Abstract
Background
Although myocardial bridging (MB) is defined as an angiographic phenomenon with a benign course, it has also been associated with adverse cardiovascular events. The effects of exercise on myocardial repolarization in patients with MB were tested in this study, with Tp-e and Tp-e/QT repolarization indexes.
Material/Methods
A total of 50 patients in whom isolated MB was diagnosed at coronary angiography (CAG) (Group I) and 48 patients with normal CAG results (Group II) were included in this study. The participants underwent treadmill exercise stress testing according to the Bruce protocol. QT dispersion (QTd) was defined as the minimum QT interval subtracted from the maximum. The Tp-e interval was defined as the difference between the QT and the QT peak time period. QTd and Tp-e intervals were calculated for all patients before and after exercise testing and differences between groups were compared.
Results
At peak exercise, QTd and cQTd showed a significant increase in comparison to baseline values in the group of patients with myocardial bridges. Significant increases were also found with exercise in the Tp-e, cTp-e durations and Tp-e/QT ratio of the MB patient group in comparison to the baseline values. On the other hand, significant differences in QTd, cQTd, Tp-e, cTp-e intervals, and Tp-e/QT ratio during peak exercise in comparison with baseline values were not detected in the control group (p>0.05).
Conclusions
Significant increases in QTd, cQTd, Tp-e and cTp-e intervals and Tp-e/QT ratio were detected in the MB patients during exercise testing.
Keywords: Arrhythmias, Cardiac; Exercise Test; Myocardial Bridging
Background
Myocardial bridging (MB) is a congenital coronary anomaly that is defined when a segment of a major epicardial coronary artery is “tunneled” in the myocardium. It extends intramurally through the myocardium beneath a muscular bridge [1,2]. The incidence of myocardial bridging is elaborated as 1.5–16% in angiographic studies and may rise up to 80% at autopsy [3,4]. MB is most commonly located in the middle left anterior descending artery (LAD) [5] and the prominent angiographic finding is systolic compression of the involved epicardial coronary artery [6].
Although MB is a phenomenon with a benign course, it has also been reported to cause myocardial ischemia [7], acute coronary syndrome [8], coronary vasospasm [9], atrioventricular block [10], transient ventricular dysfunction [11], ventricular septal rupture [12], ventricular tachycardia [13], and sudden cardiac death [14]. These reports suggest that at least some of the patients with MB may have a predisposition for major cardiac events. It has been postulated that the adverse events in the reported cases are related to the effect of MB on the coronary blood flow. On the other hand, the electrophysiological effects and arrhythmogenic potential of these effects on the coronary flow are not well-described.
Previous research has shown the importance of detecting myocardial repolarization abnormalities in the prediction of arrhythmogenic potential. QT dispersion (QTd) is defined as the minimum QT interval subtracted from the maximum and cQTd is defined as the QTd corrected for the rate. These parameters have been associated with adverse outcomes [15,16]. On the other hand, the recently defined parameters of Tp-e interval (defined as the difference between the QT interval and the QT-peak time period) and Tp-e/QT index were reported to indicate myocardial repolarization abnormalities better than QTd and cQTd parameters [17,18]. Increased Tp-e interval has been associated with cardiovascular mortality and ventricular tachyarrhythmias [19,20]. In this study, we analyzed the effects of exercise on myocardial repolarization parameters in patients with and without myocardial bridging.
Material and Methods
Study population
Patients enrolled in this prospective study were consecutive patients who underwent diagnostic coronary angiography for suspected coronary artery disease (CAD) at Ondokuz Mayıs University Hospital between January 2011 and April 2014. Fifty patients who were diagnosed as having isolated MB at coronary angiography (Group I) and 48 patients with normal coronary angiograms (Group II) were included in this study. Patients with coronary atherosclerosis, those with acute coronary syndromes, left ventricular systolic dysfunction (LVEF <50%), significant valvular heart disease, renal failure (creatinine-based estimated GFR <90 mL/min/1.73 m2 calculated by the Cockcroft-Gault formula), bundle branch block and atrioventricular conduction abnormalities on the electrocardiography (ECG), thyroid dysfunction, pulmonary disease, chronic infections or inflammatory diseases, electrolyte imbalance, and those with ECG’s without clearly analyzable QT and Tp-e intervals were excluded from the study. All the patients were in sinus rhythm, and none of them were taking antiarrhythmic medications, tricyclic antidepressants, antihistamines, or antipsychotics.
Biochemical measurements
Biochemical parameters were measured with an Abbott ARCHITECT c8000 (Abbott Laboratories, Abbott Park, IL, USA) autoanalyzer using commercial kits. Hematologic parameters were measured with an Abbott CellDyn 3700 (Abbott Laboratories, Abbott Park, IL, USA) device with laser and impedance method. High sensitive C-reactive protein (hs-CRP) (CardioPhase, hs-CRP) was measured quantitatively in BN II System Nephelometer (Dade Behring, Marburg, Germany) by immunonephelometric method from patient serum and results were reported in mg/L.
