Abstract
Background
Red blood cell (RBC) folate concentrations are a potential biomarker of folate-sensitive neural tube defect (NTD) risk in the population. The purpose of this analysis was to describe women in the U.S. population with RBC folate concentrations below those associated with optimal NTD prevention.
Methods
We used data from the 2007 to 2012 National Health and Nutrition Examination Survey (NHANES) to assess the RBC folate status of U.S. women of childbearing age relative to risk categories for NTD risk based on RBC folate concentrations. We defined suboptimal RBC folate concentrations as those associated with a prevalence of _9 NTDs per 10,000 live births.
Results
Among nonpregnant women age 12 to 49 years, 22.8% (95% Confidence Interval: 21.1, 24.6) had suboptimal RBC folate concentrations. Women had greater odds of having a suboptimal RBC folate concentration if they did not use dietary supplements containing folic acid; had mandatorily fortified enriched cereal grain products as their only source of folic acid; were non-Hispanic black or Hispanic; or were current smokers.
Conclusion
Based on RBC folate concentrations, we would predict that the majority of U.S. women of reproductive age are not at increased risk for folate sensitive NTDs in the presence of mandatory folic acid fortification. Prevention policies and programs can be aimed at population subgroups identified as having higher predicted risk for folate-sensitive NTDs based on RBC folate concentrations.
Keywords: neural tube defects, optimal RBC folate concentration, folic acid, fortification, NHANES
Introduction
Periconceptional folic acid intake has been shown to prevent neural tube defects (NTDs), including spina bifida, anencephaly and encephalocele, in multiple settings, including randomized controlled trials, community prevention programs and through the evaluation of the impact of mandatory fortification of staple grains (MRC Vitamin Study Research Group, 1991; Czeizel and Dudas, 1992; Berry et al., 1999; Williams et al., 2005; De Wals et al., 2007; Sayed et al., 2008). In the United States, folic acid intake comes from three sources: enriched cereal grain products (ECGP); ready-to-eat (RTE) cereals, and folic acid-containing dietary supplements. ECGP are grain products that are labeled “enriched” and are required to be fortified with 140 mg of folic acid per 100 g (U.S. Food and Drug Administration, 1996b). It has been estimated that mandatory fortification of ECGP increased the average daily usual intake of folic acid by _138 mg/day among U.S. adults (Yang et al., 2010). RTE cereal is permitted but not required to contain up to 400 mg of folic acid per serving (U.S. Food and Drug Administration, 1996a). In the United States, standard multivitamins generally contain 400 mg to 800 mg of folic acid, but doses up to 1000 mg are allowed without a prescription (Hendler and Rorvik, 2001). Recently, red blood cell (RBC) folate concentrations have been shown to be a generalizable biomarker of folate-sensitive NTD risk in populations. Studies in Ireland and China have shown that the risk of NTD-affected pregnancy increases substantially as RBC folate concentrations decrease (Daly et al., 1995; Crider et al., 2014). The purpose of our analysis was to describe the population of U.S. women of childbearing age with RBC folate concentrations below those associated with optimal NTD prevention (WHO, 2015).
Materials and Methods
NATIONAL HEALTH AND NUTRITION EXAMINATION SURVEY (NHANES), 2007 TO 2012
NHANES data are collected in 2-year phases using a stratified multistage probability design to capture a nationally representative sample of the noninstitutionalized civilian U.S. population. We used data from the 2007 to 2008, 2009 to 2010, and 2011 to 2012 phases for this analysis. NHANES methods are described in detail elsewhere (National Center for Health Statistics; National Center for Health Statistics; National Center for Health Statistics); briefly, NHANES includes a questionnaire administered in person at the home and a physical examination at a Mobile Examination Center (MEC). Our analysis focused on nonpregnant women of childbearing age, which we defined as 12 to 49 years. There were a total of 6433 women aged 12 to 49 years in NHANES 2007 to 2012. We excluded 182 for positive pregnancy status, an additional 164 who did not attend the MEC, an additional 416 for whom RBC folate concentration was missing, and 1 who reported that she did not know the number of supplements that she took, leaving 5670 women available for most analyses. In the analyses in which folic acid sources were considered, an additional 296 were excluded for unreliable (N58) or missing (N = 5288) day 1 dietary recall information and an additional 591 were excluded based on unreliable (N = 516) or missing (N = 5575) day 2 dietary recall information, leaving 4783 women available for analyses.
We analyzed survey data using MEC sampling weights, with the exception of data for folic acid intake sources, for which we used day 2 dietary weights, as recommended by the National Center for Health Statistics (Johnson et al., 2013). NHANES is approved by the National Center for Health Statistics Research Ethics Review Board. All participants in NHANES provide written informed consent.
RED BLOOD CELL (RBC) FOLATE CONCENTRATIONS
Data on RBC folate concentrations came from analysis of blood samples collected at the MEC. RBC folate concentrations were measured in NHANES using the microbiologic assay method from 2007 to 2012 (National Center for Health Statistics; National Center for Health Statistics; National Center for Health Statistics). RBC folate concentrations are not normally distributed; therefore, we log transformed these values before analysis.
