.2 | 9.3 ± 17.8 | 8.9 ± 17.7 |
| Physical activity, MET-h/wk | 10.7 ± 12.8 | 13.1 ± 14.1 | 13.8 ± 14.1 | 14.9 ± 14.5 | 16.4 ± 15.5 |
MET-h, metabolic equivalent task hours; NSAID, nonsteroidal anti-inflammatory drug; WHI, Women’s Health Initiative.
Sum of dietary putrescine, spermidine, and spermine.
Frequency; percentage of total in parentheses (all such values for categorical variables).
Mean ± SD (all such values for continuous variables).
Any personal history of colonoscopy or sigmoidoscopy.
Only participants with a history of colonoscopy or sigmoidoscopy were considered for this variable.
Not including aspirin.
Dietary polyamine sources and correlates at baseline
Citrus fruit was the major source of total dietary polyamines. Top contributors included orange and grapefruit juice (20.6%) and oranges and grapefruit (11.0%) followed by cheddar, Swiss, and cream cheeses (8.0%); bananas and plantains (4.8%); and tomatoes and tomato products (3.3%; Supplemental Table 1). Because other dietary components increased along with intake of total polyamines (Table 2), we estimated correlations with other key dietary components (Supplemental Table 2). Particularly high correlations were observed between total polyamine intake and dietary fiber (r = 0.669, P < 0.001) and folate (r = 0.820, P < 0.001), which are notable because of the hypothesized roles of both fiber (35, 54) and folate (44) in CRC risk reduction.
TABLE 2.
Participant dietary characteristics at baseline across quintiles of total dietary polyamine intake in the WHI Observational Study (1995-2012; n = 87,602)1
| Total dietary polyamine intake,2 μmol/d |
|||||
| Dietary characteristics at baseline | Quintile 1 (21.8–179.6) | Quintile 2 (179.7–243.0) | Quintile 3 (243.1–304.9) | Quintile 4 (305.0–384.7) | Quintile 5 (384.8–1293.7) |
| Total polyamine, μmol/d2 | 136.1 ± 30.83 | 211.9 ± 118.3 | 273.5 ± 17.7 | 342.0 ± 22.8 | 482.5 ± 95.7 |
| Putrescine, μmol/d | 74.8 ± 24.5 | 127.9 ± 26.3 | 176.4 ± 30.7 | 229.2 ± 35.5 | 339.2 ± 90.2 |
| Spermidine, μmol/d | 38.4 ± 11.4 | 53.7 ± 14.5 | 62.7 ± 17.8 | 73.7 ± 20.3 | 95.2 ± 30.8 |
| Spermine, μmol/d | 22.8 ± 8.3 | 30.3 ± 10.3 | 34.3 ± 12.0 | 39.0 ± 13.3 | 48.1 ± 18.5 |
| Total energy, kcal/d | 1128.0 ± 369.7 | 1388.0 ± 436.6 | 1545.3 ± 477.3 | 1730.9 ± 518.1 | 2078.8 ± 677.8 |
| Total fat, g/d | 41.9 ± 20.5 | 49.2 ± 25.0 | 53.3 ± 27.3 | 58.2 ± 29.9 | 68.2 ± 37.0 |
| Total saturated fat, g/d | 14.0 ± 7.5 | 16.4 ± 9.1 | 17.7 ± 9.8 | 19.5 ± 10.8 | 23.0 ± 13.4 |
| Red meat, medium servings/d | 0.5 ± 0.4 | 0.6 ± 0.5 | 0.6 ± 0.6 | 0.7 ± 0.7 | 0.8 ± 0.7 |
| Total protein, g/d | 45.9 ± 16.2 | 58.3 ± 19.7 | 65.4 ± 21.9 | 73.7 ± 24.0 | 88.9 ± 32.4 |
| Arginine, g/d | 2.4 ± 0.9 | 3.0 ± 1.1 | 3.4 ± 1.2 | 3.8 ± 1.3 | 4.6 ± 1.7 |
| Methionine, g/d | 1.1 ± 0.4 | 1.3 ± 0.5 | 1.5 ± 0.5 | 1.7 ± 0.6 | 2.0 ± 0.8 |
| Total fiber, g/d | 10.3 ± 3.7 | 14.0 ± 4.5 | 16.2 ± 5.1 | 18.8 ± 5.6 | 23.6 ± 7.3 |
