Abstract
One of the hallmarks of cancer is the ability to generate and withstand unusual levels of oxidative stress. In part, this property of tumor cells is conferred by elevation of the cellular redox buffer glutathione. Though enzymes of the glutathione synthesis and salvage pathways have been characterized for several decades, we still lack a comprehensive understanding of their independent and coordinate regulatory mechanisms. Recent studies have further revealed that overall central metabolic pathways are frequently altered in various tumor types, resulting in significant increases in biosynthetic capacity, and feeding into glutathione synthesis. In this review, we will discuss the enzymes and pathways affecting glutathione flux in cancer, and summarize current models for regulating cellular glutathione through both de novo synthesis and efficient salvage. In addition, we examine the integration of glutathione metabolism with other altered fates of intermediary metabolites, and highlight remaining questions about molecular details of the accepted regulatory modes.
Keywords: glutathione, γ-glutamyl cycle, glutamate cysteine ligase, 5-oxoprolinase, γ-glutamylcyclotransferase, γ-glutamyltranspeptidase, glutathione synthetase, ChaC1, redox homeostasis
Introduction
Glutathione is a critical low molecular weight thiol that participates in numerous cellular functions in mammalian systems (Figure 1). Intracellular glutathione concentrations range between 0.5 and 10 mM whereas extracellular glutathione concentrations are significantly lower, with estimated values in the micromolar range (Meister and Anderson, 1983). Glutathione is predominantly found in its reduced state (GSH) or as its most commonly observed oxidation product, GSSG, which is formed from two molecules of glutathione linked by a disulfide bond. The GSH/GSSG redox system maintains an overall reducing environment in the cell, with GSH/GSSG ratios ranging from 30:1 to >100:1 in the cytosol, nucleus, and mitochondria. This ratio is considerably lower in the endoplasmic reticulum with values of 1:1 to 3:1 reported, conditions in which disulfide bonds are generated in proteins traversing the secretory pathway (Dickinson and Forman, 2002; Lu, 2009, 2013; Meister and Anderson, 1983). GSSG is converted to its reduced state by glutathione reductase, using NADPH generated primarily by the pentose phosphate pathway (Figure 2).
Figure 1. Structure of glutathione.
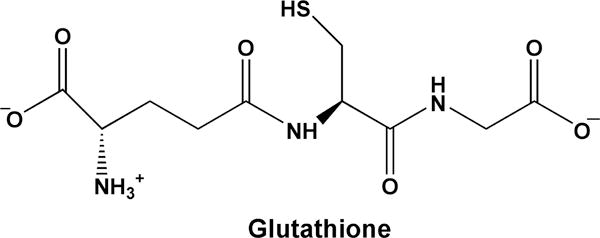
GSH is a tripeptide composed of glutamate, cysteine, and glycine. Its γ-glutamyl peptide bond makes it resistant to non-specific proteolytic cleavage.
Figure 2. Functions of glutathione and enzymes that maintain redox homeostasis.
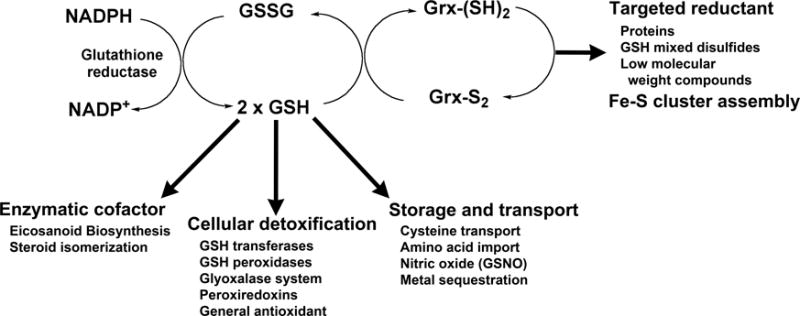
Glutathione is maintained in a reduced state by the action of NADPH-dependent glutathione reductase. Besides its role as a general antioxidant, GSH participates in biosynthetic pathways, signaling processes, detoxification, storage and transport of key metabolites, and sustains glutaredoxin (grx)-mediated reductive processes. Additional functions are described in the text.
One of the primary functions of glutathione is cellular detoxification. Glutathione transferases (GST) conjugate the tripeptide via its thiol group to endogenous and exogenous electrophilic compounds (Hayes et al., 2005; Mannervik, 2012; Townsend and Tew, 2003), reducing their reactivity and facilitating their clearance from the cell via members of the multidrug resistance associated protein family (Bachhawat et al., 2013). A comprehensive review of this topic can be found in this volume. The selenocysteine-containing glutathione peroxidases use glutathione to reduce H2O2 or lipid peroxides to water or their corresponding alcohols respectively, generating oxidized glutathione (Brigelius-Flohe and Maiorino, 2013). The glyoxalase system, composed of glyoxalase I and glyoxalase II, also requires GSH to detoxify methylglyoxal, glyoxal and other α-oxoaldehydes (Sousa Silva et al., 2013). However, there is no net oxidation of the cofactor. GSH has also been shown to be involved in the sequestration and transport of metals, including mercury, zinc and copper (Wang and Ballatori, 1998).
In addition to cellular detoxification, members of MAPEG (membrane-associated proteins in eicosanoid and glutathione metabolism) family, which are distantly related to glutathione transferases, contribute to eicosanoid biosynthesis (Hayes et al., 2005). Prostaglandin E synthase converts prostaglandin H2 to prostaglandin E2, using GSH as a cofactor. Leukotriene C4 synthase adds GSH to leukotriene A4 to produce leukotriene C4 (Lam et al., 1994; Welsch et al., 1994; Yoshimoto et al., 1985). The γ-glutamyl peptide bond of the attached glutathione is cleaved by another glutathione metabolic enzyme, γ-glutamyltranspeptidase (GGT5), to generate leukotriene D4 (Carter et al., 1998). Glutathione is also used in the storage and transport of cysteine (Lieberman et al., 1996). A different isozyme of γ-glutamyltranspeptidase (GGT1) initiates the enzymatic cleavage of extracellular glutathione, leading to the degradation of glutathione to its component amino acids (Hanigan and Ricketts, 1993). Cysteine derived from extracellular glutathione is then imported into the cell and used for protein and intracellular glutathione production (discussed below).
The glutaredoxin enzyme family (Lillig et al., 2008) is dependent on the GSH pools maintained by glutathione reductase. Glutaredoxins, also known as thiol transferases, catalyze the reversible glutathionylation of protein thiol groups. Dithiol glutaredoxins have an active site Cys-X-X-Cys motif and reduce selected protein disulfides at the expense of two molecules of GSH, generating GSSG. Monothiol glutaredoxins, such Grx5 in humans, retain the N-terminal cysteine residue of this motif. Glutaredoxins have been shown to be critical in iron-sulfur cluster assembly, and to catalyze the reduction of dehydroascorbate to ascorbate.
Given the diverse functions of glutathione, from involvement in signaling pathways to cellular detoxification, aberrant glutathione metabolism has been observed in multiple disease states. GSH levels are known to diminish with increased age and these reduced GSH levels are correlated with increased oxidative damage. In the human eye, GSH levels have been shown to drop significantly and this diminution may contribute to cataract formation (Fan et al., 2012; Harding, 1970). Similarly, brain GSH levels are lower in patients suffering from neurodegenerative diseases including Parkinson’s and Alzheimer’s and psychiatric disorders such as schizophrenia (Currais and Maher, 2013).
