Abstract
Four bodybuilders who injected anabolic steroids and ingested commercial protein (78–104 g/day) and creatine (15 g/day) products presented with serum creatinine levels between 229.84 and 335.92 µmol/L (2.6–3.8 mg/dL). Renal biopsies revealed acute tubular necrosis. Four weeks after discontinuing injections and supplements, serum creatinine was in the normal range and estimated glomerular filtration rate > 1.00 mL/s (60 mL/min), including two patients with biopsies showing >30% interstitial fibrosis and tubular atrophy. The findings highlight a risk for acute and potentially chronic kidney injury among young men abusing anabolic steroids and using excessive amounts of nutritional supplements.
Keywords: acute kidney injury, anabolic steroids, body building, chronic kidney disease, nutritional supplements
Introduction
Bodybuilders frequently use anabolic steroids and dietary supplements to acquire strength and body bulk [1]. The principal nonhormonal supplements are protein, creatine and vitamins [2]. Herlitz et al. [3] reported 10 body builders who developed renal insufficiency and focal segmental glomerulosclerosis (FSGS) while taking anabolic steroids and protein and creatine supplements with a daily protein intake of 300–550 g/day. The high-protein intake has been of concern to nephrologists because it increases glomerular filtration rates and is experimentally associated with glomerular hyperfiltration and FSGS [2]. Recent evidence indicates that anabolic steroids are directly toxic to glomeruli and that segmental sclerosis is the result of podocyte loss mediated by apoptosis through a podocyte androgen receptor [4].
Creatine powder is marketed as a muscle building supplement. Loading doses of 20–25 g/day for 5 days and then maintenance doses of 5 g/day are considered safe and to effectively contribute to exercise endurance and muscle strength [5, 6]. Creatine is used by a large number of competitive as well as casual athletes [7]. The number of reported adverse events is small and usually associated with exercise-induced acute renal failure and rhabdomyolysis during intense training but even with moderate exercise [8]. We can find three case reports of creatine-associated acute kidney injury not related to rhabdomyolysis [9–12]. One body builder developed acute tubular necrosis after taking loading doses of 20 g/day for 5 days [10]. In two patients, kidney injury was associated with acute interstitial nephritis [11, 12].
Vitamins are compounded into capsules or tablets containing several times their recommended daily allowances (RDA). Some sports nutritionists recommend modest increases of vitamin D over the RDA to 400–800 IU a day to enhance bone building [13, 14]. A sustained intake of over 50 000 IU a day can increase serum 25-hydroxyvitamin D levels and cause hypercalcemia with the potential for metastatic renal calcification [13]. This has occurred as a result of accidental overdoses in hospitalized infants, but the risk of vitamin D toxicity is considered minimal in normal adults [14, 15].
Over the past 2 years, we have seen four bodybuilders presenting with acute renal insufficiency who injected anabolic steroids and consumed moderately increased amounts of protein and creatine in doses exceeding 5 g/day on a regular basis. The findings are presented to alert nephrologists and general physicians to bodybuilding behaviors that may place young men at risk for kidney injury.
Case report
Four bodybuilders were referred to the Hawler University College of Medicine Nephrology Department complaining of weakness and lethargy. All patients injected testosterone proprionate and/or nandrolone deconate intramuscularly at weekly doses that exceeded 400 mg. Commercial protein and creatine products were used that were imported from the USA. Clinical findings are listed in Table 1 together with the type of supplement and usage as estimated by patient recall. All of the young men were well muscled but none had a body mass index (BMI) ≥ 30 kg/m2. The clinical events occurred in June (Patient 4), July (Patients 2 and 3) and February (Patient 1).
Table 1.