We defined cut-points for NTD risk categories based on RBC folate concentrations from publications by Daly et al. (1995) and Crider et al. (2014) (Table 1). The technique used for the microbiologic assay in these studies differed from that used in NHANES. We applied a conversion formula (To convert from Molloy method used by Daly et al. (1995) and Crider et al. (2014) to Pfeiffer/NHANES method, the following conversion formula should be used: Pfeiffer/NHANES RBC folate [nmol/L] = (Molloy RBC folate [nmol/L] * 0.7876) + 34.2802 [nmol/L] (correlation r=0.92, n = 2613 paired samples) (Crider et al., 2014, Pfeiffer et al., 2011)) to allow comparison of NHANES values with the risk categories defined in the Daly et al. and Crider et al. studies (Pfeiffer et al., 2011; Crider et al., 2014). Optimal RBC folate concentrations for NTD prevention have been established by the World Health organization to be >906 nmol/L (400 ng/L), for purposes of this analysis we considered an optimal RBC folate concentration to be 748 nmol/L NHANES assay (~906 nmol/L Molloy assay), the concentrations associated with an NTD risk of <9 NTDs per 10,000 live births based on the results of the Daly et al. and Crider et al. studies (WHO, 2015).
TABLE 1.
Neural tube defect (NTD) risk category by red blood cell (RBC) folate concentrations for different blood folate assay methods1
| Risk category | NTD prevalence per 10,000 live births2 |
RBC folate concentration (nmol/L) | |
|---|---|---|---|
| Molloy method | Pfeiffer/NHANES method3 |
||
| High | >14 | ≤699 | ≤585 |
| Elevated | 9 – 14 | 700 – 905 | 586 – 747 |
| Optimal | 4 – <9 | 906 – 1499 | 748 – 1215 |
| Limited additional benefit | Outside of estimable range | ≥1500 | ≥1216 |
Derived from Crider et al., 2014 (1) and Daly et al., 1995 (2)
NTD prevalence estimates are based on the median estimated NTD prevalence for an entire population with a given RBC folate concentration, corresponding to the risk category cut points; rounded to the nearest whole number
To convert from Molloy method to Pfeiffer/NHANES method, the following conversion formula should be used: Pfeiffer/NHANES RBC folate [nmol/L] = (Molloy RBC folate [nmol/L] * 0.7876) + 34.2802 [nmol/L] (1)
COVARIATES
Supplement use was defined based on whether the participant reported consuming a folic acid-containing supplement in the past 30 days. Participants who reported supplement use were asked to provide the name of the supplement, frequency of use, and typical dose. NHANES staff matched the reported products to their ingredients using in-house databases and communication with supplement manufacturers. They then compiled this information and provided an estimated daily average dose of several nutrients, including folic acid (National Center for Health Statistics; National Center for Health Statistics; National Center for Health Statistics). Among women who reported consuming folic acid from supplements, we categorized them based on their average daily folic acid intake from supplements: ≥400 µg and<400 µg; and among women who reported <400 µg, <200 µg and 200 to<400 µg. These values were chosen based on the recommendations that women of childbearing potential consume at least 400 µg/day for NTD prevention (Centers for Disease Control and Prevention, 1992; Institute of Medicine, 1998; U.S. Preventive Services Task Force, 2009) and to understand the distribution of average daily intake from supplements below the recommended level.
Dietary information on folic acid intake was collected with two 24-hr dietary recalls. Each food reported by each participant was linked to the U.S. Department of Agriculture’s (USDA) Food and Nutrient Database for Dietary Studies (Agricultural Research Service and Group, 2010; Ahuja et al., 2012; U.S. Department of Agriculture and Agricultrual Research Service, 2014). We created four mutually exclusive folic acid intake groups: (1) those who reported only consuming folic acid from enriched cereal grain products (ECGP only), (2) those who reported consuming ECGP and ready-to-eat (RTE) cereals that contained folic acid (ECGP + RTE), (3) those who reported consuming ECGP and dietary supplements (SUPP) that contained folic acid (ECGP + SUPP), and (4) those who reported consuming all three sources (ECGP + RTE + SUPP). Participants who reported consuming any RTE cereal (USDA food codes: 57000000–57419000) that contained folic acid on either day 1 or day 2 of the dietary recall were classified as RTE cereal consumers. If no consumption of RTE cereals or supplements containing folic acid was reported, the participant was assumed to consume folic acid only from ECGP, as it is the only other major source of folic acid in the United States.
Data on age, race/ethnicity, education, and poverty income ratio (PIR) were also collected during the in-home interview. We created three age group categories: 12 to 24 years, 25 to 34 years, and 35 to 49 years. Participants were categorized by NHANES based on their self-reported race/ethnicity as either non-Hispanic white, non-Hispanic black, Mexican American, Other Hispanic, or Other race/ethnicity. For our analysis, participants who classified themselves as either Mexican American or Other Hispanic were combined to form a Hispanic category. We did not analyze data for the Other race/ethnicity category because of the heterogeneity of that group; women in this category were included in all analyses except those stratified by race/ethnicity. We created three categories for educational attainment (<high school, high school graduate or equivalent, >high school). We created four categories of poverty income ratio (PIR), which is defined as the ratio of self-reported family income to federal poverty threshold, accounting for family size, year, and state; higher values correspond to higher socioeconomic (National Center for Health Statistics; National Center for Health Statistics).
Weight and height were measured during the physical examination at the MEC and BMI was calculated (weight (kg)/height (m)2). Individuals were classified as underweight (BMI<18.5), normal weight (18.5≤BMI<25), overweight (25≤BMI<30), or obese (BMI≥30) (National Heart Lung and Blood Institute, 2000). Smoking status was determined using serum cotinine concentrations from samples taken at the MEC. Women with cotinine concentrations >10 ng/ml were classified as smokers (Pirkle et al., 1996).
