Abstract
Background
Tenofovir disoproxil fumarate (TDF) may cause acute kidney injury and proximal tubular dysfunction. However, no detailed studies document urinary phosphate wasting as a marker of TDF-induced tubulopathy.
Methods
Records of HIV-infected patients with presumed TDF toxicity were reviewed. We describe the characteristics and clinical course of 15 patients who had documented elevated (>20%) fractional excretion of phosphate (FEphos).
Results
Patients were predominantly Caucasian and male (73 and 80%, respectively), with a mean age of 56 years (range 38–76). Of the 15 patients, 11 had a estimated glomerular filtration rate (eGFR) of >90 mL/min/1.732 at time of TDF initiation. The mean duration of TDF therapy prior to diagnosis of TDF toxicity was 64 months. Mean FEphos was 34% (range 20–62). The mean eGFR at TDF initiation was 104 mL/min/1.73 m2 [standard deviation (SD) 17.0] with a gradual decline to 69 mL/min/1.73 m2 (SD 19.0) by the time of TDF discontinuation. Of 10 patients with repeated FEphos after TDF discontinuation, 9 had improvement of their FEphos. Of these individuals, 6 had normalization of their FEphos. Estimated GFR improved in 12 patients after discontinuation of TDF, though importantly, none returned to their baseline eGFR.
Conclusions
Urinary phosphate wasting is a sensitive marker for TDF-induced proximal tubulopathy and is associated with unrecognized and permanent renal function decline. Tubular dysfunction can develop after years of TDF therapy in those with normal kidney function at the time of drug initiation. This suggests that continuing vigilance be maintained in all those on TDF.
Keywords: HIV, kidney injury, phosphate wasting, proximal tubular dysfunction, tenofovir
Introduction
Tenofovir disoproxil fumarate (TDF), the oral prodrug of tenofovir, is an acyclic nucleotide phosphonate, which is frequently used as a first-line therapy in HIV-infected patients [1]. It can be conveniently dosed once a day and has a favorable side effect profile, making it one of the most commonly prescribed drugs in this patient population [2]. The excretion of TDF is primarily through the kidneys. While most of this renal excretion is through filtration, 20–30% of TDF is actively transported in the proximal tubule through organic anion transporter 1 (OAT-1) [3].
Initial studies showed TDF to be a safe drug in HIV-infected patients, with a post-marketing survey of 10 343 patients demonstrating renal side effects in only 0.5% of patients [4]. However, after FDA approval, case reports of proximal tubular dysfunction and acute kidney injury emerged [5, 6]. Multiple observational cohorts have demonstrated the association of TDF use with a decline in estimated glomerular filtration rate (eGFR) and development of chronic kidney disease (CKD) [7–10]. Moreover, TDF use is associated with Fanconi syndrome characterized by proximal tubular dysfunction with urinary wasting of phosphate, glucose, amino acids and bicarbonate [11]. Apoptosis of tubular cells and inhibition of mitochondrial DNA replication in proximal tubular cells may be involved in the pathogenesis of TDF-induced nephropathy [12, 13]. Risk factors for development of TDF-associated nephrotoxicity include polymorphism in genes encoding proximal tubular transporters [14, 15], concomitant use of protease inhibitors [16–18], preexisting kidney disease [19], low body mass index, older age, advanced HIV infection, concomitant hepatitis C virus (HCV) infection and concurrent use of other nephrotoxic drugs [4, 18, 20].
Despite the widely recognized association of TDF with proximal tubular dysfunction, the clinical course of patients with TDF-induced proximal tubular dysfunction after discontinuation of TDF remains unclear. In this study, we describe the characteristics of patients with overt proximal tubular toxicity, with documented urinary phosphate wasting attributed to TDF and their course after the discontinuation of this drug.