Patient clinical data, duration of weight lifting activity, serum creatinine and MDRD eGFR estimates, serum calcium and estimated daily amounts of supplemental protein, creatine, and vitamin C and vitamin D consumed
| Patient | Age | Height/weight, BMI | Activity, months | Creatinine, eGFR | Calcium | Protein (g) | Creatine (g) | Vitamin C Vitamin D |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 175 cm/88 kg 28.7 |
36 | 229.84 µmol/L (2.6 mg/dL) 0.57 mL/s (34 mL/min) |
2.50 mmol/L (10.0 mg/dL) |
78 | 15 | 200 mg 400 IU |
| 2 | 21 | 178/85 kg 26.8 |
36 | 335.92 µmol/L (3.8 mg/dL) 0.37 mL/s (22 mL/min) |
2.50 mmol/L (10.0 mg/dL) |
78–104 | 15 | 200 mg 400 IU |
| 3 | 23 | 178/86 kg 27.1 |
48 | 282.88 µmol/L (3.2 mg/dL) 0.43 mL/s (26 mL/min) |
2.45 mmol/L (9.8 mg/dL) |
78–104 | 15 | 200 mg 400 IU |
| 4 | 26 | 180/95 kg 29.3 |
84 | 247.52 µmol/L (2.8 mg/dL) 0.50 mL/s (30 mL/min) |
2.53 mmol/L (10.1 mg/dL) |
78 | 15 | 200 mg 400 IU |
MDRD, modification of diet in renal disease; eGFR, estimated glomerular filtration rate. BMI, body mass index (kg/m2).
Serum creatinine levels were between 229.84 and 335.92 µmol/L (2.6–3.8 mg/dL) with Modification of Diet in Renal Disease calculated estimated glomerular filtration rate (eGFR) between 0.37 and 0.57 mL/s (22–34 mL/min). Microscopic urinalyses were unremarkable and urine reagent strip testing was negative for glucose, protein, hemoglobin and nitrates.
Renal biopsies contained 12 to 16 glomeruli, and all biopsies revealed foci of flattened tubular epithelium with loss of nuclei and epithelial desquamation and blebbing. Hematoxylin staining amorphous deposits and occasional dense concretions were present within the injured tubules and adjacent interstitium (Figures 1 and 2). The tubulointerstitial deposits were negative with a von Kossa silver stain that reacts primarily with phosphate ions in calcium phosphate deposits.
Fig. 1.
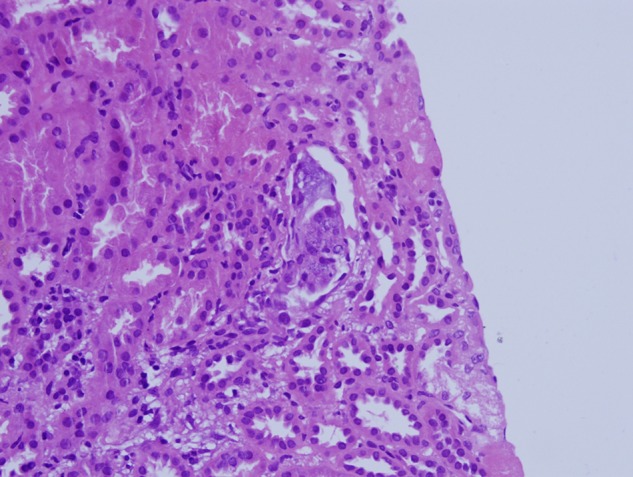
Biopsy findings, Patient 2. Degenerate and regenerative tubular epithelium is present together with amorphous intratubular calcium-like deposits. Hematoxylin and Eosin stain ×400.
Fig. 2.
