Abstract
Background
The infusion of microbubbles as a side effect of haemodialysis was repeatedly demonstrated in recent publications, but the knowledge on the source of microbubbles and on microbubble formation is scarce.
Methods
Microbubbles in the range of 10–500 µm were measured by a non-invasive bubble counter based on a pulsed ultrasonic Doppler system in a non-interventional study of a single centre. Totally, 29 measurements were performed in standard treatments covering a broad range of patient and treatment conditions (types of blood access, treatment modes, blood flow rates and arterial pressures).
Results
Several possible sources of microbubbles could be identified such as an arterial luer lock connector at negative pressure and remnant bubbles from insufficient priming, but some sources of microbubbles remain unknown. Microbubbles were found in all treatments, haemodialysis (HD) and online haemodiafiltration. The lowest average microbubble rates (17 ± 16 microbubbles per minute) were observed in patients treated by online haemodiafiltration at medium blood flow rates and moderate arterial pressures and the highest average microbubble rates (117 ± 63 microbubbles per minute) at high blood flow rates (550 mL/min) and low arterial pressures (−210 mmHg). Generally, the microbubble rate correlated to both blood flow rate (correlation coefficient r = 0.45) and negative arterial pressure (r = 0.67).
Conclusions
Microbubbles are a general side effect of HD; origin and pathophysiologic consequences of this phenomenon are not well understood, and deserve further study.
Keywords: haemodiafiltration, haemodialysis, microbubbles, microemboli, standard dialysis
Introduction
Gaseous microemboli are a concern during surgery with cardiopulmonary bypass as potential cause of neurocognitive deficit since these microbubbles were verified by ultrasonic microbubble counters operating with blood flow rates up to 6 L/min [1, 2].
In haemodialysis (HD) with much lower blood flow rates (up to 0.6 L/min), microbubbles have also been a concern, but proper measurement was difficult. Microemboli were detected in the subclavian vein downstream from the arteriovenous fistula of HD patients a decade ago [3, 4]. It was speculated that microbubbles lodge in the capillary bed of various organs, mainly the lungs, and that they obstruct the blood flow in the capillaries, causing tissue ischaemia and micro-infarct, followed by inflammatory reaction, complement activation and tissue damage [5].
Patients who undergo surgery with cardiopulmonary bypass are usually treated only once, whereas HD patients are repeatedly treated, in total >600 h each year. Any infusion of microbubbles during HD may become significant for the patient's health due to the cumulative exposure time.
When it became technically possible to measure microbubbles in HD, it was demonstrated that they may pass the venous bubble catcher and the safety system against air infusion without triggering an alarm, in vitro [6] and during treatments [7].
Microbubbles were detected in patient fistulas and carotid arteries by Swedish investigators [8]. They observed a significantly higher number of patients with elevated embolic counts on the arterial side than would be expected if an open foramen ovale was the only option, and concluded that a considerable number of microemboli seem to pass the lung [8].
Two years later, a case report of a chronic HD patient was published: he suffered from cardiac arrest and died. The autopsy revealed a closed foramen ovale; the microscopic investigation verified microembolies of air that were surrounded by fibrin in the lungs, the heart and the brain [9]. The investigators concluded that microbubbles were present before death, passed the cardiopulmonary circulation, entered the left heart and were distributed throughout the body. Autopsies of five patients (died either during or up to 2.3 days after the HD session) revealed pulmonary fibrosis and pulmonary microemboli (fibrin around air bubbles) [10].
A study by British investigators confirmed microemboli in the arteriovenous fistulae of HD patients during their treatment, but found no evidence of microembolization in the middle cerebral artery [11].
Microbubbles were determined at the site of the arteriovenous access for different blood levels in the venous bubble catcher in a randomized, double-blinded study: the lowest number of microbubbles was observed during high blood levels [12], confirmed in a second study [13].
Recently, an Australian group published a computational analysis of microbubbles in HD: most bubbles with a diameter of <50 µm, and many bubbles in the range of 50–200 µm pass through the venous bubble catcher [14].
