Abstract
Aneurysms are a common and often difficult complication seen with arteriovenous vascular access for haemodialysis. The purpose of this narrative review is to define and describe the scale of the problem and suggested therapeutic strategies. A narrative review of the published literature illustrated by individual cases is presented with the aim of summarising the relevant literature. The definitions of aneurysm are inconsistent throughout the literature and therefore systematic review is impossible. They vary from qualitative descriptions to quantitative definitions using absolute size, relative size and also size plus characteristics. The incidence and aetiology are also ill defined but separation into true aneurysms and false, or pseudoaneurysms may be helpful in planning treatment, which may be conservative, surgical or radiological. The lack of useful definitions and classification along with the multitude of management strategies proposed make firm evidence based conclusions difficult to draw. Further robust well designed studies are required to define best practice for this common problem.
Keywords: arteriovenous fistula, arteriovenous graft, haemodialysis, thrombosis, ultrasonography
Introduction
To ensure adequate haemodialysis treatments, a well-functioning vascular access (VA) is a prerequisite in dialysis patients. Complications of VA are common and represent a major cause of hospitalization in haemodialysis patients with 36–39% of admissions related to dialysis access [1, 2]. The mean length of stay was 5.3 ± 4.6 days, with a mean hospital costs of 41 896 ± 20 318 US$ [3]. Of the many complications that may threaten the longevity of VA, aneurysms represent a significant challenge which threatens the both the arteriovenous fistula (AVF) and the patient.
Definitions
Since the aim of VA creation is to establish abnormally dilated vasculature, the definition of aneurysmal development in this setting is difficult. Subdividing aneurysms into true and false aneurysms based on the wall structure may be helpful. False or pseudo aneurysms generally occur at the site of needling/puncture or at the anastomoses and may be thought of simplistically as haematomas communicating with the lumen of the access. With time, they may develop a fibrotic sac but this is devoid of endothelium or vascular wall structure.
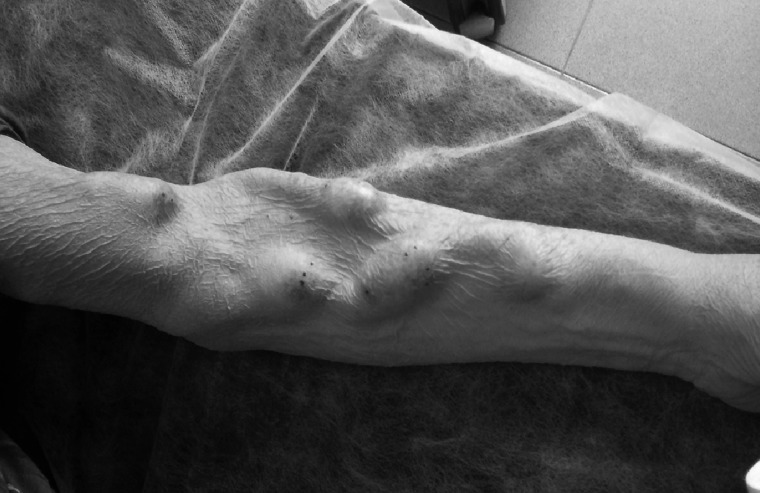
True aneurysms may be more difficult to define. The artery proximal to an AVF routinely dilates as it remodels in response to the increased blood flow [4]. The vein of an AVF typically increases in diameter greater than 3-fold to mature enough for needling. Most autologous in situ AVFs are often irregular and dilate asymmetrically (Figure 1). In practice, this is desirable and may give areas that facilitate needling; however, in classifying aneurysms and determining treatment, there is a requirement for clear definitions.
Fig. 1.
Typical appearance of a longstanding mature distal radiocephalic AVF: the dilated cephalic vein is distended irregularly along its serpentine course and other draining veins are also dilated. The presence of localized aneurysmal dilatation can be seen at the sites of needling.