A recent comprehensive study evaluated previously reported GSH levels in numerous cancer types relative to normal controls (Gamcsik et al., 2012). In general, GSH levels were found to be higher in many cancer types, including head and neck, breast, ovarian, colorectal, and lung. This in agreement with the long held hypothesis that rapidly dividing tumor cells experience increased levels of oxidative stress and require higher levels of GSH. In addition, elevated GSH in concert with higher glutathione transferase expression protects these cells against administered chemotherapeutic agents (Hayes et al., 2005; Townsend and Tew, 2003). However, GSH levels tended to be lower in other cancers, including liver and brain, suggesting the contributions of GSH to disease progression may be more complex than anticipated (Gamcsik et al., 2012).
Glutathione Homeostasis
GSH biosynthesis requires sufficient quantities of glutamate, cysteine, and glycine to maintain appropriate levels of the tripeptide. As discussed below, availability of these precursors reflects the overall metabolic status of the cell. Meister and co-workers proposed a γ-glutamyl cycle (Figure 3) to highlight the central role of GSH in amino acid uptake (Meister, 1973, 1974; Orlowski and Meister, 1970). In this proposed pathway, GSH is synthesized by the sequential action of two ATP-dependent ligases, glutamate cysteine ligase (GCL) and glutathione synthetase (GS). Human GCL, also known as γ-glutamylcysteine synthetase, is composed of a 73 kDa catalytic subunit (GCLC) and a 31 kDa regulatory subunit (GCLM). GCL catalyzes the formation of a peptide bond between the γ-carboxylate of glutamate and the α-amino group of cysteine (Orlowski and Meister, 1971) and is the rate-limiting enzyme in GSH biosynthesis. GS then couples the α-carboxylate of γ-glutamylcysteine to the α-amino group of glycine to produce glutathione (Snoke and Bloch, 1955).
Figure 3. Canonical γ-glutamyl cycle.
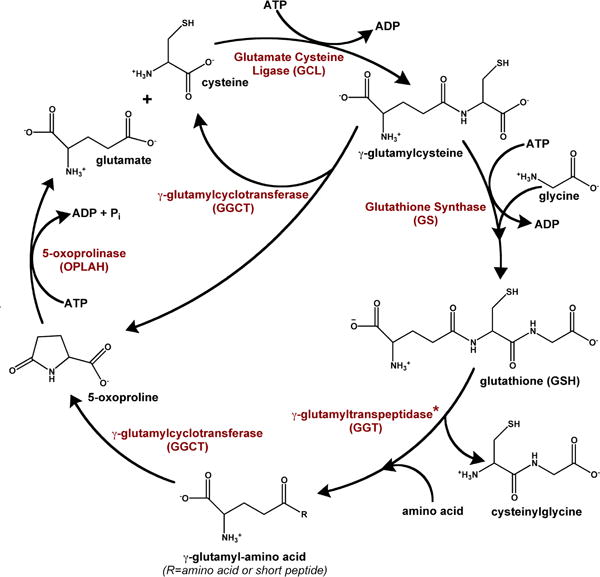
The γ-glutamyl cycle was initially proposed to describe the involvement of glutathione in transpeptidation-dependent amino acid transport, as discussed in the text (Orlowski and Meister, 1970). Since its initial description, GGCT has been suggested to use accumulated γ-glutamylcysteine as a substrate as well. A newly characterized enzyme, ChaC1, may provide a route for the intracellular degradation of glutathione, producing 5-oxoproline and cysteinyl glycine (not shown). *Please note that GGT is an ectoenzyme located at the plasma membrane.
Although several plasma membrane transporters capable of importing intact glutathione have been identified (reviewed in (Bachhawat et al., 2013)), the primary means of importing glutathione into a cell appears to be through a scavenging pathway (Lieberman et al., 1996; Lu, 2013). The salvage of extracellular glutathione is initiated by γ-glutamyltranspeptidase (GGT), a membrane anchored glycosylated enzyme that cleaves the γ-glutamyl peptide bond of glutathione (Ikeda and Taniguchi, 2005; Keillor et al., 2005; Kinlough et al., 2005). The enzyme reaction proceeds via a γ-glutamyl enzyme intermediate, in which the γ-glutamyl moiety is covalently linked to the threonine nucleophile at the active site. The released cysteinylglycine is then cleaved to its component amino acids by a dipeptidase (Dickinson and Forman, 2002; Lu, 2013), and imported into the cell. The acyl-enzyme intermediate of γ-glutamyltranspeptidase is resolved by the nucleophilic attack of water or another amino acid/peptide to generate glutamate or a new γ-glutamyl peptide, respectively. Studies by Meister and colleagues suggested that the transpeptidation reaction was preferred and facilitated the import of amino acids into the cell (Meister, 1974; Orlowski and Meister, 1970; Thompson and Meister, 1975). γ-Glutamylcyclotransferase (GGCT) cyclizes the γ-glutamyl moiety of the imported peptides to produce 5-oxoproline, also known as pyroglutamate or pyrrolidone carboxylate, and liberates the coupled amino acid or peptide (Board et al., 1978; Oakley et al., 2008; Orlowski et al., 1969). To complete the proposed cycle, human 5-oxoprolinase (OPLAH), a 275 kDa homodimer, couples ATP hydrolysis to the opening of the ring structure to generate glutamate (Van der Werf et al., 1971; Van Der Werf et al., 1974).
Now that each of the enzymes of the cycle has been identified and characterized, studies that address the concerted functionality of this pathway can be undertaken. Although the requirement of GCL and GS for glutathione biosynthesis is well documented, the contributions of the salvage pathway enzymes remain open for debate. Genome sequencing has led to the identification of close homologues of glutathione salvage enzymes in organisms that do not appear to synthesize glutathione and therefore may have alternate functions, as discussed below. Other studies suggest the cycle is not as straightforward as proposed. For example, although the transpeptidase activity of GGT was proposed to be required for amino acid import (Meister, 1973; Orlowski and Meister, 1970; Sekura and Meister, 1974), GGT null mice produced viable offspring (Lieberman et al., 1996). In addition, the observed phenotypic changes could be ameliorated by supplementation with N-acetylcysteine, a cysteine surrogate, suggesting that the primary function of GGT is to liberate cysteine from glutathione produced in the liver for biosynthetic purposes in peripheral tissue. Thus, regulation of glutathione metabolism is more complex than originally suggested and further work is needed to understand the contributions of glutathione to disease progression.
