Abstract
OBJECTIVE
To evaluate trends of monozygotic twinning after single embryo transfer and its association with patient and treatment factors.
METHODS
Our retrospective cohort study included 28,596 pregnancies after fresh, nondonor single embryo transfer during 2003–2012 reported to the National ART Surveillance System. We examined trends of monozygotic twin pregnancies (number of fetal heart tones on first-trimester ultrasonography more than one or number of neonates born more than one) and assessed patient and treatment factors for monozygotic twin compared with singleton pregnancies. Modified Poisson regression models were used to estimate adjusted risk ratios (RRs) and 95% confidence intervals (CIs) for association between monozygotic twinning and selected factors stratified by day 2–3 and day 5–6 transfer.
RESULTS
During 2003–2012, the incidence of monozygotic twinning after single embryo transfer was lower for day 2–3 transfers than for day 5–6 transfers (1.71%, 95% CI 1.45–1.98, n=162 compared with 2.50%, 95% CI 2.28–2.73, n=472); the incidence did not change significantly over the study period. Among day 2–3 transfers, assisted hatching increased the risk for monozygotic twinning compared with singletons (adjusted RR 2.16, 95% CI 1.53–3.06); use of intracytoplasmic sperm injection decreased the risk (adjusted RR 0.60, 95% CI 0.42–0.85). Having one or more prior pregnancies increased the risk for monozygotic twinning among day 5–6 transfers (adjusted RR 1.26, 95% CI 1.03–1.53).
CONCLUSION
Monozygotic twinning after single embryo transfers was more common among day 5–6 embryo transfers than day 2–3 transfers. Use of assisted hatching was associated with increased risk for monozygotic twinning for day 2–3 transfers.
Multiple gestation pregnancies are associated with higher risk of maternal and fetal morbidity and mortality compared with singleton gestation,1 and population twinning incidence has increased 76% over the past three decades.2,3 Although twin pregnancies after in vitro fertilization (IVF) are primarily dizygotic, there is also an increased incidence of monozygotic twins from two to 12 times the population incidence of 0.4%.4–6 As compared with dizygotic twins, monozygotic twins have a higher risk of complications and poor outcomes.4
As a result of low monozygotic twinning incidence, studies evaluating proposed risk factors are frequently underpowered or contradictory.5,7–13 The Centers for Disease Control and Prevention’s National ART Surveillance System, a nationally mandated surveillance system containing information about United States assisted reproductive technology (ART) cycles, is large enough to examine these factors. Although previous population-based studies assessed the risks of monozygotic twinning,13–15 the prevalence of blastocyst transfer, a risk factor for monozygotic twinning,13,15 has increased over time, particularly among single embryo transfers.16 Furthermore, findings from earlier studies may be subject to misclassification bias as a result of the indirect calculation of zygosity, particularly when more than one embryo was transferred.14,15
In the current study, we aim to quantify trends in monozygotic twinning after single embryo transfer in the United States from 2003 to 2012 and to identify risk factors for monozygotic twinning among women undergoing IVF stratified by day of transfer.
MATERIALS AND METHODS
The Fertility Clinic Success Rates and Certification Act of 1992 requires that every ART program annually report data about all ART procedures to the Centers for Disease Control and Prevention.17 These data are transmitted to the Centers for Disease Control and Prevention’s National ART Surveillance System and are used to produce an annual report containing clinic-specific and national pregnancy success rates. In the National ART Surveillance System, an ART cycle is defined as fertility treatments in which eggs and sperm or embryos are handled for the purpose of establishing a pregnancy. These data contain one record per cycle; multiple cycles from an individual patient are not linked. The Centers for Disease Control and Prevention estimates that the National ART Surveillance System includes more than 95% of all ART cycles performed in the United States.18
We selected data from reporting years 2003–2012 for this retrospective cohort study. Data were restricted to fresh, nondonor IVF cycles in which one embryo was transferred and resulted in clinical intrauterine pregnancy. Cycles with missing information on the number of embryos transferred to the uterus, number of fetal heart tones at first-trimester ultrasonogram, or maternal age were excluded (n=211). Because we determined a priori that day of embryo transfer was likely to be an important effect-measure modifier, cycles with embryo transfers occurring on days other than 2–3 (cleavage stage) or 5–6 (blastocyst stage) were also excluded (2.3%) because they could not be accurately categorized as either cleavage or blastocyst stage.