STATISTICAL ANALYSIS
We estimated the weighted percentage of our total study population that was categorized into each of the previously defined groups. We estimated geometric mean RBC folate concentrations for each of these groups, adjusting for age, race/ethnicity, and BMI categories. We used chi-square tests and t tests to assess statistical differences; we considered p-values <0.05 to be statistically significant. We used multivariable logistic regression to estimate adjusted odds ratios for factors potentially associated with having a suboptimal RBC folate concentration for NTD prevention (i.e., <748 nmol/L), again adjusting for age, race/ethnicity, and BMI. We used SAS-Callable SUDAAN Version 11.0 (Research Triangle Park, NC) to account for the complex sampling design of NHANES. The estimates accounted for weighting and design factors according to NCHS guidelines (National Center for Health Statistics, 2006).
Results
Use of a dietary supplement containing folic acid was reported by 28.5% (95% confidence interval [CI]: 26.2, 30.8) of women (Table 2). Among women who reported consuming a dietary supplement containing folic acid, approximately half reported an average dose of ≥400 mg folic acid/day, representing 13.8% of all women (95% CI, 12.5–15.3). Almost half of women reported consuming folic acid only from ECGP (48.4%; 95% CI, 46.3–50.6). Only 10.3% of the women reported consuming all three sources of folic acid (95% CI, 8.8–11.9).
TABLE 2.
Characteristics of women aged 12–49 years by selected dietary, demographic, and lifestyle factors, NHANES 2007–2010
| Unweighted count | Weighted percentage (95% Confidence Interval) |
||
|---|---|---|---|
| Total1 | 3,861 | ||
| Use of supplements containing folic acid | |||
| None | 2,965 | 71.2 (68.2, 73.9) | |
| Any | 896 | 28.8 (26.1, 31.8) | |
| Average daily dose <400µg | 454 | 14.0 (12.3, 15.8) | |
| Average daily dose<200 µg | 236 | 7.2 (6.0, 8.7) | |
| Average daily dose 200 - <400 µg | 218 | 6.8 (5.7, 8.0) | |
| Average daily dose ≥400 µg | 442 | 14.9 (13.0, 16.9) | |
| Folic acid source(s)2 | |||
| ECGP only | 1,728 | 47.5 (44.8, 50.3) | |
| ECGP + RTE | 921 | 24.1 (22.1, 26.4) | |
| ECGP + SUPP | 516 | 17.8 (15.9, 19.8) | |
| ECGP + RTE + SUPP | 290 | 10.6 (8.7, 12.8) | |
| Age group | |||
| 12 – 24 years | 1,488 | 32.0 (29.9, 34.2) | |
| 25 – 34 years | 868 | 24.4 (22.5, 26.4) | |
| 35 – 49 years | 1,505 | 43.6 (41.4, 45.6) | |
| Race/ethnicity | |||
| Non-Hispanic white | 1,555 | 63.1 (57.3, 68.4) | |
| Non-Hispanic black | 767 | 13.2 (10.9, 15.9) | |
| Hispanic | 1,325 | 16.8 (13.1, 21.3) | |
| Other race/ethnicity3 | 214 | 7.0 (5.4, 8.9) | |
| Education level | |||
| < High school | 1,591 | 30.9 (29.0, 32.9) | |
| High school graduate/GED | 666 | 17.7 (16.2, 19.2) | |
| At least some college | 1,600 | 51.5 (48.8, 54.1) | |
| Poverty income ratio4 | |||
| <1.0 | 1,029 | 20.4 (18.1, 23.0) | |
| 1.0 – 1.9 | 998 | 21.7 (19.4, 24.2) | |
| 2.0 – 3.9 | 817 | 27.2 (25.1, 29.5) | |
| ≥4.0 | 712 | 30.6 (27.1, 34.5) | |
| Body Mass Index (BMI) [kg/m2] | |||
| Underweight (BMI<18.5) | 196 | 4.8 (3.9, 5.9) | |
| Normal weight (18.5≤BMI<25) | 1,449 | 41.3 (39.2, 43.4) | |
| Overweight (25≤BMI<30) | 967 | 24.9 (23.0, 26.8) | |
| Obese (BMI≥30) | 1,202 | 29.1 (27.3, 30.9) | |
| Smoking status5 | |||
| Non-smoker | 3,014 | 77.8 (75.1, 80.3) | |
| Smoker | 817 | 22.2 (19.7, 24.9) | |
NHANES: National Health and Nutrition Examination Survey; ECGP: Enriched cereal grain products; RTE: ready-to-eat cereals; SUPP: dietary supplements containing folic acid; GED: General Education Development, equivalent to high school degree
Excludes those who did not attend the Mobile Examination Center (N = 93), those for whom RBC folate concentration was missing (N=293), and those who reported that they did not know how many supplements they had taken (N = 1).