| Alcohol, servings/wk | 1.9 ± 4.2 | 2.4 ± 4.9 | 2.6 ± 5.5 | 3.0 ± 5.5 | 3.1 ± 6.0 |
| Dietary folate,4 μg/d | 144.1 ± 42.6 | 195.7 ± 46.2 | 231.4 ± 49.4 | 270.6 ± 54.2 | 347.2 ± 82.7 |
| Dietary folic acid,5 μg/d | 112.6 ± 76.7 | 136.4 ± 85.1 | 149.0 ± 87.3 | 163.5 ± 94.0 | 185.5 ± 106.7 |
| Supplemental folic acid use, n (%) | 7656 (43.8)6 | 8432 (48.2) | 8750 (50.0) | 9100 (52.0) | 9197 (52.6) |
| Total calcium,7 mg/d | 906.6 ± 653.4 | 1107.4 ± 750.6 | 1217.2 ± 806.0 | 1363.9 ± 690.4 | 1587.3 ± 769.0 |
| Dietary calcium,4 mg/d | 559.6 ± 323.7 | 718.1 ± 364.7 | 819.3 ± 388.0 | 943.8 ± 427.9 | 1156.9 ± 531.4 |
| Supplemental calcium use, n (%) | 9404 (53.7) | 10,406 (59.4) | 10,624 (60.6) | 11,080 (63.2) | 11,227 (64.1) |
WHI, Women’s Health Initiative.
Sum of dietary putrescine, spermidine, and spermine.
Mean ± SD (all such values for continuous variables).
From food sources only.
From food sources only (including fortified foods).
Frequency; percentage of total in parentheses (all such values for categorical variables).
Sum of dietary calcium and supplemental calcium.
Dietary polyamine intake and risk of CRC and CRC-specific death
Cumulative incidence by quintile of dietary polyamine intake is shown in Supplemental Table 3. In the fully adjusted model, midrange dietary polyamine intake was significantly associated with 18% and 19% decreased risk of CRC in quintiles 2 and 3, respectively (Table 3). Point estimates for quintiles 4 and 5 did not change appreciably across models, but significance was not consistently achieved. No evidence of a linear dose-response effect was shown; all reduced risk was achieved in the second quintile with no additional benefit with higher exposure. The addition of dietary polyamines did not significantly improve the model fit over and above other covariates as assessed by using a likelihood ratio test with versus without total polyamines (P > 0.05). When we excluded cancers that occurred early during the observational period (<1 y) or participants with a personal history of CRC, a similar association with decreased risk starting in the second quintile was observed (data not shown). A separate analysis of putrescine alone (diamine) and spermine plus spermidine (triamines) revealed no differential effects by amine type and no dose effect of either putrescine alone or spermidine plus spermine (data not shown).
TABLE 3.
Dietary polyamine intake at baseline and CRC (in the whole population) and CRC-specific death (only in subjects who had a staged local, regional, or distant CRC event on study) outcomes during the observation period1
| CRC2 |
CRC-specific death3 |
|||||||||||
| Model 1 |
Model 2 |
Model 3 |
Model 1 + stage at diagnosis |
Model 2 + stage at diagnosis |
Model 3 + stage at diagnosis |
|||||||