Pregnancies containing a monozygotic pair were defined as those in which more than one fetal heart tone was reported at the first-trimester ultrasonogram or those in which the number of liveborn or stillborn neonates was more than one. The comparison group was composed of singleton pregnancies. The number of gestational sacs is not reported for clinical pregnancies; therefore, we were unable to account for twin clinical pregnancies (having two gestational sacs) where only one heartbeat was reported at first-trimester ultrasonography or only one neonate was liveborn or stillborn.
We calculated the incidence of monozygotic twinning for 2-year intervals among all pregnancies reported during 2003–2012 that resulted from fresh, nondonor single embryo transfers; we also assessed trends stratified by day of transfer (day 2–3 or 5–6) using the Cochrane-Armitage test. Data were grouped into 2-year intervals as a result of small numerators (less than 20) when individual years were considered, particularly for day 2–3 transfers. We also examined linear trends in the absolute number of day 2–3 and day 5–6 single embryo transfers and the absolute number of monozygotic twin pregnancies over the study period using ordinary least squares regression. We used two-tailed χ2 tests to compare the distribution of patient and cycle characteristics among the monozygotic twin pregnancy group and the singleton pregnancy group. We further stratified by day 2–3 and day 5–6 transfers and used modified Poisson regression models to estimate adjusted risk ratios (RRs) for the association between monozygotic twinning and selected patient and cycle characteristics. These models were adjusted for factors that were determined a priori to be potential confounders: maternal age (younger than 30, 30–34, 35–39, 40 years or older), infertility diagnosis (tubal factor, endometriosis, uterine factor, ovulatory disorder, diminished ovarian reserve, male factor), number of prior pregnancies (zero, one or more), number of prior ART cycles (zero, one or more), number of oocytes retrieved (one to four, five or more), use of intracytoplasmic sperm injection, use of assisted hatching (the purposeful disruption of an embryo’s zona pellucida by laser, mechanical, or chemical means), and having one or more supernumerary embryo(s) available for cryopreservation. Maternal race or ethnicity was not included in the final models because more than 38% of values were missing; findings from a sensitivity analysis indicated that the magnitude and direction of results did not change significantly with and without their inclusion in the model. Numbers of prior spontaneous abortions and prior live births were not included in the adjusted models because of colinearity with number of prior pregnancies. Because both younger age and assisted hatching are known to increase the risk for monozygotic twinning, we included an interaction term for age and assisted hatching in the models; however, the interaction was not statistically significant and was not retained in the final models. We also evaluated the association between monozygotic twinning and the outcomes of interest using the more restrictive definition of monozygotic twinning based on liveborn and stillborn neonates only. The findings were not substantively different from those using the definition including fetal hearts and variations were largely the result of diminished sample size; thus, the more inclusive definition was used. P<.05 were considered statistically significant.
This research was approved by the institutional review board at the Centers for Disease Control and Prevention.