It seems that microbubbles generally occur in HD treatments. Up to now, the available information on the potentially harmful microbubbles in HD is not consistent. The aim of our investigation was to detect possible sources of the generated microbubbles and to increase the knowledge on microbubble formation.
Materials and methods
The study was designed as purely observational, non-interventional investigation in a single centre, without documentation of patient data. Twenty-nine treatments of 21 patients were observed, covering a broad range of patient and treatment conditions (types of blood access, treatment modes, blood flow rates and arterial pressures).
The applied diagnostic medical device was the non-invasive, Bubble Counter BCC200 (GAMPT GmbH, Merseburg, Germany), a pulsed ultrasonic Doppler system with a transmission frequency of 2 MHz, measuring microbubbles in the range of 10–500 µm [15].
The applied dialysis machines were 5008 Haemodialysis Systems (Fresenius Medical Care, Germany). Two types of dialysers were applied: Xevonta Hi23 (BBraun, Germany) and FX1000 (Fresenius Medical Care, Germany).
The standard position of the two sensor heads of the microbubble counter was on the arterial line (between arterial access and blood pump) and on the venous line (between venous bubble catcher and venous access).
For a consistency check, the arterial and the venous sensor heads of the BCC200 were positioned during a stable treatment at the same blood line in a row. Then, their position was interchanged. The number of measured microbubbles differed by 8% in the normal and in reversed orders; thus, the two sensor heads give identical information with a deviation below 10%.
Results
Observation of visible bubbles
The blood tubing was searched for possible microbubble sources. Some air reservoirs in the blood tubing were obvious, such as in the venous bubble catcher. Other potential air reservoirs were magnitudes smaller; in rare cases, air bubbles with a diameter of a few millimetres were seen, and in most cases, air bubbles were found only after attentive search due to their extremely small size.
The largest air volume was in the venous bubble catcher. It was not observed that this air contributed to the formation of microbubbles. The usually high level of blood was not changed due to the purely observational character of our study.
Bubbles were found
– at the upper part of the dialyser on the dialysate side,
– at the dialyser caps on the blood side of the dialyser,
– in the blood line segment of the roller pump,
– in syringes with fluids for injections (such as heparin or iron), partly injected into the blood line during the treatments.
Detection of microbubbles
Measurements with the bubble counter usually revealed a broad range of sizes, with bubble diameters between 10 and 300 µm, mostly between 10 and 200 µm.
The microbubble count was usually larger in the beginning than during the further treatment.
It was repeatedly observed that the start of the blood pump was accompanied by an increase in the bubble count, higher than the level when the blood pump was running continuously.
In a single case, we could follow a small visible air volume moving through the heparin line into the main blood line; this volume vanished downstream of the dialyser, but instead 33 microbubbles were detected by the microbubble counter. Obviously, the visible volume had become the source of many microbubbles.
In a single case, the two sensor heads were positioned up- and downstream of the arterial luer lock connector. The downstream sensor head measured 46 microbubbles and the upstream sensor head only 2, indicating that small amounts of air were entering the blood line at this connection. At this part of the tubing system, the pressure is negative due to the suction of the blood pump.
As planned, a broad range of treatment conditions could be investigated, including the treatment modes online haemodiafiltration and HD, one single-needle treatment, access via Cimino fistula, teflon shunt or catheter. The blood flow rates covered the range from 200 to 550 mL/min. The treatment durations were between 200 and 550 min. Arterial pressures were observed in the range between −230 and −80 mmHg.
In total, measurements with complete data sets were obtained from 29 treatments, including 21 patients, with an observed treatment time summed up to 7871 min (≈131 h).