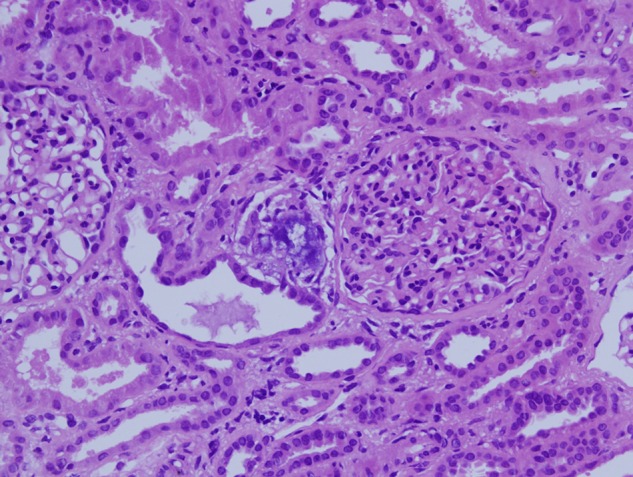
Biopsy findings, Patient 2. Denser interstitial concretions are found in an area of mild interstitial fibrosis and tubular atrophy. Hematoxylin and eosin stain ×200.
The biopsies of Patients 1 and 4 demonstrated interstitial fibrosis and tubular atrophy (IF/TA) involving 30–40% of the cortex (Figures 3 and 4). In Patient 4, the IF/TA contained clusters of obsolete glomeruli and lymphocytic inflammation. The inflammation was not present in cortex without IF/TA and was considered as a nonspecific reaction to cortical atrophy. In the biopsy of Patient 2, IF/TA involved 10–15% of the cortex. Conspicuous calcium-like concretions were not found in areas of IF/TA of any biopsy. No pigmented casts suggesting rhabdomyolysis were identified. In cryostat sections for immunofluorescence microscopy, oxalate crystals as evidence for vitamin C-associated renal oxylosis were not seen by light refraction or by polarization microscopy [16, 17]. Serum calcium levels were normal.
Fig. 3.
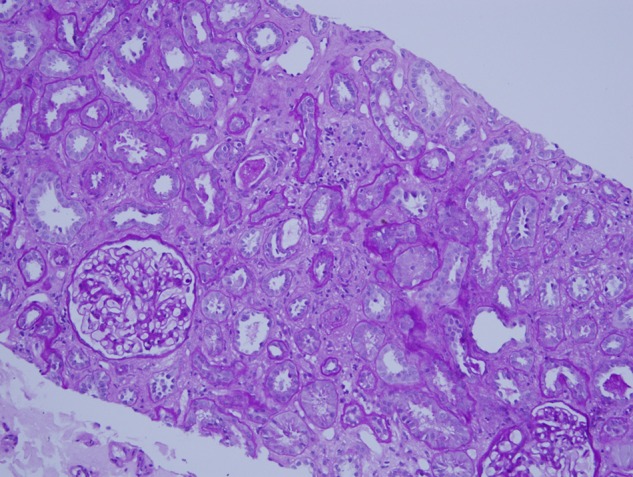
Biopsy findings, Patient 1. Cortical fibrosis and tubular atrophy involve 30–40% of the cortex in a biopsy with additional evidence of acute tubular injury. Periodic acid Schiff hematoxylin stain ×100.
Fig. 4.
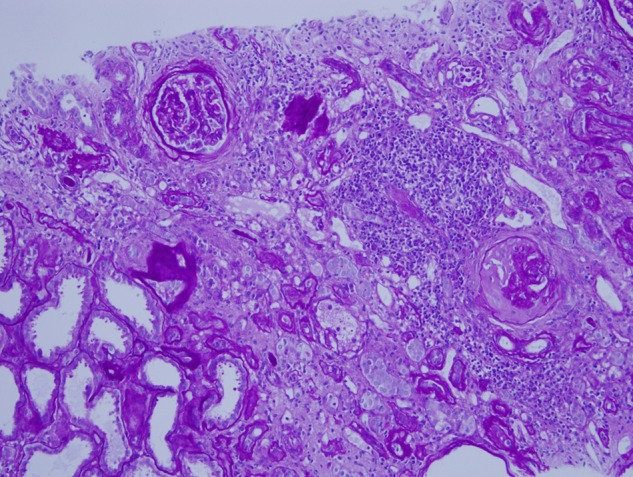
Biopsy findings, Patient 4. A broad area of subcapsular fibrosis and tubular atrophy contains chronic inflammation and obsolescent glomeruli. The inflammation is lymphocytic and considered a nonspecific reaction to cortical atrophy. Periodic acid Schiff hematoxylin stain ×100.