Additionally excludes those for whom the day 1 dietary recall was not reliable (N = 2) or missing (N = 147) and additionally those for whom the day 2 dietary recall was not reliable (N = 14) or missing (N = 438)
Other non-Hispanic race/ethnicity category not considered in subsequent analysis stratified by race/ethnicity due to heterogeneity within the category; women in this category are not excluded from other analyses
Poverty income ratio is defined as the ratio of self-reported family income to federal poverty threshold, accounting for family size, year, and state; higher values correspond to higher socioeconomic status
Smoking status is defined as non-smoker for serum cotinine concentrations ≤10 ng/mL and smoker for serum cotinine concentrations >10 ng/mL
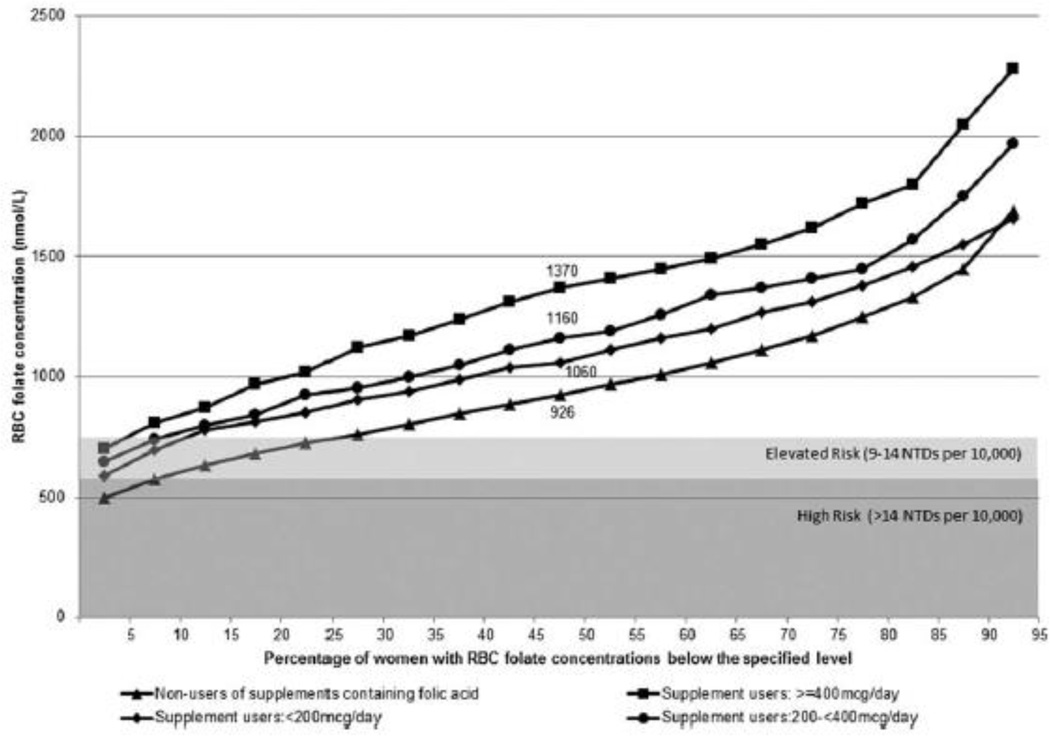
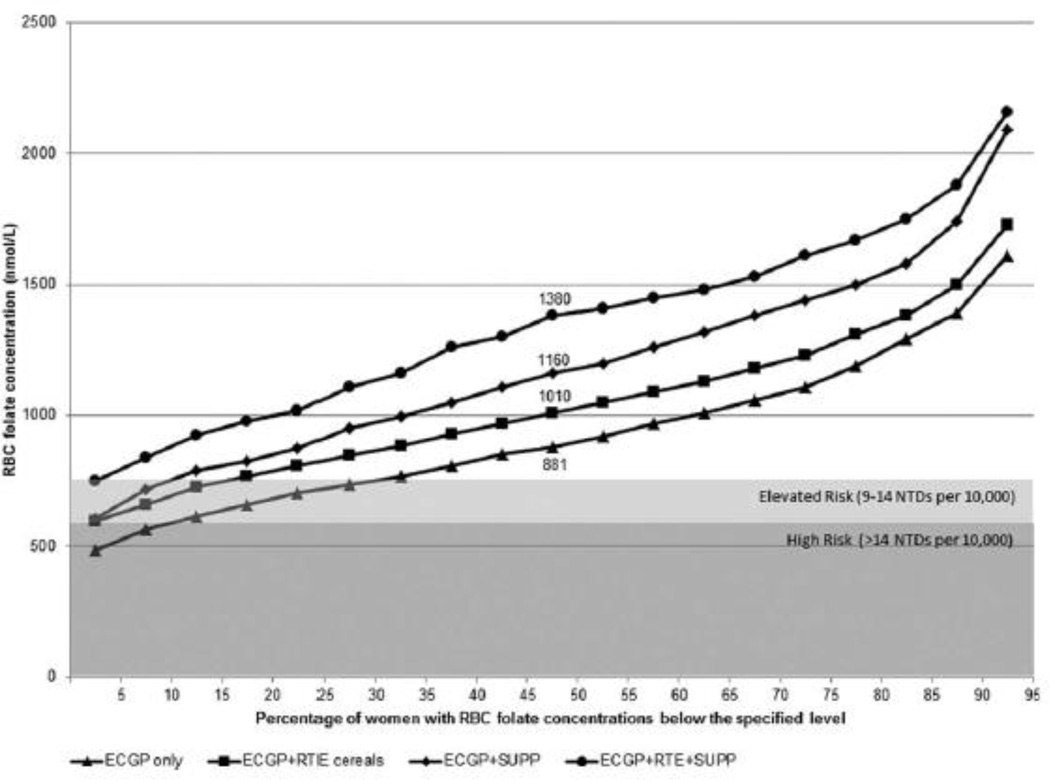
Women who did not report taking a supplement containing folic acid had lower RBC folate concentrations than those who reported taking them, and higher average daily folic acid dose from supplements was associated with higher concentrations (Fig. 1). Approximately 10% of women who did not report use of a supplement containing folic acid had RBC concentrations corresponding to the high risk category for population NTD risk; with an additional 18% with RBC concentrations consistent with an elevated risk for NTDs (exact percentages are available in Supplementary Table S1, which is available online). Women who reported intake of folic acid only from ECGP had lower RBC concentrations than women who consumed folic acid from multiple sources (Fig. 2). Approximately 12% of women who consumed folic acid only from ECGP had RBC folate concentrations in the high risk categories, and an additional 20% had concentrations in the elevated risk category (Supplementary Table S1).