Methods
Study design and population
We conducted a retrospective observational study of HIV-infected adults evaluated at the Johns Hopkins Nephrology Clinic with documented phosphate wasting in the context of TDF use. We identified 63 patients with presumed TDF toxicity that had discontinued TDF or had adjustment in the TDF dose. Only 15 patients had measurements of urinary phosphate prior to drug discontinuation and were included. Patients who did not have a fractional excretion of phosphate (FEphos) at the time of presumed TDF toxicity were excluded. Other potential causes of proximal tubule dysfunction, including multiple myeloma, amyloidosis, aminoglycoside exposure or other drug exposures were excluded in all cases [21]. The Johns Hopkins University Institutional Review Board approved the study.
Data collection
Demographic, clinical, laboratory and pharmacologic data were abstracted from electronic medical records at the time of the patients' initial evaluation in the Nephrology Clinic. Patients were classified as having diabetes mellitus or hypertension if they had a chart diagnosis of either or were on medications for the treatment of these conditions, respectively. Diagnosis of hepatitis B or HCV infection was based on the presence of antibodies or detectable viral levels by PCR.
Definitions
Fractional excretion of phosphate was calculated by the following equation:
Phosphate wasting was defined as a FEphos of >20% among patients with normal serum phosphate levels (2.7–4.5 mg/dL) or >10% among patients with hypophosphatemia (serum phosphates of <2.7 mg/dL) [22, 23]. Ratio of renal tubular maximal reabsorption of phosphate to GFR was calculated as follows [24]:
A TmP/GFR of <2.6 mg/dL was considered to be low. Hypokalemia was defined as a serum potassium level of <3.5 meq/L, and low bicarbonate was defined as a bicarbonate level of <22 meq/L. Estimated GFR was calculated using the Chronic Kidney Disease Epidemiology (CKD-EPI) equation [25, 26]. Rapid progression of kidney disease was defined as a fall in GFR of >5 mL/min/1.73 m2 per year [27].
Results
Baseline characteristics
At the time of diagnosis of TDF toxicity, the mean age of the cohort was 56 years (range 38–76) with an SD of 11.3 years as shown in Table 1. The patients were predominantly Caucasian and male (73 and 80%, respectively). Three patients (20%) had concurrent HCV infection. The mean duration of TDF therapy prior to diagnosis of TDF toxicity was 64 months (SD 21.3, range 28–103). The mean FEphos was 34% (range 20–62%). The mean serum phosphate concentration was 2.3 mg/dL (range 1.0–3.6 mg/dL), with 11 (73%) patients experiencing hypophosphatemia. Ten patients had vitamin D level checked, and all of them had a 25-OH vitamin D level of >15 ng/mL (mean 25-OH vitamin D level 36 ng/mL).
Table 1.
Baseline characteristics of 15 patients with TDF-induced proximal tubulopathy at the time of diagnosis
| Characteristics | Values |
|---|---|
| Mean age, years (range) | 56 (38–76) |
| Male gender, n (%) | 12 (80) |
| Body Mass Index, Kg/m2, mean (SD) | 23.2 (4.0) |
| Caucasian, n (%) | 11 (73) |
| Hypertension, n (%) | 9 (60) |
| Diabetes mellitus, n (%) | 6 (40) |
| HCV infection, n (%) | 3 (20) |
| Current smoker, n (%) | 10 (67) |
| Duration of TDF use, months mean (range) | 64 (28–103) |
| Baseline serum creatinine, mg/dL Mean (SD)(prior to TDF initiation) | 0.8 (0.2) |
| Baseline eGFR, mL/min/1.73 m2 mean (SD) (prior to TDF initiation) | 104 (17.0) |
| Serum creatinine, mg/dL mean (SD) (at the time of TDF discontinuation) | 1.22 (0.3) |
| eGFR, mL/min/1.73 m2 mean (SD) (at the time of TDF discontinuation) | 69 (19.0) |
| Change in eGFR from baseline/1.73 m2, mean (SD) | 35 (18.4) |
| Serum phosphate, mg/dL mean (SD) | 2.3 (0.7) |
| Serum potassium, mg/dL mean (SD) | 3.9 (0.6) |
| Serum bicarbonate, mg/dL mean (SD) | 24.5 (3.2) |
| FEphos, %, mean (range) | 34 (20–62) |
| TmP/GFR, mean (range) | 1.6 (0.6–2.8) |
| Glycosuria, n (%) | 7 (46) |
eGFR, estimated GFR; FEphos, fractional excretion of phosphate; TDF, tenofovir disoproxil fumarate; TmP, tubular maximal reabsorption of phosphate.
