Abstract
With the increasing utility of hematopoietic stem cell transplantation (SCT) as a treatment for cancer and noncancerous disorders, more challenges and complications associated with SCT have emerged. Renal injury immediately after transplant is common and well understood, but long-term renal injury is becoming more evident. Chronic graft-versus-host disease (GVHD) is a known long-term complication of SCT, and membranous nephropathy (MN) is emerging as the most common cause of SCT-associated glomerular pathology. In this case report, we present a patient who developed features of anti-PLA2R antibody-negative MN following autologous SCT. The renal injury responded well to steroids and further response to rituximab therapy was noted, suggesting antibody-mediated autoimmune glomerular disease. We also present a review of the literature on autologous GVHD and the role of T and B cells in induction of autoimmunity by SCT.
Keywords: anti-PLA2R, autologous stem cell transplant, GVHD, membranous nephropathy
Introduction
Acute or chronic renal failure is a known complication in stem cell transplant (SCT) patients. The causes of renal failure in these patients are multifactorial and include medication-induced nephrotoxicity, tumor lysis syndrome, septic or ischemic tubular necrosis, polyoma virus (BK nephropathy), radiation nephropathy, nephrotoxicity due to calcineurin inhibitor for graft-versus-host disease (GVHD) prophylaxis, hepatic veno-occlusive disease and hemolytic uremic syndrome. This paper will present for the first time autoimmune-mediated injury as another cause of kidney injury in SCT patients.
With the identification and typing of human leukocyte antigen major histocompatibility complex, allogeneic transplantation became feasible in the early 1960s. It was and still is used to treat nonmalignant hematologic diseases, metabolic disorders and immune deficiencies, many of which were once incurable or fatal. Allogeneic grafts initiate immune reactions related to histocompatibility. Recipient T cells recognize foreign donor antigens and can reject grafts; donor T cells recognize recipient antigens and can cause GVHD or graft-versus-tumor effects [1]. GVHD is a serious complication of allogeneic HCT noted in 60% of the recipients, which can affect skin, eyes, mouth, serous membranes, liver, gastrointestinal and respiratory tracts, and the musculoskeletal, hematopoietic and immune systems.
Autologous stem cell transplant and GVHD
With the emergence of ‘autologous’ SCT, GVHD and its complications, quite common in allogeneic transplantation, were expected to be eliminated. However, several cases of chronic cutaneous GVHD were described in patients with autologous SCT in the 1970s [2, 3]. It is speculated that inducing in autologous SCT patients, an autoimmune reaction similar to GVHD in allogeneic transplants would reduce this increased relapse risk. Such autoimmunity (autologous GVHD) could be achieved by the use of cyclosporine A (CsA) in up to 80% of patients [4]. Autologous GVHD has been thought of as an autoimmune syndrome and a milder form of GVHD than its counterpart in allogeneic transplantation. Autologous GVHD has been described in up to 10% of patients after autologous hematopoietic SCT [5], with involvement of skin, gastrointestinal tract or liver [2, 5–7].
Pathogenesis of autologous GVHD
T-cell mechanism
The pathogenesis of this autoimmunity, the so called ‘autoaggression syndrome’, is not understood. However, recent studies have indicated that two major factors are necessary for the induction of autologous GVHD: (i) disruption of thymic-dependent immune reconstitution and (ii) failure to re-establish peripheral self-tolerance. The thymus, which is responsible for T-cell development, is affected via either damage to the thymic epithelium by the HSCT or BMT preparative regimen and/or targeted and destroyed by alloreactive T cells. This will compromise the role of the thymus in deleting autoreactive T cells [3]. The use of cyclosporin A (CsA) [8] to successfully induce autologous GVHD further substantiated the pathogenetic role of disruption of the thymic function. Once the alloreactive T cells are released to the periphery, they can be eliminated using a T-cell-dependent regulatory system, however, due to the lymphoablative preparative regimen that SCT patients undergo, this system is not functional [9, 10].