Figure 1.
Cumulative distribution of red blood cell (RBC) folate concentrations among nonpregnant women aged 12 to 49 years, by supplement use and folic acid dose, NHANES 2007 to 2012.
NTDs, neural tube defects.
Figure 2.
Cumulative distribution of red blood cell (RBC) folate concentrations among nonpregnant women aged 12 to 49 years, by folic acid source(s), NHANES 2007 to 2010. NTDs, neural tube defects; ECGP, enriched cereal grain products; RTE, ready-to-eat cereals; SUPP, dietary supplements containing folic acid.
The adjusted geometric mean RBC folate concentration for nonpregnant women aged 12 to 49 years of age was 992 nmol/L (95% CI, 973–1012) (Table 3). Although the majority of women had an RBC folate concentration corresponding to an NTD prevalence of <9 per 10,000 live births, 22.8% (95% CI, 21.1–24.6) had RBC folate concentrations associated with higher NTD prevalence. We observed a substantial, statistically significant difference in the adjusted geometric mean RBC folate concentrations between women who did and did not report use of a dietary supplement containing folic acid (1176 nmol/L and 925 nmol/L, respectively). This difference translated to an almost 65% reduction in the odds of having suboptimal RBC folate concentrations for women who reported consuming any folic acid-containing supplement (adjusted odds ratio [aOR]: 0.36; 95% CI, 0.30–0.44).
TABLE 3.
Adjusted geometric mean red blood cell (RBC) folate concentrations and the percentage of and odds of having an RBC folate concentration associated with specific prevalence of neural tube defects (NTDs) among women aged 12 – 49 years, stratified by selected dietary, demographic, and lifestyle factors, NHANES 2007 – 2010
| Unweighted N | Adjusted1geometric mean, nmol/L (95% CI) |
Percentage with RBC folate concentration associated with an NTD prevalence of ≥9 per 10,000 live births [<748 nmol/L] (95% CI) |
Adjusted2 odds of having an RBC folate concentration associated with an NTD prevalence of ≥9 per 10,000 live births [<748 nmol/L] (95% CI) |
||
|---|---|---|---|---|---|
| Total3 | 3861 | 1002 (973, 1022) | 22.9 (20.8, 25.2) | ||
| Use of supplements containing folic acid | |||||
| None | 2965 | 925 (907, 953)a | 28.5 (25.8, 31.3) | ref | |
| Any | 896 | 1200 (1164, 1236)b | 9.3 (7.2, 11.8) | 0.23 (0.15, 0.36) | |
| Average daily dose <400µg | 454 | 1119 (1075, 1153) | 11.9 (8.8, 15.9) | 0.22 (0.12, 0.40) | |
| Average daily dose<200 µg | 236 | 1075 (1022, 1130) | 12.8 (8.8, 18.2) | 0.15 (0.06, 0.36) | |
| Average daily dose 200 - <400 µg | 218 | 1164 (1108, 1224) | 11.0 (6.9, 17.2) | 0.28 (0.14, 0.58) | |
| Average daily dose ≥400 µg | 442 | 1287 (1236, 1339) | 6.8 (4.8, 9.5) | 0.23 (0.13, 0.42) | |
| Folic acid source(s)4 | |||||
| ECGP only | 1621 | 889 (854, 925)a | 33.4 (28.9, 38.2) | ref | |
| ECGP + RTE | 859 | 1022 (982, 1054)b | 17.7 (14.2, 21.9) | 0.44 (0.30, 0.66) | |
| ECGP + SUPP | 498 | 1141 (1086, 1200)c | 11.6 (8.1, 16.4) | 0.22 (0.13, 0.37) | |
| ECGP + RTE + SUPP | 282 | 1300 (1236, 1366)d | 4.5 (2.4, 8.3) | 0.12 (0.05, 0.30) | |
| Age group | |||||
| 12 – 24 years | 1488 | 944 (907, 982)a | 25.9 (22.4, 29.8) | 1.00 (0.66, 1.51) | |
| 25 – 34 years | 868 | 992 (953, 1022)a | 23.5 (19.5, 28.1) | REF | |
| 35 – 49 years | 1505 | 1043 (1012, 1086)b | 20.4 (17.3, 23.9) | 0.90 (0.58, 1.42) | |
| Race/ethnicity | |||||
| Non-Hispanic white | 1555 | 1054 (1022, 1097)a | 18.6 (16.0, 21.5) | ref | |
| Non-Hispanic black | 767 | 837 (804, 871)b | 38.6 (35.5, 41.7) | 2.31 (1.65, 3.23) | |
| Hispanic | 1325 | 963 (925, 992)c | 22.7 (19.7, 26.1) | 1.29 (0.86, 1.94) | |
| Education level | |||||
| < High school | 1591 | 963 (934, 992)a | 26.7 (23.0, 30.7) | 0.80 (0.56, 1.14) | |
| High school graduate/GED | 666 | 944 (916, 982)a | 26.9 (23.3, 30.9) | ref | |
| At least some college | 1600 | 1043 (1002, 1075)b | 19.3 (16.9, 22.1) | 0.89 (0.58, 1.35) | |
| Poverty income ratio5 | |||||
| <1.0 | 1029 | 944 (898, 992)a | 29.0 (24.0, 34.5) | ref | |
| 1.0 – 1.9 | 998 | 992 (953, 1033)a,b | 24.8 (20.6, 29.6) | 0.75 (0.50, 1.13) | |
| 2.0 – 3.9 | 817 | 1012 (973, 1054)b | 22.5 (19.0, 26.4) | 0.81 (0.54, 1.20) | |
| ≥4.0 | 712 | 1033 (992, 1075)b | 17.6 (14.4, 21.4) | 0.71 (0.47, 1.08) | |
| Body Mass Index (BMI) [kg/m2] | |||||
| Underweight (BMI<18.5) | 196 | 953 (880, 1033)a | 25.2 (18.1, 33.9) | 0.69 (0.24, 1.98) | |
| Normal weight (18.5≤BMI<25) | 1449 | 973 (944, 1002)a | 24.2 (21.6, 26.9) | ref | |
| Overweight (25≤BMI<30) | 967 | 982 (944, 1022)a | 23.8 (20.7, 27.3) | 0.99 (0.66, 1.49) | |
| Obese (BMI≥30) | 1202 | 1064 (1022, 1097)b | 20.2 (17.5, 23.1) | 0.68 (0.51, 0.91) | |
| Smoking status6 | |||||
| Non-smoker | 3014 | 1033 (1012, 1064)a | 19.5 (17.5, 21.7) | ref | |
| Smoker | 817 | 871 (837, 916)b | 34.5 (29.9, 39.5) | 1.80 (1.25, 2.61) | |
NHANES: National Health and Nutrition Examination Survey; CI: Confidence Interval; ECGP: Enriched cereal grain products; RTE: ready-to-eat cereal; SUPP: dietary supplement containing folic acid.
