Abstract
Background
In idiopathic membranous nephropathy (MN), antibodies directed towards the glomerular phospholipase A2 receptor (PLA2R) have mainly been reported to be of IgG4 subclass. However, the role of the different IgG subclasses in the pathogenesis of MN, both in idiopathic MN and in secondary cases, is still unclear. In this retrospective study, we test the hypothesis that the absence of glomerular IgG4 and PLA2R in patients with MN indicates malignant disease.
Methods
The distribution pattern of glomerular IgG subclasses and PLA2R was studied in 69 patients with idiopathic MN and 16 patients with malignancy-associated MN who were followed up for a mean of 83 months.
Results
A significant correlation between the absence of IgG4 and PLA2R and malignancy-associated MN was found. Thus, IgG4 was positive in 45 of 69 patients (65%) with idiopathic MN but only in 5 of 16 patients (31%) with malignancy-associated MN. The other IgG subclasses did not differ statistically between the groups, IgG2-positivity being present in more than 94% of patients in both groups. Thirty-five of 63 patients (56%) with idiopathic MN and 3 of 16 (19%) patients with malignancy-associated MN had glomerular deposits of PLA2R.
Conclusions
We have found that the absence of glomerular IgG4 and PLA2R is common in patients with malignancy-associated MN. In our material, IgG2 could not be used as a marker of underlying malignant disease. Finally, neither IgG1 nor IgG3 seems to be involved in the pathogenesis of MN.
Keywords: cancer, glomerulonephritis, kidney, membranous nephropathy, proteinuria
Introduction
Membranous nephropathy (MN) is one of the most common causes of adult-onset nephrotic syndrome [1]. In developed countries, the majority of the cases of MN are idiopathic and ∼25% of the cases are secondary to underlying disease such as malignancy, hepatitis B or C, autoimmune disease, or due to toxicity of drugs [2]. Previous studies report that 5–20% of all cases of MN are malignancy associated [3]. In ∼ 45% of these cases, the renal diagnosis antedates diagnose of malignancy, in 40% there is a simultaneous presentation of malignancy and nephrotic syndrome, and in the remaining 10% of the cases the renal diagnosis appears after diagnosis of malignancy. Thus, both diseases often present within 1 year of each other [3]. Discrimination between idiopathic MN and malignancy-associated MN is of great clinical importance as treatment of secondary forms of MN is directed towards the underlying disease, while immunosuppressive medication is recommended in idiopathic MN [4]. Since clinical presentation and routine laboratory examinations in idiopathic MN and malignancy-associated MN do not differ, investigation to exclude malignancy has to be performed [5]. The question is whether all patients with MN should be screened, or how to identify patients at risk. Recently the M-type phospholipase A2 receptor, PLA2R, was identified as a major antigen involved in the development of idiopathic MN [6]. Indeed, 70–80% of patients with idiopathic MN seem to have serum anti-PLA2R antibodies. There could be several reasons why the sensitivity is <100%, such as influence of immunosuppressive drugs, involvement of other antigens than PLA2R in the pathogenesis of idiopathic MN, misclassification of patients with idiopathic MN, or absence of immunologic disease at the time of blood analysis [6–8]. Initially, anti-PLA2R antibodies were not found in patients with secondary MN, but now they have been detected in patients with hepatitis B, malignancy and sarcoidosis-associated MN [9, 10]. Expression of PLA2R in glomerular deposits has been proposed to correlate with the presence of anti-PLA2R antibodies in serum in patients with idiopathic MN [7]. It has also been suggested that the sensitivity might be even higher for the deposits than for the presence of serum PLA2R antibodies [11]. The IgG antibodies that bind to PLA2R have been reported to be predominantly of IgG4 subclass, but the involvement of the different IgG subclasses in the pathogenesis of MN is still not fully elucidated [12]. A recent report suggests an IgG subclass switch in the antibody response, from IgG1 to IgG4 in the later stages of idiopathic MN [13]. Previous studies on the glomerular distribution of IgG subclasses present partly contradicting results [14, 15], although the absence of IgG4 may indicate malignancy-associated MN [15]. We analysed consecutive patients with MN that had been carefully monitored over a period of 1–12 years. The follow-up period allowed us to distinguish between patients with idiopathic MN and malignancy-associated MN with reasonable accuracy. Patients with other forms of secondary MN, or unclear cases, were excluded, resulting in two well-defined patient groups. The pathologist examining the renal biopsies had no knowledge of which form of MN the patient had. Hereby, it was possible to test the hypothesis that patients with malignancy-associated MN could be identified based on the lack of glomerular staining for IgG4 and PLA2R.