Despite evidence of phosphate wasting in all the patients, only 53% had concurrent glycosuria. Seven (47%) of the 15 patients had >500 mg/g of protein on a spot urine collection. Eleven (73%) patients had an eGFR of >90 mL/min/1.73 m2, and the other four had a GFR of 80–90 mL/min/1.73 m2, with a mean serum creatinine of 0.8 mg/dL (range 0.5–1.0, SD 0.16). Eleven patients (73%) demonstrated rapid progression of kidney disease.
Individual laboratory values of the 15 patients are shown in Table 2. In assessing other features of proximal tubule dysfunction, only four patients had a low serum bicarbonate level (<22 meq/L) with mean bicarbonate of 24 meq/L, with no bicarbonate <20 meq/L for the cohort. Similarly, only four patients had concurrent hypokalemia, but all of those who had hypokalemia had potassium of >3.0 meq/L. Only one patient (#2) had a normal TmP/GFR in the setting of a high FePhos. He had concurrent glucosuria and a significant drop in his GFR on TDF therapy suggesting renal tubular dysfunction.
Table 2.
Individual characteristics of 15 patients with proximal tubular dysfunction at the time of diagnosis of TDF-induced proximal tubulopathy
| Baselinea serum creatinine (mg/dL) | Baselinea GFR (mL/min/1.73 m2) | Months of TDF therapy | Creatinine at TDF stop (mg/dL) | GFR at TDF stop (mL/min/1.73 m2) | Change in GFR (mL/min/1.73 m2) | Serum phosphate (mg/dL) | Fractional excretion of phosphate | TmP/GFR (mg/dL) | Urine glucose | Serum potassium (meq/L) | Serum bicarbonate (meq/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.8 | 97 | 51 | 1.3 | 58 | −39 | 2.4 | 20 | 1.9 | − | 3.1 | 20 |
| 2 | 0.7 | 126 | 103 | 1.1 | 69 | −57 | 3.6 | 21 | 2.8 | + | 3.8 | 28 |
| 3 | 1.0 | 86 | 54 | 1.3 | 74 | −12 | 2.0 | 21 | 1.6 | − | 5.0 | 28 |
| 4 | 0.8 | 121 | 48 | 1.2 | 75 | −46 | 2.9 | 24 | 2.2 | + | 4.1 | 23 |
| 5 | 1.0 | 83 | 59 | 1.2 | 64 | −19 | 2.4 | 27 | 1.8 | − | 3.3 | 30 |
| 6 | 0.8 | 106 | 72 | 1.5 | 52 | −54 | 2.0 | 30 | 1.4 | − | 4.2 | 25 |
| 7 | 1.0 | 88 | 70 | 1.5 | 52 | −36 | 2.0 | 30 | 1.4 | − | 4.2 | 25 |
| 8 | 0.6 | 122 | 94 | 1.2 | 70 | −52 | 3.1 | 30 | 2.2 | − | 4.0 | 26 |
| 9 | 0.8 | 103 | 62 | 1.0 | 77 | −26 | 3.1 | 33 | 2.1 | + | 4.4 | 28 |
| 10 | 0.6 | 115 | 53 | 1.0 | 83 | −32 | 2.2 | 33 | 1.5 | + | 4.3 | 23 |
| 11 | 0.5 | 136 | 56 | 1.1 | 69 | −67 | 1.0 | 42 | 0.6 | + | 3.8 | 21 |
| 12 | 0.8 | 100 | 53 | 2.1 | 30 | −70 | 2.2 | 44 | 1.1 | − | 3.7 | 21 |
| 13 | 0.9 | 111 | 28 | 1.1 | 85 | −26 | 1.0 | 45 | 0.6 | + | 3.3 | 22 |
| 14 | 1.0 | 81 | 50 | 1.2 | 63 | −18 | 2.6 | 48 | 1.4 | − | 4.5 | 27 |
| 15 | 1.0 | 92 | 103 | 0.5 | 116 | 24b | 2.0 | 62 | 0.8 | + | 3.1 | 21 |
GFR, estimated glomerular filtration rate; TDF, tenofovir disoproxil fumarate; TmP, tubular maximal reabsorption of phosphate.
