Abstract
Purpose
To determine if sarpogrelate, a selective 5-HT2A receptor antagonist, is protective against light-induced retinopathy in BALB/c mice.
Methods
BALB/c mice were dosed intraperitoneally with 5, 15, 30, 40, or 50 mg/kg sarpogrelate 48, 24, and 0 hours prior to bright light exposure (10,000 lux) as well as 24 and 48 hours after exposure. Additionally, a single injection regimen was evaluated by injecting mice with 50 mg/kg sarpogrelate once immediately prior to light exposure. To investigate the potential for additive effects of serotonin receptor agents, a combination therapy consisting of sarpogrelate (15 mg/kg) and 8-OH-DPAT (1 mg/kg) was evaluated with the 5-day treatment regimen. Neuroprotection was characterized by the preservation of retinal thickness and function, measured by spectral-domain optical coherence tomography (SD-OCT) and electroretinography (ERG), respectively.
Results
Mice that were light damaged and injected with saline had significantly reduced outer retinal thickness, total retinal thickness, and ERG amplitudes compared with naïve mice. A 5-day administration of 15, 30, or 40 mg/kg of sarpogrelate was able to partially protect retinal morphology and full protection of retinal morphology was achieved with a 50 mg/kg dose. Both 15 and 30 mg/kg doses of sarpogrelate partially preserved retinal function measured by ERG, whereas 40 and 50 mg/kg doses fully preserved retinal function. Additionally, a single administration of 50 mg/kg sarpogrelate was able to fully preserve both retinal morphology and function. Administration of 15 mg/kg of sarpogrelate and 1 mg/kg of 8-OH-DPAT together demonstrated an additive effect and fully preserved retinal morphology.
Conclusions
A 5- or 1-day treatment with 50 mg/kg sarpogrelate can completely protect the retina of BALB/c mice from light-induced retinopathy. Partial protection can be achieved with lower doses starting at 15 mg/kg and protection increases in a dose-dependent manner. Treatment with low doses of sarpogrelate and 8-OH-DPAT elicits an additive effect that results in full protection of retinal morphology.
Keywords: neuroprotection, light-induced retinopathy, retinal degeneration, serotonin, 5-HT2A receptor, sarpogrelate, Anplag, MCI-9042
Sarpogrelate, a 5-HT2A receptor antagonist, can protect the retina of albino mice from light-induced retinopathy.
Extended bright light exposure after complete dark-adaptation results in retinal degeneration and reduced retinal function in albino BALB/c mice. The brightness and duration of light exposure have varied in previous studies, but the outcome consistently induces severe thinning of the outer nuclear layer and reduced photoreceptor function.1–9 Due to the acute, quantifiable degeneration observed after bright light exposure, light-induced retinopathy has been utilized as a screening tool for potential therapies that can protect the retina from outer retinal degeneration. Multiple studies using light-induced retinopathy models have stratified the potential efficacy of neuroprotective agents.1–9
Serotonin (5-hydroxytryptamine or 5-HT) is an ancient neurotransmitter that is closely regulated in the mammalian retina by a combination of limited production and aggressive uptake.10–14 Currently, there are seven known 5-HT receptor families (5-HT1 to 5-HT7), which have further been divided into subtypes based on specific molecular properties. Thus far, at least 17 subtypes have been described.15 The role of serotonin in the retina is not fully understood, but recent studies suggest that certain serotonin receptor agonists and antagonists can elicit neuroprotective effects.16–20 For example, Collier et al.16 showed that two 5-HT1A receptor agonists, 8-OH-DPAT and AL-8309B, can completely protect albino rat retinas, both structurally and functionally, from blue light-induced photo-oxidative damage. In addition, Chen et al.20 demonstrated that a 5-HT2A receptor antagonist, ketanserin, could protect the morphology of Abca4−/−Rdh8−/− mouse retinas from light-induced all-trans-retinal-mediated photoreceptor degeneration.
Sarpogrelate hydrochloride, marketed as ANPLAG, is a 5-HT2A receptor antagonist that has been used to treat patients with peripheral arterial disease in Japan, China, and South Korea.21,22 Sarpogrelate was found to prevent thrombus formation in an experimental thrombosis mouse model, and a clinical pharmacologic study has shown that it inhibits platelet aggregation in patients with ischemic stroke.21,22 Inoue-Matsuhisa et al.23 investigated sarpogrelate's neuroprotective effects on an in vitro glutamate–induced retinal ganglion cell death model and an in vivo rat retinal ischemia model. A 30 mg/kg dose of sarpogrelate was able to partially protect rat retinas, both structurally and functionally, from inner retinal ischemia.23 Because sarpogrelate has an established safety profile in humans and has shown potential as a neuroprotective agent for the inner retina, we wanted to further investigate sarpogrelate's ability to protect the outer retina in a light-induced retinopathy model, which would establish sarpogrelate as a potential candidate for treating outer retinal degeneration.
The present study aimed to determine if sarpogrelate, a selective 5-HT2A receptor antagonist, could protect the retina from light-induced retinopathy. A dose escalation study was performed and two treatment regimens were evaluated. To explore the potential for additive effects of different serotonin receptor modulators, a subtherapeutic dose of sarpogrelate—a 5-HT2A antagonist—was administered with a subtherapeutic dose of 8-OH-DPAT, a 5-HT1A agonist. Neuroprotection was characterized by preservation of retinal thickness, measured by spectral-domain optical coherence tomography (SD-OCT), and functional preservation, measured by ERG.
Methods
Animals
Eight-week-old albino BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Upon delivery, mice were housed in a 12-hour alternating light/dark cycle room (∼15 lux with lights on from 9 PM to 9 AM, and lights off from 9 AM to 9 PM) for at least 2 weeks prior to injections. All experiments were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Light-Induced Retinopathy
A custom light box was built for the induction of retinopathy. It was able to hold up to 16 mice and produced approximately 10,000 lux of uniform light. After 2 hours of dark adaptation, retinopathy was induced by exposing BALB/c mice to either 6 hours or 1 hour of light emitted by four CFL bulbs (42 W, 6500 K color temperature). Room temperature was maintained within the box using an air conditioning unit. During bright light exposure, mice were monitored and had access to food and water. For each experiment, mice injected with 0.9% sodium chloride were placed in the light box to serve as controls and to ensure retinopathy was induced.