Several authors have defined aneurysms in different ways. Jankovic et al. developed a score based on the sum of the length and width in centimetres [5]. Pasklinsky and colleagues used a definition of dilatation to more than three times the native vessel diameter, with the minimum size being 2 cm. This is slightly artificial as many AVFs will be from veins of 2 mm and above. Few native veins for AVF formation will be >7 mm and in fact the author's state in their discussion that the comparison is to the ‘normal’ adjacent AVF rather than the native vein prior to formation [6]. Rajput et al. also use relative size to the adjacent AVF and defined an aneurysm as a focal dilatation of the outflow vein diameter greater than two times the normal calibre of the adjacent normal fistula vein segment [7] although Watson and colleagues suggest size alone may not be useful in predicting behaviour [8].
Valenti et al. recently proposed a classification system based on fistula morphology recognizing three types of aneurysms, Types 1 to 3 being true aneurysms and Type 4 being pseudoaneurysms. Type 1 aneurysms were subdivided into Type 1a, which involves the length of the vein and Type 1b being post-anastomotic. Type 2 aneurysms are more localized and Type 3 are classified as complex. The incidence in this series was high with 43.5% of dialysed patients having aneurysmal fistulae albeit most of these are Type 1 [9].
A working and practical clinical solution may be to define an aneurysm in an AVF as an abnormal localized dilatation of the vessel and classify it on the basis of true aneurysm or false (pseudo) aneurysm, location (arterial/venous/graft) and site (anastomotic/puncture site/native vessel and whole outflow vein).
Incidence
Without a standardized definition, it is difficult to review the literature systematically, but the incidence of aneurysms is highly variable with reports of 5% to over 60% [6, 9–12]. This is likely to represent variation in classification of aneurysms and subdivision into clinically relevant aneurysms or those that are larger AVFs with more uniform dilatation but not clinically relevant localized swellings. The incidence is higher in proximal than distal AVFs and in AVF involving the cephalic vein rather than the basilic vein [6].
Aetiology
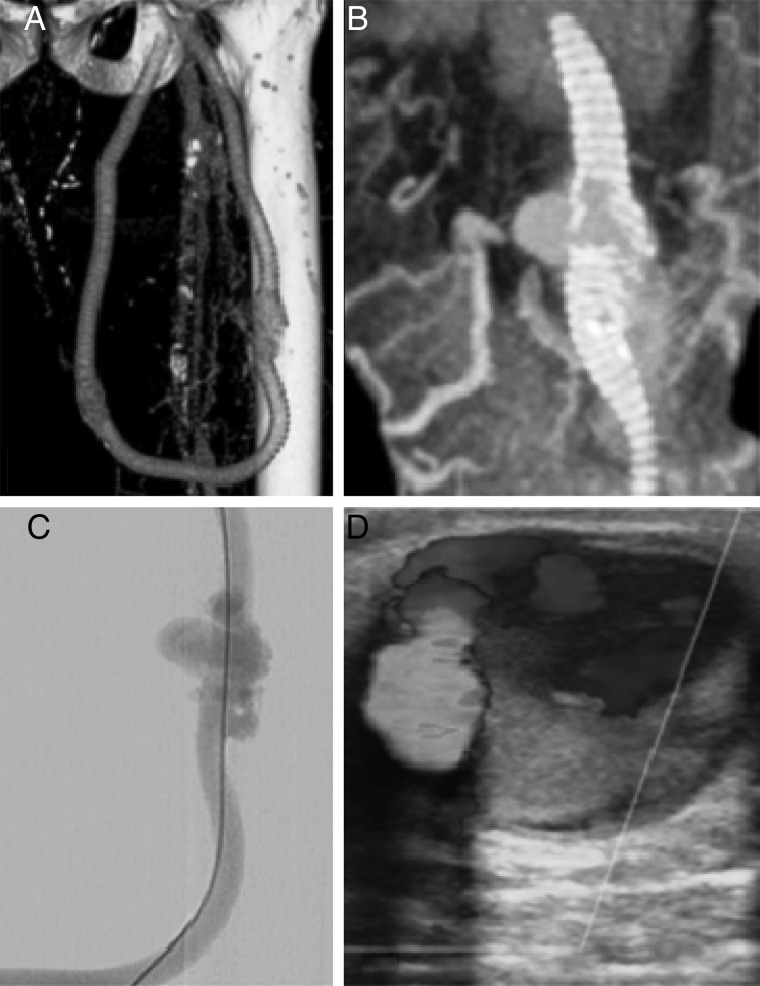
The aetiology of aneurysms is also ill defined. Pseudoaneurysms may be from the anastomosis and reflect leaking of blood outside the lumen perioperatively as a result of surgical technique or occur later as a complication of infection. Puncture of an AVF or arteriovenous graft (AVG) either as part of standard dialysis needling or from intervention can result in prolonged bleeding and pseudoaneurysm formation [13]. AVGs in particular are at risk if needling is too localized (Figure 2).