| Quintiles of total dietary polyamine intake, μmol/d | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | Sub-HR (95% CI) | P | Sub-HR (95% CI) | P | Sub-HR (95% CI) | P |
| 21.8–179.6 | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — |
| 179.7–243.0 | 0.87 (0.73, 1.03) | 0.114 | 0.83 (0.68, 1.00) | 0.048 | 0.82 (0.68, 1.00) | 0.048 | 1.01 (0.69, 1.48) | 0.963 | 1.19 (0.76, 1.84) | 0.447 | 1.14 (0.73, 1.78) | 0.563 |
| 243.1–304.9 | 0.82 (0.69, 0.98) | 0.026 | 0.81 (0.67, 0.99) | 0.036 | 0.81 (0.66, 0.99) | 0.040 | 0.97 (0.63, 1.48) | 0.870 | 1.33 (0.81, 2.16) | 0.257 | 1.27 (0.76, 2.12) | 0.353 |
| 305.0–384.7 | 0.90 (0.76, 1.07) | 0.233 | 0.91 (0.75, 1.11) | 0.369 | 0.91 (0.74, 1.12) | 0.379 | 0.78 (0.52, 1.17) | 0.229 | 1.00 (0.62, 1.62) | 0.999 | 0.95 (0.57, 1.58) | 0.838 |
| 384.8–1293.7 | 0.83 (0.70, 0.99) | 0.035 | 0.80 (0.64, 1.00) | 0.050 | 0.80 (0.62, 1.02) | 0.073 | 0.47 (0.30, 0.74) | 0.001 | 0.64 (0.36, 1.14) | 0.130 | 0.57 (0.31, 1.06) | 0.076 |
| Overall test4 | — | 0.059 | — | 0.135 | — | 0.152 | — | — | — | — | — | — |
Model 1 was adjusted for age only (n = 87,602 for CRC outcome; n = 1084 for CRC-specific death outcome). Model 2 was adjusted as for model 1 and for energy, income, smoking, physical activity, race-ethnicity, BMI, ever use of hormone replacement therapy, and history of bowel screening (n = 76,066 for CRC outcome; n = 955 for CRC-specific death outcome). Model 3 was adjusted as for model 2 and for dietary fat, dietary arginine, dietary fiber, dietary folate equivalents, and dietary calcium (n = 75,924 for CRC outcome; n = 955 for CRC-specific death outcome). CRC, colorectal cancer.
Values were generated by using Cox regression with Breslow’s method for ties.
Values were generated by using competing risk regression [as outlined by Fine and Gray (25)]. The stage at diagnosis was as follows: model 1, localized CRC (n = 479); regional CRC (n = 463); and distant CRC (n = 141); models 2 and 3, localized CRC (n = 425); regional CRC (n = 405); and distant CRC (n = 126).
Results of a likelihood ratio test for the comparison of Cox regression models with and without dietary polyamines as quintiles.
Among participants who developed CRC during observation, participants with high polyamine intake (quintile 5) experienced 53% reduced risk of CRC-specific death when adjusted for only the stage at diagnosis (Table 3, model 1). This effect was no longer present in the fully adjusted model although data on CRC treatment was unavailable in this cohort.
Dietary polyamine intake and risk of CRC by population subgroups
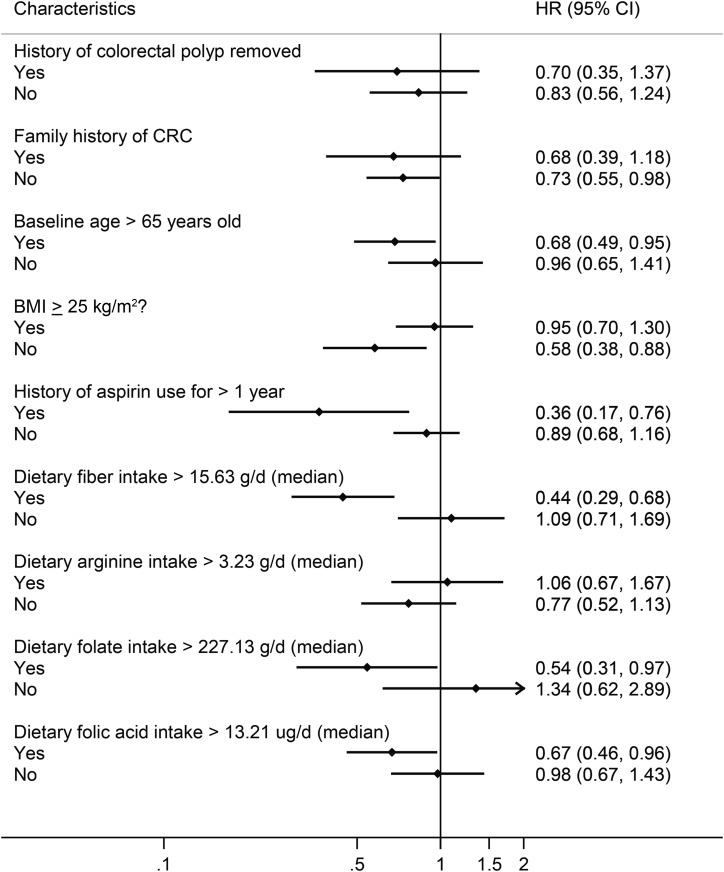