Protein supplementation consisted of 78–104 g of whey powder that when added to regular dietary protein including 2–3 L of milk reached 278–354 g daily or 3.2–4.2 g/kg/day. Creatine consumption was regularly 15 g a day including 5 g ‘maintenance’ doses with breakfast and 5 g pre- and post-exercise ‘loading and recovery’ doses. The manufacturer recommends no more than 6 g a day for long-term use. One and rarely two daily multi-vitamin tablets were taken. No additional calcium was added to the diet.
After the biopsy diagnoses, the patients complied with advice that steroid injections and supplements be discontinued. At 4 weeks, serum creatinine levels were below 123.76 µmol/L (1.4 mg/dL). At 6 months, serum creatinine levels were 118.92, 88.40, 97.24 and 106.08 µmol/L (1.3, 1.0, 1.1 and 1.2 mg/dL) for patients 1–4, respectively, and eGFR was >1.00 mL/s (60 mL/min) for all. Random urine collections from all patients demonstrated acidification with a pH of 5–6.5. None of the patients had any personal or family history of renal stones.
We have examined protein and creatine products sold in local gymnasiums. They have verified shipping documents, and high-pressure liquid chromatography by the regional Quality Assurance Laboratory showed no evidence of adulteration. In addition to patients, we interviewed other bodybuilders at gymnasiums in the region. They admit the illegal purchase of anabolic steroids but know of no unusual vitamin D use either orally or by injection. Creatine is referred to as ‘magic’ powder. Trainers recommend no more than 3–5 g on a regular basis but increased consumption is common. Trainers favor water for hydration, but colas and energy drinks are preferred by trainees. The gymnasiums are cooled by air conditioning or evaporative fans but are rarely <25°C.
Discussion
Four bodybuilders developed acute renal insufficiency while using commercial nutritional supplements consisting of protein and creatine combined with anabolic steroid injections. Biopsies demonstrated acute tubular injury, and when the injections and supplement use were stopped, serum creatinine levels became normal within 4 weeks.
The findings raised the possibility of the hypervitaminosis D-induced nephrocalcinosis recently encountered in four Brazilian bodybuilders [18–20]. These subjects developed hypercalcemia and renal failure with nephrocalcinosis while injecting veterinary grade vitamin D with estimated doses of >10 million IU annually. Of note was that the injections were not for vitamin D but for the silicone-like effect of the oily carrying medium that was used to add bulk to specific muscle groups. Our findings differ from those of the Brazilians in that none of our patients were hypercalcemic or took large doses of vitamin D, and none of our biopsies could be considered to show nephrocalcinosis.
Closely related to hypervitaminosis D is the milk- or calcium-alkali syndrome [21–23]. This is caused by an excessive consumption of milk or anti-acid calcium carbonate compounds. Currently, the calcium-alkali syndrome is primarily seen in post-menopausal women taking supplemental calcium and vitamin D for osteoporosis. The occurrence in bodybuilders is not specifically reported apart from hypervitaminosis D, and among our patients, milk and other calcium consumption could not be considered greatly excessive.
Acute phosphate nephropathy with intratubular calcium phosphate deposition that somewhat resembles the pathology of our patients is caused by oral sodium phosphates used for bowel preparation before colonoscopy [24]. Protein powder contains little inorganic phosphate with three daily supplement servings providing <0.5 g [25]. Creatine is sold mainly in the form of a monohydrate also with little phosphorus. Daily 2–3 L milk consumption will add 1.6–2.4 g to a normal dietary phosphorus intake of 1.0–1.5 g [25]. This is well below the 11 g oral intake used in bowel preparations [24]. In addition, the negative von Kossa stains indicates that the mainly amorphous tubular concretions found in the kidneys of our patients were acutely precipitated and had not complexed into crystalline hydroxyapatite with prominently stained phosphates that are seen in hyperphosphatemic nephropathy as well as other forms of neprocalcinosis [26].