Coronary angiography
All patients underwent coronary angiography with Judkins technique and femoral approach. Images were recorded at a digital angiographic system (ACOM.PC; Siemens AG, Germany) at a speed of 15 frames/second during the procedure. Iopromide (Ultravist 370, Schering AG, Berlin, Germany) was used as contrast material. The cine-angiograms were evaluated by two independent cardiologists and MB was identified as 50% or more systolic narrowing by visual estimation. Quantitative measurements of the coronary arteries were performed on the digital angiographic system (ACOM.PC; Siemens AG, Germany). The length of MB and percentage of diameter reduction in the bridged segments were calculated by quantitative coronary angiography (QCA).
Electrocardiography and Calculation of ventricular repolarization parameters
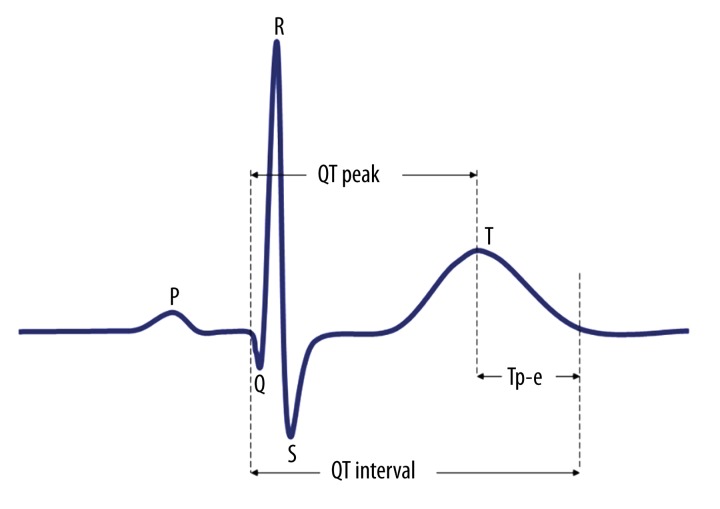
The 12-lead ECG recording was performed after 10 minute of rest at supine position at 50 mm/s speed and 20 mm/mV amplitude (Nihon Kohden, Tokyo, Japan). ECG measurements of QT and Tp-e intervals were performed by two cardiologists who were blinded to the patient data. In order to lessen errors in QT and Tp-e interval analyses, each interval was measured manually with calipers and magnifying glass. In order to improve accuracy, average value of three readings were calculated and used as data. We measured the QT interval from the beginning of the QRS complex to the end of the T wave. The QT maximum (QTmax) and QT minimum (QTmin) were calculated in all leads of a 12-lead ECG. QTd was defined as the maximum minus minimum QT interval and corrected QTd (cQTd) was calculated according to Bazett’s Formula adjusted according to heart rate [21]. QT peak interval was defined as the time from QRS complex onset to the peak of the T wave, whereas Tp-e interval was defined as the time from the peak to the end of the T wave. The measurements of Tp-e interval were performed from precordial leads and were corrected according to heart rate [19]. The Tp-e/QT ratios were subsequently calculated (Figure 1).
Figure 1.
Diagram demonstrating ventricular repolarization parameters measured from surface ECG.
The reproducibility of ECG repolarization indices was assessed by coefficients of variation (standard deviation of differences between the repeated measurements divided by the mean value and expressed as a percentage) between measurements. The intra-observer variability was calculated in 34 randomly selected study participants (18 patients with myocardial bridging and 16 control subjects) by repeating the measurements under the same basal conditions. Intra-observer and inter-observer variation was found to be <5%.
Stress electrocardiography
Treadmill exercise stress test was applied to subjects according to the Bruce protocol (Customed Cardio 100, Germany). The reproducibility of ECG repolarization indexes was assessed by coefficients of variation (standard deviation of differences between the repeated measurements divided by the mean value and expressed as a percentage) between measurements. Intra-observer and inter-observer variation were <5%. The abnormal response (positive test) to exercise testing included a horizontal or downsloping ST-segment depression equal to or greater than 1 mm (0.1 mV) at 60–80 ms after J-point [22]. Functional capacity was assessed by the peak metabolic equivalent (METs peak), indirectly obtained by formulas, according to the maximum slope and speed achieved in an incremental exercise treadmill test, with the ramp protocol adjusted to the individual [23].
Standard echocardiography
Transthoracic echocardiography was performed in all patients at left lateral decubitus position with a GE Vingmed Vivid 7 (GE Vingmed Ultrasound, Horten, Norway) echocardiography device. Images at the parasternal longitudinal axis, short axis, apical four chambers and two chambers were obtained and evaluated by M-mode, 2-D, continuous wave Doppler, pulsed wave Doppler methods based on American Echocardiography Association criteria [24]. Values were measured on three separate beats and then the average was calculated for all parameters. Left ventricular mass (LVM) was calculated by Devereux et al. using the equation previously described [25]. LVM was indexed to body surface area to obtain the LV mass index (LVMI). Relative wall thickness (RWth) was measured at end-diastole as the ratio of [2× posterior wall thickness (PWth)/left ventricular end-diastolic diameter (LVEDD)].