Previously, we showed that, in patients with a history of colorectal adenoma (i.e., a subgroup of the population at higher risk of CRC), high polyamine intake was associated with increased odds of adenoma at follow-up (4). One explanation for the lack of a positive association between polyamines and CRC in postmenopausal women is that polyamines have different effects depending on background risk of CRC. Thus, to explore the potential heterogeneity of polyamine effect by CRC risk, we stratified analyses on established CRC risk factors at baseline. We observed a significant protective effect of dietary polyamines in women with CRC risk–lowering characteristics, specifically subjects with BMI ≤25 (P-interaction = 0.012) or who reported higher dietary fiber consumption (P-interaction = 0.015; Figure 1, Table 4). A baseline history of polyp removal (P-interaction = 0.635), family history of CRC (P-interaction = 0.687), age >65 y (P-interaction = 0.764), aspirin use >1 y (P-interaction = 0.100), and dietary arginine, folate, and folic acid (P-interaction = 0.129, 0.607, and 0.890, respectively) did not significantly modify the association between polyamines and CRC risk (Figure 1).
FIGURE 1.
Risk of CRC associated with total dietary polyamine intake (quintile 5 compared with quintile 1) stratified by established risk factors for CRC assessed at baseline. HRs (♦), 95% CIs (line), and P values were generated by using Cox regression with Breslow’s method for ties. The results were adjusted for all model 3 covariates (age, energy intake, income, smoking, physical activity, race-ethnicity, BMI, hormone replacement therapy, history of bowel screening, dietary fat, dietary arginine, dietary fiber, dietary folate equivalents, and dietary calcium; n = 76,066). CRC, colorectal cancer.
TABLE 4.
Stratified models that describe the relation between BMI or dietary fiber, dietary polyamines, and colorectal cancer risk after adjustment for all model 3 covariates1
| Quintile 2 |
Quintile 3 |
Quintile 4 |
Quintile 5 |
||||||
| n/total person-years | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| BMI, kg/m2 | |||||||||
| ≤25 | 379/393,982 | 0.56 (0.40, 0.77) | 0.001 | 0.66 (0.48, 0.92) | 0.014 | 0.73 (0.52, 1.03) | 0.077 | 0.58 (0.38, 0.88) | 0.011 |
| >25 | 715/547,985 | 1.01 (0.80, 1.29) | 0.915 | 0.91 (0.71, 1.18) | 0.486 | 1.04 (0.79, 1.35) | 0.792 | 0.95 (0.70, 1.30) | 0.754 |
| Dietary fiber,2 g/d | |||||||||
| ≤15.63 | 555/459,121 | 0.96 (0.76, 1.20) | 0.700 | 0.86 (0.66, 1.11) | 0.244 | 1.37 (1.04, 1.82) | 0.027 | 1.09 (0.71, 1.69) | 0.683 |
| >15.63 | 539/482,845 | 0.51 (0.33, 0.79) | 0.003 | 0.55 (0.36, 0.82) | 0.004 | 0.49 (0.33, 0.74) | 0.001 | 0.44 (0.29, 0.68) | <0.001 |
Model 3 as adjusted for age, energy intake, income, smoking, physical activity, race-ethnicity, BMI, hormone replacement therapy, history of bowel screening, dietary fat, dietary arginine, dietary fiber, dietary folate equivalents, and dietary calcium. Quintile 1 of total dietary polyamine intake was the reference group. HRs (95% CIs) and P values for quintiles of total dietary polyamine intake were generated by using Cox regression with Breslow’s method for ties.
Split at the median.