RESULTS
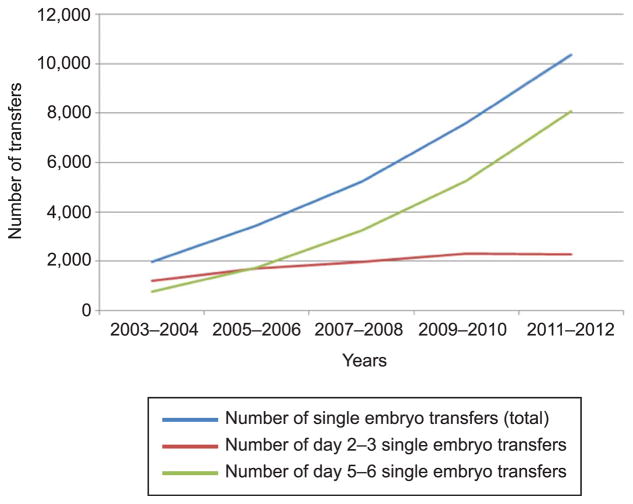
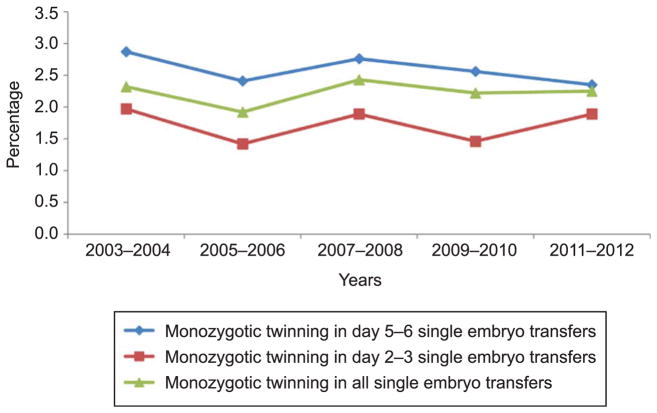
We identified 28,596 pregnancies after a single embryo transfer during January 2003 to December 2012. Of those, 641 pregnancies contained a monozygotic pair and 27,955 were singleton pregnancies. When the definition of monozygotic pregnancy was restricted to the presence of more than one liveborn or stillborn neonate irrespective of the number of fetal hearts, the total number of monozygotic pregnancies was 442. The absolute number of fresh, nondonor single embryo transfers increased over the study period for day 2–3 and day 5–6 transfers (Fig. 1). The overall incidence of monozygotic twinning after single embryo transfer during 2003–2012 was 2.24% (95% confidence interval [CI] 2.07–2.41); the incidence did not change over the study period (Fig. 2; P=.80). The absolute number of monozygotic twin pregnancies was 46, 66, 127, 169, and 233 for 2003–2004, 2005–2006, 2007–2008, 2009–2010, and 2011–2012, respectively, and increased linearly over the study period (P=.001). When stratified by day of transfer, the overall incidence of monozygotic twinning was lower in day 2–3 transfers than in day 5–6 transfers (1.71%, 95% CI 1.45–1.97, n=162 compared with 2.50%, 95% CI 2.28–2.73, n=472, χ2 P<.001). The incidence of monozygotic twinning among day 2–3 and day 5–6 transfers was also unchanged over the study period (Cochran-Armitage P=.95 and P=.29, respectively).
Fig. 1.
Absolute number of fresh, nondonor, single embryo transfer cycles by day of transfer, United States, 2003–2012. P<.05 for all linear trends.
Fig. 2.
Trends in monozygotic twinning for fresh, non-donor, single embryo transfer cycles by day of transfer, United States, 2003–2012. P=.80 for Cochrane-Armitage trend test.
Women with monozygotic twin pregnancies had a higher frequency of ovulatory disorders and lower frequencies of diminished ovarian reserve and prior pregnancies than women with singleton pregnancies (Table 1). The monozygotic twin pregnancy group also had a higher proportion of prior pregnancies and day 5–6 transfers and used intracytoplasmic sperm injection less frequently than the singleton pregnancy group. For all variables, the amount of missing data was less than 0.50%.
Table 1.