Fig. 2.
Pseudoaneurysm: (A) CT, (B) 3D CT reconstruction, (C) CT, (D) colour Doppler Ultrasound and angiographic images of graft rupture due to repeated localized needling and subsequent pseudoaneurysm.
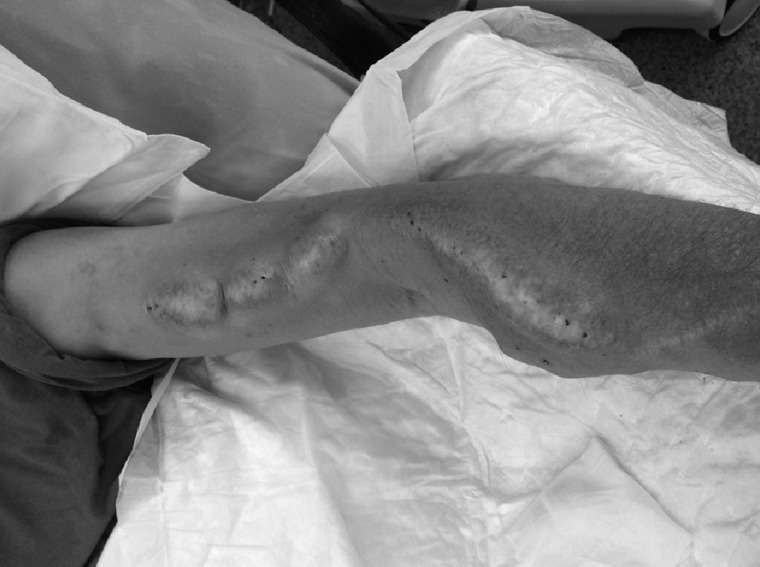
The aetiology of true aneurysms in AVFs is less clear. Repeated needling results in multiple small fibrous scars in the vessel wall, which may expand with time and result in localized aneurysmal areas (Figure 3). In areas where needling has not been performed, aneurysmal dilation can occur and the high flow though the vessel may result in abnormal shear stress, which promotes outward remodelling and gradual dilation with grossly increased calibre of the vessel [14, 15]. Histological examination of resected fistula aneurysms shows extensive infiltration of collagen with thickening and altered architecture of the vessel wall [6]. Stenoses, which exacerbate abnormal haemodynamics, may be associated with aneurysm formation. Aneurysms can occur downstream of a stenosis, which may be due to the increased transmural pressure or be related to disturbed flow patterns [7]. Upstream of a stenosis marked changes in flow patterns occur and can result in pathological remodelling of the vessel.
Fig. 3.
Aneurysms in the site of repeated venipuncture: longstanding radiocephalic fistula with presence of aneurysms in the site of repeated venipuncture. The scares area from the needle sites are clearly visible.
The nature of the vessel wall may by implicated, and some patients may have a predisposition to aneurysm formation. This is seen in other aneurysms with connective tissue disorders [16], and higher aneurysm formation in VA has been described in Alport's syndrome [17]. Other disease states are associated with aneurysms elsewhere for example adult polycystic kidney disease, which is associated with intracerebral aneurysms, have been shown to increase the diameter of AVFs [6, 18]. Pregnancy is associated with aneurysm formation and splenic artery aneurysms although this is less frequent than is cited [19]. The role of this in VA is unreported, presumably due to the rarity of the situation.