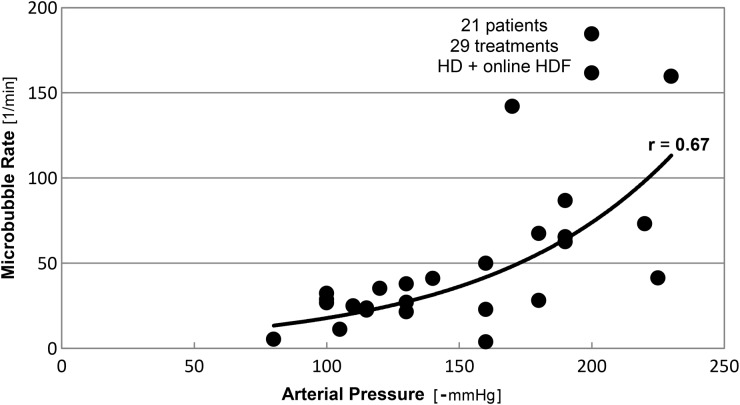
We observed a correlation between the count of microbubbles and the blood flow rate and the arterial pressure, see Figures 1 and 2. The microbubble rate increased with the blood flow rate and the negative arterial pressure.
Fig. 1.
Microbubble rates as function of the blood flow rate, measured during 29 treatments (haemodialysis and online haemodiafiltration) in 21 patients.
Fig. 2.
Microbubble rates as function of the negative arterial pressure, measured during 29 treatments (haemodialysis and online haemodiafiltration) in 21 patients.
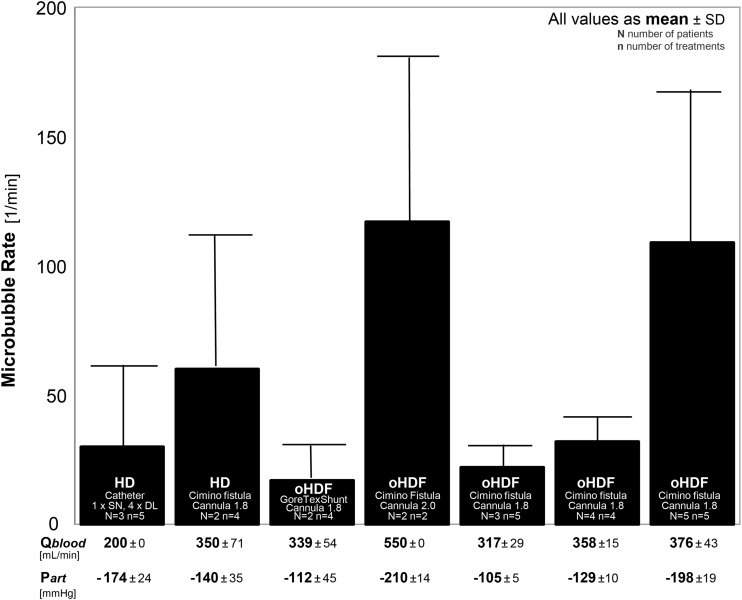
An overview of our observational results concerning microbubble rate is shown in Figure 3. The data were grouped first according to the treatment mode, HD or online haemodiafiltration, and then according to blood access and cannula diameter. Additionally, the largest group of 14 treatments with identical blood access, cannula diameter and treatment mode was divided into three groups by arterial pressure.
Fig. 3.
Microbubble rates for different treatments: haemodialysis (HD) and online haemodiafiltration (oHDF), blood access by catheter [single needle (SN) catheter or double lumen (DL) catheter], Gore Tex shunt or Cimino fistula, with cannula diameters of 1.8 mm or 2.0 mm. The number of patients (N) as well as the number of treatments (n) per group is noted. For all groups the blood flow (Qblood) and the arterial pressure (Part) are listed below. All values are presented as mean value with standard deviation.
Generally, a large difference between the groups was seen with a spread in microbubble rate up to a factor of 5.
Microbubble rates below 40 per minute were observed for HD treatments with catheters at blood flow rates of 200 mL/min (1st bar), and online haemodiafiltration treatments in patients with GoreTex shunts (3rd bar) or Cimino fistulas with mean blood flow rates <360 mL/min (5th and 6th bars).