Percentage Distribution of Patient and Cycle Characteristics in Monozygotic Twin Pregnancies and Singleton Pregnancies After Single Embryo Transfer, United States, 2003–2012
| Characteristic | Monozygotic Twin Pregnancies* (n=641) | Singleton Pregnancies† (n=27,955) | P |
|---|---|---|---|
| Maternal age (y) | .37 | ||
| Younger than 30 | 131 (20.4) | 5,258 (18.9) | |
| 30–34 | 288 (44.9) | 12,159 (43.5) | |
| 35–39 | 172 (26.8) | 8,352 (29.9) | |
| 40 or older | 50 (7.8) | 2,186 (7.8) | |
| Race or ethnicity | .61 | ||
| Non-Hispanic white | 284 (44.3) | 13,214 (47.3) | |
| Non-Hispanic black | 22 (3.4) | 951 (3.4) | |
| Asian or Pacific Islander | 55 (8.6) | 2,106 (7.5) | |
| Hispanic | 29 (4.5) | 1,186 (4.2) | |
| Other race | 0 | 39 (0.1) | |
| Unknown or missing | 251 (39.2) | 10,459 (37.4) | |
| Infertility diagnosis | |||
| Tubal factor | 75 (11.7) | 4,013 (14.4) | .06 |
| Endometriosis | 62 (9.7) | 2,752 (9.8) | .89 |
| Uterine factor | 21 (3.3) | 1,315 (4.7) | .09 |
| Ovulatory disorder (polycystic ovary syndrome) | 145 (22.6) | 5,212 (18.6) | .01 |
| Diminished ovarian reserve | 68 (10.6) | 3,745 (13.4) | .04 |
| Male factor | 219 (34.2) | 10,341 (37.0) | .14 |
| Unexplained | 100 (15.6) | 4,185 (15.0) | .66 |
| No. of prior pregnancies | .02 | ||
| 0 | 272 (42.5) | 13,181 (47.3) | |
| 1 or more | 368 (57.5) | 14,707 (52.7) | |
| No. of prior spontaneous abortions | .04 | ||
| 0 | 451 (70.4) | 20,678 (74.0) | |
| 1 or more | 190 (29.6) | 7,277 (26.0) | |
| No. of prior live births | 1.00 | ||
| 0 | 429 (67.0) | 18,674 (67.0) | |
| 1 or more | 211 (33.0) | 9,184 (33.0) | |
| No. of prior ART cycles | .99 | ||
| 0 | 441 (68.8) | 19,239 (68.8) | |
| 1 or more | 200 (31.2) | 8,715 (31.2) | |
| Day of transfer | <.001 | ||
| 2–3 | 162 (25.3) | 9,309 (33.3) | |
| 5–6 | 479 (74.7) | 18,646 (66.7) | |
| No. of oocytes retrieved | .07 | ||
| 1–4 | 91 (14.2) | 4,734 (16.9) | |
| 5 or more | 550 (85.8) | 23,221 (83.1) | |
| Use of ICSI | .02 | ||
| Did not use ICSI | 222 (34.8) | 8,540 (30.6) | |
| Used ICSI | 416 (65.2) | 19,366 (69.4) | |
| Use of assisted hatching | .46 | ||
| Did not use assisted hatching | 465 (72.5) | 20,639 (73.8) | |
| Used assisted hatching | 176 (27.5) | 7,316 (26.2) | |
| Supernumerary embryos available and cryopreserved | .21 | ||
| None | 271 (42.4) | 12,493 (44.9) | |
| 1 or more cryopreserved | 368 (57.6) | 15,342 (55.1) | |
ART, assisted reproductive technology; ICSI, intracytoplasmic sperm injection.
Data are n (%) unless otherwise specified.
Missing data less than 0.50% for all variables.
One embryo transferred and more than one fetal heart tone reported or more than one liveborn or stillborn neonate reported.
One embryo transferred and one fetal heart tone reported or one liveborn or stillborn neonate reported.
Among day 2–3 transfers, maternal age younger than 30 years (adjusted RR 1.68, 95% CI 1.05–2.71) and assisted hatching (adjusted RR 2.16, 95% CI 1.53–3.06) were associated with an increased risk of monozygotic twin pregnancy when compared with singleton pregnancies; use of intracytoplasmic sperm injection (adjusted RR 0.60, 95% CI 0.42–0.85) was associated with decreased risk (Table 2).
Table 2.