Injections
Sarpogrelate hydrochloride (Sigma-Aldrich Corp., St. Louis, MO, USA) and (±)-8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT; TOCRIS, Bristol, UK) were dissolved in 0.9% sodium chloride (Hospira, Inc., Lake Forest, IL, USA). Injections were performed in dim red light 2 hours into the 12-hour dark cycle. Care was taken to not expose mice to any illumination other than dim red light during the 2 hours prior to light damage. Mice received intraperitoneal injections of 0.9% sodium chloride, 5 mg/kg, 15 mg/kg, 30 mg/kg, 40 mg/kg or 50 mg/kg sarpogrelate 48, 24, and 0 hours prior to a 6-hour bright light exposure as well as 24 and 48 hours after bright light exposure. To evaluate a 1-day time course, a single 50 mg/kg dose of sarpogrelate was delivered intraperitoneally right before 6 hours of light exposure. Additional mice received intraperitoneal injections of 1 mg/kg or 10 mg/kg 8-OH-DPAT 48, 24, and 0 hours prior to a 1-hour bright light exposure as well as 24 and 48 hours after bright light exposure. A combination treatment consisting of 8-OH-DPAT (1 mg/kg) and sarpogrelate (15 mg/kg) was administered following the 5-day time course and mice were exposed to 1 hour of bright light. Naïve mice did not receive injections or bright light exposure.
Imaging
Retinas were imaged using SD-OCT 7 days after bright light exposure as previously reported.24 Briefly, mice were sedated using 1.5% isoflurane, corneas were anesthetized with proparacaine, and pupils were dilated with 1% tropicamide and 2.5% phenylephrine. Artificial tears were used throughout the procedure to maintain corneal clarity. Images taken with SD-OCT were acquired with a commercial device (Envisu R2200-HR SD-OCT; Bioptigen, Durham, NC, USA). Two linear horizontal scans (temporal and nasal) and two linear vertical scans (superior and inferior) were obtained for each eye.
Image Processing and Segmentation
We processed and segmented SD-OCT scans as previously reported.24 Scans were exported as audio video interleaved files (from InVivoVue; Bioptigen, Inc.). These files were loaded into ImageJ (version 1.45; http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) where they were registered using the Stackreg plug-in and averaged as a z-stack. A custom designed SD-OCT segmentation program built in graphing and data analysis software (IGOR Pro version 6.35A; WaveMetrics, Inc., Lake Oswego, OR, USA) was used to profile and measure the average thickness of retinal layers seen in the SD-OCT images.24,25 For segmentation analysis, total retinal (TR) thickness and receptor plus (REC+) thicknesses were obtained and reported. Total retinal thickness defines retinal thickness from Bruch's membrane to the vitreous/retinal nerve fiber layer interface. Receptor plus thickness was defined as the distance from Bruch's membrane to the inner nuclear layer/outer plexiform layer interface.24
ERGs
Electroretinograms were recorded 2 to 20 days post–SD-OCT imaging. Prior to ERGs, fully dark-adapted mice were anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg). Mouse pupils were dilated with 1% tropicamide and 2.5% phenylephrine and lubricated during the procedure with hypromellose 2.5% ophthalmic lubricant (Goniovisc; Dynamic Diagnostics, Westland, MI, USA). Anesthetized mice were positioned on a heated platform (37°C) inside of a Ganzfeld dome coated with highly reflective white paint. Flash ERGs were recorded from a platinum electrode positioned on the center of the cornea. Similar platinum reference and ground electrodes were placed in the forehead and tail. Signals were amplified with a preamplifier (Model CP511, Grass Instruments, West Warwick, RI, USA). Traces were averaged and analyzed with custom software. Flashes were calibrated as previously described.26,27 A mecablitz xenon flash (Metz, Germany) was modulated with neutral density filters and spectrally filtered with a 500-nm interference filter (Edmund Industrial Optics, Barrington, NJ, USA) to achieve light intensities ranging from −4.34 to 1.52 log cd·s/m2. The maximum light intensity of 3.55 log cd·s/m2 was achieved without the use of a neutral density filter. As the intensity of the flash increased, the number of trials was decreased to prevent light adaptation and the time between each flash was increased to allow for pigment regeneration. The minimum interstimulus interval was 7 seconds and the maximum interstimulus interval was 70 seconds.
Statistical Analysis
For analysis with SD-OCT, right eye and left eye TR thicknesses and REC+ thicknesses were averaged together to give one TR thickness and one REC+ thickness for each animal in both the temporal and nasal regions of the retinas. Then, individual TR thicknesses and REC+ thicknesses were averaged for each group. Similarly, for ERG analysis, right eye and left eye a- and b-wave amplitudes were averaged together to give one a- and one b-wave amplitude per animal, per light intensity. Then, individual a- and b-wave amplitudes were averaged for each group at each light intensity. Standard deviations and standard errors were calculated for all averaged data. One-way ANOVA was performed to compare TR and REC+ thickness differences and ERG amplitude differences between the groups. Electroretinogram amplitude comparisons were performed on data collected at light intensities of −2.14 log cd·s/m2 (bmax,rod) and 3.55 log cd·s/m2 (a/bmax,rod+cone) in order to evaluate differences at maximum rod only response and a maximum rod and cone response. A value of P less than or equal to 0.05 was considered significant. To analyze the trend of the dose-response data, two linear regressions were performed. One linear regression compared the right eye REC+ thicknesses (averaged temporal and nasal thicknesses) to the dose of sarpogrelate administered. The second compared each animal's a-wave amplitude (averaged right and left eye) to the dose of sarpogrelate administered.