Statistical analysis
All data were loaded to the SPSS 15 program. Subsequently, the normal distribution of the data was tested using the Kolmogorov–Smirnov test. The t-test was used to compare two groups of variables demonstrating normal distribution, while groups of variables without normal distribution were compared using the Mann-Whitney U test. Comparison of categorical variables was carried out by the chi-square test. Wilcoxon signed-rank test or paired sample t test were used to analyze the change (in individual subjects) of measurements between baseline and peak exercise according to the variables distribution. Any correlation between data was tested with the Spearman and Pearson correlation analysis. While the continuous data were expressed as “mean ±SD” (standard deviation), the categorical data were expressed as percentage values and a p value of <0.05 was accepted as statistically significant. Multivariate stepwise logistic analysis was performed to assess the parameters that are associated with prolonged cTp-e interval and age, LVMI, length of MB and percentage of diameter reduction were included as the covariates in the multivariate regression model.
Results
A total of 50 patients with MB (Group I, 21 males; mean age 48.2±9.8 years) and age and sex-matched 48 healthy subjects (Group II, 22 males; mean age 49.1±8.4 years) were included in this study. The baseline clinical and laboratory characteristics of the patients are presented in Table 1. There were no significant differences between the groups in terms of baseline laboratory and clinical characteristics. In addition, no significant difference was found between the patient groups in terms of drug usage. The length of MB was found to be 15.3±4.5 mm in the MB patient group and the percentage of diameter reduction in the bridged segments was found to be 65.1±9.7%. Also, no significant differences were observed between the conventional echocardiographic measurements of the groups (p>0.05) (Table 2).
Table 1.
Baseline clinical and laboratory characteristics of study population and comparison between groups.
| Variable | MB group (n=50) | Control group (n=48) | P value |
|---|---|---|---|
| Age (years) | 48.2±9.8 | 49.1±8.4 | 0.610 |
| Male, n (%) | 21 (42) | 22 (46) | 0.702 |
| Body mass index (kg/m2) | 26.8±3.7 | 28.3±3.1 | 0.187 |
| Hypertension, n (%) | 17 (34) | 15 (31) | 0.772 |
| Smoking, n (%) | 18 (36) | 16 (33) | 0.782 |
| Hemoglobin (g/L) | 13.9±1.6 | 13.3±1.7 | 0.785 |
| White blood cell count, 103/mm3 | 7.19±1.78 | 6.89±1.54 | 0.383 |
| Platelet, 103/mm3 | 251.2±60.9 | 239.1±55.7 | 0.308 |
| Creatinine, mg/dL | 0.83±0.16 | 0.83±0.18 | 0.848 |
| Fasting glucose, mg/dL | 88.1±10.9 | 85.2±10.8 | 0.199 |
| Total cholesterol, mg/dL | 195.4±38 | 184.4±42.6 | 0.181 |
| Low-density lipoprotein cholesterol, mg/dL | 122.3±31.5 | 122.1±30.1 | 0.983 |
| High-density lipoprotein cholesterol, mg/dL | 42.8±7.4 | 45.5±9.5 | 0.118 |
| Triglyceride, mg/dL | 158.6±55.3 | 164.5±70.2 | 0.642 |
| AST, U/L | 21.8±12.5 | 23.8±11.9 | 0.432 |
| ALT, U/L | 20.7±11.5 | 20.1±10.9 | 0.822 |
| Na, mmol/L | 141.3±2.7 | 141.2±2.8 | 0.763 |
| K, mmol/L | 4.2±0.3 | 4.1±0.3 | 0.564 |
| Ca, mg/dL | 9.0±0.6 | 8.9±0.6 | 0.582 |
| TSH, uIU/mL | 2.3±1.0 | 2.5±1 | 0.355 |
| Hs-CRP (mg/L) | 1.1±0.5 | 1.0±0.3 | 0.856 |
| eGFR* (ml/min/1.73 m2) | 108.1±12.6 | 111.5±10.7 | 0.255 |
| Cardiovascular medication | |||
| ACE-I/ARB, n (%) | 12 (24) | 10 (20.8) | 0.108 |
| Beta-blocker | 11 (22) | 8 (16.6) | 0.195 |
| Calcium channel blocker, n (%) | 4 (8) | 5 (10.4) | 0.755 |
| Nitrate, n (%) | 3 (6) | 2 (4.1) | 0.169 |
| Statin, n (%) | 7 (14) | 5 (10.4) | 0.296 |
| Bridging segment characteristics | |||
| Length of MB (mm) | 15.3±4.5 | ||
| Percentage of diameter reduction (%) | 65.1±9.7 | ||
| Site of MB | |||
| LAD, n (%) | 47 (94) | ||
| LCX, n (%) | 2 (4) | ||
| RCA, n (%) | 1 (2) | ||
| Degree of narrowing | |||
| 50–74%, n (%) | 46 (92) | ||
| ≥75%, n (%) | 4 (8) | ||
AST – aspartate aminotransferase; ALT – alanine aminotransferase; TSH – thyroid-stimulating hormone; LAD – Left anterior descending artery; LCX – left circumflex coronary artery; RCA – right coronary artery; MB – myocardial bridge; Hs-CRP – high sensitive C-reactive protein; ACE-I – angiotensin converting enzyme inhibitor; ARB – angiotensin receptor blocker; GFR – glomerular filtration rate.
e-GFR was calculated based on the Cockcroft-Gault equation: estimated creatinine clearance = (140-age) × (weight in kg) × (0.85 if female)/72 × serum creatinine.