Relative Risk of Monozygotic Twinning by Cycle Characteristics for Day 2–3 Single-Embryo Transfers, 2003–2012
| Characteristic | Comparison Group: Singleton Pregnancies
|
||
|---|---|---|---|
| Monozygotic Twinning (%) | RR (95% CI) | Adjusted RR* (95% CI) | |
| Maternal age (y) | |||
| Younger than 30 | 2.2 | 1.64 (1.02–2.63) | 1.68 (1.05–2.71) |
| 30–34 | 1.3 | Reference | Reference |
| 35–39 | 2.0 | 1.46 (1.03–2.17) | 1.33 (0.90–1.97) |
| 40 or older | 1.6 | 1.18 (0.70–1.99) | 0.99 (0.56–1.76) |
| Infertility diagnosis | |||
| Tubal factor | 1.4 | 0.77 (0.48–1.22) | 0.68 (0.42–1.09) |
| Endometriosis | 1.9 | 1.13 (0.72–1.78) | 1.05 (0.66–1.67) |
| Uterine factor | 2.0 | 1.18 (0.60–2.29) | 1.14 (0.59–2.21) |
| Ovulatory disorder | 1.9 | 1.13 (0.74–1.74) | 1.05 (0.67–1.65) |
| Diminished ovarian reserve | 1.6 | 0.89 (0.62–1.29) | 0.77 (0.51–1.16) |
| Male factor | 1.5 | 0.83 (0.60–1.15) | 0.98 (0.68–1.42) |
| No. of prior pregnancies | |||
| 0 | 1.7 | Reference | Reference |
| 1 or more | 1.8 | 1.07 (0.79–1.46) | 1.15 (0.83–1.59) |
| No. of prior ART cycles | |||
| 0 | 1.8 | Reference | Reference |
| 1 or more | 1.5 | 0.84 (0.61–1.15) | 0.81 (0.58–1.13) |
| No. of oocytes retrieved | |||
| 1–4 | 1.9 | Reference | Reference |
| 5 or more | 1.6 | 0.85 (0.63–1.15) | 0.80 (0.56–1.13) |
| Use of ICSI | |||
| Did not use ICSI | 2.2 | Reference | Reference |
| Used ICSI | 1.5 | 0.69 (0.51–0.94) | 0.60 (0.42–0.85) |
| Use of assisted hatching | |||
| Did not use assisted hatching | 1.2 | Reference | Reference |
| Used assisted hatching | 2.3 | 1.81 (1.33–2.48) | 2.16 (1.53–3.06) |
| Supernumerary embryos available and cryopreserved | |||
| None | 1.7 | Reference | Reference |
| 1 or more cryopreserved | 1.7 | 1.00 (0.69–1.45) | 1.19 (0.78–1.82) |
RR, relative risk; CI, confidence interval; ART, assisted reproductive technology; ICSI, intracytoplasmic sperm injection.
Adjusted for all characteristics in the table.
Among day 5–6 transfers, maternal age 35–39 years (adjusted RR 0.73, 95% CI 0.58–0.93) and uterine factor infertility (adjusted RR 0.50, 95% CI 0.28–0.90) were associated with a decreased risk for monozygotic pregnancy compared with singleton pregnancies (Table 3).
Table 3.
Relative Risk of Monozygotic Twinning by Cycle Characteristics for Day 5–6 Single Embryo Transfers, 2003–2012
| Characteristic | Comparison Group: Singleton Pregnancies
|
||
|---|---|---|---|
| Monozygotic Twinning (%) | RR (95% CI) | Adjusted RR* (95% CI) | |
| Maternal age (y) | |||
| Younger than 30 | 2.5 | 0.93 (0.74–1.17) | 0.97 (0.77–1.23) |
| 30–34 | 2.7 | Reference | Reference |
| 35–39 | 2.1 | 0.76 (0.61–0.96) | 0.73 (0.58–0.93) |
| 40 or older | 3.1 | 1.17 (0.80–1.69) | 1.17 (0.73–1.79) |
| Infertility diagnosis | |||
| Tubal factor | 2.1 | 0.82 (0.62–1.08) | 0.81 (0.61–1.08) |
| Endometriosis | 2.4 | 0.95 (0.70–1.31) | 1.00 (0.72–1.37) |
| Uterine factor | 1.4 | 0.53 (0.30–0.94) | 0.50 (0.28–0.90) |
| Ovulatory disorder | 3.0 | 1.13 (0.74–1.74) | 1.17 (0.95–1.45) |
| Diminished ovarian reserve | 2.1 | 0.83 (0.58–1.19) | 0.78 (0.53–1.16) |
| Male factor | 2.4 | 0.91 (0.76–1.10) | 0.97 (0.79–1.20) |
| No. of prior pregnancies | |||
| 0 | 2.2 | Reference | Reference |
| 1 or more | 2.8 | 1.25 (1.04–1.49) | 1.26 (1.03–1.53) |
| No. of prior ART cycles | |||
| 0 | 2.4 | Reference | Reference |
| 1 or more | 2.8 | 1.16 (0.95–1.40) | 1.12 (0.91–1.38) |
| No. of oocytes retrieved | |||
| 1–4 | 2.0 | Reference | Reference |
| 5 or more | 2.5 | 1.30 (0.79–2.13) | 1.25 (0.74–2.10) |
| Use of ICSI | |||
| Did not use ICSI | 2.7 | Reference | Reference |
| Used ICSI | 2.4 | 0.87 (0.72–1.05) | 0.88 (0.71–1.09) |
| Use of assisted hatching | |||
| Did not use assisted hatching | 2.5 | Reference | Reference |
| Used assisted hatching | 2.5 | 0.99 (0.78–1.26) | 1.03 (0.80–1.33) |
| Supernumerary embryos available and cryopreserved | |||
| None | 2.7 | Reference | Reference |
| 1 or more cryopreserved | 2.4 | 0.91 (0.75–1.10) | 0.91 (0.73–1.12) |
RR, relative risk; CI, confidence interval; ART, assisted reproductive technology; ICSI, intracytoplasmic sperm injection.