Materials and methods
Recruitment of patients
Patients were identified on the basis of the renal biopsy files at the Department of Pathology at Sahlgrenska University Hospital. All adult patients (>15 years old) that were diagnosed with MN between January 2000 and April 2012 were considered for inclusion, a total of 210 patients. Thirty-four patients were excluded since they had moved to other parts of Sweden, 11 patients had missing medical records and 10 patients declined to participate in the study. Among the remaining 155 patients, 44 had systemic lupus erythematosus, 10 patients had other forms of secondary MN and 73 patients had idiopathic MN. Twenty-eight patients had a prior history of malignant disease or a malignancy at the time of or after the appearance of kidney disease. This study was approved by the regional ethical review board in Gothenburg, approval number 432-09. Written informed consent was obtained from patients before collecting clinical data and performing biopsy examinations. The youngest patient was 15 years old at the time of diagnosis, but all patients were >18 years old at the time of inclusion in the study. This study adheres to the Declaration of Helsinki.
Diagnosis of MN
The definition of idiopathic MN was (i) renal biopsy MN, (ii) absence of other forms of MN such as systemic lupus erythematosus, hepatitis B and C, and toxic drug reaction, and (iii) absence of malignancy during the follow-up period that was at least 12 months (82 ± 5 months). The definition of malignancy-associated MN was (i) the existence of malignancy discovered at the time of, or after, the biopsy, as well as in treated cases; or (ii) reduction or disappearance of proteinuria after therapy for malignancy and (iii) no evidence of other secondary forms of MN. In 3 of the 28 patients with malignancy, the time between MN and cancer was 138, 168 and 180 months and the nephrotic syndrome did not relapse when cancer was later diagnosed. The probability of developing cancer at a high age was assumed more likely than a connection between malignancy and MN in these three cases. In three additional cases, the nephrotic syndrome was in complete remission despite the fact that the malignant disease was uncured and the patients had metastatic disease. In five cases, there was more than 3 years between the occurrence of malignancy and kidney disease, and a connection between the two entities was difficult to determine. In our aim to include clearly defined patients with idiopathic MN and malignancy-associated MN, we considered these 11 patients not to be typical for neither idiopathic nor malignancy-associated MN and therefore excluded them from the study.
Histological examination of immune deposits
One renal pathologist (J.M.), blinded to the clinical and laboratory data, reviewed all renal biopsies from the included patients. All biopsy specimens were examined using light microscopy, immunohistochemistry and electron microscopy. The kidney tissue was adequately preserved and available for staining for IgG immune deposits except in five cases (four idiopathic MN and one malignancy-associated MN).
Standard light microscopy examination generally demonstrated a membranous pattern and excluded other glomerulonephritis including lupus. Immune deposits (IgG, IgA, IgM, light chains, C1q, C3c and C5b-9, all Dako, Copenhagen, Denmark) were examined using a standardized immunoperoxidase method. The EnVision™ Flex high pH (Link) detection kit (Dako K8000) was used. This is an indirect immunohistochemical technique using unlabelled primary antibodies. The procedure is standardized with a total processing time of 2.5 h. The most important steps are as follows. Consecutive series of paraffin sections are produced at a 4-µm constant thickness setting, floated on a 37°C water bath, and collected on serially numbered polylysin-coated glass slides (Dako). Sections are de-paraffinized in xylene–ethanol at room temperature and rehydrated in phosphate-buffered saline (PBS) before antigen retrieval using protease XXIV (Sigma-Aldrich, St Louis, MO, USA) for 30 min (IgG4). Endogenous peroxidase activity was blocked by immersion in peroxidase-blocking solution (Dako K8000) for 5 min at room temperature and immunostaining performed in a computer-assisted Autostainer Plus processor (Dako). Incubation time for primary antibodies was 30 min at room temperature, terminated by repeated washings, followed by incubation with a dextran polymer coated with secondary antibodies and horseradish peroxidase for another 30 min. Slides were transferred to fresh hydrogen peroxide plus 3,3′-diaminobenzidine tetrahydrochloride (DAB) solutions for 4 min. Finally, slides were stained with Mayer's haematoxylin and permanently mounted under cover slips. Omitting or replacing the primary antibodies produced negative controls. IgG subclass antibody expression was studied using the same immunohistochemical protocol. IgG1 (clone9052-01, Southern Biotech, Birmingham, AL, USA) was used at a final concentration of 1:100, IgG2 (clone 9080-01, Southern Biotech) at 1:100, IgG3 at 1:500, IgG3 (clone 9210, Southern Biotech) at 1:100 and IgG4 (clone MRQ-55, Cell Marque, Rocklin, CA, USA) at 1:100. Optimal primary antibody dilutions were obtained using serial dilutions of each antibody on human tonsils and five cases of MN.
