Abstract
This case report presents fluoconazole efficacy to reduce hypercalcaemia and increased urinary calcium excretion in a patient with nephrocalcinosis after a long history of recurrent renal stones caused by a loss-of-function mutation of the CYP24A1 gene. The CYP24A1 gene codes for a key enzyme in the vitamin D endocrine system that protects against vitamin D toxicity by degrading the circulating excess of both 1,25-dihydroxyvitamin D, the hormonal form of vitamin D, and its precursor, 25-hydroxyvitamin D. In order to expedite the identification of this rare disorder and improve therapies to avoid its progression to nephrocalcinosis, this editorial updates the current knowledge on the frequency of CYP24A1-inactivating mutations, the features of their early clinical presentation and progression, and the pathophysiology of vitamin D activation in health and in granulomatous disorders that may help improve current treatment.
Keywords: albumin, calcaemia, calcium, gene expression, vitamin D
The integrity of the vitamin D endocrine system is essential to maintain serum calcium levels within the narrow limits required to protect individuals from the adverse effects of hypercalcaemia and increased urinary calcium excretion, which increase the propensity for kidney stones, nephrocalcinosis and renal insufficiency [1, 2].
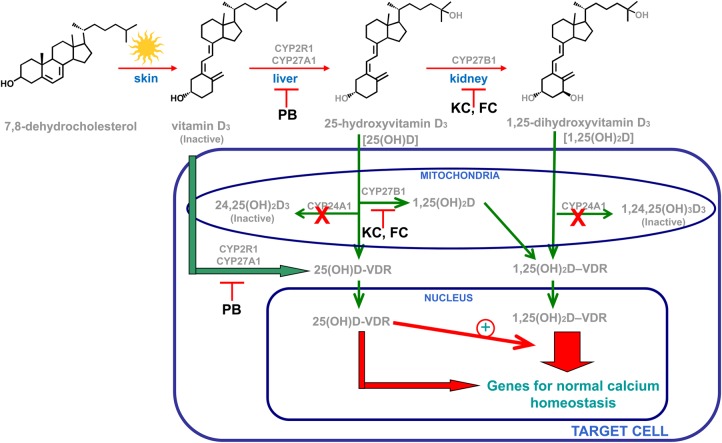
Figure 1 summarizes the current understanding of the tight systemic and local control of vitamin D activation, inactivation and biological actions. The vitamin D hormone 1,25-dihydroxyvitamin D (1,25D) is the most potent endogenous activator of the vitamin D receptor (VDR) to exert the plethora of vitamin D biological actions, including the maintenance of mineral homeostasis and skeletal health. However, recent studies in Cyp27b1-null mice [3, 4], which lack the enzyme that converts 25-hydroxyvitamin D (25D) to 1,25D, and also in vitro, using 25D analogues chemically modified to prevent their 1-hydroxylation, have demonstrated that 25D can activate the VDR directly and can also synergize with 1,25D for VDR activation.
Fig. 1.
Systemic and local vitamin D bioactivation and actions. Genetic inactivation of the CYP24A1 gene (red X) compromises the tightly controlled balance between synthesis and catabolism increasing serum and intracellular 1,25D and 25D levels. These elevations cause hypercalcaemia by simultaneous exacerbation of 1,25D/VDR calcitropic actions, 25D/1,25D synergy for VDR activation and direct 25D activation of the VDR. Interventions with ketoconazole (KC) and fluconazole (FC) or phenobarbital (PB) should effectively inhibit 25D and 1,25D syntheses through a direct targeting of the key converting enzymes.
Importantly, many cells possess CYP27A1 and CYP2R1, the two main cytochrome P450s that convert vitamin D to 25D. Since this reaction is very loosely regulated, it is clear that a tight regulation of 25D conversion to 1,25D is essential to maintain serum 1,25D within the narrow limits required to avoid hypercalcaemia.