aBaseline values are those recorded at the time of TDF initiation.
bPatient had an improvement in his GFR after starting of TDF.
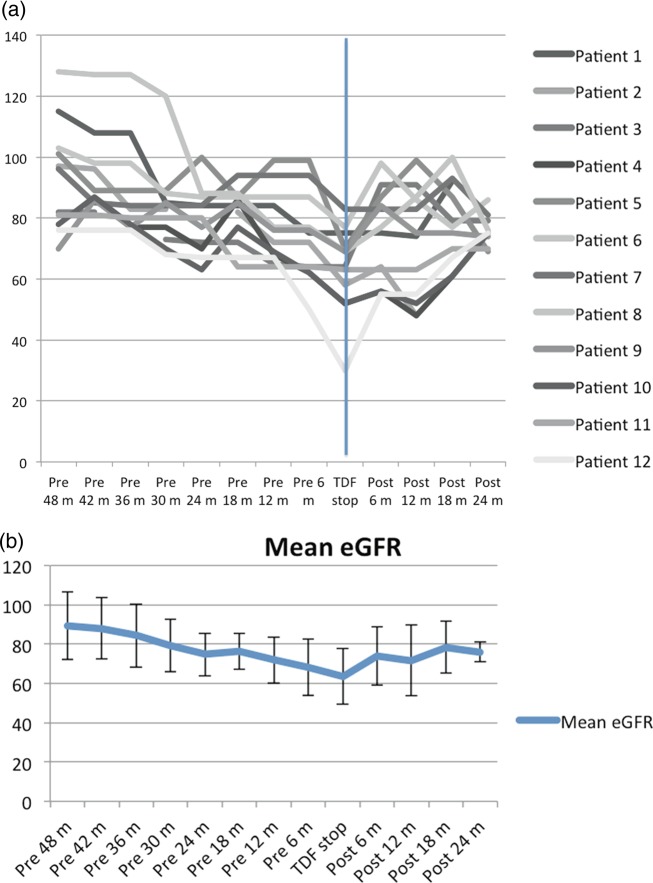
The mean eGFR for the 15 patients included at the start of TDF therapy was 104 mL/min/1.73 m2 (SD 17.0, range 81–136) and decreased to a mean of 69 mL/min/1.73 m2 (SD 19.0, range 30–116) with a mean eGFR decline of 35 mL/min/1.73 m2 by the time of TDF discontinuation. Only 12 of the 15 patients had multiple eGFR measurements before and after discontinuation of TDF at 6-month intervals. The trend for their GFR based on their point eGFR at 6-month intervals and the changes in their eGFR at 6-month interval after discontinuation of TDF are shown in Figure 1a. If the patient did not have an eGFR measurement at the exact 6-month interval, we used the one which was closest to the 6-month mark within a 2-month interval. Figure 1b depicts the mean eGFR of patients in relation to discontinuation of TDF and highlights the slow decline in eGFR of patients prior to TDF discontinuation. One patient (Patient 15) in our group had apparent improvement in eGFR after initiation of TDF. However, this may have been due to a decline in this patient's serum creatinine as a result of poor nutrition and muscle atrophy prior to the patient's death, which occurred within a month of TDF discontinuation, rather than an actual improvement in renal function; his weight decreased from 76 to 62 kg in the 4 months prior to discontinuation of the TDF.