Geometric means in a column with superscript letters without a common letter differ, Student’s t test P< 0.05.
Adjusted for age, race/ethnicity, and BMI
Adjusted for age, race/ethnicity, poverty income ratio, education, folic acid exposure group, BMI, smoking, and alcohol use, except for results stratified by folic acid exposure group
Excludes those who did not attend the Mobile Examination Center (N=93), those who reported that they did not know how many supplements they had taken (N=1), and those with missing RBC folate concentrations (N=293)
Additionally excludes those for whom the day 1 dietary recall was not reliable (N=2) or missing (N=147) and additionally those for whom the day 2 dietary recall was not reliable (N=14) or missing (N=438)
Poverty income ratio is defined as the ratio of self-reported family income to federal poverty threshold, accounting for family size, year, and state; higher values correspond to higher socioeconomic status
Smoking status is defined as smoker (cotinine >10 ng/mL) and non-smoker (≤ 10 ng/mL)
The adjusted geometric mean RBC folate concentrations were higher for women with higher average daily folic acid dose from dietary supplements (Table 3). Large, statistically significant differences in adjusted geometric mean RBC folate concentrations were also observed when we considered groups defined by folic acid intake source. Women who reported consuming folic acid through only ECGP had an adjusted geometric mean RBC folate concentration of 889 nmol/L (95% CI, 863–916), while those who consumed folic acid from ECGP, RTE cereals, and supplements had an adjusted geometric mean of 1274 nmol/L (95% CI, 1224–1326). The odds of having suboptimal RBC folate concentrations were significantly lower for women who reported consuming sources of folic acid in addition to ECGP, ranging from 46% lower for women who reported consuming ECGP and RTE cereals (aOR: 0.54; 95% CI, 0.44–0.66) to 82% lower for women who reported consuming ECGP, RTE cereals, and supplements (aOR: 0.18; 95% CI, 0.11–0.31), compared with women who consumed ECGP only.
Non-Hispanic white women had an adjusted geometric mean RBC folate concentration of 1043 nmol/L (95% CI, 1022–1075), which was significantly higher than that of Hispanics (963 nmol/L; 95% CI, 934–982) and non-Hispanic blacks (829 nmol/L; 95% CI, 804–854) (Table 2). The adjusted geometric mean RBC folate concentration was significantly higher among older women; women with at least some college education; women with higher PIR; obese women; and nonsmokers. Use of supplements containing folic acid, intake of folic acid from sources in addition to ECGP, having at least some college educational experience, having a high PIR, and being obese were associated with significantly reduced odds of having suboptimal RBC folate concentrations (Table 3). Non-Hispanic black and Hispanic race/ethnicity and being a current smoker were associated with significantly increased odds of having suboptimal RBC folate concentrations. The distribution into NTD risk categories (i.e., high, elevated, optimal, limited additional benefit) based on RBC folate concentrations by dietary, demographic, and lifestyle factors is available in Supplementary Table S1.
Discussion
Our findings suggest that the majority of nonpregnant women aged 12 to 49 years have RBC folate concentrations associated with a risk of <9 NTDs per 10,000 live births. The proportion of women with suboptimal RBC folate concentrations differs by supplement use, sources of folic acid intake, race/ethnicity, socioeconomic status, BMI category, and smoking status. Overall, nearly a quarter of women (22.8%; 95% CI, 21.1–24.6) of childbearing age have suboptimal folate concentrations for NTD prevention. Among women who report consuming ECGP only, a third (32.5%; 95% CI, 28.7–36.6) have suboptimal RBC folate concentrations. Given that almost half of women report consuming ECGP only, this finding could have significant implications on the potential risk of NTD-affected pregnancies.