Table 2.
Conventional echocardiographic parameters.
| Variable | MB group (n=50) | Control group (n=48) | P value |
|---|---|---|---|
| LVEDD (mm) | 47.4±3.4 | 46.9±3.2 | 0.460 |
| LVESD (mm) | 26.7±3.5 | 26.4±4 | 0.713 |
| IVS thickness (mm) | 9.7±1.4 | 9.7±1.4 | 0.919 |
| PW thickness (mm) | 9.2±1.0 | 9.2±1.0 | 0.957 |
| LA dimension (mm) | 34.0±3.4 | 34.1 ± 4.6 | 0.894 |
| LVEF (%) | 59.2±4.7 | 59.8±4.6 | 0.490 |
| RV dimesion (mm) | 22.4±2.9 | 21.9±3.5 | 0.466 |
| E (m/sn) | 0.73±0.19 | 0.78±0.18 | 0.211 |
| A (m/sn) | 0.75±0.17 | 0.82±0.19 | 0.076 |
| E/A | 1.03±0.38 | 1.0±0.33 | 0.699 |
| LVMI (gr/m2) | 87.9±16.1 | 85.7±17.8 | 0.245 |
| RWth | 0.37±0.03 | 0.36±0.04 | 0.386 |
IVS – ventrıcular septal thickness; PW – posterior wall thickness; LVEDD – left ventrıcular end-diastolic dimension; LVESD – left ventrıcular end-systolic dimensions; LA – left atrium; LVEF – left ventrıcular ejection fraction; RV – right ventrıcular; E – early diastolic flow; A – atrial contraction signal; LVMI – left ventricular mass index; RWth – relative wall thickness.
The results of exercise testing are shown in Table 3. No significant differences were observed between the result of exercise testing of the groups (p>0.05). No rhythm abnormalities or hemodynamic deteriorations were detected in the two groups during exercise testing. Additionally, the exercise testing yielded a positive result in 9 patients (18%) in the MB group, while it was negative in 41 patients (82%).
Table 3.
The results of exercise testing.
| Variable | MB group (n=50) | Control group (n=48) | P value |
|---|---|---|---|
| METs | 9.6±0.6 | 9.8±0.5 | 0.131 |
| Baseline systolic blood pressure, mm Hg | 126.3±9.1 | 125.9±9.1 | 0.828 |
| Baseline diastolic blood pressure, mm Hg | 73.6±6.6 | 73.3±6.0 | 0.836 |
| Peak systolic blood pressure, mm Hg | 169.7±9.7 | 167.5±7.9 | 0.218 |
| Peak diastolic blood pressure, mm Hg | 83.8±9.9 | 83.1±9.8 | 0.730 |
| Total exercise duration (min) | 8.7±1.1 | 8.8±1.2 | 0.671 |
| % heart rate achieved | 91.5±4.3 | 90.5±4.2 | 0.257 |
METs – metabolic equivalents.
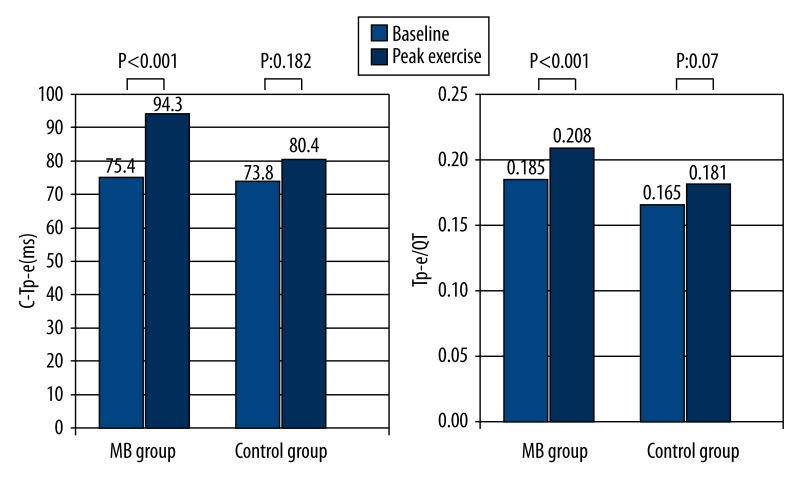
The changes in the ventricular repolarization parameters of the patients during exercise are shown in Table 4. QT max and QT min intervals during peak exercise showed a significant decrease in comparison with the baseline values in the two groups (372.3±12.1 vs. 327.8±10.7 ms, p<0.001; 335.8±14.1 vs. 285.1±10.5 ms, p<0.001; 368.3±11.5 vs. 304.5±15.1 ms, p<0.001; 344.9±13.2 vs. 275.4±14.9 ms, p<0.001, respectively). However, QTd and cQTd at peak exercise increased significantly in comparison to baseline values in the MB patient group (36.4±10.3 vs. 42.6±14.1 ms, p=0.003 and 39.3±10.1 vs. 65.4±16.7 ms, p<0.001 respectively). Additionally, significant increases were detected in Tp-e, cTp-e intervals and Tp-e/QT ratio during peak exercise in comparison to baseline values in patients with MB (69±5.7 vs. 81.1±8.4 ms, p<0.001; 75.2±6.6 vs. 94.5±7.4 ms, p<0.001; 0.18±0.01 vs. 0.20±0.02, p<0.001, respectively) (Figure 2).