Histological examination of PLA2R
Consecutive series of paraffin sections were produced at a 4-µm constant thickness setting, floated on a 37°C water bath and collected on serially numbered polylysin-coated glass slides (Dako). Sections were de-paraffinized in xylene–ethanol at room temperature with an endogenous peroxidase-blocking step. The sections were rehydrated in PBS, and heat-induced epitope retrieval using citrate buffer pH 6.2 was performed, followed by a blocking step and incubation with a polyclonal rabbit anti-PLA2R (Atlas Antibodies, Sweden) at a dilution of 1:8000 overnight at 4°C. POLAP (Zytomed, Germany) was used as the detection system. Labelled sections were analysed by three independent scientists in a blinded fashion by using a scoring method, where 0 = negative and 1 = positive staining for PLA2R.
Statistical analysis
Chi-square test (Fisher's exact test), Spearman's rank-sum coefficient of correlation and ANOVA was used for statistical analysis and P ≤ 0.05 was considered as being significant. Descriptive statistics are presented as the means (±SEM).
Results
Background of patients with malignancy-associated MN
The total prevalence of cancer among patients with MN in our material was 8.1% (17 out of 210) based on the extensive clinical records at hand. That number is fairly accurate, but we do acknowledge that some of the 210 patients were lost to follow-up, or had missing files, which excluded them from the study. The prevalence of 8.1% in the MN population is 10 times higher than in the general population according to the Swedish cancer register in 2010. Table 1 (and Supplementary Figure S1) gives an overview of the characteristics of the patient population with malignancy. In half of the cases, the malignant disease was already known at the time of biopsy, or discovered within a month from biopsy. In the rest of the cases, the malignant disease was diagnosed within 2 years from kidney biopsy, except for one patient with prostate cancer (31 months after kidney biopsy). In this case, serum prostate-specific antigen was elevated at least 6 months before prostate biopsy was performed and the prostate cancer diagnosis was confirmed. In total, three patients did not receive treatment for cancer, due to metastatic disease, the proteinuria persisted and they died due to the malignant disease. Thirteen patients received treatment for the malignant disease with complete resection and cure of tumour; remission of proteinuria was seen in seven of these cases (six complete remission/one partial remission). In the remaining six cases, the malignancy was not cured and five of these patients died due to the malignant disease. Proteinuria persisted in three of these cases, while a partial remission of proteinuria was noted in two cases. One patient received heavy treatment for leukaemia, including bone marrow transplantation, and achieved complete remission of both leukaemia and nephrotic syndrome. This patient later had a recurrence of leukaemia and died due to complications of the malignancy. It is unclear whether proteinuria reappeared or not because of lack of urinary test for protein at that point.
Table 1.