Results
Sarpogrelate
Figure 1 contains representative SD-OCT scans from a naïve mouse and light-damaged mice injected with a 5-day course of saline, 5, 15, 30, 40, or 50 mg/kg sarpogrelate. The first panel in Figure 1 demonstrates the ability of SD-OCT to delineate multiple retinal layers including the ganglion cells (GC), the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), the outer nuclear layer (ONL), the outer limiting membrane (OLM), the photoreceptor inner segments (IS), the photoreceptor outer segments (OS), and the RPE. The retinas of saline-injected mice were degenerated due to bright light exposure, which was demonstrated by the lack of the ONL, IS, and OS. Similarly, the retinas of 5 mg/kg sarpogrelate-injected mice were degenerated and lacked the ONL, IS, and OS. The retinas of mice injected with 15, 30, 40, or 50 mg/kg sarpogrelate retained the OLM, OS, IS, and RPE. However, the thickness of the ONL increased in a dose-dependent manner (Fig. 1).
Figure 1.
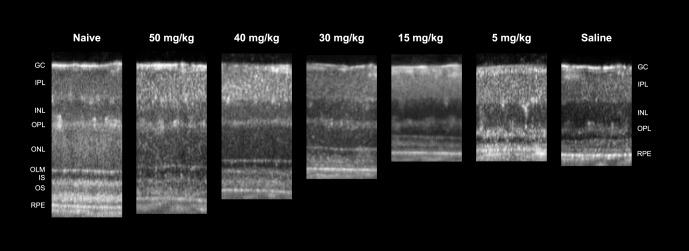
Sarpogrelate protects retinal morphology in a dose-dependent manner. Representative linear SD-OCT scans of nasal retina from a naïve mouse or mice injected with either 50, 40, 30, 15, or 5 mg/kg of sarpogrelate or saline. Injections followed the 5-day time course with a 6-hour light damage.
Nasal and temporal SD-OCT images were segmented to calculate outer retinal thickness (REC+) and TR thickness. Table 1 reports average REC+ and TR thicknesses for each group. The 50 mg/kg treatment of sarpogrelate completely protected retinal morphology and average REC+ and TR thicknesses were not statistically different from the naïve group (P > 0.05). Average TR thickness of the 40 mg/kg group was not significantly different from the naïve group (P > 0.05), but average REC+ thickness was thinner (P < 0.05). The 30 and 15 mg/kg treatments of sarpogrelate partially protected retinal morphology. Average REC+ and TR thicknesses were significantly increased compared to the saline group, but were significantly decreased from the naïve group (P < 0.05). Lastly, the 5 mg/kg treatment did not protect the retina from light-induced retinopathy as REC+ and TR thicknesses were not statistically different from the saline group (P > 0.05).
Table 1.
Summary of OCT Findings
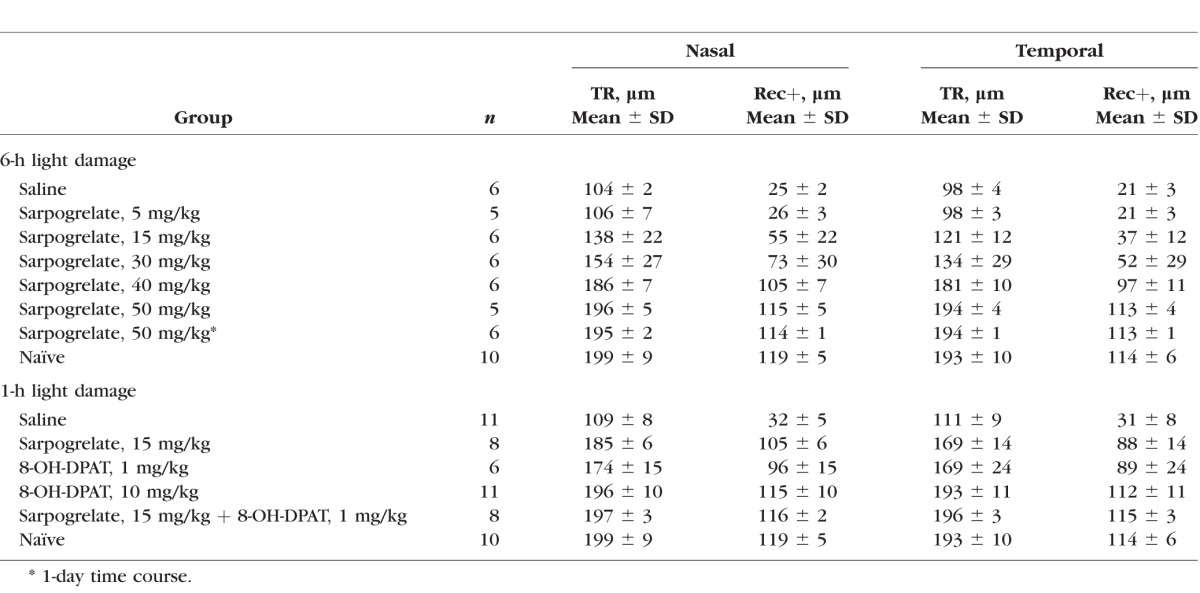
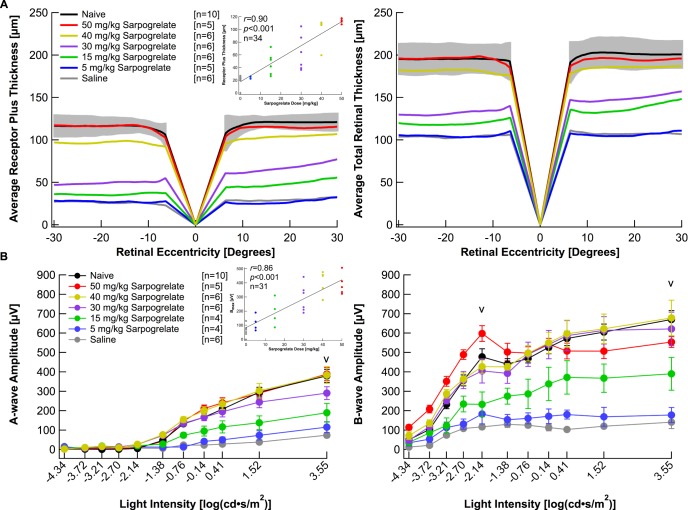
Figure 2A plots average topographic distribution of REC+ and TR thicknesses centered at the optic nerve from naïve, 50, 40, 30, 15, and 5 mg/kg sarpogrelate-injected mice as well as saline-injected mice. For the naïve data, gray shading was used to indicate ±2 SD from the average. The raw data highlights the variable protection achieved in each group (Supplementary Fig. S1A). The averaged data shows that saline-injected mice and 5 mg/kg sarpogrelate-injected mice exhibited severe loss of REC+ and TR thickness compared to naïve mice (Fig. 2A). Both the 15 and 30 mg/kg groups showed a distinguishable preservation in REC+ and TR thickness compared with the saline group. The 40 mg/kg group had a significant preservation in REC+ thickness compared with the saline group and the TR thickness was within 2 SD of the naïve group. However, the 50 mg/kg group showed maximum protection of retinal morphology and maintained normal REC+ and TR thicknesses (Fig. 2A). Quantification of the segmentation data from the horizontal scans did not reveal any significant asymmetries in retinal thickness between nasal or temporal retina (Table 1, Fig. 2A).