Table 4.
Electrocardiographic repolarization parametres in patients.
| Variable | MB group (n=50) | Control group (n=48) | ||||
|---|---|---|---|---|---|---|
| Baseline | Peak exercise | P value | Baseline | Peak exercise | P value | |
| Heart rate(beats/minute) | 74.3±6.3 | 145.9±11.5 | <0.001 | 72.1±5.4 | 144.6±10.6 | <0.001 |
| QT max (ms) | 372.3±12.1 | 327.8±10.7 | <0.001 | 368.3±11.5 | 304.5±15.1 | <0.001 |
| QT min (ms) | 335.8±14.1 | 285.1±10.5 | <0.001 | 344.9±13.2 | 275.4±14.9 | <0.001 |
| QTd (ms) | 36.4±10.3 | 42.6±14.1 | 0.003 | 27.1±5.4 | 29.1±8.1 | 0.178 |
| cQTd (ms) | 39.3±10.1 | 65.4±16.7 | <0.001 | 28.1±5.5 | 30.1±5.6 | 0.071 |
| Tp-e (ms) | 69±5.7 | 81.1±8.4 | <0.001 | 66.6±7.8 | 65±5.4 | 0.065 |
| cTp-e (ms) | 75.2±6.6 | 94.5±7.4 | <0.001 | 73.2±6.8 | 74.9±8.5 | 0.182 |
| Tp-e/QT | 0.18±0.01 | 0.20±0.02 | <0.001 | 0.18±0.02 | 0.17±0.01 | 0.070 |
QTmax – QT maximum; QTmin – QT minimum; QTd – QT dispersion; cQTd – corrected QT dispersion; Tp-e – transmural dispersion of repolarization; cTp-e – corrected transmural dispersion of repolarization.
Figure 2.
Comparison of cTp-e interval and Tp-e/QT ratio at baseline and peak exercise in study groups.
On the other hand, significant differences in QTd, cQTd, Tp-e, cTp-e intervals and Tp-e/QT ratio during peak exercise in comparison with baseline values were not detected in the control group (p=0.178, p=0.071, p=0.065, p=0.182, p=0.07, respectively) (Table 4).
Multivariate analysis demonstrated that the length of MB (standardized β coefficient=0.446, p<0.001) and percentage of diameter reduction (standardized β coefficient=0.510, p<0.001) were independent predictors of a prolonged cTp-e interval in the multivariate stepwise logistic regression model (Table 5). Standardized β coefficient and P values were 0.125 and 0.110 for age, −0.109 and 0.128 for LVMI, respectively.
Table 5.
Multivariate logistic regression analysis to demonstrate independent predictors of prolonged cTp-e interval.
| Variables | β coefficient | SE | OR (95%CI) | p |
|---|---|---|---|---|
| Age | 0.125 | 0.043 | 0.071 (−0.017–0.159) | 0.110 |
| Length of MB (mm) | 0.446 | 0.148 | 0.797 (0.498–1.096) | <0.001 |
| Percentage of diameter reduction (%) | 0.510 | 0.059 | 0.343 (0.223–0.462) | <0.001 |
| LVMI (gr/m2) | −0.109 | 0.026 | −0.041 (−0.093–0.012) | 0.128 |
MB – myocardial bridge; LVMI – left ventricular mass index.
Discussion
Although myocardial bridge phenomenon is a condition which runs a benign course, cases associated with myocardial ischemia [7], acute coronary syndrome [8], coronary vasospasm [9], cardiac arrhythmia [10,13], and sudden cardiac death [14] were reported. Thus, some patients with MB may be considered at risk for major cardiac events. We detected a significant increase in ventricular repolarization parameters (QTd, cQTd, Tp-e, Tp-e/QT) in patients with MB in the present study.
QT dispersion is a simple and effective marker of electrical heterogeneity in myocardial cells. An increase in this heterogeneity is associated with an increase in the potential for cardiac arrhythmias [26]. Several novel repolarization parameters (Tp-e and Tp-e/QT) were defined in recent studies. These parameters were reported to be more sensitive in detecting cardiac repolarization dispersion than conventional parameters [16,18]. Indeed, an increase in Tp-e interval was shown to be associated with long QT syndrome [27], Brugada syndrome [19], hypertrophic cardiomyopathy [28], and development of malignant ventricular arrhythmias during the course of myocardial infarctions [20].