For most athletes, sports nutritionists recommend a daily protein intake of 1.4–1.7 g/kg/day, less than half of what was used by our patients [2, 27]. There is substantial experimental and clinical data supporting the safety of creatine supplementation when it is used in the recommended amounts, but there is concern that excess dietary protein and creatine that is not accompanied by increased fluid intake may lead to a relative hypovolemia [6–9]. The clinical presentation of our three of our patients was in the summer. Iraqi summers are very hot, and by Western standards, the regional gymnasiums are warm and trainee hydration less than optimal.
In previously reported cases of acute kidney injury in creatine users, one demonstrated acute tubular necrosis, but two were classified as acute interstitial nephritis suggesting idiosyncratic allergic reactions [10–12]. Our patients' biopsies showed acute tubular necrosis that could be nephrotoxic or ischemic [28, 29]. In either case, this type of kidney injury among otherwise healthy young men is a rare event and points to a causal relationship with supplement and steroid use. Two of our patients had significant chronicity in their renal biopsies. This raises the possibility of preexisting chronic kidney disease that may or not be related to supplement use, but more likely reflects the risk that acute kidney injury of any type carries for chronic kidney disease [28, 29].
We must emphasize that a specific offending agent cannot be identified in this case material, and it may be the combination of excess creatine and protein with steroid injections that compounds risk not incurred with the individual substances. Our working hypothesis is that under hydration rather than direct toxicity precipitated the kidney injury.
Informed consent
Written informed consent was obtained from the patients for publication of these case reports and the accompanying images.
Authors’ contributions
S.E.A. was the attending nephrologist for all patients. He collected clinical information and wrote the first draft of the manuscript. A.A.A. investigated the supplements used by the patients, performed literature reviews, and wrote the description of the case material. M.D.H. contributed to the analysis of the literature and edited the several drafts of the manuscript. D.A.M. with M.D.H. conducted interviews with trainers and trainees in the regional gymnasiums and verified product invoices and quality control testing.
Conflict of interest statement
None declared.
References
- 1.Fink HH, Burgoon LA, Mikesky ARE. Practical Applications in Sports Nutrition, 2nd edn Sudbury, MA: Jones and Bartlett, 2009, pp. 220–254 [Google Scholar]
- 2.Martin WF, Armstrong LE, Rodriguez NR. Dietary protein intake and renal function. Nutr Metab 2005; 2: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herlitz LC, Markowitz GS, Farris AB, et al. Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 2010; 21: 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendergraft WF, III, Herlitz LC, Thornley-Brown D, et al. Nephrotoxic effects of common and emerging drugs. Clin J Am Soc Nephrol 2014; 9: 1996–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelhardt M, Neumann G, Berbalk A, et al. Creatine supplementation in endurance sports. Med. Sci. Sports Exerc 1998; 30: 1123–1129 [DOI] [PubMed] [Google Scholar]
- 6.Deldicque L, Décombaz J, Zbinden FH, et al. Kinetics of creatine ingested as a food ingredient. Eur J App Physiol 2007; 102: 133–143 [DOI] [PubMed] [Google Scholar]
- 7.Cooper R, Naclerio F, Allgrove J, et al. Creatine supplementation with specific view to exercise/sports performance: an update. J Int Soc Sports Nutr 2012; 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandhu RS, Como JJ, Scalea TS, et al. Renal failure and exercise-induced rhabdomyolysis in patients taking performance-enhancing compounds. J Trauma 2002; 53: 761–763 [DOI] [PubMed] [Google Scholar]