Figure 2.
Sarpogrelate elicits neuroprotective effects in a dose-dependent manner. (A) Receptor plus thicknesses and total retinal thicknesses obtained from segmented horizontal SD-OCT scans were averaged and plotted versus retinal eccentricity from the optic nerve head. The gray area indicates ±2 SD of the naïve averaged data. The correlation coefficient, r, was determined by comparing right eye REC+ thicknesses to the dose of sarpogrelate administered. (B) Electroretinogram a- and b-wave amplitudes from mice in each group were extracted from individual waveforms, averaged and plotted versus the light intensity of each flash. Averages are represented as mean ± standard error. “V” indicates at which light intensity the statistical analysis was performed. The correlation coefficient, r, was determined by comparing each animal's amax amplitude to the dose of sarpogrelate administered. Three animals died after acquiring OCT images, but prior to ERG recordings. Injections followed the 5-day time course with a 6-hour light damage. Black: Naïve. Red: 50 mg/kg sarpogrelate. Yellow: 40 mg/kg sarpogrelate. Purple: 30 mg/kg sarpogrelate. Green: 15 mg/kg sarpogrelate. Blue: 5 mg/kg sarpogrelate. Gray: Saline.
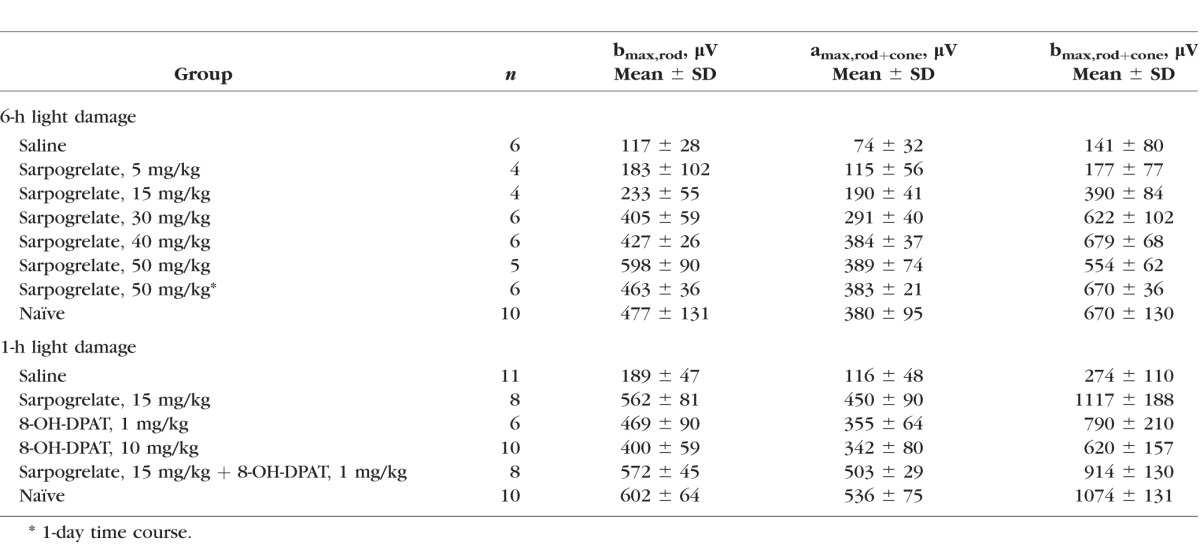
Electroretinograms recorded from saline-injected mice demonstrated severe attenuation of both the scotopic a- and b-waves compared with naïve mice. Waveforms recorded from 5 mg/kg sarpogrelate-injected mice were slightly larger than saline-injected mice, but significantly reduced compared with 50 with/kg sarpogrelate-injected mice or naïve mice (Supplementary Fig. S2). Raw a- and b-wave amplitudes highlight the variable protection achieved in each treatment group (Supplementary Fig. S1B). Raw data was then averaged for each group and reported in Figure 2B and Table 2. Average a- and b-wave ERG amplitudes of the 50, 40, and 30 mg/kg group were not statistically different from the naïve group (Fig. 2B, P > 0.05). The 15 mg/kg treatment elicited partial protection in retinal function as the average a- and b-wave ERG amplitudes of bmax,rod+cone were significantly increased compared with the saline group, but significantly decreased from the naïve group (Fig. 2B, P < 0.05). A 5 mg/kg treatment of sarpogrelate was unable to preserve retinal function (Fig. 2B).
Table 2.