B-cell mechanism and rituximab
The role of B cells in ‘allogenic’ GVHD has been investigated in the last few years. Because of the similar clinical presentation of GVHD and autoimmune diseases such as scleroderma and lupus, B cells could be a key common player. Development of autoantibodies, such as antinuclear antibodies in association with a chronic allogeneic GVHD or immune recovery has been reported [11]. A possible theory on how B cells can contribute to chronic GVHD is that the reconstituted B cells after myeloablative conditioning may have impaired immune tolerance of peripheral B cells, leading to the production of autoantibody in chronic GVHD. It has been shown in autoimmune disease that more than half of the developing B cells in the bone marrow express autoreactive B-cell receptors [12]. These observations support the use of B-cell depletion agents such as rituximab to treat refractory GVHD [13]. Rituximab is a chimeric monoclonal antibody directed against the B-cell-specific cluster of differentiation 20 (CD20) antigen, which is expressed only on pre-B lymphocytes and immature and mature B cells. Interestingly, in SLE patients, rituximab helps to reconstitute a normal B- and T-cell homeostasis and decrease the number of autoreactive memory B cells [14]. Prophylactic rituximab therapy delivered 2 months after transplantation decreased allogeneic donor B-cell immunity and the incidence of chronic GVHD [15]. Autologous GVHD is more like an autoimmune syndrome and has been well documented in the gastrointestinal tract and skin. In recent studies, the presence of autoantibodies after SCT in association with cGVHD provides evidence for B-cell involvement. In this paper, we will present a case of a multiple myeloma patient with a history of autologous SCT and secondary immune-mediated glomerulonephritis, with some features suggestive of membranous nephropathy (MN).
Case report
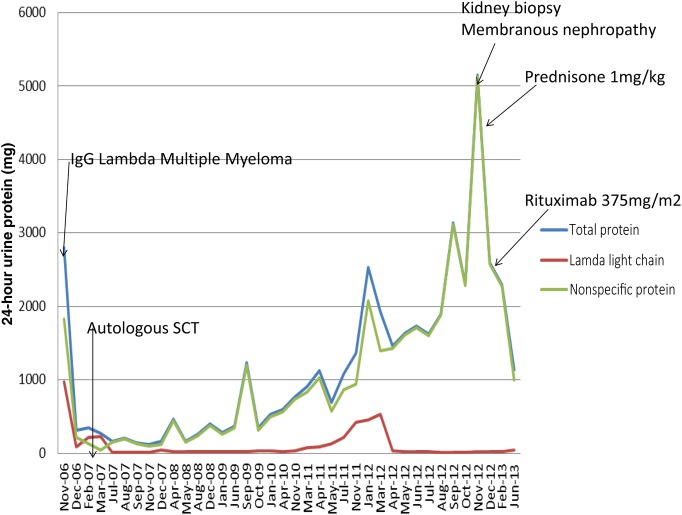
A 62-year-old man was diagnosed with lambda light chain multiple myeloma in November 2006. Bone marrow biopsy and aspiration showed 100% lambda light chain-restricted plasma cells. Urine studies showed a protein excretion of 3.4 g daily with lambda light chain Bence-Jones proteinuria of 2.082 g (Figure 1). In November 2006, he was induced with bortezomib, thalidomide and dexamethasone with good response. Due to myeloma cell infiltration in the bone marrow in early May 2007, he underwent autologous SCT with high-dose melphalan. Starting in May 2012, however, he was noted to have progressive nonspecific proteinuria (5 g/day) despite continued remission from the myeloma. The patient was referred to nephrology for further evaluation of his proteinuria and a kidney biopsy was performed with a diagnosis of immune-mediated glomerulonephritis with features suggestive of early MN (Figure 2). No secondary causes were present to explain the MN. He had undergone testing for hepatitis, syphilis, anti-thyroid antibodies, colonoscopy, PSA screen, CT scan of lung, abdomen and pelvis, all of which were negative. In addition, the patient's serum was tested for anti-PLA2R autoantibodies, which were also negative.
Fig. 1.
Trend of proteinuria in 24-h urine collections over the time course of treatment. The decreased lambda light chains in comparison to the total proteinuria illustrate the persistence of proteinuria in the setting of multiple myeloma being in remission. Dramatic improvement in proteinuria after steroid initiation with further improvement after rituximab treatment is also illustrated.