Several authors have attempted to identify other factors, which may predispose to a higher risk of developing aneurysms [20] with one series quoting lower incidence of aneurysmal development using buttonhole cannulation techniques [21]. Repeated localized puncture in prosthetic grafts results in pseudoaneurysms although true aneurysms can occur in the arterial inflow [22].
Management
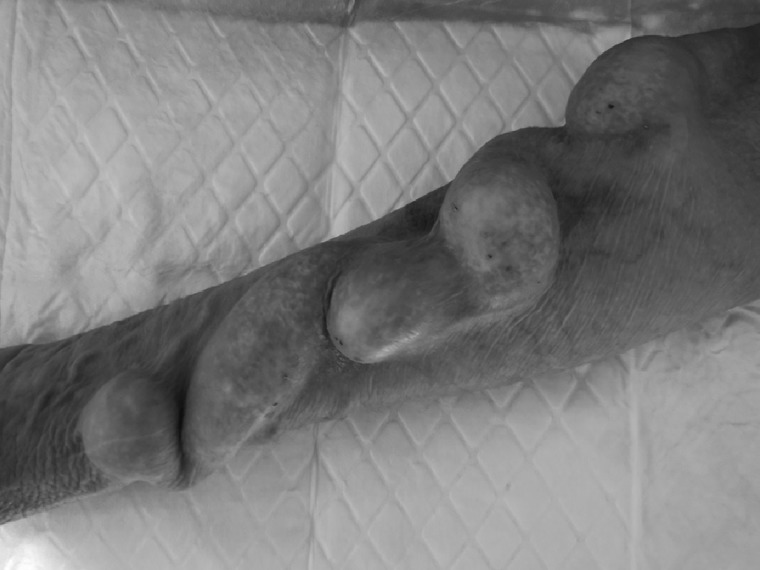
The natural history of VA aneurysms is most commonly a benign process where patients remain stable and asymptomatic without jeopardizing the functioning of the access or the haemodialysis. Many patients may dialyse for years without problem on large and tortuous AVFs that would be classified as aneurysms (Figure 4).
Fig. 4.
A 10-year-old radiocephalic AVF: showing marked dilatation throughout but with excellent function and otherwise asymptomatic.
These stable aneurysms can be observed, and following baseline measurements may require simple surveillance, both clinically and with ultrasound. Sonographic assessment should include transverse and longitudinal measurements and assessment of flow and thrombus In addition, the inflow and outflow vessels should be assessed. Wall characteristics such as thickness and infection may also be possible [8, 23].
Proximal and distal stenoses should be identified, although formal venography may be required, particularly in more central stenosis. These should be treated to prevent progression and instability of aneurysmal segments. Sites of suitable wall thickness should be identified, needling sites changed and blunt needles used if possible [24].
The risk of rupture of small stable aneurysms is low as they are thick walled and covered with skin. Clinical concerns are raised when an aneurysm presents with a rapid increase in size, pain, thinning and degeneration of the overlying skin and/or infection. This situation can lead to rupture subcutaneously or a free rupture through the skin [25].
If an aneurysm has ruptured or there is a risk of imminent rupture (skin ulceration and scab/infection), emergency ligation of the aneurysm is required. Where the site is perianastomotic and involves the artery then reconstruction may be required. On occasions, the fistula may be salvaged [26] but the priority in such situations is to prevent and control life threatening haemorrhage as flow volumes are high in aneurysmal VA.
In less-emergent situations, the indications for surgery are not defined. An increasing rate of growth may be a trigger for surgery [27] although rate of growth and risk of rupture is unclear. In other aneurysms, a growth of >10% per year increases the risk of rupture [28]. Skin thinning and shiny atrophic skin are indicators for intervention. Some patients may request treatment for aesthetic reasons and also in high-flow situations, treatment may be required.
The options for treatment are surgical or radiological, and the desired outcome should be preservation of definitive access where possible.