DISCUSSION
To our knowledge, this is the first study of the association between dietary polyamines and risk of CRC and CRC-specific mortality in a large, average-risk population of postmenopausal women. Contrary to our a priori hypothesis, we observed no significant dose-response relation between dietary polyamines and risk of CRC or CRC-specific mortality in fully adjusted models. Before full adjustment, total polyamine intake ≥179.67 μmol/d (quintiles 2–5) was consistently associated with 9–20% decreased risk of CRC. After adjustment for dietary covariates, these associations were not always significant, although point estimates were consistent across all models and quintiles. These dietary covariates included dietary folate equivalents and fiber, whose food sources strongly overlapped with those of polyamines and may have resulted in overadjustment of the model. Overall, our data suggest modest, nonsignificant decreased risk of CRC in individuals with average-to-high polyamine intakes or, conversely, modest increased risk of individuals with very-low polyamine intakes.
Our findings are supported by animal and human studies that suggested effects of dietary polyamines are dependent on background risk of CRC. Similar to previous dietary polyamine studies (4, 18), we showed that citrus fruits are the largest contributor of polyamines to the diet of postmenopausal US women. In ecological studies, the Mediterranean diet, which includes high citrus fruit intake, is associated with protection against the development of diseases including CRC (55). In experimental animal models, high-polyamine diets delayed aging (56), and putrescine was shown to mediate the effect of probiotics on longevity in mice through direct inhibitory effects on colonic cell senescence (17, 53). In contrast, low-polyamine diets were shown to delay wound healing (57) and inhibit growth (13). In humans at high risk of CRC (adenomatous polyp formers), we previously showed that dietary polyamines increased risk of adenomatous polyp recurrence (4). Similarly, mice initiated with a chemical colorectal carcinogen showed an increase in the severity of premalignant colorectal aberrant crypt foci and adenomatous polyps when exposed to a high-polyamine diet (45, 46, 56). However, these carcinogen-treated animals had fewer aberrant crypt foci in the intestinal tract when they kept receiving low-polyamine diets (13, 46), and a high-polyamine diet before carcinogen treatment decreases tumor incidence (56). Overall, our observations are consistent with the notion suggested by the literature that the effect of dietary polyamines is to promote health in low-risk colorectums, and polyamines may promote carcinogenesis in colorectums that experienced an initiating event.
Although the multiple subgroup analyses reported should be interpreted cautiously, our findings suggest that dietary polyamines may be particularly beneficial in lowering CRC risk in women with normal BMI and in those who consumed higher than average fiber in this population (>15.63 g/d). Fiber (58) was not protective against CRC in the WHI cohort overall, but fiber intake has been shown to reduce risk of CRC in previous studies (35). Obesity has been consistently linked with CRC risk, more so in men (33), and the effect of polyamines could be dependent on body size (49, 50). Thus, these observations also support a differential effect of dietary polyamines dependent on background CRC risk. Although the exact mechanisms by which BMI or fiber act with polyamine intake in a protective fashion are unknown, we speculate that one mechanism may be through effects on the gut microbiome. Specifically, BMI (49, 50), fiber (51), and polyamines (17, 52, 53) all interact with the gut flora, which is a major secondary source of polyamine exposure to the colonic epithelium (11, 12). This plausible mechanism of action, and others, warrants exploration in both high- and low-risk CRC model systems to better understand by what mechanism exogenous polyamines affect gut function and tumorigenesis.
In contrast with our previous findings for adenoma outcomes (4), we did not show evidence that women with a history of colorectal polyp removal had increased risk of CRC from dietary polyamine exposure. However, the WHI cohort did not differentiate between histories of colorectal polyps compared with adenomas. In addition, we failed to identify significant associations between polyamines and CRC risk in participants with some high-risk characteristics (e.g., family history of CRC or advanced age). These results may reflect differences related to past screening behaviors in these groups for which we lacked sufficient detail before enrollment in WHI. We could not discount the possibility that our findings were biased because of residual confounding because women of lower socioeconomic status are more often in the lowest quintile of dietary polyamine consumption.
The potential for misclassification of a dietary exposure, the presence of correlated nutrients, and the lack of more-precise biomarkers of polyamine intake were important limitations in this and other similar studies of dietary factors (18). The polyamine content depends on the ripeness, bacterial activity, and age of foods (4, 18, 59), which makes estimation challenging. For example, the considerable overlap of polyamine-, fiber- and folate-rich foods may preclude a conclusive distinction of individual effects of any of these dietary exposures in the observational setting. This possibility raises the prospect that previous findings of associations between dietary folate and fiber with CRC may have been confounded by the biological effects of polyamines or that our study suffered from residual confounding. Nonetheless, the large sample size, quantity of measured variables, adjudicated CRC outcomes, and long follow-up period in this study were considerable strengths.