The characteristic angiographic finding of myocardial bridging is a narrowing during systole (milking effect). On the other hand, intracoronary ultrasound and Doppler studies have shown that coronary obstruction may also involve the diastolic period [29,30]. This may be the main triggering mechanism in MB patients with severe ischemia. Exercise and stress may increase the myocardial oxygen demand while shortening the diastolic duration by increasing the heart rate and contractility. For this reason, higher heart rates may be expected to cause a predisposition for ischemia in patients with MB. Huang et al. have detected a reversible perfusion defect in patients with myocardial bridging in 201T1 single photon emission computed tomography after exercise stress testing [31]. In another study, malignant ventricular arrhythmias induced by exercise were reported in patients with MB with evidence for concomitant ischemia [13].
The relationship between MB and atherosclerotic process has not been fully elucidated. The segment proximal to the region of the myocardial bridge has been associated with atherosclerosis rather than the MB segment itself [32]. Previously, the disturbance of blood flow and high wall stress proximal to myocardial bridge have been identified as main contributors for the development of atherosclerosis in the segment proximal to the bridge [33]. In addition, vasoactive agents (endothelin-1, endothelial nitric oxide synthase, angiotensin-converting enzyme) have been shown to be present in higher concentrations in the proximal portion of the MB artery in comparison to the MB segment, further shedding light into the mechanisms of atherosclerosis found in the proximal segment [34].
Another probable mechanism of ischemia observed in patients with MB may be coronary vasospasm [35]. The mechanical stress caused by systolic narrowing at the MB segment may result in endothelial damage, which in turn may lead to platelet aggregation, coronary vasospasm and eventually ACS [3]. Indeed, coronary vasospasm has been described in the myocardial bridge artery segment before [36]. As a conclusion, endothelial damage, vasospasm, and atherosclerotic processes developing in the proximal portion of the MB artery segment are among other causes of ischemia that develops in patients with myocardial bridging.
Myocardial fibrosis and interstitial edema development were shown in the myocardial bridge artery area in a recent histopathological study [37]. Similarly, Hostiuc et al. [38] have reported significant myocardial fibrosis and interstitial edema in the MB area in patients who experienced sudden death due to MB. Also, death in these patients was attributed to electrical myocardial instability, which had developed in a background of myocardial fibrosis.
The close association between prolongation of QT dispersion and development of ventricular arrhythmias was shown, but it may also be a predictor in the diagnosis of coronary artery disease (CAD). Stoletniy et al. have shown that [39] a QTd longer than 60 ms during exercise has 70% sensitivity and 95% specificity in diagnosing CAD, with a predictive value superior than ≥1 mm ST segment depression. An association between QTd and ischemia was reported in other studies previously [40,41]. Similarly, the association between Tp-e interval and Tp-e/ QT ratio with myocardial ischemia was also investigated. Tatlısu et al. [42] have shown that Tp-e interval measured after a primary percutaneous coronary intervention (pPCI) in patients with ST elevation myocardial infarction (STEMI) is a predictor of death and target vessel revascularization. Xiao et al. [43] have detected a significant decrease in QTc, cTp-e intervals and Tp-e/QT ratio after successful thrombolysis for STEMI. Also, a decreased Tp-e interval was shown to be a predictor of successful reperfusion in patients undergoing primary PCI [44]. In our study, positive ischemia findings were found during peak exercise in 9 patients (18%) in the MB patient group, while no ischemia finding was found in 41 (82%) patients. However, we think that absence of ST segment depression in most of our patients during peak exercise did not mean absence of ischemia in these patients in the light of the information mentioned above and considering the inadequate sensitivity and specificity of exercise test in terms of detecting ischemia. Although positive ischemia findings were not observed during peak exercise, prolongation in QT dispersion, Tp-e interval and Tp-e/QT ratio in MB patients might develop as a result of ischemia in the area supplied by the MB artery during peak exercise. Hence, in the multivariate analysis we performed, we found a close relation between the length of MB and percentage of diameter reduction which might be markers of the severity of ischemia observed in the area of MB artery and prolonged cTp-e interval and we concluded that the length of MB and percentage of diameter reduction were an independent predictor of prolonged cTp-e interval. Based on this information, the increased repolarization dispersion indexes in our patients may both be associated with chronic ischemia and myocardial changes developing in this background.
Study limitations
We were not able to interpret the potential prognostic role of the exercise-induced changes of the repolarization indexes in correlation to future adverse events. To determine the predictive value of prolonged Tp-e interval and increased Tp-e/QT ratio, longer follow-up and large-scale prospective studies in patients with myocardial bridging are needed.
Conclusions
A significant increase in QTd, cQTd, Tp-e, and cTp-e intervals and Tp-e/QT ratio during exercise testing was detected in patients with myocardial bridges. The observed increase in ventricular repolarization dispersion indexes may possibly develop on an ischemic background induced by exercise in the MB artery area.
Footnotes
Source of support: Departmental sources
Conflict of Interest
None.