Surgical options to salvage the AVF should be considered. If the aneurysm is small and discrete in a tortuous AVF, it may be possible to excise it, mobilize the ends and reconstruct the AVF with a direct end-to-end anastomosis [29]. This may be done via a small flap ‘trap door’ technique [30]. With more extensive aneurysms where this is not possible, either a bypass with a graft or an aneurysmorrhaphy may be performed [31, 32]. Various surgical techniques have been described including sutures and stapling devices to re-fashion the aneurysm and reduce the volume of the sac [33–35]. The advantages of this are to preserve the autologous AVF and avoid prosthetic material and may allow the fistula to be used for needling in the unaffected segments. If an AVG is used, one allowing early cannulation may prevent the requirement for a central venous dialysis catheter.
Endovascular techniques either alone or combined with surgery have been used to treat AVF aneurysms [36–38]. The aneurysm can be treated with a covered stent or stent graft to exclude the sac, although this may compromise future needling. This technique may be better reserved for use in patients considered to have ‘high surgical risk’ or as an interim measure to extend the interval before surgery. The advantage of endovascular treatment is that associated stenosis can be treated simultaneously.
The management of pseudoaneurysmal AVFs is similar to true aneurysms although small saccular pseudoaneurysms with narrow necks may be amenable to thrombin injection [39, 40] or ultrasound compression [41]. This non-invasive technique may be attempted before resorting to surgical or endovascular alternatives.
In the acute setting, perianastomotic and large non-resolving puncture-related pseudoaneurysms generally require surgical repair to prevent local complications or enlargement. Small pseudoaneurysms may spontaneously thrombose, but this is unpredictable and surgical treatment in the acute phase is often minimal and successful.
Pseudoaneurysms related to infection, particularly with grafts, require surgery that is more extensive and the probability of rupture is high. If associated with the anastomosis, the graft should be removed and the native vessels repaired. If the infected aneurysm is localized and in the mid graft, some authors have suggested excision of the affected area and repair with a biological or biosynthetic graft although this has been described in bypass rather than in VA [42].
Conclusion
Regular puncture treatment with anticoagulation and abnormal haemodynamics make psuedoaneurysms and true aneurysms a relatively common complication in patients with VA for heamodialysis. The lack of robust definitions and classification result in little supporting evidence to base conclusions on either the true incidence or best practice for management.
Regular surveillance of VA may allow earlier detection of aneurysms and allow timely treatment to prevent life-threatening complications; however, evidence is lacking. Studies defining the natural history and progression of aneurysms are required to identify the best timing of intervention as well as the optimum treatment modality.
Conflict of interest statement
None declared.
References
- 1.Rayner HC, Pisoni RL, Bommer J, et al. Mortality and hospitalization in haemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2004; 19: 108–120 [DOI] [PubMed] [Google Scholar]
- 2.Arora P, Kausz AT, Obrador GT, et al. Hospital utilization among chronic dialysis patients. J Am Soc Nephrol 2000; 11: 740–746 [DOI] [PubMed] [Google Scholar]
- 3.Sawant A, Mills PK, Dhingra H. Increased length of stay and costs associated with inpatient management of vascular access failures. Semin Dial 2013; 26: 106–110 [DOI] [PubMed] [Google Scholar]