Characteristics of the patient population with malignancy-associated MN
| Cases | Sex | Age | Malignancy | Time from onset of proteinuria to biopsy (months) | Time from biopsy to identification of malignancy (months) | Treatment of tumour | Remission |
Glomerular |
Follow-up time after biopsy (months) | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumour | Proteinuria | IgG4 | PLA2R | |||||||||
| 1 | M | 61 | Prostate cancer | 6 | 31 | S | Yes | PR | Neg | Neg | 148 | Alive |
| 2 | M | 56 | Lung cancer | 8 | 11 | Cs + R | No | No | Neg | Neg | 23 | Dead |
| 3 | F | 70 | Uterus cancer | 2 | 9 | No | No | No | Neg | Neg | 10 | Dead |
| 4 | M | 76 | Lymphoma | 1 | 6 | Cs + Ch | No | PR | Neg | Neg | 30 | Dead |
| 5 | M | 83 | Prostate cancer | 1 | 0 | H | No | PR | Neg | Neg | 8 | Dead |
| 6 | M | 65 | Prostate cancer | 4 | 0 | S | Yes | CR | Neg | Neg | 72 | Alive |
| 7 | F | 68 | Buccal cancer | 1 | 12 | S + R | Yes | CR | Neg | Neg | 62 | Alive |
| 8 | M | 58 | Lymphoma | 1 | 0 | Cs + Ch + BMtx | Yes | CR | Neg | Pos | 60 | Alive |
| 9 | M | 78 | Prostate cancer | 9 | 1 | H | No | No | Pos | Neg | 2 | Dead |
| 10 | F | 49 | Leukaemia | 3 | 0 | Cs + Ch + BMtx | No | CR | Neg | Neg | 36 | Dead |
| 11 | M | 60 | Prostate cancer | 0,5 | 0 | H + R | No | No | Pos | Pos | 33 | Alive |
| 12 | F | 66 | Lung cancer | 1 | 18 | No | No | No | Pos | Neg | 27 | Dead |
| 13 | F | 65 | Breast cancer | 7 | 17 | S + H | Yes | CR | Pos | Pos | 33 | Alive |
| 14 | M | 79 | Prostate cancer | 2 | 1 | No | No | No | Neg | Neg | 8 | Dead |
| 15 | M | 73 | Lung cancer | 2 | 4 | S | Yes | CR | Neg | Neg | 18 | Alive |
| 16 | F | 80 | Colon cancer | 5 | 0 | S | Yes | CR | Pos | Neg | 16 | Alive |
CR, complete remission of proteinuria (<300 mg/24 h); PR, partial remission of proteinuria (<3.5 g/24 h and 50% reduction in proteinuria); S, surgery; R, radiation; H, hormonal therapy; Cs, chemotherapy including steroids; Ch, chemotherapy including alkylating agents; BMtx, bone marrow transplantation; Ig, immunoglobulin; PLA2R, phospholipase A2 receptor.
Baseline characteristics
Table 2 gives an overview of the clinical and laboratory data of all the patients at the time of renal biopsy. The patients with malignancy-associated MN were significantly older (67 ± 2 years) than those with idiopathic disease (52 ± 2 years), P < 0.001. Other baseline data did not differ statistically between the groups.
Table 2.
Baseline characteristics of all patients
| Idiopathic MN (n = 69) | Malignancy-associated MN (n = 16) | Significance, P-value | |
|---|---|---|---|
| Sex (male/female) | 45/24 | 10/6 | NS |
| Smoking (yes/no), missing data 3 patients | 40/26 | 9/7 | NS |
| Age (years) | 52 ± 16 | 68 ± 10 | <0.001 |
| Serum-albumin (g/L) | 24 ± 8 | 21 ± 7 | NS |
| Urine-albumin (g/day) | 5.4 ± 3 | 5.5 ± 3 | NS |
| Urine-protein (g/day) | 5.9 ± 3 | 6.0 ± 3 | NS |
| eGFR (mL/min/m2) | 82 ± 32 | 76 ± 24 | NS |
| Time from symptom to biopsy, months (range) | 15 ± 6 (5–360) | 3 ± 1 (0.5–9) | NS |
| Length of follow-up, months (range) | 82 ± 5 (12–164) | 37 ± 9 (2–164) | <0.05 |
Histological findings
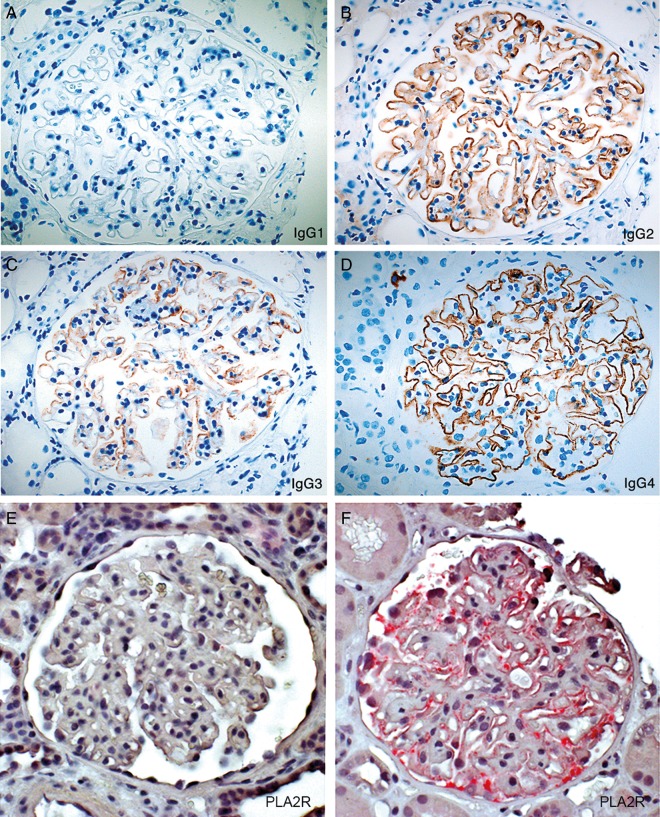
The MN cases displayed everything from faint irregularities in the glomerular capillaries to a distinct membranous pattern, but few other histological changes in the glomeruli (no proliferation, no crescents). Diagnosis was confirmed using immunoperoxidase and electron microscopy, and other forms of glomerulonephritis, including lupus, were excluded. Figure 1A–D shows immunoperoxidase detection of IgG subclasses exemplified in one typical patient with MN.