In conclusion and in contrast to our original hypothesis, we showed no evidence for a positive association between dietary polyamines and CRC risk. Instead, this study provides the first evidence, to our knowledge, that diets high in polyamines may decrease risk of CRC in average-risk postmenopausal women, particularly in those with other CRC risk–lowering habits such as consumption of higher fiber diets and maintenance of a normal body weight. This observation is consistent with emerging evidence of beneficial effects of polyamines on longevity and gastrointestinal health and argues for differential effects by risk status, thereby warranting additional study on the potential health benefits of polyamine-rich foods.
Acknowledgments
The short list of WHI investigators is as follows—program office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller; clinical coordinating center: (Fred Hutchinson Cancer Research Center, Seattle, Washington) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg; investigators and academic centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts) JoAnn E Manson, (MedStar Health Research Institute/Howard University, Washington, District of Columbia) Barbara V Howard, (Stanford Prevention Research Center, Stanford, California) Marcia L Stefanick, (The Ohio State University, Columbus, Ohio) Rebecca Jackson, (University of Arizona, Tucson/Phoenix, Arizona) Cynthia A Thomson, (University at Buffalo, Buffalo, New York) Jean Wactawski-Wende, (University of Florida, Gainesville/Jacksonville, Florida) Marian Limacher, (University of Iowa, Iowa City/Davenport, Iowa) Robert Wallace, (University of Pittsburgh, Pittsburgh, Pennsylvania) Lewis Kuller, (Wake Forest University School of Medicine, Winston-Salem, North Carolina) Sally Shumaker; and the WHI Memory Study: (Wake Forest University School of Medicine, Winston-Salem, North Carolina) Sally Shumaker. For a list of all investigators who contributed to WHI science, please visit https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
The authors’ responsibilities were as follows—AJV, RBW, MLN, CAT, and PAT: designed the research; AJV, ELA, and BCW: conducted the statistical research and analyses; RBW, MLN, and CAT: provided access to WHI data sets; AJV, ELA, CAT, and PAT: wrote the manuscript; and AJV: had primary responsibility for the final content of the manuscript. None of the authors reported a conflict of interest related to the study.
REFERENCES
- 1.Gerner EW, Meyskens FL Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 2004;4:781–92. [DOI] [PubMed] [Google Scholar]
- 2.Gerner EW. Cancer chemoprevention locks onto a new polyamine metabolic target. Cancer Prev Res 2010;3:125–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basuroy UK, Gerner EW. Emerging concepts in targeting the polyamine metabolic pathway in epithelial cancer chemoprevention and chemotherapy. J Biochem 2006;139:27–33. [DOI] [PubMed] [Google Scholar]
- 4.Vargas AJ, Wertheim BC, Gerner EW, Thomson CA, Rock CL, Thompson PA. Dietary polyamine intake and risk of colorectal adenomatous polyps. Am J Clin Nutr 2012;96:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen SS. A guide to the polyamines. New York: Oxford Universities Press; 1997. [Google Scholar]
- 6.Hixson LJ, Garewal HS, McGee DL, Sloan D, Fennerty MB, Sampliner RE, Gerner EW. Ornithine decarboxylase and polyamines in colorectal neoplasia and mucosa. Cancer Epidemiol Biomarkers Prev 1993;2:369–74. [PubMed] [Google Scholar]
- 7.Hixson LJ, Emerson SS, Shassetz LR, Gerner EW. Sources of variability in estimating ornithine decarboxylase activity and polyamine contents in human colorectal mucosa. Cancer Epidemiol Biomarkers Prev 1994;3:317–23. [PubMed] [Google Scholar]
- 8.Horn Y, Schechter PJ, Marton LJ. Phase I-II clinical trial with alpha-difluoromethylornithine–an inhibitor of polyamine biosynthesis. Eur J Cancer Clin Oncol 1987;23:1103–7. [DOI] [PubMed] [Google Scholar]