Summary of ERG Parameters
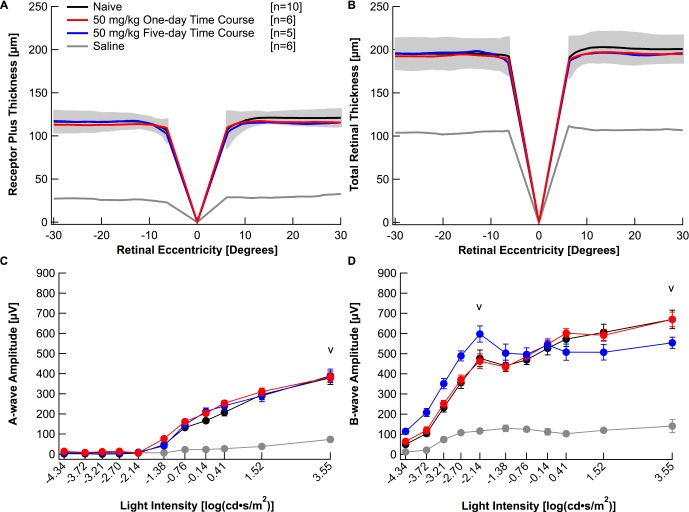
To investigate if sarpogrelate is a fast-acting drug that can elicit neuroprotective effects after transient delivery, a 1-day time course was evaluated. A single 50 mg/kg dose of sarpogrelate was delivered intraperitoneally right before light damage. Figure 3A plots the average topographic distribution of REC+ and TR thickness centered at the optic nerve from naïve mice, mice administered 50 mg/kg of sarpogrelate for 1 day, mice administered 50 mg/kg of sarpogrelate for 5 days, and saline-injected mice. Interestingly, a single injection of 50 mg/kg sarpogrelate completely protected retinal morphology (Fig. 3A). Furthermore, average REC+ and TR thicknesses in both nasal and temporal areas of the retina were not statistically different from naïve mice (Table 1, P > 0.05). A single injection of 50 mg/kg sarpogrelate also completely protected retinal function. Average a- and b-wave amplitudes were not statistically different from the naïve group (Fig. 3B, P > 0.05). Average bmax,rod, amax,rod+cone and bmax,rod+cone amplitudes are listed in Table 2.
Figure 3.
A 1-day time course of 50 mg/kg sarpogrelate completely protects retinal morphology and function. Receptor plus thicknesses (A) and total retinal thicknesses (B) obtained from segmented horizontal SD-OCT scans were averaged and plotted versus retinal eccentricity from the optic nerve head. The gray area indicates ±2 SD of the naïve averaged data. Electroretinogram a- (C) and b-wave (D) amplitudes for all mice in each group were extracted from individual waveforms, averaged and plotted versus the light intensity of each flash. Averages are represented as mean ± standard error. “V” indicates at which light intensity the statistical analysis was performed. Black: Naïve. Red: 50 mg/kg 1-day time course. Blue: 50 mg/kg 5-day time course. Gray: Saline.
8-OH-DPAT
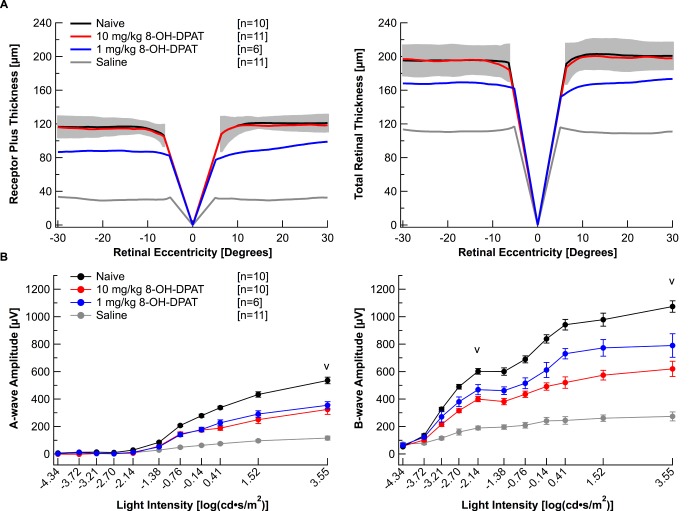
Previous studies have reported the neuroprotective effects produced by activating the 5-HT1A receptor with 8-OH-DPAT in albino rats.16 In order to elucidate the protection achieved with 8-OH-DPAT in albino BALB/c mice, mice were injected with either 1 or 10 mg/kg 8-OH-DPAT following the 5-day regimen. On average, mice injected with 1 mg/kg 8-OH-DPAT had only partially protected retinal morphology (Fig. 4A; Table 1). However, the raw data illustrates that individual protection was variable between each mouse (Supplementary Fig. S3A). In contrast, mice injected with 10 mg/kg 8-OH-DPAT had fully protected retinal morphology (Fig. 4A; Table 1) and variation between each animal was minimal (Supplementary Fig. S3A). Both the 1 and the 10 mg/kg 8-OH-DPAT treatments elicited partial protection in retinal function as the average a- and b-wave ERG amplitudes were significantly increased compared with the saline group, but significantly decreased from the naïve group (Fig. 4B; Table 2; P < 0.05).
Figure 4.
Chemical 8-OH-DPAT elicits neuroprotective effects in BALB/c mice. (A) Receptor plus thicknesses and total retinal thicknesses obtained from segmented horizontal SD-OCT scans were averaged and plotted versus retinal eccentricity from the optic nerve head. The gray area indicates ±2 SD of the naïve averaged data. (B) Electroretinogram a- and b-wave amplitudes from mice in each group were extracted from individual waveforms, averaged, and plotted versus the light intensity of each flash. Averages are represented as mean ± standard error. “V” indicates at which light intensity the statistical analysis was performed. One animal died after acquiring OCT images, but prior to ERG recordings. Injections followed the 5-day time course with a 1-hour light damage. Black: Naïve. Red: 10 mg/kg 8-OH-DPAT. Blue: 1 mg/kg 8-OH-DPAT. Gray: Saline.