- 4.Eugster T, Wigger P, Bölter S, et al. Brachial artery dilatation after arteriovenous fistulae in patients after renal transplantation: a 10-year follow- up with ultrasound scan. J Vasc Surg 2003; 37: 564–567 [DOI] [PubMed] [Google Scholar]
- 5.Jankovic A, Donfrid B, Adam J, et al. Arteriovenous fistula aneurysm in patients on regular hemodialysis: prevalence and risk factors. Nephron Clin Pract 2013; 124: 94–98 [DOI] [PubMed] [Google Scholar]
- 6.Pasklinsky G, Meisner RJ, Labropoulos N, et al. Management of true aneurysms of hemodialysis access fistulas. J Vasc Surg 2011; 53: 1291–1297 [DOI] [PubMed] [Google Scholar]
- 7.Rajput A, Rajan DK, Simons ME, et al. Venous aneurysms in autogenous hemodialysis fistulas: is there an association with venous outflow stenosis. J Vasc Access 2013; 14: 126–130 [DOI] [PubMed] [Google Scholar]
- 8.Watson KR, Gallagher M, Ross R, et al. The aneurysmal arteriovenous fistula: morphological study and assessment of clinical implications. A pilot study. Vascular 2014; doi:10.1177/1708538114557069 [DOI] [PubMed] [Google Scholar]
- 9.Valenti D, Mistry H, Stephenson M. A novel classification system for autogenous arteriovenous fistula aneurysms in renal access patients. Vasc Endovascular Surg 2014; 48: 491–496 [DOI] [PubMed] [Google Scholar]
- 10.Fokou M, Teyang A, Ashuntantang G, et al. Complications of arteriovenous fistula for hemodialysis: an 8-year study. Ann Vasc Surg 2012; 26: 680–684 [DOI] [PubMed] [Google Scholar]
- 11.Lo HY, Tan SG. Arteriovenous fistula aneurysm--plicate, not ligate. Ann Acad Med Singapore 2007; 36: 851–853 [PubMed] [Google Scholar]
- 12.Zibari GB, Rohr MS, Landreneau MD, et al. Complications from permanent hemodialysis vascular access. Surgery 1988; 104: 681–686 [PubMed] [Google Scholar]
- 13.Lazarides MK, Georgiadis GS, Argyriou C. Aneurysm formation and infection in AV prosthesis. J Vasc Access 2014; 15 Suppl 7: S120–S124 [DOI] [PubMed] [Google Scholar]
- 14.Corpataux JM, Haesler E, Silacci P, et al. Low-pressure environment and remodelling of the forearm vein in Brescia-Cimino haemodialysis access. Nephrol Dial Transplant 2002; 17: 1057–1062 [DOI] [PubMed] [Google Scholar]
- 15.Dixon BS. Why don't fistulas mature? Kidney Int 2006; 70: 1413–1422 [DOI] [PubMed] [Google Scholar]
- 16.Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013; 2013: 267215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Field MA, McGrogan DG, Tullet K, et al. Arteriovenous fistula aneurysms in patients with Alport's. J Vasc Access 2013; 14: 397–399 [DOI] [PubMed] [Google Scholar]
- 18.Hadimeri H, Hadimeri U, Attman PO, et al. Dimensions of arteriovenous fistulas in patients with autosomal dominant polycystic kidney disease. Nephron 2000; 85: 50–53 [DOI] [PubMed] [Google Scholar]
- 19.Nanez L, Knowles M, Modrall JG, et al. Ruptured splenic artery aneurysms are exceedingly rare in pregnant women. J Vasc Surg 2014; 60: 1520–1523 [DOI] [PubMed] [Google Scholar]
- 20.Gallieni M, Brenna I, Brunini F, et al. Which cannulation technique for which patient. J Vasc Access 2014; 15 Suppl 7: S85–S90 [DOI] [PubMed] [Google Scholar]
- 21.van Loon MM, Goovaerts T, Kessels AG, et al. Buttonhole needling of haemodialysis arteriovenous fistulae results in less complications and interventions compared to the rope-ladder technique. Nephrol Dial Transplant 2010; 25: 225–230 [DOI] [PubMed] [Google Scholar]
- 22.Mestres G, Fontsere N, Yugueros X, et al. Aneurysmal degeneration of the inflow artery after arteriovenous access for hemodialysis. Eur J Vasc Endovasc Surg 2014; 48: 592–596 [DOI] [PubMed] [Google Scholar]
- 23.Arshad FH, Sutijono D, Moore CL, et al. Emergency ultrasound diagnosis of a pseudoaneurysm associated with an arteriovenous fistula. Acad Emerg Med 2010; 17: e43–e45 [DOI] [PubMed] [Google Scholar]