Fig. 1.
Immunoperoxidase detection of IgG subclasses exemplified in one typical patient with MN (A–D) and POLAP detection of phospholipase receptor 2 (PLA2R) exemplified in two representative cases of idiopathic MN (E–F.) (A) (IgG1) Completely negative (B) (IgG2) staining in a membranous granular pattern; (C) (IgG3) slightly uneven membranous granular pattern and (D) (IgG4) strong IgG staining in an almost linear membranous pattern. (E) Case lacking positive staining for PLA2R and (F) case positive for PLA2R. Magnification ×40.
Comparison of glomerular IgG subclasses between patients with idiopathic MN and malignancy-associated MN
IgG4 subclass deposits were found in the renal biopsy in 45 of 69 patients with idiopathic MN and in 5 of 16 patients with malignancy, a statistically significant difference (P < 0.05). The positive predictive value for IgG4 as an indicator of idiopathic MN was 90% (95% CI 78.19–96.67). For more details, see Table 3. There was no difference in staining pattern for IgG1, IgG2 or IgG3 between the two groups. To see whether we could find a sharper tool in distinguishing between idiopathic and malignancy-associated MN, we created a recognition category score based on the four distinct categories of staining pattern that are possible, Supplementary Figure S2. In the malignant group, 63% of the patients were positive for IgG2 and negative for IgG4 (category 2) compared with 25% of the idiopathic patients. Patients with idiopathic MN were in 55% of the cases positive for IgG2 and IgG4 (category 3) compared with 31% in the malignant group. Supplementary Figure S2 illustrates the marked difference in the distribution pattern between patients with idiopathic MN and malignancy-associated MN. However, neither IgG2 nor IgG4 can be used to identify patients with malignancy with any reasonable precision.
Table 3.
Result of staining for glomerular IgG subclasses and PLA2R
| Idiopathic MN (n = 69) |
Malignancy-associated MN (n = 16) |
Difference between groups, P-value | |||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | % Positive | Positive | Negative | % Positive | ||
| IgG4 | 45 | 24 | 65 | 5 | 11 | 31 | <0.05 |
| IgG3 | 15 | 54 | 22 | 3 | 13 | 19 | NS |
| IgG2 | 56 | 13 | 81 | 15 | 1 | 94 | NS |
| IgG1 | 1 | 68 | 1 | 1 | 15 | 6 | NS |
| PLA2R | 35 | 28 | 56 | 3 | 13 | 19 | <0.05 |
Ig, immunoglobulin; I-MN, idiopathic MN; M-MN, malignancy-associated MN.
PLA2R staining
Glomerular PLA2R was detected in 35 of 63 patients with idiopathic MN and in 3 of 16 patients with malignancy-associated MN, a statistically significant difference (P < 0.05) (Table 3). In six patients with idiopathic MN, there was not enough material left to perform staining for PLA2R. Figure 1E and F shows the detection of PLA2R in two representative cases of MN. 86% (30 of 35) of the patients with positive staining for glomerular PLA2R were also positive for IgG4 subclass. However, in a large percentage (29 of 35 patients), IgG2 was also detectable. No patient was positive for IgG1, Table 4. In the malignant group, PLA2R was positive in three patients; two of these were also positive for IgG4. There was a significant positive correlation in the idiopathic group between the presence of IgG4 and PLA2R, but not in the malignant group (P < 0.05).