Combination Therapy
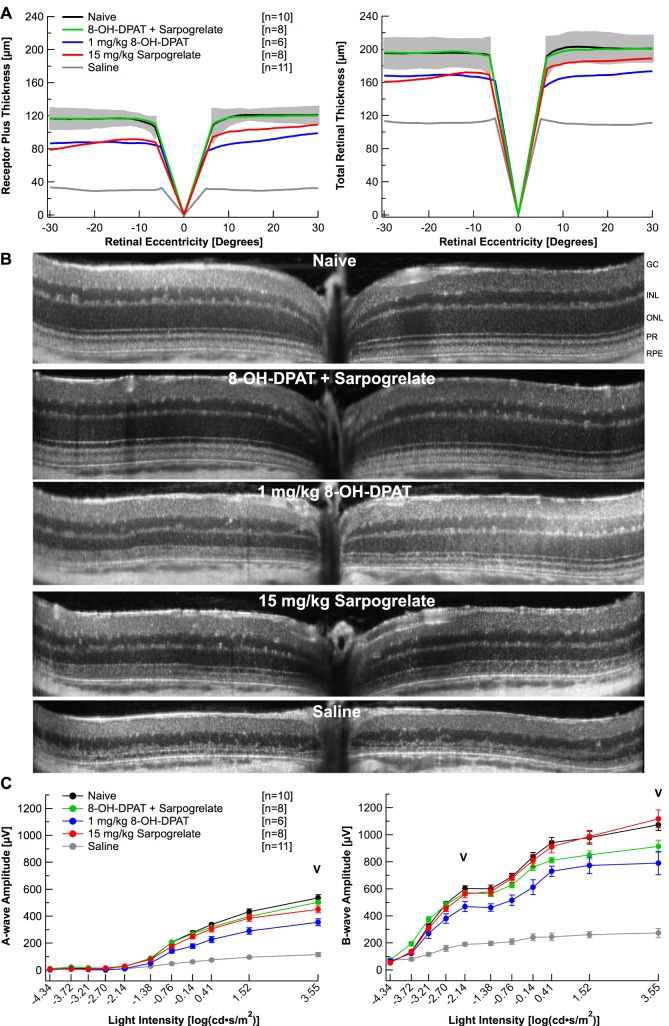
We hypothesize that the 8-OH-DPAT mediated protection is due to the activation of the Gi pathway through the 5-HT1A receptor, while the sarpogrelate-mediated protection is due to inhibition of the Gq pathway through the 5-HT2A receptor. Chen et al.19 showed that simultaneous activation of the Gi and inhibition of the Gq pathways with an α2-adrenergic receptor agonist and antagonist protect Abca4−/−Rdh8−/− mouse retinas from light-induced retinopathy in an additive manner. If our serotonin receptor agonist, 8-OH-DPAT, and antagonist, sarpogrelate, are protecting the retina by activating the Gi pathway and inhibiting the Gq pathway, then an additive effect should be observed when these drugs are administered together. Thus, BALB/c mice were administered 8-OH-DPAT (1 mg/kg) and sarpogrelate (15 mg/kg) together following the 5-day time course. Figure 5A plots average topographic distribution of REC+ and TR thickness centered at the optic nerve from naïve mice, mice injected with 8-OH-DPAT (1 mg/kg); and sarpogrelate (15 mg/kg); 8-OH-DPAT alone (1 mg/kg); sarpogrelate alone (15 mg/kg); or saline. Treatment with 1 mg/kg 8-OH-DPAT alone or 15 mg/kg sarpogrelate alone only partially protected retinal morphology, whereas administration of both 8-OH-DPAT and sarpogrelate together fully protected retinal morphology (Fig. 5A; Table 1). Average REC+ and TR thicknesses of 8-OH-DPAT + sarpogrelate-injected mice were not statistically different from the naïve group (P > 0.05), but average REC+ and TR thicknesses of mice injected with 8-OH-DPAT alone or sarpogrelate alone were significantly reduced from the naïve group (P < 0.05). The additive retinal morphology preservation achieved by the 8-OH-DPAT + sarpogrelate treatment is clearly represented with the SD-OCT scans in Figure 5B.
Figure 5.
A combination therapy of sarpogrelate and 8-OH-DPAT provide additive neuroprotection. (A) Receptor plus thicknesses and total retinal thicknesses obtained from segmented horizontal SD-OCT scans were averaged and plotted versus retinal eccentricity from the optic nerve head. The gray area indicates ±2 SD of the naïve averaged data. (B) Representative linear SD-OCT scans. (C) Electroretinogram a- and b-wave amplitudes from mice in each group were extracted from individual waveforms, averaged and plotted versus the light intensity of each flash. Averages are represented as mean ± standard error. “V” indicates at which light intensity the statistical analysis was performed. Injections followed the 5-day time course with a 1-hour light damage. Black: Naïve. Green: 8-OH-DPAT + Sarpogrelate. Blue: 1 mg/kg 8-OH-DPAT. Red: 15 mg/kg Sarpogrelate. Gray: Saline.
Discussion
This study provides the first evidence that sarpogrelate can fully protect albino BALB/c retinas, both structurally and functionally, from light-induced retinopathy. Photoreceptor degeneration that occurs within 7 days of bright light exposure was fully prevented with a 50 mg/kg dose of sarpogrelate, whether delivered for 5 days or 1 day immediately before light exposure. Partial protection was observed after a 5-day 15 mg/kg treatment of sarpogrelate and protection increased in a dose-dependent manner.