At similar conditions concerning blood access, cannula diameter, blood flow rate and arterial pressure, a much higher microbubble rate was observed during HD than during online haemodiafiltration (2nd bar versus 6th bar). This observation was possibly due to the substitution fluid applied during online haemodiafiltration, prepared from degassed dialysate.
The highest microbubble rates were observed for high blood flow rates and low arterial pressures (4th and 7th bars). A low arterial pressure (−210 mmHg) was observed (although a large cannula diameter was applied: 2.0 mm) at the very high blood flow rate (550 mL/min). At a lower blood flow rate (376 mL/min) with a lower cannula diameter (1.8 mm), the arterial pressure was also low (−198 mmHg).
These observations indicate that a high blood flow rate and a low arterial pressure may lead to a high microbubble rate.
A large spread was observed also concerning the number and volume of the injected microbubbles.
The lowest microbubble rate was observed in patients with GoreTex shunts, a cannula diameter of 1.8 mm, treated by online haemodiafiltration at a medium blood flow rate (339 mL/min) and a moderate arterial pressure (−112 mmHg), resulting in a mean value of 3723 microbubbles per treatment with a total volume of 17 µL.
The highest microbubble rate was observed in patients with Cimino fistula, a cannula diameter of 2.0 mm, treated by online haemodiafiltration at a high blood flow rate (550 mL/min) and a low arterial pressure (−210 mmHg), resulting in a mean of 29 889 microbubbles per treatment with a total volume of 232 µL.
Discussion
Our observations increased the knowledge on formation kinetic and quantification of microbubbles in HD.
In interventional studies, more microbubbles were observed for a low blood level in the venous bubble catcher than for a high level [7, 12, 13]. Air might be dragged below the blood level, and at low blood levels, the buoyant force might not be sufficient to separate small bubbles from downwards flowing blood. Then, small bubbles may be injected into the patient [6, 7, 12–14].
Due to the non-interventional character of our study, the blood level in the venous bubble catcher remained without change; we could not observe that the air above the high blood level contributes to the formation of microbubbles.
The persistent air bubbles found on the dialysate side of the dialyser did not vanish possibly due to a slow degradation of large bubbles at the air fluid interface by the degassed dialysate (the dissolution time for air bubbles in water strongly increases with larger bubble diameter [5]).
The observed split of a visible bubble into smaller fragments that were no longer visible gave cause for three concerns concerning patient safety.
First, as already demonstrated [6, 7, 12–14], microbubbles may pass the safety system against air infusion without triggering an alarm.
Second, microbubbles may be transported into the patient, and if small enough, pass the lung capillaries, and be distributed throughout the body. It is well known that meeting gas bubbles tend to combine. Larger gas bubbles have a higher dissolution time and a higher chance to get stuck in a capillary. Moreover, microbubbles may increase or decrease depending on surrounding pressure (e.g. sparkling water effect). Consequently, different scenarios concerning the lifetime of microbubbles inside the human body are possible. The microbubbles measured upstream of the arterial luer lock connector were possibly a remnant of these microbubbles inside the patient's body.
Third, the total surface area increases. If a bubble is divided into smaller bubbles, then the surface area increases (e.g. for a split into 64 bubbles, the surface area increases by the factor of 4). Each increase in the surface area may also mean an increase in interaction of air with blood and the endothelium of the capillaries.
Microbubbles may originate from the remaining air of an incomplete initial filling with insufficient rinsing of the extracorporeal circuit. If the remaining air was the only source of the microbubbles, then the microbubble count should monotonously decrease.
In the beginning of the treatments, the mean microbubble count was an order of magnitude larger than during the rest of the treatment.
The microbubble count decrease was only observed in the beginning. Even in long treatments (e.g. nocturnal dialysis), the number of microbubbles was not decreasing towards the end of the treatment, indicating either other sources of microbubble generation or large ‘stores’ of initially not removed air somewhere in the blood tubing.