- 9.Ignatenko NA, Gerner EW, Besselsen DG. Defining the role of polyamines in colon carcinogenesis using mouse models. J Carcinog 2011;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyskens FL Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res 2008;1:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milovic V. Polyamines in the gut lumen: bioavailability and biodistribution. Eur J Gastroenterol Hepatol 2001;13:1021–5. [DOI] [PubMed] [Google Scholar]
- 12.Sawada Y, Pereira SP, Murphy GM, Dowling RH. Origins of intestinal luminal polyamines in man. British Society of Gastroenterology. Gut 1994;35(Suppl 5):S20. [Google Scholar]
- 13.Duranton B, Nsi-Emvo E, Schleiffer R, Gosse F, Galluser M, Raul F. Suppression of preneoplastic changes in the intestine of rats fed low levels of polyamines. Cancer Res 1997;57:573–5. [PubMed] [Google Scholar]
- 14.Loser C, Eisel A, Harms D, Folsch UR. Dietary polyamines are essential luminal growth factors for small intestinal and colonic mucosal growth and development. Gut 1999;44:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab 2008;294:E995–1010. [DOI] [PubMed] [Google Scholar]
- 16.Frostesjo L, Holm I, Grahn B, Page W, Bestor TH, Heby O. Interference with DNA methyltransferase activity and genome methylation during F9 teratocarcinoma stem cell differentiation induced by polyamine depletion. J Biol Chem 1997;272:4359–66. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto M, Kurihara S, Kibe R, Ashida H, Benno Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS One 2011;6:e23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoumas-Morse C, Rock CL, Quintana EL, Neuhouser ML, Gerner EW, Meyskens FL Jr. Development of a polyamine database for assessing dietary intake. J Am Diet Assoc 2007;107:1024–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali M, Poortvliet E, Stromberg R, Yngve A. Polyamines in foods: development of a food database. Food Nutr Res 2011;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali MA, Poortvliet E, Stromberg R, Yngve A. Polyamines: total daily intake in adolescents compared to the intake estimated from the Swedish Nutrition Recommendations Objectified (SNO). Food Nutr Res 2011;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj KP, Zell JA, Rock CL, McLaren CE, Zoumas-Morse C, Gerner EW, Meyskens FL. Role of dietary polyamines in a phase III clinical trial of difluoromethylornithine (DFMO) and sulindac for prevention of sporadic colorectal adenomas. Br J Cancer 2013;108:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003;13(9 Suppl):S107–21. [DOI] [PubMed] [Google Scholar]
- 23.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 24.Curb JD, Mctiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol 2003;13(9 Suppl):S122–8. [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. 2014;94:496–509.
- 26.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY) 2011;3:716–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–78. [DOI] [PubMed] [Google Scholar]
- 28.Paskett ED, Reeves KW, Rohan TE, Allison MA, Williams CD, Messina CR, Whitlock E, Sato A, Hunt JR. Association between cigarette smoking and colorectal cancer in the Women’s Health Initiative. J Natl Cancer Inst 2007;99:1729–35. [DOI] [PubMed] [Google Scholar]
- 29.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer 2009;125:171–80. [DOI] [PubMed] [Google Scholar]
- 30.Simon MS, Thomson CA, Pettijohn E, Kato I, Rodabough RJ, Lane D, Hubbell FA, O’Sullivan MJ, Adams-Campbell L, Mouton CP, et al. Racial differences in colorectal cancer incidence and mortality in the Women’s Health Initiative. Cancer Epidemiol Biomarkers Prev 2011;20:1368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, Rosenberg CA, Taylor VM, Harris R, Chen C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004;350:991–1004. [DOI] [PubMed] [Google Scholar]
- 32.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:132–41. [DOI] [PubMed] [Google Scholar]
- 33.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556–65. [DOI] [PubMed] [Google Scholar]
- 34.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684–96. [DOI] [PubMed] [Google Scholar]
- 35.WCRF/AICR. Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington (DC): AICR; 2007. [Google Scholar]