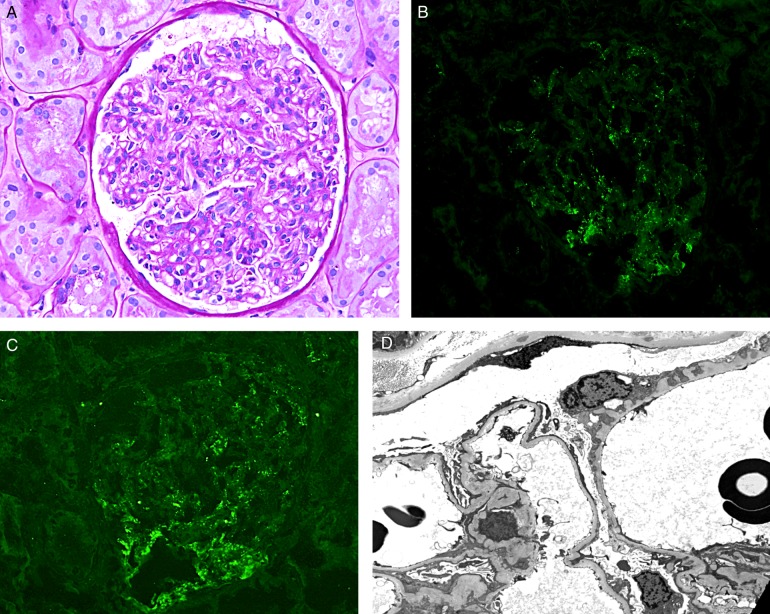
Fig. 2.
Kidney biopsy: (A) Light microscopy: A glomerulus with mild mesangial sclerosis and hypercellularity, and mild thickening of glomerular capillary wall (Periodic acid–Schiff stain, ×400). (C) Immunofluorescence studies: Punctate staining of IgG (B) and C4d (C) along the glomerular capillaries and in mesangial areas (direct immunofluorescence, ×400). (D): Electron microscopy: Rare small electron-dense deposits are noted in the subendothelial and mesangial locations. There is mild mesangial sclerosis. Indentation of the glomerular basement membrane is also noted in a rare glomerular capillary (×3000).
With the biopsy evidence of an immune-mediated process, he was started on prednisone 60 mg/day and tapered down with significant improvement of his proteinuria to a level of 2.0 g/day. Due to the reported success of rituximab in treating MN and the involvement of humoral immunity, the patient received four weekly doses of 375 mg/m2 of rituximab infusion and his proteinuria was reduced further to 1.1 g/day (Figure 1).
Biopsy findings
The kidney biopsy showed cortical tissue with up to 10 glomeruli (7 glomeruli in the portion for light microscopy; in addition, there are also 2 glomeruli in the portion for immunofluorescence and 1 glomerulus in the portion for Electron microscopy). These glomeruli displayed global diffuse mild mesangial sclerosis and hypercellularity and mild thickening of glomerular capillary wall (Figure 2A).
The immunofluorescence studies showed two glomeruli, both of which displayed punctate 2/3+ staining for IgG, C3, kappa light chain, lambda light chain and C4d in mesangial areas and along the glomerular capillaries (Figure 2B and C). There was no staining for IgA, IgM, C4 or C1q.
Electron microscopic study showed a few electron-dense deposits, suggestive of immune-type deposits, in the subepithelial location of a rare glomerular capillary, and also in some mesangial areas (Figure 2D).
The findings were most consistent with an immune-mediated glomerulonephritis, with features suggestive of early membranous glomerulonephritis.
Discussion
Recently, nephrotic syndrome occurring in allogeneic SCT patients has been considered to be a possible presentation of chronic GVHD of the kidney. A decrease in immunosuppressive medication after SCT was associated with the occurrence of nephrotic syndrome within 9 months in 63% of patients [16, 17]. MN was the most common cause of glomerular pathology post-HSCT, comprising 64% of all reported cases, followed by minimal change disease at 19% [17]. There are few described cases of chronic GVHD with manifestations similar to SLE and ANA positivity, further confirming a role of autoimmunity in the pathogenesis of GVHD [18].