Table 4.
IgG subclasses in patients with positive staining for glomerular PLA2R
| Glomerular IgG subclasses | Number of patients (% of PLA2R positive) |
|---|---|
| IgG4 | 30 (86) |
| IgG3 | 13 (37) |
| IgG2 | 29 (83) |
| IgG1 | 0 |
Discussion
In our material, the prevalence of malignancy in patients with MN was close to 10%, which is within the wide range published in previous studies [3]. The most frequent cancer in our cohort was located in the prostate (six) and in the lung (three). It is noteworthy that there was no case of skin cancer despite the fact that skin cancer is quite common in Sweden. Our data support previous findings of cancer prevalence in MN [5], as well as the fact that the existing few case reports of correlation between skin cancer and MN include melanoma and Kaposi's sarcoma [16, 17].
The trigger of MN in patients with malignancy is unclear. Different mechanisms have been postulated [2], such as formation of in situ and/or circulating immune complexes, tumour antigens, or extrinsic factors such as viral infection. There might be different pathogenic mechanisms involved depending on the type of malignancy and the patient's immune system. However, it is likely that the malignancy-associated MN could also be induced by reactions to secreted PLA2 proteins. Thus, extensive studies of PLA2 have revealed that the human genome contains nine secretory PLA2 genes [18]. Group IIA secretory PLA2 seems to accumulate during inflammatory conditions such as arthritis. The enzyme has also been found to have a direct antibacterial activity against many Gram-positive bacteria. Group IIA and IB secretory PLA2 are also proposed to play a role in the development of cancer, although the exact mechanism on cell proliferation is unknown. It seems that the inflammatory effect of secretory PLA2 does not always require lipolytic enzymatic activity but can be secondary to direct binding to membrane receptors on the target cells. One could speculate that certain cancer cells release secretory PLA2 that affects the kidneys and leads to the development of MN through an immune response. However, in the case of malignancy-associated MN, the immune response less often seems to involve antibodies of the IgG4 subclass.
We found a significant correlation between the absence of glomerular IgG4 and PLA2R and malignancy-associated MN, a result that is consistent with previous reports [7, 15]. Furthermore, 45 of 69 (65%) patients with idiopathic MN were positive for IgG4. Analysis of IgG1–3 was performed and IgG1 and IgG3 were present in a low number of cases while IgG2 was found in a high number of cases. However, in our material, a positive staining for IgG2 could not be used as an indicator of underlying malignancy.
Of 63 (56%) patients with idiopathic MN, 35 had PLA2R in glomerular deposits. This is lower than previous reports [7, 11], which could possibly be due to aged biopsy materials in this retrospective study. Three patients in the malignant group had glomerular PLA2R, and it cannot be ruled out that the presence of MN and malignancy in these cases was coincidental.
The dominance of IgG2 and IgG4 antibodies in MN fits well with the notion that these two subclasses are less prone to complement activation than IgG1 and IgG3 [19, 20]. Thus, the patients with MN have little inflammation such as infiltrating inflammatory cells or crescents. Recently, it has been proposed that there is a subclass switch from IgG1 to IgG4 during the progression of idiopathic MN [13]—a phenomenon for which we could not find any evidence. IgG4 antibodies possess an ability of exchanging Fab arms, a mechanism that provides the base for their anti-inflammatory activity with poor ability to activate complement through the classical pathway and a low affinity of Fc receptors [21]. The effect of IgG4 on PLA2R, as well as the normal function of PLA2R, is still not fully understood [2]. In addition, it is still not clear how the deposition of immune complexes induces the glomerular damage and podocyte loss. One hypothesis is that the podocytes are exposed to increased oxidative stress, and the anti-oxidative defence system indeed has been shown to be down-regulated in MN [22].
Since malignant disease can appear years after diagnosis of MN, early differentiation between idiopathic MN and malignancy-associated MN is crucial to avoid a delay in finding and treating malignancy. In our study, baseline laboratory data did not differ between the groups, while older age indicated a risk for malignancy-associated MN. Also, the absence of glomerular IgG4 and PLA2R strongly indicates underlying malignant disease.