Glutathione Biosynthesis
Glutamate cysteine ligase
Human GCL (Figure 4) catalyzes the rate-limiting step in glutathione biosynthesis and its activity is subject to multiple levels of regulation (Griffith and Mulcahy, 1999; Lu, 2013). Transcriptional regulation of the catalytic and regulatory subunits of the enzyme is mediated through several established pathways including Nrf2-Keap1 and NFκB (Griffith and Mulcahy, 1999; Lu, 2013). GCL is also extensively regulated at the protein level. The enzyme is feedback inhibited by glutathione (Richman and Meister, 1975), and is allosterically activated by addition of the regulatory subunit (GCLM) to the catalytic subunit (GCLC). The resulting heterodimer is 2 – 5 fold more efficient in the production of glutathione, due to improved catalytic efficiency of the enzyme and alleviation of feedback inhibition by glutathione (Backos et al., 2011; Lu, 2013). The relative ratio of GCLM/GCLC differs considerably from tissue to tissue, and this site specific ratio may largely dictate glutathione production (Chen et al., 2005). Formation of the GCL heterodimer has been examined in human and closely related systems, but the molecular details of how complex formation improves flux through the biosynthetic pathway are not clear. This section summarizes published regulatory models and addresses associated limitations that leave the models open to further interpretation.
Figure 4. Homology model of human GCL.
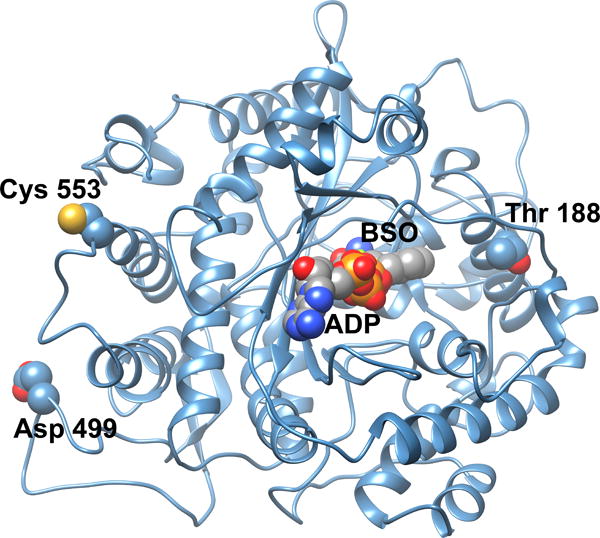
A homology model of human GCLC was generated from the closely related S. cerevisiae GCL structure (Biterova and Barycki, 2009). Potential sites of post-translational modifications as discussed in the text are illustrated in space filling representation. Docked into the enzyme active site are ADP and the mechanism based inhibitor L-S,R-buthionine sulfoximine (BSO), also in space filling representation. Colored atoms are as follows: yellow, sulfur; red, oxygen; deep blue, nitrogen; orange, phosphorus; gray, carbon.
Redox regulation
An attractive model of redox regulation of complex formation was postulated based on the observation of a disulfide linked species in the early preparations of the enzyme purified from rat kidney (Huang et al., 1993; Seelig et al., 1984). The model suggested that at lower reduced glutathione concentration, a disulfide bond would form between the catalytic and modifier subunits and increase activity to produce more glutathione. Once glutathione levels were restored, the disulfide bond would be reduced, the complex would no longer be stabilized, and flux through the glutathione biosynthetic pathway would slow. However, the identities of these cysteine residues have not been established. Studies of Drosophila GCL indicate that an intersubunit disulfide bond can be formed but it is not required for allosteric activation of the enzyme (Fraser et al., 2003). The disulfide linked species is a relatively minor fraction of the total enzyme in mouse tissue, and the holoenzyme forms readily without any oxidation (Krejsa et al., 2010). Unpublished work from our group using recombinant GCLC and GCLM suggests that although a disulfide can form, it is not required for stabilization of the human GCLC/GCLM complex or activation of the enzyme. However, agents that stimulate oxidative stress (e.g. pherone, H2O2) have been shown to rapidly increase GCL activity independently of increased enzyme expression suggesting that other cysteine oxidation events may indeed regulate GCL activity (Krejsa et al., 2010; Ochi, 1995, 1996). However, oxidized species of GCL have not been detected.
Phosphorylation
Additional post-translational modifications of human GCL have been reported. Hormone-mediated phosphorylation of rat liver GCL resulted in modest but significant reductions of enzymatic activity and correspondingly lower levels of glutathione (Lu et al., 1991). Subsequent studies indicated that protein kinase A (PKA), protein kinase C (PKC) and Ca2+/calmodulin-dependent kinase II (CKII) could each phosphorylate the catalytic subunit of GCL in vitro, resulting in an approximately 20% reduction in Vmax (Sun et al., 1996). Although the precise site(s) of phosphorylation were not identified, the authors suggested that Thr 132, Thr 188, Ser 331, Thr 506, Ser 567 and Ser 591 are potential CKII target sites. Examination of a homology model of GCLC generated using the Phyre2 webserver (Kelley and Sternberg, 2009) suggests that, with the exception of Ser 591, each of these residues is relatively solvent accessible and thus a possible candidate for phosphorylation. Of the remaining residues, Thr 188 (Figure 4) is located on the periphery of the enzyme active site and its phosphorylation could impact substrate binding and/or catalysis. However, direct identification of specific phosphorylation sites has not been done to establish the biological significance of phosphorylation given the relatively modest, yet significant, reduction in enzymatic activity.
Proteolysis
During apoptosis, caspase-3 cleaves the catalytic subunit of GCL into 60 kDa and 13 kDa fragments, and this modification may contribute to reduced cellular GSH levels observed during this process (Siitonen et al., 1999). The site of proteolytic cleavage was mapped to Asp 499 by mutational analysis (Franklin et al., 2002). In a recent review, the authors indicated that in vitro cleavage of recombinant GCLC by caspase-3 did not impact the overall structure of GCLC or its enzymatic activity (Franklin et al., 2009). Examination of a homology model of human GCL (Willis et al., 2011) indicates that Asp 499 is predicted to be in an extended surface exposed loop remote from the enzyme active site and that cleavage would not directly impact the core structural features (Figure 4). Thus, it is unclear how significant this observation is, with respect to maintenance of glutathione levels.
Lipid adducts
A reactive product of lipid peroxidation, 4-hydroxy-2-nonenal, was shown to rapidly increase GCL activity and GSH levels in the lung adenocarcinoma line, A549 (Backos et al., 2011). A corresponding increase in isolated catalytic subunit activity was observed with 4-hydroxy-2-nonenal treatment. Modification of Cys 553 by 4-hydroxy-2-nonenal at a 100-fold molar excess resulted in an approximately two-fold increase in Vmax and modest reductions in the Km values for glutamate and ATP. Comparable results were observed with another cysteine modifying agent, N-ethylmaleimide, suggesting that any number of modifications of this cysteine residue may activate the enzyme. Within the regulatory subunit, Cys 35 was identified as the primary site of modification by 4-hydroxy-2-nonenal. Isolated subunits when pre-incubated with 4-hydroxy-2-nonenal had impaired ability to form the holoenzyme. Modification of Cys 553 of GCLC impaired but did not completely block complex formation whereas modification of Cys 35 of GCLM precluded subunit association. A telling observation was that in the preformed complex, only Cys 35 was reactive with 4-hydroxy-2-nonenal, suggesting that Cys 553 may be inaccessible to chemical modification in the complex. Conversely, Cys 35 is solvent accessible in either case and is considerably more reactive than any of the other 5 cysteine residues of GCLM. These studies clearly indicate that Cys 553 of GCLC and Cys 35 of GCLM are reactive towards alkylating agents and these modifications impact GCL function in vitro, but direct adduct formation was not demonstrated in cell lysates or tissue samples.