References
- 1.Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449–54. doi: 10.1161/01.cir.0000016175.49835.57. [DOI] [PubMed] [Google Scholar]
- 2.Angelini P, Trivellato M, Donis J, Leachman RD. Myocardial bridges: a review. Prog Cardiovasc Dis. 1983;26:75–88. doi: 10.1016/0033-0620(83)90019-1. [DOI] [PubMed] [Google Scholar]
- 3.Alegria JR, Herrmann J, Holmes DR, Jr, et al. Myocardial bridging. Eur Heart J. 2005;26:1159–68. doi: 10.1093/eurheartj/ehi203. [DOI] [PubMed] [Google Scholar]
- 4.Mohlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002;106:2616–22. doi: 10.1161/01.cir.0000038420.14867.7a. [DOI] [PubMed] [Google Scholar]
- 5.Channer KS, Bukis E, Hartnell G, Rees JR. Myocardial bridging of the coronary arteries. Clin Radiol. 1989;40:355–59. doi: 10.1016/s0009-9260(89)80118-7. [DOI] [PubMed] [Google Scholar]
- 6.Portmann WC, Iwig J. Die intramurale koronarie im angiogramm. Fortschr Rontgenstr. 1960;92:129–32. [in German] [PubMed] [Google Scholar]
- 7.Tauth J, Sullebarger T. Myocardial infarction associated with myocardial bridging: Case history and review of the literature. Cathet Cardiovasc Diagn. 1997;40:364–67. doi: 10.1002/(sici)1097-0304(199704)40:4<364::aid-ccd9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Gowda RM, Khan IA, Ansari AW, Cohen RA. Acute ST segment elevation myocardial infarction from myocardial bridging of left anterior descending coronary artery. Int J Cardiol. 2003;90:117–18. doi: 10.1016/s0167-5273(02)00518-1. [DOI] [PubMed] [Google Scholar]
- 9.Berry JF, von Mering GO, Schmalfuss C, et al. Systolic compression of the left anterior descending coronary artery: A case series, review of the literature, and therapeutic options including stenting. Catheter Cardiovasc Interv. 2002;56:58–63. doi: 10.1002/ccd.10151. [DOI] [PubMed] [Google Scholar]
- 10.Den Dulk K, Brugada P, Braat S, et al. Myocardial bridging as a cause of paroxysmal atrioventricular block. J Am Coll Cardiol. 1983;1:965–69. doi: 10.1016/s0735-1097(83)80218-6. [DOI] [PubMed] [Google Scholar]
- 11.Galli M, Politi A, Zerboni S. “Functional myocardial bridging” and “hyperkinetic state”: A rare association as a cause of acute myocardial infarction. G Ital Cardiol. 1997;27:1286–89. [PubMed] [Google Scholar]
- 12.Tio RA, Ebels T. Ventricular septal rupture caused by myocardial bridging. Ann Thorac Surg. 2001;72:1369–70. doi: 10.1016/s0003-4975(01)02562-0. [DOI] [PubMed] [Google Scholar]
- 13.Feld H, Guadanino V, Hollander G, et al. Exercise-induced ventricular tachycardia in association with a myocardial bridge. Chest. 1991;99:1295–96. doi: 10.1378/chest.99.5.1295. [DOI] [PubMed] [Google Scholar]
- 14.Cutler D, Wallace JM. Myocardial bridging in a young patient with sudden death. Clin Cardiol. 1997;20:581–83. doi: 10.1002/clc.4960200614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antzelevitch C, Shimizu W, Yan GX, Sicouri S. Cellular basis for QT dispersion. J Electrocardiol. 1998;30:168–75. doi: 10.1016/s0022-0736(98)80070-8. [DOI] [PubMed] [Google Scholar]
- 16.Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J Electrocardiol. 2008;41:575–80. doi: 10.1016/j.jelectrocard.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe N, Kobayashi Y, Tanno K, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004;37:191–200. doi: 10.1016/j.jelectrocard.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Gupta P, Patel C, Patel H, et al. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–74. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, et al. Tpeak- Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828–34. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erikssen G, Liestol K, Gullestad L, et al. The terminal part of the QT interval (T peak to T end): a predictor of mortality after acute myocardial infarction. Ann Noninvasive Electrocardiol. 2012;17:85–94. doi: 10.1111/j.1542-474X.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day CP, McComb JM, Campbell RW. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br Heart J. 1990;63:342–44. doi: 10.1136/hrt.63.6.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okin PM, Chen J, Kligfield P. Effect of baseline ST segment elevation on test performance of standard and heart rate-adjusted ST segment depression criteria. Am Heart J. 1990;119:1280–86. doi: 10.1016/s0002-8703(05)80176-0. [DOI] [PubMed] [Google Scholar]
- 23.Franklin B, Whaley M, Howley E. Guidelines for exercise testing and prescription. 6th ed. Baltimore: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 24.Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–58. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 26.Perkiomaki JS, Koistinen J, Yli-Mayry S, Huikuri HV. Dispersion of QT interval in patients with and without susceptibility to ventricular taschyarrhytmias after previous myocardial infarction. J Am Coll Cardiol. 1995;26:174–79. doi: 10.1016/0735-1097(95)00122-g. [DOI] [PubMed] [Google Scholar]