For many years, it was believed that 1,25D tightly controlled its own levels through dual mechanisms: suppressing its own synthesis and stimulating its own degradation through the induction of CYP24A1 gene expression in most vitamin D-responsive tissues [1]. However, the phenotype of markedly enhanced 1,25D levels, severe hypercalcaemia and nephrocalcinosis in the Cyp24a1-null mice [5], underscored not only the importance of CYP24A1 in preventing vitamin D toxicity, but also that 1,25D suppression of its own synthesis had little, if any, pathophysiological relevance to prevent elevations in serum 1,25D above normal levels.
Recently, several loss-of-function mutations of the CYP24A1 gene were identified [6–11] with a very low frequency in the general population of 0.06% for the pathogenic pE143del mutation initially described by Schlingmann [11]. A more recent report based on single nucleotide polymorphisms estimated, however, the frequency of predicted bi-allelic mutations of CYP24A1 in the general population to be as high as 4–20% [9]. Regardless of their actual frequency, these mutations were sufficient to cause infantile hypercalcaemia in children [6–11], followed by a long-lasting history of hypercalcaemic episodes and kidney stone formation [6–11], which could eventually progress to nephrocalcinosis and renal insufficiency, as described in this case report.
The absence of concurrent primary hyperparathyroidism, malignancy or a granulomatous disorder (sarcoidosis or tuberculosis) exacerbating 1,25D production and hypercalcaemia in individuals carrying the CYP24A1 mutation has conclusively corroborated the essential role of CYP24A1 in 1,25D degradation demonstrated in the Cyp24a1-null mice. Furthermore, the hypercalcaemia of tuberculosis and several granulomatoses results not only from γ-interferon induction of macrophage 1,25D production but also from γ-interferon impairment of 1,25D induction of CYP24A1 expression. Indeed, physical interactions of the DNA binding site of the VDR with Stat1, the protein that mediates γ-interferon signalling, impede 1,25D–VDR binding to the CYP24A1 gene promoter to induce transcription [12, 13].
Not every hospital has the resources for genetic analyses to conclusively identify a CYP24A1 mutation. However, the concurrence of persistent hypercalcaemia, hypercalciuria, suppressed parathyroid hormone (PTH), and elevated serum 1,25D levels, with normal or mildly elevated 25D in the absence of any malignancy or granulomatous disorder, should make a clinician suspicious of this rare disorder and ready to implement the best therapeutic strategy to avoid persistent hypercalcaemia and prevent disease progression to nephrocalcinosis or renal damage. Important considerations to improve current strategies are that some patients with inactivating CYP24A1 mutations responded to ketoconazole but not to corticosteroids [6] and also the worsening of the hypercalcaemic episodes upon elevations in the dosage of hydrochlorothiazide [6].
The cytochrome P450 inhibitor ketoconazole has been used alone or in combination with corticosteroids to attenuate macrophage 1,25D production and hypercalcaemia in granulomatous disorders [14]. Indeed, in some of the newly reported cases of Cyp24a1-inactivating mutations, ketoconazole administration effectively lowered serum 1,25D and calcium levels. However, there is concern about the potential renal and hepatic toxicity of the high doses of ketoconazole required and also in a prolonged duration of treatment with ketoconazole for persistent hypercalcaemia. An important contribution of this case report is that it presents fluconazole as a potentially safer alternative to ketoconazole to attenuate 1,25D synthesis and effectively correct hypercalcaemia in this rare disorder.
Another important consideration to improve therapy is that the incidence of hypercalcaemic episodes in patients with CYP24A1 mutations also associated with periods of higher exposure to sunlight [8]. This could be explained in part by the described synergy between 25D and 1,25D for VDR activation, as sunlight exposure will increase circulating vitamin D levels that will be converted to 25D by the loosely regulated CYP27A1 and CYP2R1 enzymes. In this case report, the patient was instructed to avoid tanning beds.
Theoretically, inhibition of vitamin D conversion to 25D should help attenuate the hypercalcaemic episodes following sunlight exposure or vitamin D supplementation. One well-recognized inhibitor of vitamin D conversion to 25D is the antiepileptic drug phentobarbital [15] suggesting its potential as a co-adjuvant to ketoconazole, or the safer fluconazole, to expand the therapeutic strategy to target simultaneously vitamin D conversion to 25D and 25D conversion to 1,25D.