Glycation
Recently, glycation of GCLM and GCLC has also been reported and this post-translational modification was shown to impact heterodimer formation, with modest effects on kinetic constants (Backos et al., 2013). Pre-treatment of individual subunits with 2-deoxy-D-ribose as a chemical modifier impaired heterodimer formation, but treatment of the holoenzyme had no apparent effect on oligomeric state. Extended incubations with 30 mM 2-deoxy-D-ribose (24 h) resulted in decreased activities for GCLC alone and the holoenzyme complex with no significant effects on the Km values for each substrate. Similarly, Ki values for glutathione remained unchanged. These results suggest that glycation inactivated the enzyme directly but slowly over time. As with 4-hydroxy-2-nonenal modification, glycation products of GCLC and GCLM were not identified in biological samples. Similarly, attempts to identify the sites of modification using recombinant protein were unsuccessful so it is difficult to assign functional significance to this observation. Cys 553 has been previously implicated in GCL complex formation (Tu and Anders, 1998) and thiol modification at this position may be an important mode of regulation. However, direct evidence for modification at Cys 553 under physiologically relevant conditions was not shown.
Glutathione Synthetase
GS is largely overlooked when considering the regulation of cellular glutathione levels. Human GS is a homodimeric enzyme that has been extensively characterized using both structural (Gogos and Shapiro, 2002) and kinetic approaches (Brown et al., 2011; Dinescu et al., 2010; Dinescu et al., 2004; Luo et al., 2000; Slavens et al., 2011; Snoke and Bloch, 1955). It is a member of the ATP-grasp superfamily (Fawaz et al., 2011) and rapidly catalyzes the ligation of γ-glutamylcysteine and glycine to generate glutathione. Based on these kinetic studies, flux through the glutathione biosynthetic pathway is thought to be largely controlled by GCL activity (Backos et al., 2011; Lu, 2013). Unlike GCL, GS is not feedback inhibited by GSH nor does it have an associated regulatory subunit. Furthermore, there have been no validated reports of post-translational modification of GS having any impact on enzymatic activity. As such, GS activity appears to be primarily controlled at the level of transcription and substrate availability.
GSH in development
Targeted disruption of either glutathione synthetase (Winkler et al., 2011) or the catalytic subunit of GCL (Dalton et al., 2000; Shi et al., 2000) results in an embryonic lethal phenotype in null homozygous mice, demonstrating the central importance of glutathione in development. Although GS null mice were not viable, heterozygous mice had no discernable phenotype other than a 50% reduction both in protein levels and enzymatic activity. Glutathione levels were normal and γ-glutamylcysteine did not accumulate, supporting the assertion that GCL activity is typically the limiting factor in glutathione production (Winkler et al., 2011). Similarly, heterozygous GCLC-deficient mice had no overt phenotype, but had approximately two-fold reductions in GCLC levels and GCLC activity (Dalton et al., 2000; Shi et al., 2000). However, glutathione levels were only reduced by approximately 20%, suggesting other compensatory mechanisms in heterozygous mice (Dalton et al., 2000). A liver-specific targeted disruption of GCLC did not impact embryogenesis or development, but severe abnormalities of the liver manifested within four weeks of birth (Chen et al., 2007). Plasma glutathione levels were reduced dramatically, although glutathione levels in peripheral tissues remained relatively unchanged. In contrast, GCLM deficient mice have less dramatic phenotypes (Yang et al., 2002). Without its regulatory subunit, GCLC activity and glutathione levels are reduced to between 10 – 40% of wild-type mice depending on the tissue, but no overt phenotype is observed in the absence of an applied stress.
GSH and cancer
Increased glutathione synthesis and the resulting lower levels of reactive oxygen species (ROS) have been suggested to confer a growth advantage to cancer cells (Traverso et al., 2013). In several human cancer types, including liver, skin, colorectal, lung, head and neck, breast, and prostate cancers, GCLC and glutathione levels are increased relative to normal tissue (Gamcsik et al., 2012). In a study involving human breast tumors, subpopulations of cancer stem cells were shown to have lower levels of ROS primarily through increased expression of GCLM and GS (Diehn et al., 2009). Pharmacological inhibition of glutathione synthesis by L-S,R-buthionine sulfoximine in these cancer stem cells reduced their ability to form colonies and increased their sensitivity to ionizing radiation. Similarly, L-S,R-buthionine sulfoximine treatment of MDA-MB231 human breast carcinoma cells enhanced 2-deoxy-D-glucose–induced cytotoxicity (Andringa et al., 2006). Experimentally induced reductions of GCLC protein levels and corresponding reductions in intracellular glutathione sensitized human colon cancer cells to cisplatin (Iida et al., 2001). Prenatal exposure to benzo[a]pyrene, a polycyclic aromatic hydrocarbon, led to increased premature ovarian failure and ovarian tumorigenesis in GCLM null mice relative to controls, due to their diminished glutathione levels (Lim et al., 2013). This subset of studies highlights the therapeutic potential of targeting GSH homeostasis in a variety of cancer types.
Glutathione Salvage
γ-Glutamyltranspeptidase
GGT initiates the degradation of extracellular glutathione. There are 7 potential full-length isoforms of the enzyme but only GGT1 and GGT5 have been validated as active transeptidases (Heisterkamp et al., 2008). GGT1 is the canonical GGT, found at the surface of epithelial cells and involved in glutathione salvage, whereas GGT5 appears to be primarily involved in leukotriene biosynthesis as well as the degradation of select glutathione conjugates (Carter et al., 1998). GGT is a member of the N-terminal nucleophile (Ntn) superfamily, which is functionally defined by an autocatalytic cleavage reaction that generates a new N-terminal residue within the protein (Oinonen and Rouvinen, 2000). This residue then serves as the nucleophile in the enzyme catalyzed reaction. Considerable detail is known about this autoprocessing and activation event based on studies of GΓT from several model organisms (Boanca et al., 2006; Boanca et al., 2007; Castonguay et al., 2003; Okada et al., 2006, 2007; Suzuki and Kumagai, 2002). Interestingly, organisms that do not maintain an intracellular pool of glutathione (e.g. H. pylori) express GΓT, suggesting that its main function is salvage of glutathione to provide a growth advantage (Shibayama et al., 2007). Recently, the crystal structure of human GGT1 was determined which will aid in detailed mechanistic and inhibitor design studies (West et al., 2013). A comprehensive review of GΓT can be found within this volume.
In response to oxidative stress, glutathione and glutathione conjugates are actively transported out the cell by multidrug resistance-associated proteins (Bachhawat et al., 2013). To recover the rapidly exported glutathione and glutathione conjugates, GGT initiates salvage, cleaving the γ-glutamyl peptide bond to generate cysteinylglycine and transferring the γ-glutamyl group to another amino acid or peptide. Intriguingly, Meister and co-workers provided evidence that the γ-glutamyl group could be transferred to cystine, imported into the cell, and reduced intracellularly to generate γ-glutamylcysteine independently of GCL (Thompson and Meister, 1975). The cysteinylglycine liberated is proposed to be cleaved by non-specific dipeptidases and cysteine and glycine imported into the cell. PEPT2, a di- and tripeptide transporter, has also been demonstrated to contribute to uptake of the intact cysteinylglycine (Frey et al., 2007).