- 27.Lubinski A, Leweick-Nowak E, Kempa M, et al. New insight into repolarization abnormalities in patients with congenital long QT syndrome. The increased transmural dispersion of repolarization. Pace. 1998;21:172–75. doi: 10.1111/j.1540-8159.1998.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu M, Ino H, Okeie K, et al. T-peak to T-end may be a better predictor of high-risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol. 2002;25:335–39. doi: 10.1002/clc.4950250706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarz ER, Klues HG, vom Dahl J, et al. Functional characteristics of myocardial bridging: a combined angiographic and intracoronary Doppler flow study. Eur Heart J. 1997;18(3):434–42. doi: 10.1093/oxfordjournals.eurheartj.a015263. [DOI] [PubMed] [Google Scholar]
- 30.Bourassa MG, Butnaru A, Lesperance J, Tardif JC. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351–59. doi: 10.1016/s0735-1097(02)02768-7. [DOI] [PubMed] [Google Scholar]
- 31.Huang WS, Chang HD, Yang SP, et al. Abnormal 201Tl myocardial single photon emission computed tomography in energetic male patients with myocardial bridge. Nucl Med Commun. 2002;23:1123–28. doi: 10.1097/00006231-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Ishii T, Asuwa N, Masuda S, Ishikawa Y. The effects of a myocardial bridge on coronary atherosclerosis and ischemia. J Pathol. 1998;185:4–9. doi: 10.1002/(SICI)1096-9896(199805)185:1<4::AID-PATH50>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Ge J, Eebel R, Görge G, et al. High wall shear stress proximal to myocardial bridging and atherosclerosis: intracoronary ultrasound and pressure measurements. Br Heart J. 1995;73(5):462–65. doi: 10.1136/hrt.73.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuda T, Ishikawa Y, Akasaka Y, et al. The effect of myocardial bridging of the coronary artery on vasoactive agents and atherosclerosis localization. J Pathol. 2001;193:408–14. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH792>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 35.Maseri A, Beltrame JF, Shimokawa H. Role of coronary vasoconstriction in ischemic heart disease and search for novel therapeutic targets. Circ J. 2009;73:394–403. doi: 10.1253/circj.cj-09-0033. [DOI] [PubMed] [Google Scholar]
- 36.Ciampricotti R, el Gamal M. Vasospastic coronary occlusion associated with a myocardial bridge. Catheter Cardiovasc Diagn. 1988;14:118–20. doi: 10.1002/ccd.1810140213. [DOI] [PubMed] [Google Scholar]
- 37.Brodsky SV, Roh L, Ashar K, et al. Myocardial bridging of coronary arteries: a risk factor for myocardial fibrosis? Int J Cardiol. 2008;124:391–92. doi: 10.1016/j.ijcard.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 38.Hostiuc S, Curca GC, Dermangiu D, et al. Morphological changes associated with hemodynamically significant myocardial bridges in sudden cardiac death. Thorac Cardiovasc Surg. 2011;59(7):393–98. doi: 10.1055/s-0030-1270703. [DOI] [PubMed] [Google Scholar]
- 39.Stoletniy MD, Pai RG. Value of QT dispersion in the interpretation of exercise stress test in women. Circulation. 1997;96:904–10. doi: 10.1161/01.cir.96.3.904. [DOI] [PubMed] [Google Scholar]
- 40.Sporton SC, Taggart P, Sutton PM, et al. Acute ischemia: a dynamic influence on QT dispersion. Lancet. 1997;349:306–9. doi: 10.1016/S0140-6736(96)06143-0. [DOI] [PubMed] [Google Scholar]
- 41.Koide Y, Yotsukura M, Yoshino H, Ishikawa K. Usefulness of QT dispersion immediately after exercise as an indicator of coronary stenosis independent of gender or exercise-induced ST-segment depression. Am J Cardiol. 2000;86(12):1312–17. doi: 10.1016/s0002-9149(00)01233-9. [DOI] [PubMed] [Google Scholar]
- 42.Tatlısu MA, Özcan KS, Güngör B, et al. Can the T-peak to T-end interval be a predictor of mortality in patients with ST-elevation myocardial infarction? Coron Artery Dis. 2014;25(5):399–404. doi: 10.1097/MCA.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 43.Xiao WT, Wang XP, Gao CY, et al. Predictive value of corrected QT interval, corrected Tp-e interval and Tp-e/QT ratio on malignant arrhythmia events in acute ST-segment elevation myocardial infarction patients undergoing thrombolysis. Zhonghua Xin Xue Guan Bing Za Zhi. 2012;40(6):473–76. [PubMed] [Google Scholar]
- 44.Eslami V, Safi M, Taherkhani M, et al. Evaluation of QT, QT dispersion, and T-wave peak to end time changes after primary percutaneous coronary intervention in patients presenting with acute ST-elevation myocardial infarction. J Invasive Cardiol. 2013;25:232–34. [PubMed] [Google Scholar]