An increase in bubble count was repeatedly observed at the start of the blood pump, possibly due to a sudden displacement of the remaining bubbles in the blood line, suction of bubbles from a sideline (e.g. heparin line) or from the roller pump segment into the main line.
Our simultaneous measurement up- and downstream of the arterial luer lock connector indicated that, in the negative pressure region, small amounts of air may enter the blood line. This single observation at the arterial connector did not allow generalization concerning prevalence of incomplete tightness. The involved volume was so small that the safety system against air infusion did not react. Generally, the tightness of luer lock connectors is under discussion [17–20].
Our data also suggested that the microbubble rate increased with substitution flow rate. The substitution fluid is made from degassed dialysis fluid, thus should be counteracting the microbubbles (e.g. by diluting). On the other hand, online haemodiafiltration is often performed with a high blood flow rate and a low negative arterial pressure. Possibly, a high microbubble rate may not be due to the online haemodiafiltration per se, but due to a low negative arterial pressure (e.g. by small cannula diameter). A large randomized clinical trial demonstrated the survival benefit of online haemodiafiltration; a major influence of microbubbles as a confounding factor on the primary outcome parameter all-cause mortality was unlikely, because the blood flow rate was similar in both the study groups (differences only between 2 and 5%) [21].
We did not observe treatments without microbubbles, although the measurements covered different conditions; thus, it seems that microbubbles are always present during a standard HD treatment. This observation is in line with other investigations, and microbubbles were observed during treatments with the dialysis machines of all manufacturers [4, 8, 11, 12], indicating that microbubbles are a general side effect of HD. But the range of the microbubble counts was large; obviously, in the standard HD setting, there are treatments with a low generation of microbubbles.
There are several limitations of this non-interventional study: The study was performed in a single centre with a small number of patients and treatments. Its reproducibility is limited due to the modest number of repeated measurements.
Nevertheless, some preventive actions were identified (partially mentioned in earlier studies):
– The cannula diameter should be adjusted to the blood flow rate.
– The arterial luer lock connector should be tightened.
– Careful filling and sufficient rinsing in the preparation phase might reduce remaining gas bubbles.
– Syringes filled with fluids for injections (such as heparin or iron) and side lines should be given special care.
– The blood in the venous bubble catcher should be kept at a high level [6, 7, 12, 13].
– Extreme blood flow rates and arterial pressures should be avoided.
From the visible bubbles (at blood pump segment, dialyser cap, venous chamber and heparin line), the total volume was estimated to a few microliters, obviously not enough to be the only source of microbubbles. A general air leakage at the arterial luer lock was not assumed [16].
Therefore, the source of microbubbles is at least partly unknown.
Systematic investigations in this field are scarce. Considering the clinical observations, one might hypothesize that a degassing or cavitation phenomenon of blood may contribute in generating microbubbles, even if the threshold pressure gradient is not achieved [16]. The results should be further investigated in sound laboratory experiments, in vitro and clinical studies.
Generally, during HD, microbubbles are infused in large numbers into the patients, but the involved volume is extremely small. If there is an impact on the patient's health, it seems scarcely due to the miniscule volume of an individual microbubble, because this volume is many orders of magnitude below any volume inducing air embolism. The large surface areas of the microbubbles and their enormous number appear to be more eligible candidates.
Conclusion
During HD, a large number of microbubbles may reach the patients, but the involved volume is extremely small. Origin and pathophysiologic consequences of this phenomenon are not well understood but deserve further study and, if necessary, a new blood line design aiming to minimize blood air interfaces.
Conflict of interest statement
R.W. and B.C. are employees of Fresenius Medical Care.