γ-Glutamylcyclotransferase
Imported γ-glutamylpeptides are processed in the cell by GGCT, which cyclizes glutamate, generating 5-oxoproline and the free amino acid or peptide. Although GGCT was first purified nearly 40 years ago (Board et al., 1978; Orlowski et al., 1969; Palekar et al., 1974), the gene encoding this enzyme has only recently been identified (Oakley et al., 2008). It shares sequence and structural similarity to γ-glutamylamine cyclotransferase, which degrades γ-glutamyl-ɛ-lysine liberated from proteins covalently crosslinked by transglutaminases (Oakley et al., 2010). Furthermore, this enzyme family is broadly represented in bacteria, plants, and other higher eukaryotes. Initial studies by Meister and co-workers indicated that GGCT purified from rat liver has relatively broad substrate specificity, consistent with the relatively non-specific transfer of a γ-glutamyl moiety to acceptor substrates by GGT. These observations led to the proposal of a GGT/GGCT pathway for import of amino acids, as a component function of the γ-glutamyl cycle (Orlowski et al., 1969; Palekar et al., 1974). However, GGCT involvement in amino acid salvage has not been directly demonstrated using systems in which enzyme expression has been experimentally altered. Since the substrate specificity of human GGCT has not been extensively characterized, it remains unclear if the enzyme is involved in amino acid import, intracellular cleavage of γ-glutamylcysteine (Oakley et al., 2008), or apoptotic signaling (Masuda et al., 2006).
Several recent reports have suggested that increased expression of GGCT, initially designated as C7orf24, may have utility as a tumor marker. Using a proteomics approach, GGCT was found at higher levels in bladder urothelial carcinoma samples relative to normal controls. Manipulation of GGCT expression indicated that the enzyme provided a growth advantage to cancerous cells by an unknown mechanism (Kageyama et al., 2007). In human osteosarcoma, enzyme levels were considerably higher both in cell lines and primary tumors (Uejima et al., 2011). Knockdown of GGCT by siRNA resulted in cells with slower cell growth rates, increased clustering, and reduced invasiveness and motility as measured by standard in vitro methods, consistent with GGCT being involved in tumor progression (Uejima et al., 2011). Similarly, reduction of GGCT levels by siRNA inhibited the growth of human lung squamous cell carcinoma in a mouse model (Hama et al., 2012). GGCT is an effective diagnostic marker in esophageal squamous cell carcinoma, where higher levels of expression correlate with a more aggressive phenotype (Takemura et al., 2014). Unfortunately, the impacts on GSH levels within the cell were not reported in those studies in which GGCT levels were manipulated. Promoter analysis of GGCT suggests that its expression is cell-cycle dependent, consistent with a potential role in cancer cell proliferation (Ohno et al., 2011). In contrast, GGCT was initially characterized as a novel cytochrome c releasing factor, and overexpression in HeLa cells resulted in apoptosis, suggesting that GGCT may have alternate metabolic functions in different cell types (Masuda et al., 2006).
5-Oxoprolinase
Historically, the primary functions of 5-oxoprolinase (OPLAH) have been associated with glutathione salvage, with the enzyme catalyzing the ATP-dependent cleavage of 5-oxoproline to generate glutamate to complete the pathway (Griffith et al., 1978; Orlowski and Meister, 1970; Van der Werf et al., 1971). Recently, studies of the γ-glutamyl cycle suggest that 5-oxoproline levels may reflect nutritional status, particularly with respect to glycine availability (Friesen et al., 2007). When glycine becomes limiting, increased levels of 5-oxoproline are observed, stimulating amino acid uptake. This is consistent with the observation that 5-oxoproline levels increase dramatically in GS deficiency (Dahl et al., 1997; Wellner et al., 1974). This led to the suggestion that the conversion of γ-glutamylcysteine to 5-oxoproline and cysteine by GGCT may be the major source of 5-oxoproline (Oakley et al., 2008). However, there are examples of patients that present with 5-oxoprolinuria who have normal circulating glutathione concentrations despite increased 5-oxoproline levels (Calpena et al., 2013). These levels show glutathione homeostasis is normal in these patients. Therefore, 5-oxoproline may have additional functions, perhaps as a glutamate reservoir. In addition, the coordinate regulation of GGCT and OPLAH may provide another level of control in glutathione biosynthesis.
OPLAH protein levels and activity have been shown to decrease in some human tumor tissues, including lung and colon, although the extent and overall pattern are not conclusive (Chen et al., 1998). The authors suggest that differential expression of OPLAH between normal and cancerous tissue may be exploited by the use of the cysteine pro-drug, L-2-oxothiazolidine-4-carboxylic acid (OTZ). OPLAH can hydrolyze OTZ, and the resulting product spontaneously decarboxylates to generate cysteine. In this fashion, GSH production would be bolstered in normal cells relative to cancer cells. However, OPLAH levels are elevated in ovarian cancer (Chien et al., 2009) and unchanged in colon cancer (Chen et al., 1998), illustrating potential pitfalls of this general approach. In searching for potential cancer biomarkers, several groups have identified 5-oxoproline as a biomolecule that is more abundant in tumor cells, such as nasopharyngeal carcinoma (Tang et al., 2011) and bladder cancer (Kim et al., 2010). In addition, comparison of genome-wide methylation status in normal versus colorectal cancer cells revealed as a potential biomarker (Naumov et al., 2013). A comparable study also identified OPLAH as a potential marker in gastric cancer (Nanjo et al., 2012). In each of these studies, there was no direct association between OPLAH and glutathione metabolism reported. Given the diversity of metabolic fates for glutamate, it is likely that the originally proposed γ-glutamyl cycle is overly simplistic and comprehensive metabolic labeling studies are needed to examine flux of intermediates through the proposed cycle.
Precursor Availability
An often overlooked determinant of cellular glutathione levels is precursor availability. Glutamate, cysteine, and glycine are amino acids that intersect with major metabolic hubs that reflect on nitrogen and sulfur metabolism, one-carbon metabolism, and overall energy levels within the cell (Figure 5). Although cysteine is generally considered the limiting component of glutathione biosynthesis, glutamate and glycine partitioning can impact glutathione levels. An emerging trend in tumor cell biology is the concept of metabolic reprogramming to meet core demands on bioenergetics, biosynthesis, and redox homeostasis (Cairns et al., 2011). For example, Nrf2 is a well-studied transcriptional regulator responsible for mounting a response to xenobiotic and oxidative stress (Jaramillo and Zhang, 2013; Sporn and Liby, 2012). In addition to its involvement in the production of detoxifying enzymes, Nrf2 has recently been shown to participate in metabolic reprogramming, diverting glucose and glutamine to biosynthetic pathways (Mitsuishi et al., 2012). As discussed below, Nrf2 promotes purine nucleotide synthesis and glutamine metabolism. Stimulation of these pathways also provides precursors for increased glutathione synthesis and reducing equivalents, generated via the pentose phosphate pathway, to maintain GSH pools.
Figure 5. Precursors of glutathione synthesis.