Fortification of ECGP has had a dramatic impact on RBC folate concentrations and rates of NTDs in the United States. RBC folate concentrations for women aged 15 to 44 years increased from 686 nmol/L in 1988 to 1994 (prefortification) to 1060 nmol/L in 1999 to 2010 (postfortification) (Pfeiffer et al., 2012). Similarly, the decline in rates of NTDs (anencephaly and spina bifida) has been estimated to be 35% since fortification, with a similar decrease observed across racial/ethnic groups (Williams et al., 2015). Yet, there are still approximately 2500 to 3000 U.S. pregnancies affected by an NTD every year (Centers for Disease Control and Prevention, 2004; Parker et al., 2010). Current U.S. recommendations for the prevention of NTDs are that women capable of becoming pregnant consume 400 mg of folic acid every day from supplements and fortified foods in addition to consuming a diet that is high in folate-rich foods (Centers for Disease Control and Prevention, 1992; Institute of Medicine, 1998; U.S. Preventive Services Task Force, 2009). However, evidence has suggested that on average nonpregnant women aged 15 to 44 years report consuming a median daily usual intake of 245 mg of total folic acid and less than a quarter report a usual intake ≥400 mg of folic acid every day (Tinker et al., 2010).
Results from two large U.S. retrospective case-control studies of NTDs conducted after the implementation of folic acid fortification suggest that folic acid supplement use in the presence of fortification of ECGP and RTE cereals is not associated with additional decreases in the risk of NTDs (Mosley et al., 2009; Ahrens et al., 2011). Our results are consistent with these findings. The majority of U.S. women of childbearing age, including those who do not take supplements containing folic acid, have RBC folate concentrations associated with the lowest (optimal) NTD risk. There is still a group of women with less-than-optimal RBC folate concentrations, but it is relatively small and the majority of these women are in the elevated (not high) risk group, such that studies would need extremely large sample sizes to detect the residual impact of supplements in the presence of consumption of fortified ECGP for these women.
We observed that non-Hispanic black women had significantly lower RBC folate concentrations than non-Hispanic white women, which is consistent with previous data showing that non-Hispanic black women have lower estimated average daily usual total folic acid intake than non-Hispanic white women (Tinker et al., 2010). Despite lower folic acid intake and lower RBC folate concentrations, non-Hispanic black women have not been observed to be at higher risk for an NTD-affected pregnancy (Canfield et al., 2006; Williams et al., 2015). This apparent paradox has generally been attributed to a lack of genetic susceptibility (very low MTHFR genotype frequency <3%) (Yang et al., 2008) and high B12 concentrations (20% higher than non-Hispanic whites) (Sternberg et al., 2013) which could allow for more efficient use of folate and possibly lower folate requirements and/or less folate vitamers present in the folate pools, making measurement of folate status more difficult. The categories of NTD risk defined by RBC folate concentration used in our analysis were based on data from Irish and Chinese populations, and it is possible that these categories may not be appropriate for all racial/ethnic groups.
LIMITATIONS
Our analyses are subject to several additional limitations. The NTD risk categories based on RBC folate concentrations were derived from the Daly et al. and Crider et al. studies (Daly et al., 1995; Crider et al., 2014). These risk estimates have uncertainty associated with them and although mutually exclusive categories of NTD risk (Table 1) are useful for descriptive analysis, they fail to convey this imprecision. Additionally, the microbiologic assay technique used by NHANES differs from that used in the Daly et al. (1995) and Crider et al. (2014) studies and results in nonequivalent RBC folate concentrations; we therefore converted the RBC concentration values between the studies to ensure comparability. Although a conversion equation is available and correlation between the assays, based on comparison of >2600 samples, is high (r = 50.92) (Pfeiffer et al., 2011; Crider et al., 2014) we were not able to account for uncertainty in this conversion in our analysis. In addition, we categorized women by their reported average daily dose of folic acid from supplements; however, supplement use was based on the previous 30 days and might not be reflective of long-term supplement use across the population. We also might have misclassified some RTE cereal consumers if they did not report consuming these on either day 1 or day 2 of the dietary recall. The use of data from NHANES is a major strength of this study; NHANES data are nationally representative and provide information on dietary, demographic, and lifestyle factors as well as biological samples.
CONCLUSIONS
In the presence of mandatory folic acid fortification and current folic acid supplementation patterns we would predict that the majority of U.S. women of reproductive age are not at increased risk for folate-sensitive NTDs based on RBC folate concentrations. However, the data presented here allow us to identify U.S. women with different dietary, lifestyle and sociodemographic characteristics that put them at higher risk for having a pregnancy affected by a folate sensitive NTD. Policies and programs aimed at these at-risk groups can help increase RBC folate concentrations through a variety of methods, including promoting the consumption of fortified foods or increasing awareness of the importance of taking a supplement containing folic acid, in addition to consuming a diet that is high in naturally folate-rich foods.
Supplementary Material
Footnotes
Presented at the 47th Annual Society for Epidemiologic Research Meeting and the 27th Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research. Seattle, Washington; June 2014.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Ethics approval received from the National Center for Health Statistics Research Ethics Review Board for Protocols #2005-06 (continuations for the 2007 to 2008 and 2009 to 2010 cycles) and #2011-17 (continuation for the 2011 to 2012 cycle).
S.C.T., H.C.H., and K.S.C. designed research; S.C.T., H.C.H., and Y.P.Q. analyzed data; S.C.T., H.C.H., Y.P.Q., and K.S.C. wrote the study; S.C.T. had primary responsibility for final content.
The authors declare no conflicts of interest. No financial disclosures were reported by the authors of this study.