- 9.Yoshima WM, Tsourounis C. Effects of creatine supplementation on renal function. J Herb Pharmacother 2004; 4: 1–7 [PubMed] [Google Scholar]
- 10.Taner B, Aysim O, Abdulkadir U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT Plus 2011; 4: 23–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorsteinsdottir B, Grande JP, Garovic VD. Acute renal failure in a young weight lifter taking multiple food supplements. J Ren Nutr. 2006; 16: 341–345 [DOI] [PubMed] [Google Scholar]
- 12.Koshy KM, Griswold E, Schneeberger EE. Interstitial nephritis in a patient taking creatine. N Engl J Med 1999; 340: 815–815 [DOI] [PubMed] [Google Scholar]
- 13.Hathcock JN, Shao A, Vieth R, et al. Risk assessment for vitamin D. Am J Clin Nutr 2007; 85: 6–18 [DOI] [PubMed] [Google Scholar]
- 14.OM (Institute of Medicine). Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press, 2011. www.nap.edu [PubMed] [Google Scholar]
- 15.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008; 88: 582S–586S [DOI] [PubMed] [Google Scholar]
- 16.Chai W, Liebman M, Kynast-Gales S, et al. Oxalate absorption and endogenous oxalate synthesis from ascorbate in calcium oxalate stone formers and non-stone formers. Am J Kidney Dis 2004; 44: 1060–1069 [DOI] [PubMed] [Google Scholar]
- 17.Mashour S, Turner JF, Merrell R. Acute renal failure, oxalosis, and vitamin C supplementation: a case report and review of the literature. Chest 2000; 118: 561–563 [DOI] [PubMed] [Google Scholar]
- 18.Liborio AB, Nasserala JC, Gondim AS, et al. The case: renal failure in a bodybuilder athlete. Diagnosis: nephrocalcinosis secondary to exogenous vitamin D intoxication. Kidney Int 2014; 85: 1247–1248 [DOI] [PubMed] [Google Scholar]
- 19.Daher EF, Silva Júnior GB, Queiroz AL, et al. Acute kidney injury due to anabolic steroid and vitamin supplement abuse: report of two cases and a literature review. Int Urol Nephrol 2009; 4: 717–723 [DOI] [PubMed] [Google Scholar]
- 20.Rocha PN, Santos CS, Avila MO, et al. Hypercalcemia and acute kidney injury caused by abuse of a parenteral veterinary compound containing vitamins A, D, and E. J Bras Nefrol 2011; 33: 467–471 [PubMed] [Google Scholar]
- 21.Beall DP, Henslee HB, Webb HR, et al. Milk-alkali syndrome: a historical review and description of the modern version of the syndrome. Am J Med Sci 2006; 331: 233–242 [DOI] [PubMed] [Google Scholar]
- 22.Patel AM, Goldfarb S. Got calcium? Welcome to the calcium-alkali syndrome. J Am Soc Nephrol 2010; 21: 1440–1443 [DOI] [PubMed] [Google Scholar]
- 23.Khan SR. Nephrocalcinosis in animal models with and without stones. Urol Res 2010; 38: 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heher EC, Thier SO, Rennke H, et al. Adverse renal and metabolic effects associated with oral sodium phosphate bowel preparation. Clin J Am Soc Nephrol 2008; 3: 1494–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaucheron FG. The minerals of milk. Review Reprod Nutr Dev 2005; 45: 473–483 [DOI] [PubMed] [Google Scholar]
- 26.Robijin S, Vervaet BA, D'Haese PC, et al. Evaluation of intestinal binding to improve the safety profile of oral sodium phosphate bowel cleansing. PLoS One 2015; 10: e0116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarnopolsky M. Protein and amino acid needs for training and bulking up. In: Burke L, Deakin V. (eds). Clinical Sports Nutrition, 3rd edn McGraw Hill Australia Pty Ltd, 2006, pp. 73–103 [Google Scholar]
- 28.Wonnacott A, Meran S, Amphlett B, et al. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol 2014; 9: 1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talabani B, Zouwail S, Pyart RD, et al. Epidemiology and outcome of community-acquired acute kidney injury. Nephrology 2014; 19: 282–287 [DOI] [PubMed] [Google Scholar]