Adjusted for all characteristics in the table.
DISCUSSION
Monozygotic twinning incidence after single embryo transfer has not changed over the past decade and was more common among day 5–6 than day 2–3 embryo transfers. Increases in the number of day 5–6 single embryo transfers over time may be the result of advances in extended media culture,19 improvements in live birth rates after blastocyst transfer,20 and reductions in aneuploidy compared with cleavage-stage embryo transfers.21
Assisted hatching was associated with a twofold increased risk of monozygotic pregnancy among day 2–3 transfers compared with singleton pregnancy; however, no association was found among day 5–6 transfers. This finding is consistent with a recent study reporting an interaction between assisted hatching and day of transfer in models predicting the likelihood of monozygotic twinning.13 Because the incidence of monozygotic twinning among day 5–6 transfers is higher than among day 2–3 transfers, we may have been unable to detect any additional increase in risk associated with assisted hatching in cycles using day 5–6 embryos. Although previous reports of the association between assisted hatching and monozygotic twinning have been contradictory,7,10,14,22 recent studies9,13 indicated that zona pellucida manipulation during assisted hatching confers increased risk, particularly for cleavage-stage embryos, although the mechanism is unknown. Although there is some evidence that assisted hatching marginally improves clinical pregnancy rates, corresponding increases in live birth rates have not been documented.23 Indeed, a recent study found that pregnancy outcomes are not improved after the use of assisted hatching, even among patients with poor prognoses.24
We found that use of intracytoplasmic sperm injection was associated with decreased risk for monozygotic twinning in day 2–3 transfers compared with conventional IVF. Findings from one study are consistent with our results,13 whereas other studies found either no association7,9 or an increased risk for monozygotic twinning with intracytoplasmic sperm injection use compared with conventional IVF.10 A potential explanation for our finding is that use of intracytoplasmic sperm injection is a surrogate measure of the underlying indication for the procedure, and thus performance of procedure itself does not necessarily reduce the risk for monozygotic twinning. For example, if intracytoplasmic sperm injection were performed for poor sperm quality or prior failed fertilization, the resultant embryos could be of lesser quality and have lower implantation potential than embryos resulting from fertilization that did not require the procedure. Our findings also indicated that the presence of uterine factor infertility reduced the risk for monozygotic twinning in day 5–6 embryos perhaps reflecting a lower implantation rate among women with uterine factor.
The primary strength of our study is the use of a large national surveillance database with sufficient sample size to study monozygotic twinning incidence among various subgroups. Our study also had some limitations. National ART Surveillance System data do not include information on embryo quality, and we were unable to control for differences in embryo quality between the study and comparison groups. However, we controlled for cryopreservation of extra embryos, which has been shown to be a good predictor of embryo quality.25 Like with any observational study, participants were not randomized, and we were only able to adjust for patient and cycle characteristics that were available in the National ART Surveillance System. Finally, our findings are only generalizable to patients undergoing IVF in the United States.