References
- 1.Eitschberger S, Henseler A, Krasenbrink B, et al. Investigation on the ability of an ultrasound bubble detector to deliver size measurements of gaseous bubbles in fluid lines by using a glass bead model. ASAIO J 2001; 47: 18. [DOI] [PubMed] [Google Scholar]
- 2.De Somer FM, Vetrano MR, Van Beeck JP, et al. Extracorporeal bubbles: a word of caution. Interact Cardiovasc Thorac Surg 2010; 10: 995. [DOI] [PubMed] [Google Scholar]
- 3.Rollé F, Pengloan J, Abazza M, et al. Identification of microemboli during haemodialysis using Doppler ultrasound. Nephrol Dial Transplant 2000; 15: 1420. [DOI] [PubMed] [Google Scholar]
- 4.Droste DW, Kühne K, Schaefer RM, et al. Detection of microemboli in the subclavian vein of patients undergoing haemodialysis and haemodiafiltration using pulsed Doppler ultrasound. Nephrol Dial Transplant 2002; 17: 462. [DOI] [PubMed] [Google Scholar]
- 5.Barak M, Katz Y. Microbubbles: pathophysiology and clinical implications. Chest 2005; 128: 2918. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson P, Karlsson L, Forsberg U, et al. Air bubbles pass the security system of the dialysis device without alarming. Artif Organs 2007; 31: 132. [DOI] [PubMed] [Google Scholar]
- 7.Stegmayr B, Forsberg U, Jonsson P, et al. The sensor in the venous chamber does not prevent passage of air bubbles during hemodialysis. Artif Organs 2007; 31: 162. [DOI] [PubMed] [Google Scholar]
- 8.Forsberg U, Jonsson P, Stegmayr C, et al. Microemboli, developed during haemodialysis, pass the lung barrier and may cause ischaemic lesions in organs such as the brain. Nephrol Dial Transplant 2010; 25: 2691. [DOI] [PubMed] [Google Scholar]
- 9.Stegmayr B, Brännström T, Forsberg U, et al. Microbubbles of air may occur in the organs of hemodialysis patients. ASAIO J 2012; 58: 177. [DOI] [PubMed] [Google Scholar]
- 10.Brännström T, Forsberg U, Jonsson P, et al. Microembolies of air are deposited in the lungs of heamodialysis patients. Int J Artif Organs 2012; 35: 579 [Google Scholar]
- 11.George S, Holt S, Hildick-Smith D. Patent foramen ovale, dialysis and microembolization. Nephrology (Carlton) 2012; 17: 569. [DOI] [PubMed] [Google Scholar]
- 12.Forsberg U, Jonsson P, Stegmayr C, et al. A high blood level in the air trap reduces microemboli during hemodialysis. Artif Organs 2012; 36: 525. [DOI] [PubMed] [Google Scholar]
- 13.Forsberg U, Jonsson P, Stegmayr C, et al. A high blood level in the venous chamber and a wet-stored dialyzer help to reduce exposure for microemboli during hemodialysis. Hemodial Int 2013; 17: 612–617 [DOI] [PubMed] [Google Scholar]
- 14.Keshavarzi G, Barber TJ, Yeoh G, et al. Two-dimensional computational analysis of microbubbles in hemodialysis. Artif Organs 2013; 37: E139. [DOI] [PubMed] [Google Scholar]
- 15.BCC200 Manual Gesellschaft für Angew. Med. Physik u. Technik mbH (GAMPT mbH). Merseburg, Germany [Google Scholar]
- 16.Polaschegg HD. Hemodialysis machine air detectors need not detect microbubbles. Artif Organs 2007; 31: 911. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous. Two piece Luer lock may not be locked. Health Devices 2000; 29: 190. [PubMed] [Google Scholar]
- 18.Fletcher SJ. Failure of a Luer lock. Anaesthesia 2005; 60: 206. [DOI] [PubMed] [Google Scholar]
- 19.Holmes K, Snow D. More problems with luer lock connections. Anaesthesia 2006; 61: 73. [DOI] [PubMed] [Google Scholar]
- 20.Luer Connector Misconnections: Under-Recognized but Potentially Dagerous Events, MedSun KidNet Subnetwork, Nov 19, 2008. www.FDA.gov/MedicalDevices/Safety
- 21.Maduell F, Moreso F, Pons M, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013; 24: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]