Funding
This study was supported by grants from Plan Nacional de I+D+i 2008–2011, Plan Estatal de I+D+i 2013–2016, Instituto de Salud Carlos III (ISCIII)—Fondo Europeo de Desarrollo Regional (FEDER) (PI07/0893, PI10/0896 and PI13/00497), Plan de Ciencia, Tecnología e Innovación 2013–2017 del Principado de Asturias (GRUPIN14-028), Fundación para el Fomento en Asturias de la Investigación Científica Aplicada y la Tecnología (FICYT), Instituto Reina Sofía de Investigación Nefrológica, Fundación Renal Íñigo Álvarez de Toledo and by Red de Investigación Renal-RedInRen from ISCIII (RD06/0016, RD12/0021).
Conflict of interest statement
None declared.
References
- 1.Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int 2011; 79: 715–729 [DOI] [PubMed] [Google Scholar]
- 2.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008; 29: 726–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lou YR, Molnar F, Perakyla M, et al. 25-Hydroxyvitamin D(3) is an agonistic vitamin D receptor ligand. J Steroid Biochem Mol Biol 2010; 118: 162–170 [DOI] [PubMed] [Google Scholar]
- 4.Hoenderop JG, van der Kemp AW, Urben CM, et al. Effects of vitamin D compounds on renal and intestinal Ca2+ transport proteins in 25-hydroxyvitamin D3–1alpha-hydroxylase knockout mice. Kidney Int 2004; 66: 1082–1089 [DOI] [PubMed] [Google Scholar]
- 5.St-Arnaud R, Arabian A, Travers R, et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology 2000; 141: 2658–2666 [DOI] [PubMed] [Google Scholar]
- 6.Jacobs TP, Kaufman M, Jones G, et al. A lifetime of hypercalcemia and hypercalciuria, finally explained. J Clin Endocrinol Metab 2014; 99: 708–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tebben PJ, Milliner DS, Horst RL, et al. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab 2012; 97: E423–E427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueres ML, Linglart A, Bienaime F, et al. Kidney function and influence of sunlight exposure in patients with impaired 24-hydroxylation of vitamin D due to CYP24A1 mutations. Am J Kidney Dis 2015; 65: 122–126 [DOI] [PubMed] [Google Scholar]
- 9.Nesterova G, Malicdan MC, Yasuda K, et al. 1,25-(OH)2D-24 Hydroxylase (CYP24A1) deficiency as a cause of nephrolithiasis. Clin J Am Soc Nephrol 2013; 8: 649–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinour D, Beckerman P, Ganon L, et al. Loss-of-function mutations of CYP24A1, the vitamin D 24-hydroxylase gene, cause long-standing hypercalciuric nephrolithiasis and nephrocalcinosis. J Urol 2013; 190: 552–557 [DOI] [PubMed] [Google Scholar]
- 11.Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 2011; 365: 410–421 [DOI] [PubMed] [Google Scholar]
- 12.Vidal M, Ramana CV, Dusso AS. Stat1-vitamin D receptor interactions antagonize 1,25-dihydroxyvitamin D transcriptional activity and enhance stat1-mediated transcription. Mol Cell Biol 2002; 22: 2777–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dusso AS, Kamimura S, Gallieni M, et al. Gamma-interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab 1997; 82: 2222–2232 [DOI] [PubMed] [Google Scholar]
- 14.Glass AR, Cerletty JM, Elliott W, et al. Ketoconazole reduces elevated serum levels of 1,25-dihydroxyvitamin D in hypercalcemic sarcoidosis. J Endocrinol Invest 1990; 13: 407–413 [DOI] [PubMed] [Google Scholar]
- 15.Samaniego EA, Sheth RD. Bone consequences of epilepsy and antiepileptic medications. Semin Pediatr Neurol 2007; 14: 196–200 [DOI] [PubMed] [Google Scholar]