Fig. 1.
(a) Trends of eGFR values 48 months prior or 24 months after discontinuation of TDF in 12 patients. (b) Mean eGFR of patients before and after discontinuation of TDF.
Concomitant antiretroviral use included 11 patients on ritonavir boosted protease inhibitors (5 atazanavir), 8 patients on efavirenz and 4 on raltegravir.
Effects of discontinuation of TDF
All patients discontinued tenofovir. New regimens included addition of the protease inhibitor darunavir with ritonavir in 2 patients (in addition to the 11 already on a boosted protease inhibitor), raltegravir in 6 and abacavir in 5. Efavirenz was discontinued in two.
Only 10 of the 15 patients had their FEphos checked after discontinuation of TDF. Of these, six had normalization of their FEPhos between 8 and 60 weeks after TDF discontinuation. Three others had a decrease in their FEphos by the time of their last measurement between 8 and 52 weeks after drug discontinuation. All three of these had been hypophosphatemic at the time of drug discontinuation and had normalized their serum phosphate levels. One patient had an increase in FEphos measured 2 months after TDF discontinuation. At that time the serum phosphate had increased from 2.0 to 2.3 mg/dL (patient 3); no further assessments were performed.
Of the eleven (73%) hypophosphatemic patients, all patients, except one, had normalization of serum phosphate levels after discontinuation of TDF. The one patient (# 3) without normalization of his serum phosphate level had an increase from 2.0 to 2.3 after 2 months without further follow-up. Of the four patients who had a normal serum phosphate at the time diagnosis, three patients had an increase in their serum phosphate after discontinuation of TDF. Of note, in at least three hypophosphatemic patients it took >6 months before their serum phosphate normalized.
All patients with urine glucose had resolution of glycosuria. One patient (#10) had persistent glycosuria a year after stopping the TDF. Subsequent urinalyses remained free of glucose. The same patient had an elevated FEphos at the time of the glycosuria. Though the FEphos was not reassessed, the serum phosphate had normalized in this patient and has been in the normal range for over 2.5 years since stopping TDF.
Twelve (86%) of 14 with loss of kidney function on TDF had an increase in eGFR after discontinuation of TDF. The eGFR started to increase in these patients within 6 months and continued to improve beyond 6 months before stabilizing (Figure 1a,b). However, none of the patients improved to their baseline eGFR. One patient, who had an improvement in eGFR after discontinuation of TDF, later had a decline in his eGFR after 2 years as a result of biopsy-proven diabetic nephropathy.
Two of the 14 patients continued to experience a decline in eGFR despite discontinuation of TDF. One had an underlying CKD with ongoing illicit drug use and history of non-adherence. The other had only 6 months of follow-up, and this failure to recover eGFR might be explained by a residual effect from TDF use.
Discussion
The potential of TDF to cause nephrotoxicity reflected by increased serum creatinine is well described in the literature [28, 29]. Furthermore, Scherzer et al. in a large cohort study demonstrated that each year of TDF exposure was associated with a 33% increased risk CKD [10]. However, serum creatinine is a late marker of kidney dysfunction [30], and urinary phosphate wasting reflective of proximal tubular dysfunction may be a more sensitive marker for TDF-induced kidney injury. The frequency of tubulopathy reported in observational and clinical cohorts may be underestimated since most trials did not perform specific detailed analysis for the diagnosis of phosphate wasting [31–33].