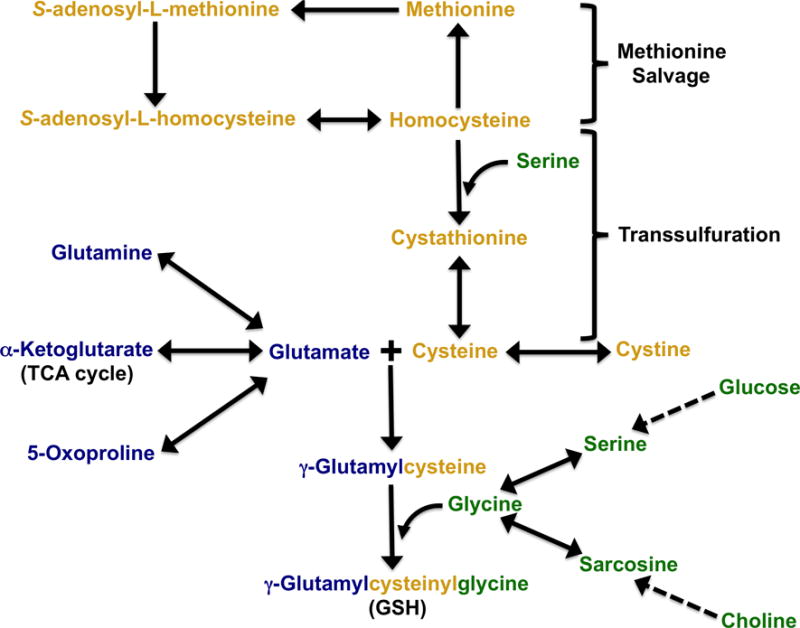
Metabolic routes for the three amino acids needed for GSH biosynthesis are outlined and color coded by amino acid provision pathways: yellow, cysteine, denotes intimate crosstalk with methionine metabolism (Lu and Mato, 2012; Zhang et al., 2005); green, glycine, is directly linked to carbohydrate, serine, and choline levels (Locasale, 2013); blue, glutamine/glutamate, indicates coordination with proline and TCA cycle metabolites (Daye and Wellen, 2012). Although not illustrated, one carbon metabolism links glycine metabolism and methionine salvage pathways.
Glutamate/Glutamine
Central to the concept of metabolic reprogramming is glutamine bioavailability, as some cancer cells have been shown to depend heavily on this amino acid for rapid proliferation and protection against ROS (Daye and Wellen, 2012; DeBerardinis et al., 2007; Le et al., 2012). Although considered a non-essential amino acid under normal metabolic conditions, glutamine becomes a conditional essential amino acid, needed for biosynthetic purposes. Glutamine can serve as a nitrogen donor in the production of amino sugars, nucleotides, and other amino acids. After donation of the γ-amino group, the remaining glutamate can be incorporated directly into glutathione or transformed into proline. Alternatively, its α-amino group can serve as a nitrogen donor via transamination to produce aspartate and alanine from oxaloacetate and pyruvate respectively, and through more complex biosynthesis pathways, integrated into threonine, serine, glycine, and cysteine. The residual carbon skeleton, α-ketoglutarate, can then feed into the citric acid cycle. As such, glutamine/glutamate availability is directly tied to biosynthesis of key biomolecules.
The complex regulatory network that controls glutamine metabolism shares considerable overlap with pathways implicated in tumor progression. Glutamine is rapidly taken up in HeLa (cervical adenocarcinoma) and RT112 (urinary bladder carcinoma) cells by SLC1A5 in a Na+-dependent manner. Accumulated intracellular glutamine drives the uptake of essential amino acids via SLC7A5/SLC3A2 mediated antiport (Nicklin et al., 2009). The resulting increase of intracellular leucine levels activates the mTORC1 pathway, and the increased availability of amino acid precursors stimulates proliferation. In contrast, mTORC1 signaling does not need to be primed by glutamine uptake in the breast cancer line, MCF-7, as sufficient levels of glutamine are present (Nicklin et al., 2009).
c-Myc has been shown to increase SLC1A5 and SLC7A5 mRNA levels and to stimulate glutaminolysis through suppression of miR-23a/b in human P-493 B lymphoma cells and PC3 prostate cancer cells (Gao et al., 2009). The resulting increase in mitochondrial glutaminase expression (GLS1) promoted flux through the tricarboxylic acid cycle (TCA) and more robust glutathione biosynthesis. siRNA-mediated knockdown of GLS1 in both the lymphoma and prostate cancer cell lines resulted in reduced ATP levels, diminished glutathione levels, and increased levels of ROS, firmly establishing glutamine metabolism as a target of c-Myc and as a potential target for cancer therapeutics.
Phosphate-activated mitochondrial glutaminase (GLS2) has also been shown to initiate glutaminolysis to protect against ROS through increased glutathione production (Suzuki et al., 2010). However, GLS2 has different kinetic constants and tissue distribution relative to GLS1, and is under the control of p53, a tumor suppressor. In this context, GLS2 is thought to contribute to tumor suppression as its overexpression inhibits tumor cell growth in cell culture models and GLS2 expression levels are down in liver tumors. In addition to stimulating GLS2 expression, p53 also activates TP53-inducible glycolysis and apoptosis regulator (TIGAR), which stimulates the pentose phosphate pathway, resulting in increased NADPH levels and, through the action of glutathione reductase, increased GSH/GSSG ratios. Although induction of GLS1 or GLS2 leads to increased antioxidant capacity as a result of increases in reduced glutathione levels, the opposing effects of GLS1 and GLS2 in tumor progression suggest that the timing of and the flux through glutaminolysis can lead to tumor promotion or suppression.
Cysteine
As discussed above, studies of GGT1 null mice suggested that one of the primary functions of glutathione was to serve as a means of conveyance for cysteine from the liver to peripheral tissue (Lieberman et al., 1996). This assertion is supported by the observation that the addition of N-acetylcysteine to the diet nearly restored the growth rate of the null animals. Thus, the GGT1-initiated degradation of glutathione ultimately leads to the liberation and import of cysteine into the cell. GGT1 expression is upregulated in several cancer types (Pompella et al., 2007; Strasak et al., 2008), and the prevailing hypothesis is that the ability to effectively scavenge extracellular glutathione confers a growth advantage to these metabolically demanding cells (Hanigan et al., 1999).
Another source of cysteine for glutathione biosynthesis originates from methionine (Lu and Mato, 2012). Methionine adenosyltransferase catalyzes the formation of S-adenosylmethionine (AdoMet, SAM) from methionine and ATP. AdoMet serves as a potent methyl donor in hundreds of biologically significant reactions with concomitant production of S-adenosylhomocysteine. Hydrolysis of S-adenosylhomocysteine produces homoscysteine that can be converted to methionine by either methionine synthase, using 5-methyltetrahydrofolate as a methyl donor, or betaine homocysteine methyltransferase, which requires the choline metabolite, betaine. Alternatively, homoscysteine can be partitioned into the transsulfuration pathway in which two PLP-dependent enzymes, cystathionine β-synthase and cystathionine γ-lyase, catalyze the condensation of homocysteine and serine to produce cystathionine, and its subsequent cleavage to α-ketobutyrate and cysteine.