There have been dramatic advancements in the diagnosis and treatment of idiopathic MN after the identification of anti-PLA2R autoantibody as the putative pathogenic factor in primary MN [19]. It was noted that a high proportion of patients with idiopathic MN have circulating antibodies to the M-type phospholipase A2 receptor (PLA2R), a transmembrane protein located on podocytes (sensitivity and specificity >75%) [20]. A recent study on anti-PLA2R antibodies in patients with allogeneic SCT-associated MN showed a uniform absence of these antibodies [21]. MN and other immune-mediated glomerulonephritis in allogeneic transplant patients may thus represent a form of ‘kidney-specific’ chronic GVHD, in which donor immune cell-induced alloantibodies may target podocyte antigens/proteins specific for the recipient but not present in the donor.
In autologous SCT populations, there have been reports of associated glomerular disease, as a result of any type of immune dysregulation. Based on a literature review published in 2010 [17], 6% of all patients with glomerulonephritis after HCT had received autologous transplants [13, 22, 23]. A systemic autoimmune syndrome resembling GVHD has been described in liver, skin and gastrointestinal tract as a milder form of GVHD. The failure to re-establish self-tolerance can lead to systemic autoimmunity (T-cell- and probably B-cell-mediated) and exacerbate or even mimic GVHD. Treatment for SCT-associated MN commonly includes steroids [24–26] and calcineurin inhibitors. Other immunosuppressants, such as cyclophosphamide, mycophenolate mofetil, chlorambucil and rituximab, have also been used [27–30]. In the case presented, corticosteroids were more beneficial in the treatment of the immune-mediated process as noted with the dramatic drop in proteinuria than with rituximab.
Conclusion
In conclusion, the case presented is an example in which autoimmunity has been induced after an autologous SCT and manifests as an immune-mediated glomerulonephritis with features suggestive of early MN. The glomerular deposition of IgG and complement components C3 and C4d further establishes the involvement of humoral immunity. The absence of anti-PLA2R antibody and the presence of electron-dense deposits in the mesangial areas render the possibility of the coincidental development of primary MN less likely. Failure to maintain self-tolerance after autologous SCT has resulted not in alloimmune antibodies as in the allogeneic SCT, but an antibody-mediated autoimmune glomerular disease.
Conflict of interest statement
All authors have read and approved the manuscript and declared no competing financial interests or other conflicts of interest.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826 [DOI] [PubMed] [Google Scholar]
- 2.Hood AF, Vogelsang GB, Black LP, et al. Acute graft-vs-host disease. Development following autologous and syngeneic bone marrow transplantation. Arch Dermatol 1987; 123: 745–750 [DOI] [PubMed] [Google Scholar]
- 3.Hess AD, Thoburn CJ. Immunobiology and immunotherapeutic implications of syngeneic/autologous graft-versus-host disease. Immunol Rev 1997; 157: 111–123 [DOI] [PubMed] [Google Scholar]
- 4.Bolanos-Meade J, Garrett-Mayer E, Luznik L, et al. Induction of autologous graft-versus-host disease: results of a randomized prospective clinical trial in patients with poor risk lymphoma. Biol Blood Marrow Transplant 2007; 13: 1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cogbill CH, Drobyski WR, Komorowski RA. Gastrointestinal pathology of autologous graft-versus-host disease following hematopoietic stem cell transplantation: a clinicopathological study of 17 cases. Mod Pathol 2011; 24: 117–125 [DOI] [PubMed] [Google Scholar]
- 6.Holmberg L, Kikuchi K, Gooley TA, et al. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 2006; 12: 226–234 [DOI] [PubMed] [Google Scholar]
- 7.Rappeport J, Mihm M, Reinherz E, et al. Acute graft-versus-host disease in recipients of bone-marrow transplants from identical twin donors. Lancet 1979; 2: 717–720 [DOI] [PubMed] [Google Scholar]