The current study demonstrates that in a series of patients with tenofovir nephrotoxicity as reflected by urine phosphate wasting the diagnosis is often late in the treatment course (average of 64 months after TDF initiation) and occurs in those who have normal kidney function at baseline. Moreover, it is usually associated with hypophosphatemia though not infrequently the serum phosphate can be normal with renal phosphate wasting. This is generally not associated with other components of Fanconi syndrome such as hypokalemia and metabolic acidosis. Glycosuria was present in only 47 percent of patients and therefore appears to be a less sensitive marker than both serum phosphate and urine phosphate wasting.
Though declining eGFR (increasing serum creatinine) was occurring slowly over time prior to the diagnosis of toxicity, this may not have been clinically recognized due to the very gradual absolute increase in serum creatinine over years. Importantly, this study suggests that vigilance for tenofovir toxicity should remain beyond the early years of treatment. In addition, since no patient showed full recovery of eGFR, earlier identification of toxicity prior to development of renal function decline is important. Certainly, close monitoring of eGFR over time, rather than serum creatinine alone (with clear documentation of a baseline level) might detect subtle decline and alert one earlier to the possibility of nephrotoxicity. In addition, only 5 of 15 patients had serum creatinine levels beyond the ‘normal’ range of 0.5–1.2 mg/dL at the time of diagnosis of nephrotoxicity. This likely also explains the significant declines in eGFR prior to detection and further emphasizes the need to use GFR estimation in the monitoring of kidney function.
The difficulty of detecting TDF nephrotoxicity in the context of subtle changes in serum creatinine further highlights the importance of monitoring phosphate excretion, serum phosphate, urinary protein and glycosuria for TDF toxicity rather than relying on serum creatinine as a lone marker of kidney injury. Earlier detection of urinary phosphate wasting or serum phosphate decline may have identified nephrotoxicity sooner. Hypophosphatemia and urinary phosphate wasting have been described in the absence of GFR loss [34]. However, urine phosphate was not previously assessed in any patient, and serum phosphate levels were not monitored in the majority of the patients.
Current guidelines by the Infectious Disease Society of America recommend monitoring of serum creatinine, serum phosphate, glycosuria and proteinuria twice a year in patients who are prescribed TDF and have an eGFR of <90 mL/min/1.73 m2 or in patients with diabetes and hypertension who have a high risk of kidney disease [35]. Despite these guidelines, only 7 (47%) of the 15 patients in this series had a serum phosphate level checked prior to their referral to the renal clinic even after years of TDF therapy. Also, since not all patients developed hypophosphatemia, the diagnosis of TDF-induced tubulopathy would have been missed based on current screening guidelines in 27% of patients who had renal phosphate wasting with normal serum phosphate levels.
As noted earlier, the average time for the detection of proximal tubular dysfunction after start of TDF therapy in our cohort was 64 months, a duration that is much longer than has been previously reported in other studies [36]. This underscores the necessity of continued monitoring of side effects in patients even if they have tolerated TDF therapy for years.
Even after discontinuation of TDF, recovery of serum phosphate and improvement of eGFR may take several months. Despite improvement in eGFR after discontinuation of TDF, none of the patients returned to their baseline eGFR after a mean follow-up of 23 months after discontinuation of TDF. Two patients continued to experience decline in eGFR despite discontinuation of TDF. As noted earlier, the implications may be important with regard to earlier identification of TDF toxicity, as CKD is associated with a long-term increase in morbidity and mortality in HIV-infected patients [37, 38]. The apparent loss of kidney function, seen here and in earlier studies [10], despite drug discontinuation, would support monitoring potentially more sensitive markers of toxicity such as serum phosphate and FEphos.
It is notable that the majority of patients were on a boosted protease inhibitor at the time of development of the proximal tubular dysfunction. Several studies have suggested increased toxicity of tenofovir due to the effects of protease inhibitors, particularly ritonavir, on apical membrane transporters involved in the elimination of tenofovir from the proximal tubular cell. Decreased elimination has been proposed to increase intracellular concentrations of the drug and hence increase toxicity. However, since no control group is assessed, it is difficult to determine the role in protease inhibitor use in this cohort.