References
- Agricultural Research Service, Group FSR. USDA Food and Nutrient Database for Dietary Studies 4.1. Beltsville, MD: U.S. Department of Agriculture; 2010. [Google Scholar]
- Ahrens K, Yazdy MM, Mitchell AA, Werler MM. Folic acid intake and spina bifida in the era of dietary folic acid fortification. Epidemiology. 2011;22:731–737. doi: 10.1097/EDE.0b013e3182227887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja JKA, Montville JB, Omolewa-Tomobi G, et al. USDA Food and Nutrient Database for Dietary Studies, 5.0. Beltsville, MD: U.S. Department of Agriculture, Agricultural Research Services, Food Surveys Research Group; 2012. [Google Scholar]
- Berry RJ, Li Z, Erickson JD, Li S, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Canfield MA, Honein MA, Yuskiv N, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Res A Clin Mol Teratol. 2006;76:747–756. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992;41(RR-14):1–7. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Spina bifida and anencephaly before and after folic acid mandate–United States, 1995–1996 and 1999–2000. MMWR Morb Mortal Wkly Rep. 2004;53:362–365. [PubMed] [Google Scholar]
- Crider KS, Devine O, Hao L, et al. Population red blood cell folate concentrations for prevention of neural tube defects: bayesian model. BMJ. 2014;349:g4554. doi: 10.1136/bmj.g4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Daly LE, Kirke PN, Molloy A, et al. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274:1698–1702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- De Wals P, Tairou F, Van Allen MI, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med. 2007;357:135–142. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- Hendler SS, Rorvik DR. PDR for Nutritional Supplements. Montvale: Medical Economics Company, Inc; 2001. [Google Scholar]
- Institute of Medicine. DRI dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- Johnson CL, Paulouse-Ram R, Ogden CL. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2:1–24. [PubMed] [Google Scholar]
- Mosley BS, Cleves MA, Siega-Riz AM, et al. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am J Epidemiol. 2009;169:9–17. doi: 10.1093/aje/kwn331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey 2007 – 2008: laboratory procedure manual – folate [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey 2009 – 2010: laboratory procedure manual – folate [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey 2011 – 2012: laboratory procedure manual – folate [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2007 – 2008 data documentation, codebook, and frequencies - demographic variables and sample weights (DEMO_E) [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2009 – 2010 Data Documentation, Codebook, and Frequencies - Demographic Variables and Sample Weights (DEMO_F) [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey: NHANES 2007–2008 [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey: NHANES 2009–2010 [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey: NHANES 2011–2012 [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey; 2007–2008 data documentation, codebook, and frequencies; dietary supplement use 30-day: total dietary supplements (DSQTOT_E) [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey; 2009–2010 data documentation, codebook, and frequencies; dietary supplement use 30-day: total dietary supplements (DSQTOT_F) [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey; 2011–2012 data documentation, codebook, and frequencies; dietary supplement use 30-day: total dietary supplements (DSQTOT_G) [Google Scholar]
- National Center for Health Statistics. Analytic and reporting guidelines: the National Health and Nutrition Examination Survey. Hyattsville, MD: 2006. [Google Scholar]
- National Heart Lung and Blood Institute. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. Report nr NIH 00-4084. 2000 [Google Scholar]
- Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C, Hughes JP, Lacher DA, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J Nutr. 2012;142:886–893. doi: 10.3945/jn.111.156919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer CM, Zhang M, Lacher DA, et al. Comparison of serum and red blood cell folate microbiologic assays for national population surveys. J Nutr. 2011;141:1402–1409. doi: 10.3945/jn.111.141515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkle JL, Flegal KM, Bernert JT, et al. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275:1233–1240. [PubMed] [Google Scholar]
- Sayed AR, Bourne D, Pattinson R, et al. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res A Clin Mol Teratol. 2008;82:211–216. doi: 10.1002/bdra.20442. [DOI] [PubMed] [Google Scholar]
- Sternberg MR, Schleicher RL, Pfeiffer CM. Regression modeling plan for 29 biochemical indicators of diet and nutrition measured in NHANES 2003–2006. J Nutr. 2013;143:948S–956S. doi: 10.3945/jn.112.172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker SC, Cogswell ME, Devine O, Berry RJ. Folic acid intake among U.S. women aged 15–44 years, National Health and Nutrition Examination Survey, 2003–2006. Am J Prev Med. 2010;38:534–542. doi: 10.1016/j.amepre.2010.01.025. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, Agricultrual Research Service. USDA Food and Nutrient Database for Dietary Studies 2011–2012. 2014 [Google Scholar]
- U.S. Food and Drug Administration. Food Additives Permitted for Direct Addition to Food for Human Consumption; Folic Acid (folacin): Final Rule. Fed Regist. 1996a;61:8797–8807. [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Food Standards: Amendment of Standards of Identity for Enriched Grain Products to Require Addition of Folic Acid: Final Rule. 21 CFR Parts 136, 137, and 139. Federal Register. 1996b;61:8781–8797. [Google Scholar]
- U.S. Preventive Services Task Force. Folic acid for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626–631. doi: 10.7326/0003-4819-150-9-200905050-00009. [DOI] [PubMed] [Google Scholar]
- WHO. Guideline: Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- Williams J, Mai CT, Mulinare J, et al. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification - United States, 1995–2011. MMWR Morb Mortal Wkly Rep. 2015;64:1–5. [PMC free article] [PubMed] [Google Scholar]
- Williams LJ, Rasmussen SA, Flores A, et al. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics. 2005;116:580–586. doi: 10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]
- Yang Q, Cogswell ME, Hamner HC, et al. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2006. Am J Clin Nutr. 2010;91:64–72. doi: 10.3945/ajcn.2009.28401. [DOI] [PubMed] [Google Scholar]
- Yang QH, Botto LD, Gallagher M, et al. Prevalence and effects of gene-gene and gene-nutrient interactions on serum folate and serum total homocysteine concentrations in the United States: findings from the third National Health and Nutrition Examination Survey DNA Bank. Am J Clin Nutr. 2008;88:232–246. doi: 10.1093/ajcn/88.1.232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.