Although we found that the rate of monozygotic twinning after single embryo transfer was unchanged over the study period, future increases in the use of blastocyst transfer may lead to elevated incidence of monozygotic twinning. Given recent increases in the use of elective single embryo transfer and the high proportion of blastocyst-stage embryos used during these transfers, rates of monozygotic twinning after single embryo transfer may increase in the future.26 Although the American Society for Reproductive Medicine recommends transfer of fewer embryos for blastocyst-compared with cleavage-stage embryos, our findings suggest that it may be important to consider the increased risk of monozygotic twinning associated with assisted hatching when deciding how many cleavage-stage embryos to transfer.27
The findings of this study reinforce the importance of determining types of placentation as early as possible for all twins, even those conceived with in vitro techniques. Monochorionic twins (twins that share the same placenta) carry increased risks for poor pregnancy outcomes, including twin–twin transfusion syndrome, twin anemia–polycythemia syndrome, and fetal anomalies, compared with dichorionic pregnancies. As such, high-risk care for monochorionic twins is important to diagnose and manage these problems should they occur.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Presented as an oral presentation at the Annual Clinical and Scientific Meeting of the American College of Obstetricians and Gynecologists, April 26–30, 2014, Chicago, Illinois.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Land JA, Evers JLH. Risks and complications in assisted reproduction techniques: Report of an ESHRE consensus meeting. Hum Reprod. 2003;18:455–7. doi: 10.1093/humrep/deg081. [DOI] [PubMed] [Google Scholar]
- 2.Martin J, Hamilton B, Osterman M. Three decades of twin births in the United States, 1980–2009. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 3.Kulkarni AD, Jamieson DJ, Jones HW, Jr, Kissin DM, Gallo MF, Macaluso M, et al. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369:2218–25. doi: 10.1056/NEJMoa1301467. [DOI] [PubMed] [Google Scholar]
- 4.Rao A, Sairam S, Shehata H. Obstetric complications of twin pregnancies. Best Pract Res Clin Obstet Gynaecol. 2004;18:557–76. doi: 10.1016/j.bpobgyn.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Aston KI, Peterson CM, Carrell DT. Monozygotic twinning associated with assisted reproductive technologies: a review. Reproduction. 2008;136:377–86. doi: 10.1530/REP-08-0206. [DOI] [PubMed] [Google Scholar]
- 6.Vitthala S, Gelbaya TA, Brison DR, Fitzgerald CT, Nardo LG. The risk of monozygotic twins after assisted reproductive technology: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:45–55. doi: 10.1093/humupd/dmn045. [DOI] [PubMed] [Google Scholar]
- 7.Abusheika N, Salha O, Sharma V, Brinsden P. Monozygotic twinning and IVF/ICSI treatment: a report of 11 cases and review of literature. Hum Reprod Update. 2000;6:396–403. doi: 10.1093/humupd/6.4.396. [DOI] [PubMed] [Google Scholar]
- 8.Kawachiya S, Bodri D, Shimada N, Kato K, Takehara Y, Kato O. Blastocyst culture is associated with an elevated incidence of monozygotic twinning after single embryo transfer. Fertil Steril. 2011;95:2140–2. doi: 10.1016/j.fertnstert.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Knopman J, Krey LC, Lee J, Fino ME, Novetsky AP, Noyes N. Monozygotic twinning: an eight-year experience at a large IVF center. Fertil Steril. 2010;94:502–10. doi: 10.1016/j.fertnstert.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 10.Skiadas CC, Missmer SA, Benson CB, Gee RE, Racowsky C. Risk factors associated with pregnancies containing a monochorionic pair following assisted reproductive technologies. Hum Reprod. 2008;23:1366–71. doi: 10.1093/humrep/den045. [DOI] [PubMed] [Google Scholar]
- 11.Tarlatzis BC, Qublan HS, Sanopoulou T, Zepiridis L, Grimbizis G, Bontis J. Increase in the monozygotic twinning rate after intracytoplasmic sperm injection and blastocyst stage embryo transfer. Fertil Steril. 2002;77:196–8. doi: 10.1016/s0015-0282(01)02958-2. [DOI] [PubMed] [Google Scholar]