Naturally, there are some limitations in our study. Firstly, it is a retrospective study, and therefore we had no possibility of measuring anti-PLA2R antibodies in serum at the time of biopsy. Secondly, although we have included a larger number of patients with malignancy-associated MN than most previous studies, the number of cases is still small, and we have reduced statistical power. Nevertheless, we conclude that there is not yet any specific diagnostic tool to identify patients with malignancies among those who display a nephrotic syndrome with a morphological pattern of MN. The absence of IgG4 antibodies and glomerular deposits of PLA2R should raise suspicion in particular in patients of higher age.
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org.
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgements
The study was supported by the Swedish Medical Research (grants 9898 and 14764), the National Association for Kidney Diseases, the John and Brit Wennerström Research Foundation, IngaBritt and Arne Lundberg Research Foundation and Sahlgrenska University Hospital (grant LUA75450). We thank Dr G Lambeau for providing staining the staining protocol for glomerular PLA2R.
References
- 1.Haas M, Meehan SM, Karrison TG, et al. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976–1979 and 1995–1997. Am J Kidney Dis 1997; 30: 621–631 [DOI] [PubMed] [Google Scholar]
- 2.Beck LH., Jr Membranous nephropathy and malignancy. Semin Nephrol 2010; 30: 635–644 [DOI] [PubMed] [Google Scholar]
- 3.Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int 1999; 56: 355–377 [DOI] [PubMed] [Google Scholar]
- 4.KDIGO. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int 2012; 2(Suppl. 2): 139–274 [Google Scholar]
- 5.Lefaucheur C, Stengel B, Nochy D, et al. Membranous nephropathy and cancer: epidemiologic evidence and determinants of high-risk cancer association. Kidney Int 2006; 70: 1510–1517 [DOI] [PubMed] [Google Scholar]
- 6.Beck LH, Jr., Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoxha E, Kneissler U, Stege G, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int 2012; 82: 797–804 [DOI] [PubMed] [Google Scholar]
- 8.Beck LH, Jr., Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int 2010; 77: 765–770 [DOI] [PubMed] [Google Scholar]
- 9.Knehtl M, Debiec H, Kamgang P, et al. A case of phospholipase A receptor-positive membranous nephropathy preceding sarcoid-associated granulomatous tubulointerstitial nephritis. Am J Kidney Dis 2011; 57: 140–143 [DOI] [PubMed] [Google Scholar]
- 10.Qin W, Beck LH, Jr., Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 2011; 22: 1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 2011; 364: 689–690 [DOI] [PubMed] [Google Scholar]
- 12.Oliveira DB. Membranous nephropathy: an IgG4-mediated disease. Lancet 1998; 351: 670–671 [DOI] [PubMed] [Google Scholar]
- 13.Huang CC, Lehman A, Albawardi A, et al. IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod Pathol 2013; 26: 799–805 [DOI] [PubMed] [Google Scholar]
- 14.Ohtani H, Wakui H, Komatsuda A, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant 2004; 19: 574–579 [DOI] [PubMed] [Google Scholar]
- 15.Qu Z, Liu G, Li J, et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transplant 2012; 27: 1931–1937 [DOI] [PubMed] [Google Scholar]
- 16.Bacchetta J, Juillard L, Cochat P, et al. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol 2009; 70: 39–58 [DOI] [PubMed] [Google Scholar]
- 17.Baris YS, Akpolat T, Akpolat I, et al. Coexistence of membranous glomerulonephritis and Kaposi's sarcoma. Nephron 1998; 79: 371–372 [DOI] [PubMed] [Google Scholar]
- 18.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem 2008; 77: 495–520 [DOI] [PubMed] [Google Scholar]
- 19.Gomez AM, Van Den Broeck J, Vrolix K, et al. Antibody effector mechanisms in myasthenia gravis-pathogenesis at the neuromuscular junction. Autoimmunity 2010; 43: 353–370 [DOI] [PubMed] [Google Scholar]
- 20.Sitaru C, Mihai S, Zillikens D. The relevance of the IgG subclass of autoantibodies for blister induction in autoimmune bullous skin diseases. Arch Dermatol Res 2007; 299: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007; 317: 1554–1557 [DOI] [PubMed] [Google Scholar]
- 22.Granqvist A, Nilsson UA, Ebefors K, et al. Impaired glomerular and tubular antioxidative defense mechanisms in nephrotic syndrome. Am J Physiol Renal Physiol 2010; 299: F898–F904 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.