The receptor 5-HT2A has become a favorable target for retinal neuroprotection. Not only is the 5-HT2A receptor present in the mammalian retina,28 but the 5-HT2A receptor transcript is expressed at higher levels than all other 5-HT receptors in the human retina.19 Furthermore, different 5-HT2A receptor antagonists, ketanserin, ritanserin and now sarpogrelate, are capable of protecting BALB/c mice from light-induced retinopathy.20
We chose to study the selective 5-HT2A antagonist sarpogrelate hydrochloride, marketed as ANPLAG, because it has already been approved for the treatment of patients with peripheral vascular disease in Japan, China, and South Korea.21,22 Sarpogrelate has specificity to the 5-HT2 receptor and lacks significant specificity to other 5-HT receptors, α-adrenergic receptors and histamine receptors.29,30 Furthermore, sarpogrelate has higher binding and functional affinity to the 5-HT2A receptor over the 5-HT2B/2C receptors, while the majority of clinically used 5-HT2A antagonists have little selectivity for the human 5-HT2A receptor over the 5-HT2B/2C receptors.29 The selectivity of 5-HT2A is most likely due to the unique chemical structure of sarpogrelate that lacks the N-ethyl piperidine.29 Because sarpogrelate can completely protect BALB/c retinas from light-induced retinopathy, it has a relatively benign side effect profile31,32 and is more 5-HT2A selective compared to other 5-HT2A antagonists, we feel that sarpogrelate should be investigated for the treatment of outer retinal degeneration. The tolerability of sarpogrelate in humans at the doses used in our study will need to be explored prior to examining the efficacy of sarpogrelate.
The mechanism by which antagonism of the 5HT2A receptor provides retinal neuroprotection is not fully understood. We hypothesize that sarpogrelate is inhibiting the Gq pathway through the 5-HT2A receptor. Upon activation of the 5-HT2A receptor, the Gq subtype activates phospholipase C, which increases the second messengers inositol triphosphate (IP3) and diacylglycerol.20,33,34 Water-soluble IP3 diffuses through the cytoplasm into the endoplasmic reticulum, where it binds to and opens calcium channels, releasing calcium stores in the cytoplasm. This ionized calcium can activate NADPH oxidase, which is capable of generating reactive oxygen species (ROS).20,33,34 Antagonism of this pathway may decrease ROS load and promote cell survival.20 It has also been reported that simultaneous activation of the Gi pathway and inhibition of the Gq pathway can provide additive neuroprotection.19 Thus, we hypothesized that if sarpogrelate is providing neuroprotection through inhibition of the Gq pathway, then activation of the Gi pathway with the well-established 5-HT1A agonist, 8-OH-DPAT, should provide additive neuroprotection. When a low dose of sarpogrelate and a low dose of 8-OH-DPAT were administered together, additive protection of retinal morphology was observed.
Our data supports the previously published data that 5-HT2A receptor antagonists can protect the outer retina from light-induced retinopathy and that simultaneous activation of the Gi pathway and inhibition of the Gq pathway can provide additive neuroprotection.19,20 We have established that another 5-HT2A antagonist, sarpogrelate, which is more selective for the 5-HT2A receptor and has an established safety profile, should be investigated for the treatment of outer retinal degeneration. Our future studies will focus on exploring the mechanisms and pathways responsible for the observed neuroprotection and evaluating sarpogrelate's neuroprotective effects in clinically relevant models of retinal degeneration.
Supplementary Material
Acknowledgments
We thank Spencer Grant, Michael Andrews, Halkene Gemechu, and Alex Chu for their assistance with segmentation; and Michelle Sorenson for her technical assistance.
Supported by K08 Career Development Award K08 EY021186 (MEP); Alcon Young Investigator Award (MEP); Foundation Fighting Blindness Enhanced Research and Clinical Training Award CD-NMT-0914-0659-OHSU (MEP); Career Development Award from Research to Prevent Blindness (MEP); unrestricted departmental funding from Research to Prevent Blindness, NIH T32 EY023211 (RCR); and Oregon Clinical and Translational Research Institute Grant TL1 RR024159 (BET).
Disclosure: B.E. Tullis, None; R.C. Ryals, None; A.S. Coyner, None; M.J. Gale, None; A. Nicholson, None; C. Ku, None; D. Regis, None; W. Sinha, None; S. Datta, None; Y. Wen, None; P. Yang, None; M.E. Pennesi, AGTC (F)
References
- 1. Ueki Y,, Wang J,, Chollangi S,, Ash J. STAT3 activation in photoreceptors by leukemia inhibitory factor is associated with protection from light damage. J Neurochem. 2008; 105: 784–796. [DOI] [PubMed] [Google Scholar]
- 2. Zhao L,, Wang C,, Song D,, et al. Systemic administration of the antioxidant/iron chelator α-lipoic acid protects against light-induced photoreceptor degeneration in the mouse retina. Invest Ophthalmol Vis Sci. 2014; 55: 5979–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song D,, Song Y,, Hadziahmetovic M,, Zhong Y,, Dunaief JL. Systemic administration of the iron chelator deferiprone protects against light-induced photoreceptor degeneration in the mouse retina. Free Radic Biol Med. 2012; 53: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imai S,, Inokuchi Y,, Nakamura S,, Tsuruma K,, Shimazawa M,, Hara H. Systemic administration of a free radical scavenger, edaravone, protects against light-induced photoreceptor degeneration in the mouse retina. Eur J Pharmacol. 2010; 642: 77–85. [DOI] [PubMed] [Google Scholar]
- 5. Razaie T,, McKercher SR,, Kosaka K,, et al. Protective effect of carnosic acid, a pro-electrophilic compound, in models of oxidative stress and light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 7847–7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ojino K,, Shimazawa M,, Ohno Y,, Otsuka T,, Tsuruma K,, Hara H. Protective effect of SUN N8075 a free radical scavenger, against excessive light-induced retinal damage in mice. Biol Pharm Bull. 2014; 37: 424–430. [DOI] [PubMed] [Google Scholar]