- 12.Toledo MG. Is there increased monozygotic twinning after assisted reproductive technology? Aust N Z J Obstet Gynecol. 2005;45:360–4. doi: 10.1111/j.1479-828X.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 13.Luke B, Brown MB, Wantman E, Stern JE. Factors associated with monozygosity in assisted reproductive technology pregnancies and the risk of recurrence using linked cycles. Fertil Steril. 2014;101:683–9. doi: 10.1016/j.fertnstert.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schieve LA, Meikle SF, Peterson HB, Jeng G, Burnett NM, Wilcox LS. Does assisted hatching pose a risk for monozygotic twinning in pregnancies conceived through in vitro fertilization. Fertil Steril. 2000;74:288–94. doi: 10.1016/s0015-0282(00)00602-6. [DOI] [PubMed] [Google Scholar]
- 15.Wright V, Schieve LA, Vahratian A, Reynolds MA. Monozygotic twinning associated with day 5 embryo transfer in pregnancies conceived after IVF. Hum Reprod. 2004;19:1831–6. doi: 10.1093/humrep/deh338. [DOI] [PubMed] [Google Scholar]
- 16.Marsh CA, Farr SL, Chang J, Kissin DM, Grainger DA, Posner SF, et al. Trends and factors associated with the Day 5 embryo transfer, assisted reproductive technology surveillance, USA, 2001–2009. Hum Reprod. 2012;27:2325–31. doi: 10.1093/humrep/des168. [DOI] [PubMed] [Google Scholar]
- 17.Fertility Clinic Success Rate and Certification Act of 1992 (FCSRCA). Pub. L. 102–493; October 24, 1992.
- 18.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for assisted reproductive Technologies. 2011 assisted reproductive technology success rates: fertility clinic success rates report. Atlanta (GA): U.S. Department of Health and Human Services; 2013. [Google Scholar]
- 19.Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocyst increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–8. doi: 10.1016/s0015-0282(97)00438-x. [DOI] [PubMed] [Google Scholar]
- 20.Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. The Cochrane Database of Systematic Reviews. 2012;(7):Art. No.: CD002118. doi: 10.1002/14651858.CD002118.pub4. [DOI] [PubMed] [Google Scholar]
- 21.Adler A, Lee H-L, McCulloh DH, Ampeloquio E, Clarke-Williams M, Wertz BH, et al. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reprod Biomed Online. 2014;28:485–91. doi: 10.1016/j.rbmo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Sills ES, Moomjy M, Zaninovic N, Veeck LL, McGee M, Palermo GD, et al. Human zona pellucida micromanipulation and monozygotic twinning frequency after IVF. Hum Reprod. 2000;15:890–5. doi: 10.1093/humrep/15.4.890. [DOI] [PubMed] [Google Scholar]
- 23.Carney SK, Das S, Blake D, Farquhar C, Seif MM, Nelson L. Assisted hatching on conception (in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) The Cochrane Database of Systematic Reviews. 2012;(12):Art. No.: CD001894. doi: 10.1002/14651858.CD001894.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kissin DM, Kawwass JF, Monsour M, Boulet SL, Session DR, Jamieson DJ National ART Surveillance System (NASS) Group. Assisted hatching: trends and pregnancy outcomes, United States, 2000–2010. Fertil Steril. 2014;102:795–801. doi: 10.1016/j.fertnstert.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern JE, Lieberman ES, Macaluso M, Racowsky C. Is cryopreservation of embryos a legitimate surrogate marker of embryo quality in studies of assisted reproductive technology conducted using national databases? Fertil Steril. 2012;97:890–3. doi: 10.1016/j.fertnstert.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg ML, Boulet S, Kissin D, Warner L, Jamieson DJ. Elective single embryo transfer trends and predictors of a good perinatal outcome—United States, 1999–2010. Fertil Steril. 2013;99:1937–43. doi: 10.1016/j.fertnstert.2013.01.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Practice Committee of American Society for Reproductive Medicine, Practice Committee of Society for Assisted Reproductive Technology. . Criteria for number of embryos to transfer: a committee opinion. Fertil Steril. 2013;99:44–6. doi: 10.1016/j.fertnstert.2012.09.038. [DOI] [PubMed] [Google Scholar]