- 8.Gao EK, Lo D, Cheney R, et al. Abnormal differentiation of thymocytes in mice treated with cyclosporin-A. Nature 1988; 336: 176–179 [DOI] [PubMed] [Google Scholar]
- 9.Hess AD, Thoburn CJ, Chen W, et al. Complexity of effector mechanisms in cyclosporine-induced syngeneic graft-versus-host disease. Biol Blood Marrow Transplant 2000; 6: 13–24 [DOI] [PubMed] [Google Scholar]
- 10.Hess AD. Reconstitution of self-tolerance after hematopoietic stem cell transplantation. Immunol Res 2010; 47: 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol 2006; 34: 389–396 [DOI] [PubMed] [Google Scholar]
- 12.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science 2003; 301: 1374–1377 [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi M, Townamchai N, Intragumtornchai T, et al. Crescentic glomerulonephritis developing 3 months after autologous peripheral blood stem cell transplantation for non-Hodgkin's lymphoma. Bone Marrow Transplant 1998; 22: 725–727 [DOI] [PubMed] [Google Scholar]
- 14.Anolik JH, Barnard J, Cappione A, et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum 2004; 50: 3580–3590 [DOI] [PubMed] [Google Scholar]
- 15.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood 2012; 119: 6145–6154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brukamp K, Doyle AM, Bloom RD, et al. Nephrotic syndrome after hematopoietic cell transplantation: do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol 2006; 1: 685–694 [DOI] [PubMed] [Google Scholar]
- 17.Hu SL. The role of graft-versus-host disease in haematopoietic cell transplantation-associated glomerular disease. Nephrol Dial Transplant 2011; 26: 2025–2031 [DOI] [PubMed] [Google Scholar]
- 18.Fraile P, Vazquez L, Garcia-Cosmes P, et al. Graft-versus-host disease of the kidney in patients with allogenic bone marrow transplant. Bone Marrow Transplant 2013; 48: S446. [DOI] [PubMed] [Google Scholar]
- 19.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck LH, Jr, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int 2010; 77: 765–770 [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Qin W, Zhang M, et al. Detection of anti-PLA2R autoantibodies and IgG subclasses in post-allogeneic hematopoietic stem cell transplantation membranous nephropathy. Am J Med Sci 2013; 346: 32–37 [DOI] [PubMed] [Google Scholar]
- 22.Navaneethan SD, Taylor J, Goldman B, et al. Anti-neutrophil cytoplasmic antibody associated crescentic IgA nephropathy in hematopoietic stem cell transplantation. Clin Nephrol 2009; 71: 59–62 [DOI] [PubMed] [Google Scholar]
- 23.Sakarcan A, Neuberg RW, McRedmond KP, et al. Membranoproliferative glomerulonephritis develops in a child with autologous stem cell transplant. Am J Kidney Dis 2002; 40: E19. [DOI] [PubMed] [Google Scholar]
- 24.Chanswangphuwana C, Tamaoki A, Tada A, et al. Glomerular diseases associated with chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation: case reports. Transplant Proc 2014; 46: 3616–3619 [DOI] [PubMed] [Google Scholar]
- 25.Fraile P, Vazquez L, Caballero D, et al. Chronic graft-versus-host disease of the kidney in patients with allogenic hematopoietic stem cell transplant. Eur J Haematol 2013; 91: 129–134 [DOI] [PubMed] [Google Scholar]
- 26.Reddy P, Johnson K, Uberti JP, et al. Nephrotic syndrome associated with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2006; 38: 351–357 [DOI] [PubMed] [Google Scholar]
- 27.Ferrannini M, Vischini G, Di Daniele N. Rituximab in membranous nephropathy after haematopoietic stem cell transplantation. Nephrol Dial Transplant 2008; 23: 2700–2701; author reply 2701 [DOI] [PubMed] [Google Scholar]
- 28.Rao PS. Nephrotic syndrome in patients with peripheral blood stem cell transplant. Am J Kidney Dis 2005; 45: 780–785 [DOI] [PubMed] [Google Scholar]
- 29.Iguchi E, Minakata T, Tsudo M. A case of membranous nephropathy associated with chronic GVHD successfully treated with rituximab. Bone Marrow Transplant 2012; 47: 132–134 [DOI] [PubMed] [Google Scholar]
- 30.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant 2003; 9: 505–511 [DOI] [PubMed] [Google Scholar]