- 7. Marchette LD,, Wang H,, Li F,, Babizhayev MA,, Kasus-Jacobi A. Carcinine has 4-hydroxynonenal scavenging property and neuroprotective effect in mouse retina. Invest Ophthalmol Vis Sci. 2012; 53: 3572–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang C,, Lei B,, Lam TT,, Yang F,, Sinha D,, Tso MO. Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2004; 45: 2753–2759. [DOI] [PubMed] [Google Scholar]
- 9. Imai S,, Shimazawa M,, Nakanishi T,, Tsuruma K,, Hara H. Calpain inhibitor protects cells against light-induced retinal degeneration. J Pharmacol Exp Ther. 2010; 335: 645–652. [DOI] [PubMed] [Google Scholar]
- 10. Sjoerdsma A,, Palfreyman M. History of serotonin and serotonin disorders. Ann N Y Acad Sci. 1990; 600: 1–8. [DOI] [PubMed] [Google Scholar]
- 11. Chanut E,, Nguyen-Legros J,, Labarthe B,, Trouvin JH,, Versaux-Botteri C. Serotonin synthesis and its light-dark variation in the rat retina. J Neurochem. 2002; 83: 863–869. [DOI] [PubMed] [Google Scholar]
- 12. Jin XT,, Brunken WJ. Serotonin receptors modulate rod signals: a neuropharmacological comparison of light- and dark-adapted retinas. Vis Neurosci. 1998; 15: 891–902. [DOI] [PubMed] [Google Scholar]
- 13. Brunken WJ,, Jin XT. A role for 5HT3 receptors in visual processing in the mammalian retina. Vis Neurosci. 1993; 10: 511–522. [DOI] [PubMed] [Google Scholar]
- 14. Mangel SC,, Brunken WJ. The effects of serotonin drugs on horizontal and ganglions cells in the rabbit retina. Vis Neurosci. 1992; 8: 213–218. [DOI] [PubMed] [Google Scholar]
- 15. Oh SJ,, Ha HJ,, Chi DY,, Lee HK. Serotonin receptor and transporter ligands-current status. Curr Med Chem. 2001; 8: 999–1034. [DOI] [PubMed] [Google Scholar]
- 16. Collier RJ,, Patel Y,, Martin EA,, et al. Agonists at the serotonin receptor (5-HT(1A)) protect the retina from severe photo-oxidative stress. Invest Ophthalmol Vis Sci. 2011; 52: 2118–2126. [DOI] [PubMed] [Google Scholar]
- 17. Collier RJ,, Wang Y,, Smith SS,, et al. Complement deposition and microglial activation in the outer retina in light-induced retinopathy: inhibition by a 5-HT1A agonist. Invest Ophthalmol Vis Sci. 2011; 52: 8108–8116. [DOI] [PubMed] [Google Scholar]
- 18. Thampi P,, Rao HV,, Mitter SK,, et al. The 5HT1a receptor agonist 8-Oh DPAT induces protection from lipofuscin accumulation and oxidative stress in the retinal pigment epithelium. PLoS One. 2012; 7: e34468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y,, Palczewska G,, Mustafi D,, et al. Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration. J Clin Invest. 2013; 123: 5119–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Y,, Okano K,, Maeda T,, et al. Mechanism of all-trans-retinal toxicity with implications for Stargardt disease and age-related macular degeneration. J Biol Chem. 2012; 287: 5059–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shinohara Y,, Nishimaru K,, Sawada T,, et al. Sarpogrelate-aspirin comparative clinical study for efficacy and safety in secondary prevention of cerebral infarction (S-ACCESS): a randomized, double-blind, aspirin-controlled trial. Stroke. 2008; 39: 1827–1833. [DOI] [PubMed] [Google Scholar]
- 22. Uchiyama S,, Ozaki Y,, Satoh K,, Kondo K,, Nishimaru K. Effect of sarpogrelate a 5-HT(2A) antagonist, on platelet aggregation in patients with ischemic stroke: clinical-pharmacological dose-response study. Cerebrovasc Dis. 2007; 24: 264–270. [DOI] [PubMed] [Google Scholar]
- 23. Inoue-Matsuhisa E,, Sogo S,, Mizota A,, Taniai M,, Takenaka H,, Mano T. Effect of MCI-9042 a 5-HT2 receptor antagonist, on retinal ganglion cell death and retinal ischemia. Exp Eye Res. 2003; 76: 445–452. [DOI] [PubMed] [Google Scholar]
- 24. Pennesi ME,, Michaels KV,, Magee SS,, et al. Long-term characterization of retinal degeneration in rd1 and rd10 mice using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 4644–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hood DC,, Lin CE,, Lazow MA,, Locke KG,, Zhang X,, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009; 50: 2328–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lyubarsky AL,, Pugh EN. Recovery phase of the murine rod photoreceptor response reconstructed from electroretinographic recordings. J Neurosci. 1996; 16: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pennesi ME,, Cho JH,, Yang Z,, et al. BETA2/NeuroD1 null mice: a new model for transcription factor-dependent photoreceptor degeneration. J Neurosci. 2003; 23: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pootanakit K,, Prior KJ,, Hunter DD,, Brunken WJ. 5-HT2a receptors in the rabbit retina: potential presynaptic modulators. Vis Neurosci. 1999; 16: 221–230. [DOI] [PubMed] [Google Scholar]
- 29. Muntasir HA,, Hossain M,, Bhuiyan MA,, et al. Selectivity of sarpogrelate to the recombinant human 5-HT2A receptors as assessed by molecular pharmacology and molecular modeling. Pharmacometrics. 2007; 72: 39–49. [Google Scholar]
- 30. Israilova M,, Suzuki F,, Tanaka T,, Nagatomo T,, Taniguchi T,, Muramatsu I. Binding and functional affinity of sarpogrelate its metabolite m-1 and ketanserin for human recombinant alpha-1-adrenoceptor subtypes. Pharmacology. 2002; 65: 69–73. [DOI] [PubMed] [Google Scholar]
- 31. Anplag [package insert]. Osaka, Japan: Mitsubishi Tanabe Pharma Corporation; 2012. Available at: http://www.shijiebiaopin.com/upload/product/201472920201816.pdf. Accessed January 30, 2013. [Google Scholar]
- 32. Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. Pharmaceuticals and medical devices safety information: revision of precautions (No. 164). Tokyo: Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour & Welfare; 2001. [Google Scholar]
- 33. Organisciak DT,, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010; 29: 113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Du Y,, Cramer M,, Lee CA,, et al. Adrenergic and serotonin receptors affect retinal superoxide generation in diabetic mice: relationship to capillary degeneration and permeability. FASEB J. 2015; 29: 2194–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.