- 36.Gerner EW. Impact of dietary amino acids and polyamines on intestinal carcinogenesis and chemoprevention in mouse models. Biochem Soc Trans 2007;35:322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zell JA, Ignatenko NA, Yerushalmi HF, Ziogas A, Besselsen DG, Gerner EW, Anton-Culver H. Risk and risk reduction involving arginine intake and meat consumption in colorectal tumorigenesis and survival. Int J Cancer 2007;120:459–68. [DOI] [PubMed] [Google Scholar]
- 38.Yerushalmi HF, Besselsen DG, Ignatenko NA, Blohm-Mangone KA, Padilla-Torres JL, Stringer DE, Cui H, Holubec H, Payne CM, Gerner EW. The role of NO synthases in arginine-dependent small intestinal and colonic carcinogenesis. Mol Carcinog 2006;45:93–105. [DOI] [PubMed] [Google Scholar]
- 39.Alexander DD, Cushing CA, Lowe KA, Sceurman B, Roberts MA. Meta-analysis of animal fat or animal protein intake and colorectal cancer. Am J Clin Nutr 2009;89:1402–9. [DOI] [PubMed] [Google Scholar]
- 40.Bistulfi G, Diegelman P, Foster BA, Kramer DL, Porter CW, Smiraglia DJ. Polyamine biosynthesis impacts cellular folate requirements necessary to maintain S-adenosylmethionine and nucleotide pools. FASEB J 2009;23:2888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract 2012;27:613–23. [DOI] [PubMed] [Google Scholar]
- 42.Alexander DD, Cushing CA. Red meat and colorectal cancer: a critical summary of prospective epidemiologic studies. Obes Rev 2011;12:e472–93. [DOI] [PubMed]
- 43.Kennedy DA, Stern SJ, Moretti M, Matok I, Sarkar M, Nickel C, Koren G. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol 2011;35:2–10. [DOI] [PubMed] [Google Scholar]
- 44.Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr 2011;93:817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ignatenko NA, Besselsen DG, Roy UK, Stringer DE, Blohm-Mangone KA, Padilla-Torres JL, Guillen RJ, Gerner EW. Dietary putrescine reduces the intestinal anticarcinogenic activity of sulindac in a murine model of familial adenomatous polyposis. Nutr Cancer 2006;56:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulsen JE, Reistad R, Eliassen KA, Sjaastad OV, Alexander J. Dietary polyamines promote the growth of azoxymethane-induced aberrant crypt foci in rat colon. Carcinogenesis 1997;18:1871–5. [DOI] [PubMed] [Google Scholar]
- 47.Yerushalmi HF, Besselsen DG, Ignatenko NA, Blohm-Mangone KA, Padilla-Torres JL, Stringer DE, Guillen JM, Holubec H, Payne CM, Gerner EW. Role of polyamines in arginine-dependent colon carcinogenesis in Apc(Min) (/+) mice. Mol Carcinog 2006;45:764–73. [DOI] [PubMed] [Google Scholar]
- 48.Sun D, Wollin A, Stephen AM. Moderate folate deficiency influences polyamine synthesis in rats. J Nutr 2002;132:2632–7. [DOI] [PubMed] [Google Scholar]
- 49.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ. Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature 2009;457:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 51.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumoto M, Benno Y. The relationship between microbiota and polyamine concentration in the human intestine: a pilot study. Microbiol Immunol 2007;51:25–35. [DOI] [PubMed] [Google Scholar]
- 53.Kibe R, Kurihara S, Sakai Y, Suzuki H, Ooga T, Sawaki E, Muramatsu K, Nakamura A, Yamashita A, Kitada Y, et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep 2014;4:4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soda K. Polyamine intake, dietary pattern, and cardiovascular disease. Med Hypotheses 2010;75:2997–301. [DOI] [PubMed]
- 56.Soda K, Kano Y, Chiba F, Koizumi K, Miyaki Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 2013;8:e64357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahuja V, Abrams JM, Tantry U, Park J, Barbul A. Effect of difluoromethylornithine, a chemotherapeutic agent, on wound healing. J Surg Res 2003;114:308–9. [Google Scholar]
- 58.Beresford SA, Johnson KC, Norman L, Assaf AR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Harrigan RC, Hays J, et al. Low-fat dietary pattern and risk of colorectal cancer. JAMA 2006;295:643–54. [DOI] [PubMed] [Google Scholar]
- 59.Kalac P, Krausova P. A review of dietary polyamines: formation, implications for growth and health and occurrence in foods. Food Chem 2005;90:219–30. [Google Scholar]