- 24.Navuluri R, Regalado S. The KDOQI 2006 vascular access update and fistula first program synopsis. Semin Intervent Radiol 2009; 26: 122–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mennes PA, Gilula LA, Anderson CB. Complications associated with arteriovenous fistulas in patients undergoing chronic hemodialysis. Arch Int Med 1978; 138: 1117–1121 [PubMed] [Google Scholar]
- 26.Sedki N, Jiber H, Zrihni Y, et al. Successful repair of a ruptured arterio-venous fistula aneurysm with femoral vein autograft. J Vasc Access 2012; 13: 267. [DOI] [PubMed] [Google Scholar]
- 27.Georgiadis GS, Lazarides MK, Panagoutsos SA, et al. Surgical revision of complicated false and true vascular access-related aneurysms. J Vasc Surg 2008; 47: 1284–1291 [DOI] [PubMed] [Google Scholar]
- 28.Bernstein EF, Chan EL. Abdominal aortic aneurysm in high-risk patients. Outcome of selective management based on size and expansion rate. Ann. Surg 1984; 200: 255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almehmi A, Wang S. Partial aneurysmectomy is effective in managing aneurysm-associated complications of arteriovenous fistulae for hemodialysis: case series and literature review. Semin Dial 2012; 25: 357–364 [DOI] [PubMed] [Google Scholar]
- 30.Kapoulas KC, Georgakarakos EI, Georgiadis GS, et al. Modification of the trap door technique to treat venous aneurysms in arteriovenous fistulae. J Vasc Access 2012; 13: 256–258 [DOI] [PubMed] [Google Scholar]
- 31.Hossny A. Partial aneurysmectomy for salvage of autogenous arteriovenous fistula with complicated venous aneurysms. J Vasc Surg 2014; 59: 1073–1077 [DOI] [PubMed] [Google Scholar]
- 32.Balaz P, Rokosny S, Klein D, et al. Aneurysmorrhaphy is an easy technique for arteriovenous fistula salvage. J Vasc Access 2008; 9: 81–84 [PubMed] [Google Scholar]
- 33.Hakim NS, Romagnoli J, Contis JC, et al. Refashioning of an aneurysmatic arteriovenous fistula by using the multifire GIA 60 surgical stapler. Int Surg 1997; 82: 376–377 [PubMed] [Google Scholar]
- 34.Woo K, Cook PR, Garg J, et al. Midterm results of a novel technique to salvage autogenous dialysis access in aneurysmal arteriovenous fistulas. J Vasc Surg 2010; 51: 921–925 [DOI] [PubMed] [Google Scholar]
- 35.Bachleda P, Utíkal P, Kalinová L, et al. Surgical remodelling of haemodialysis fistula aneurysms. Ann Acad Med Singapore 2011; 40: 136–139 [PubMed] [Google Scholar]
- 36.Keeling AN, Naughton PA, McGrath FP, et al. Successful endovascular treatment of a hemodialysis graft pseudoaneurysm by covered stent and direct percutaneous thrombin injection. Semin Dial 2008; 21: 553–556 [DOI] [PubMed] [Google Scholar]
- 37.Mantha ML, Baer R, Bailey GS, et al. Endovascular repair of a hemodialysis fistula aneurysm with covered stents. Kidney Int 2009; 76: 918. [DOI] [PubMed] [Google Scholar]
- 38.Allaria PM, Costantini E, Lucatello A, et al. Aneurysm of arteriovenous fistula in uremic patients: is endograft a viable therapeutic approach? J Vasc Access 2002; 3: 85–88 [DOI] [PubMed] [Google Scholar]
- 39.Clark TW, Abraham RJ. Thrombin injection for treatment of brachial artery pseudoaneurysm at the site of a hemodialysis fistula: report of two patients. Cardiovasc Intervent Radiol 2000; 23: 396–400 [DOI] [PubMed] [Google Scholar]
- 40.O'Neill JM, Jenkins DA, Ireland HM. Recurrence of dialysis shunt pseudoaneurysm following percutaneous thrombin embolization. Cardiovasc Intervent Radiol 2001; 24: 441–442 [DOI] [PubMed] [Google Scholar]
- 41.Witz M, Werner M, Bernheim J, et al. Ultrasound-guided compression repair of pseudoaneurysms complicating a forearm dialysis arteriovenous fistula. Nephrol Dial Transplant 2000; 15: 1453–1454 [DOI] [PubMed] [Google Scholar]
- 42.Töpel I, Betz T, Uhl C, et al. Use of biosynthetic prosthesis (Omniflow II®) to replace infected infrainguinal prosthetic grafts--first results. Vasa 2012; 41: 215–220 [DOI] [PubMed] [Google Scholar]