A significant fraction of cysteine for glutathione biosynthesis can be produced via the transsulfuration pathway. For example, approximately half of the cysteine used for glutathione biosynthesis in HepG2 cells is acquired through the transsulfuration pathway (Mosharov et al., 2000). In contrast to the conclusions drawn from the studies of GGT1 null mice, targeted disruption of GCLC in hepatocytes did not lead to dramatic reductions in cysteine or glutathione levels in extrahepatic tissue (Chen et al., 2007). This suggests that tissues can derive significant amounts of cysteine from methionine, and are not solely dependent on salvage of cysteine from circulating glutathione.
Cystine import has recently emerged as an important process in maintaining intracellular cysteine levels. System xc− is a cysteine/glutamate antiporter composed of two subunits, xCT and 4F2 heavy chain. xCT null mice have higher plasma levels of cysteine and lower circulating levels of glutathione (Sato et al., 2005). Although not required for normal development, xCT is important in maintaining plasma redox balance, and there is considerable evidence that cystine is vital for cancer cell survival. Chronic lymphocytic leukaemia cells do not efficiently uptake cystine and require co-culture with bone-marrow-derived stromal cells to maintain cellular glutathione levels (Zhang et al., 2012). The stromal cells have high levels of system xc− and rapidly assimilate cystine, which is reduced intracellularly and secreted back into the microenvironment. The chronic lymphocytic leukaemia cells use the secreted cysteine for glutathione production and improved resistance to oxidative stress. Cancer stem cells have elevated levels of CD44 variants that have been shown to stabilize xCT, increasing cystine import and leading to increased intracellular glutathione levels (Ishimoto et al., 2011). In addition, Nrf2 signaling stimulates the expression of xCT to help combat increasing levels of oxidative stress (Sasaki et al., 2002).
Glycine
Glycine represents another critical biomolecule that connects glutathione to other metabolic processes (Locasale, 2013). Metabolic profiling studies have demonstrated that significant amounts of 3-phosphoglycerate are diverted from glycolysis into serine and ultimately glycine biosynthesis in cultured cancer cell lines (Jain et al., 2012). Phosphoglycerate dehydrogenase, which catalyzes the committed step in this biosynthetic pathway, has been found to be upregulated in many human cancer samples (Locasale et al., 2011). Additional studies have shown that glycine is used for the biosynthesis of purines in rapidly dividing cancer cells and may potentially serve as a one-carbon donor for downstream methylation events. In these studies, a significant fraction of exogenously added glycine was incorporated into glutathione. Overall, it was estimated that approximately two thirds of the total intracellular glycine arose from biosynthesis and the remainder from exogenous sources (Jain et al., 2012). The partitioning of glycine into its various metabolic fates allows cancer cells to control proliferation rates and increases capacity to combat oxidative stress.
Remaining questions and emerging pathways
5-Oxoproline
Although there have been a number of correlative and observational studies that indicate dynamic variations in 5-oxoproline levels, the precise cellular functions of 5-oxoproline beyond GSH salvage remain unclear. The concentrations in normal human plasma and various tissues are between 20 and 50 μM (Van Der Werf et al., 1975), but can reach several millimolar in pathological conditions, leading to anion gap metabolic acidosis and ataxia (Ristoff and Larsson, 2007). Several studies have suggested that 5-oxoproline levels indicate the availability of glycine and/or cysteine (Metges et al., 2000). In normal individuals consuming a low protein diet, elevated 5-oxoproline levels were reduced with increased protein content or supplementation with other sources of nitrogen, such as urea (Jackson et al., 1996). When cysteine is limiting, the γ-glutamylphosphate intermediate of GCL can spontaneously cyclize to form 5-oxoproline (Orlowski and Meister, 1971). Increased urinary excretion of 5-oxoproline has been noted in children gaining weight rapidly during recovery from severe malnutrition. Glycine supplementation restored 5-oxoproline to normal levels, suggesting that excess γ-glutamylcysteine was being converted to 5-oxoproline and cysteine (Meakins et al., 1998; Persaud et al., 1996). Similarly, a study of pregnant women and their newborn infants has shown an inverse correlation between 5-oxoproline and dimethylglycine, a glycine precursor (Friesen et al., 2007). Given the number of nutritional states that lead to increased 5-oxoproline production, it is surprising that OPLAH is not more effective in hydrolyzing this metabolite.
5-oxoprolinuria has been shown to be associated with urea cycle defects, such as ornithine transcarbamylase deficiency and carbamoyl phosphate synthetase deficiency (Mayatepek, 1999). Nephropathic cystinosis mimics the effects of cysteine deficiency and results from a genetic defect in the lysosomal cystine transporter, which leads to a high level of cystine accumulation in the lysosome with corresponding low levels of cysteine in the cytosol (Kumar and Bachhawat, 2010). Similarly, defects in cystathionine synthase, which converts homocysteine to cysteine, leads to elevated 5-oxoproline levels as well. Homocysteine can replace cysteine as a substrate for GCL, forming γ-glutamylhomocysteine. However, this dipeptide is not tolerated by GS, and is efficiently processed by GGCT to generate 5-oxoproline and homocysteine (Stokke et al., 1982). Perhaps 5-oxoproline can serve as a signaling molecule to alter metabolism and limit flux through unproductive metabolic pathways.
As with GGT1, OPLAH is a highly conserved enzyme across diverse species. For example, human and Methanosarcina acetivorans 5-oxoprolinase share ~45% sequence identity over the length of this nearly 1300 amino acid protein. This striking conservation is more remarkable considering that M. acetivorans do not appear to synthesize glutathione or have close homologues of either GGT or GGCT, suggesting that the enzyme may have additional enzymatic activities. Eukaryotic 5-oxoprolinases are homodimeric enzymes with two hydantoinase domains (HyuA and HyuB) per subunit. Hydantoinases can act on a number of 5- and 6-membered ring substrates, perhaps indicating that OPLAH may hydrolyze other substrates besides 5-oxoproline. Studies on the enzymatic properties of OPLAH have been relatively limited, and a large range of kinetic constants has been observed (Kumar and Bachhawat, 2010; Ohkama-Ohtsu et al., 2008; Williamson and Meister, 1982). The enzyme appears to require both monovalent and divalent cations to maintain catalytic activity and is stabilized by the presence of 5-oxoproline. Oxidation of the protein leads to inactivation (Van der Werf et al., 1971; Watanabe et al., 2004). A large-scale study by Ischiropoulos et al. reported that 5-oxoprolinase in mouse kidney, liver, and lung is S-nitrosylated (Doulias et al., 2013). The impacts of nitrosylation on the enzyme activity and stability have yet to be elucidated, but it is possible that some of the 24 cysteine residues found in human OPLAH may be sites of post-translational regulation. Addition studies are warranted to define the breadth of reactions catalyzed by OPLAH.
ChaC1
An exciting development has been the identification of a cytosolic pathway for the degradation of glutathione (Kumar et al., 2012). Human ChaC1 has the ability to cleave the γ-glutamyl group from glutathione specifically, as the enzyme had minimal activity with γ-glutamyl dipeptide substrates. The observed kinetic constants suggest ChaC1 activity is physiologically relevant, as the measured Km for glutathione was approximately 3 mM. Homology modeling of human ChaC1 indicated that the enzyme adopts a fold similar to GΓXT, despite low sequence identity. The validity of the model was confirmed by mutational analysis of ChaC1, in which a structurally conserved glutamate, Glu 116, was demonstrated to be critical for catalysis. In an organic sulphur auxotroph strain of S.cerevisiae, ChaC1 expression conferred the ability to grow on glutathione as the sole sulfur source. Its ability to initiate glutathione degradation is consistent with the recent observation that amino acid starvation induced by bacterial pathogens leads to dramatic upregulation of ChaC1 expression (Tattoli et al., 2012).