There are several limitations to our study. The small sample size constrains the generalizations of these findings. Moreover, patients who are referred to the nephrology clinic are more likely to have severe kidney disease. The retrospective nature of the study had its inherent limitations in terms of selection bias and missing data. It is also important to note that urine phosphate measurements were not obtained on a fasting urine sample, which may make measurements less reliable. Finally, most of our patients had no urinary phosphate measurements at preset intervals of follow-up.
Conclusion
TDF-induced phosphate wasting may be a more sensitive marker for kidney dysfunction than an increased serum creatinine level. Moreover, tubular dysfunction may only become evident after years of TDF therapy, even in those who have normal kidney function at the time of starting TDF; this emphasizes the importance of continued monitoring in all patients treated with TDF.
Conflict of interest statement
None declared.
Acknowledgements
M.G.A. and D.M.F. are supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant P01DK056492. M.M.E. is supported by the NIDDK grant K23DK081317. G.M.L. was supported by the National Institute on Drug Abuse (K24 DA035684 and R01 DA026770) and by the Johns Hopkins University Center for AIDS Research (P30 AI094189).
References
- 1.Mateo L, Holgado S, Marinoso ML, et al. Hypophosphatemic osteomalacia induced by tenofovir in HIV-infected patients. Clin Rheumatol 2014; doi:10.1007/s10067-014-2627-x [DOI] [PubMed] [Google Scholar]
- 2.Jimenez-Nacher I, Garcia B, Barreiro P, et al. Trends in the prescription of antiretroviral drugs and impact on plasma HIV-RNA measurements. J Antimicrob Chemother 2008; 62: 816–822 [DOI] [PubMed] [Google Scholar]
- 3.Patel KK, Patel AK, Ranjan RR, et al. Tenofovir-associated renal dysfunction in clinical practice: an observational cohort from western India. Indian J Sex Transm Dis 2010; 31: 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson MR, Katlama C, Montaner JS, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS 2007; 21: 1273–1281 [DOI] [PubMed] [Google Scholar]
- 5.Gaspar G, Monereo A, Garcia-Reyne A, et al. Fanconi syndrome and acute renal failure in a patient treated with tenofovir: a call for caution. AIDS 2004; 18: 351–352 [DOI] [PubMed] [Google Scholar]
- 6.Karras A, Lafaurie M, Furco A, et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis 2003; 36: 1070–1073 [DOI] [PubMed] [Google Scholar]
- 7.Winston A, Amin J, Mallon P, et al. Minor changes in calculated creatinine clearance and anion-gap are associated with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy. HIV Med 2006; 7: 105–111 [DOI] [PubMed] [Google Scholar]
- 8.Gallant JE, Parish MA, Keruly JC, et al. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis 2005; 40: 1194–1198 [DOI] [PubMed] [Google Scholar]
- 9.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis 2013; 207: 1359–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 2012; 26: 867–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith KY, Patel P, Fine D, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS 2009; 23: 1547–1556 [DOI] [PubMed] [Google Scholar]
- 12.Gitman MD, Hirschwerk D, Baskin CH, et al. Tenofovir-induced kidney injury. Expert Opin Drug Saf 2007; 6: 155–164 [DOI] [PubMed] [Google Scholar]
- 13.Perazella MA. Tenofovir-induced kidney disease: an acquired renal tubular mitochondriopathy. Kidney Int 2010; 78: 1060–1063 [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Novoa S, Labarga P, Soriano V, et al. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis 2009; 48: e108–e116 [DOI] [PubMed] [Google Scholar]
- 15.Nishijima T, Komatsu H, Higasa K, et al. Single nucleotide polymorphisms in ABCC2 associate with tenofovir-induced kidney tubular dysfunction in Japanese patients with HIV-1 infection: a pharmacogenetic study. Clin Infect Dis 2012; 55: 1558–1567 [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann AE, Pizzoferrato T, Bedford J, et al. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis 2006; 42: 283–290 [DOI] [PubMed] [Google Scholar]
- 17.Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS 2008; 22: 99–103 [DOI] [PubMed] [Google Scholar]
- 18.Gupta SK, Anderson AM, Ebrahimi R, et al. Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: a prospective, case-control study of predictors and resolution in HIV-infected patients. PLoS One 2014; 9: e92717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalyesubula R, Perazella MA. Nephrotoxicity of HAART. AIDS Res Treat 2011; 2011: 562790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madeddu G, Bonfanti P, De Socio GV, et al. Tenofovir renal safety in HIV-infected patients: results from the SCOLTA project. Biomed Pharmacother 2008; 62: 6–11 [DOI] [PubMed] [Google Scholar]
- 21.Haque SK, Ariceta G, Batlle D. Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant 2012; 27: 4273–4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezinga M, Wetzels JF, Bosch ME, et al. Long-term treatment with tenofovir: prevalence of kidney tubular dysfunction and its association with tenofovir plasma concentration. Antivir Ther 2014; 19: 765–771 [DOI] [PubMed] [Google Scholar]
- 23.Pitisci L, Demeester R, Legrand JC. Prevalence and European AIDS Clinical Society (EACS) criteria evaluation for proximal renal tubular dysfunction diagnosis in patients under antiretroviral therapy in routine setting. J Int AIDS Soc 2014; 17: 19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barth JH, Jones RG, Payne RB. Calculation of renal tubular reabsorption of phosphate: the algorithm performs better than the nomogram. Ann Clin Biochem 2000; 37 (Pt 1): 79–81 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim F, Hamzah L, Jones R, et al. Comparison of CKD-EPI and MDRD to estimate baseline renal function in HIV-positive patients. Nephrol Dial Transplant 2012; 27: 2291–2297 [DOI] [PubMed] [Google Scholar]
- 27.Anonymous Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 28.Herlitz LC, Mohan S, Stokes MB, et al. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int 2010; 78: 1171–1177 [DOI] [PubMed] [Google Scholar]
- 29.Rifkin BS, Perazella MA. Tenofovir-associated nephrotoxicity: Fanconi syndrome and renal failure. Am J Med 2004; 117: 282–284 [DOI] [PubMed] [Google Scholar]
- 30.Slocum JL, Heung M, Pennathur S. Marking renal injury: can we move beyond serum creatinine? Transl Res 2012; 159: 277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol 2013; 24: 1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atta MG, Fine DM. Editorial comment: tenofovir nephrotoxicity—the disconnect between clinical trials and real-world practice. AIDS Read 2009; 19: 118–119 [PubMed] [Google Scholar]
- 33.Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr 2010; 55: 49–57 [DOI] [PubMed] [Google Scholar]
- 34.Wanner DP, Tyndall A, Walker UA. Tenofovir-induced osteomalacia. Clin Exp Rheumatol 2009; 27: 1001–1003 [PubMed] [Google Scholar]
- 35.Gupta SK, Eustace JA, Winston JA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2005; 40: 1559–1585 [DOI] [PubMed] [Google Scholar]
- 36.Peyriere H, Reynes J, Rouanet I, et al. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr 2004; 35: 269–273 [DOI] [PubMed] [Google Scholar]
- 37.Szczech LA, Hoover DR, Feldman JG, et al. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis 2004; 39: 1199–1206 [DOI] [PubMed] [Google Scholar]
- 38.Gardner LI, Holmberg SD, Williamson JM, et al. Development of proteinuria or elevated serum creatinine and mortality in HIV-infected women. J Acquir Immune Defic Syndr 2003; 32: 203–209 [DOI] [PubMed] [Google Scholar]