ChaC1 was initially identified as part of the Unfolded Protein Response (UPR) cascade, downstream of ATF4 (Mungrue et al., 2009). Overexpression of ChaC1 led to apoptosis whereas knockdown by siRNA attenuated apoptosis in HEK293 cells, consistent with its involvement in the UPR. Its ability to cleave the γ-glutamyl peptide bond of glutathione likely facilitates apoptosis, as lower GSH levels are a hallmark of this process. Similarly, in head and neck squamous cell carcinoma cells overexpression of ChaC1 resulted in slower proliferation rates and induced apoptosis (Joo et al., 2012). In contrast, neither overexpression nor reduction of ChaC1 protein levels impacted apoptosis in breast cancer and ovarian cancer lines. siRNA mediated knockdown of ChaC1 resulted in reduced migration and proliferation, whereas overexpression of ChaC1 increased migration and proliferation (Goebel et al., 2012). An analysis of human breast and ovarian tumors indicated that higher ChaC1 levels correlated with more advanced stage and poorer prognosis.
Overall, these studies highlight our limited understanding of ChaC1 function. Tumor cells generally tend to have higher levels of ROS and require elevated glutathione levels for survival. However, initial observations indicated that ChaC1 participated in apoptosis and GSH degradation. Therefore, it is surprising that breast and ovarian tumors would have higher ChaC1 levels. Perhaps variants of ChaC1 have alternate functions. There is some ambiguity as to the proper start site for translation of human ChaC1, as there is another potential initiator methionine that would result in a protein that is shorter by 43 amino acid residues. In addition, there are two isoforms of the enzyme, with variant 2 resulting in a 45 amino acid deletion near the center of the coding region. It is unclear how each of these changes would impact ChaC1 function and perhaps isoform distributions of ChaC1 may be an important consideration (Goebel et al., 2012). Currently only the standard ChaC1 isoform (Kumar et al., 2012) has been characterized in vitro and further studies are clearly needed.
Additional functions of γ-glutamylcysteine and glutathione
The contributions of γ-glutamylcysteine and glutathione to cellular function have been expanded in two recent studies. Mitochondrial γ-glutamylcysteine was shown to be sufficient to respond to oxidative stress irrespective of the cytsolic glutathione concentration (Quintana-Cabrera and Bolanos, 2013). Specifically, γ-glutamylcysteine was shown to be an enzymatic cofactor for glutathione peroxidase 1 (GPX1), and this system was able to control mitochondrial H2O2 concentrations to limit cellular damage. However, it is unclear how γ-glutamylcysteine is partitioned between detoxification and glutathione synthesis pathways and how oxidized γ-glutamylcysteine is reduced, as this process is not likely to be mediated by glutathione reductase.
Glutathione has been shown to participate in a futile cycle that depletes NADPH, leading to oxidative stress (Sullivan et al., 2013). Patients with hereditary leiomyomatosis and renal cell carcinoma (HLRCC) have reduced fumarate hydratase (fumarase) activity. As flux through the citric acid cycle is compromised, cells can accumulate fumarate, which serves as a proto-oncometabolite. Reduced glutathione adds across the double bond of fumarate to generate succinated glutathione. This recently identified metabolite is a substrate of glutathione reductase, which can regenerate reduced glutathione at the expense of NADPH. As a result, the NADPH ratio drops concomitant with an increase in oxidative stress, leading to stabilization of HIF1α. Identifying the potential contributions of succinated glutathione to disease progression in other tissue types is an exciting area of future research.
Summary
Glutathione is a critical detoxification precursor and a vital component of redox homeostasis thought to be especially beneficial in promoting growth and survival of tumor cells. Though the enzymes of the γ-glutamyl cycle have been studied over a period of several decades, new complexities have arisen through the characterization of genetically engineered mouse models that perturb glutathione levels without directly impacting disease progression. Further advances in technologies such as mass spectrometry have improved detection limits for relevant metabolites, and revealed points of regulated flux through glutathione synthesis and salvage pathways that intersect with other central metabolic pathways also subject to reprogramming in cancer. However, as these innovations have answered mechanistic questions about the roles of glutathione in cancer, new questions have come to the forefront. For example, increases in steady state glutathione within bloodborne and peripheral solid tumors are not accompanied consistently by upregulation of the biosynthetic or salvage enzymes, and liver tumors have actually shown reduced glutathione, despite the pivotal importance of glutathione to the metabolism of this tissue in general. To resolve these questions, it will be necessary to focus attention on the structural and functional regulation of the γ-glutamyl cycle enzymes and systematically assess glutathione with respect to each perturbation. It is also increasingly evident that the pathways of central metabolism that are altered to increase general biosynthetic potential in cancer (e.g.; serine and glycine metabolism, pentose phosphate pathway) are intimately related to glutathione levels. It is tempting to speculate that as tumor cells reprogram metabolism in response to microenvironment conditions, there may be co-evolution of γ-glutamyl cycle enzyme adaptations that optimally select with, or reciprocally to, the other central metabolic pathways. Detailed examination of metabolite flux in conjunction with the cross-talking pathways will be important to dissect these mechanisms and further understand the role of glutathione.
Acknowledgments
This work was made possible by National Institutes of Health Grants R01 GM077289 (J.J.B.), R01 CA165574 (M.A.S.), and P20 RR-17675 (National Center for Research Resources).
Contributor Information
Yilin Liu, Email: yliu2@unl.edu.
Annastasia S. Hyde, Email: annastasia.harris@huskers.unl.edu.
Melanie A. Simpson, Email: msimpson2@unl.edu.
Joseph J. Barycki, Email: jbarycki2@unl.edu.
References
- Andringa KK, Coleman MC, Aykin-Burns N, Hitchler MJ, Walsh SA, Domann FE, Spitz DR. Inhibition of glutamate cysteine ligase activity sensitizes human breast cancer cells to the toxicity of 2-deoxy-D-glucose. Cancer Res. 2006;66:1605–1610. doi: 10.1158/0008-5472.CAN-05-3462. [DOI] [PubMed] [Google Scholar]
- Bachhawat AK, Thakur A, Kaur J, Zulkifli M. Glutathione transporters. Biochim Biophys Acta. 2013;1830:3154–3164. doi: 10.1016/j.bbagen.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Backos DS, Fritz KS, McArthur DG, Kepa JK, Donson AM, Petersen DR, Foreman NK, Franklin CC, Reigan P. Glycation of glutamate cysteine ligase by 2-deoxy-d-ribose and its potential impact on chemoresistance in glioblastoma. Neurochemical research. 2013;38:1838–1849. doi: 10.1007/s11064-013-1090-4. [DOI] [PubMed] [Google Scholar]
- Backos DS, Fritz KS, Roede JR, Petersen DR, Franklin CC. Posttranslational modification and regulation of glutamate-cysteine ligase by the alpha,beta-unsaturated aldehyde 4-hydroxy-2-nonenal. Free Radic Biol Med. 2011;50:14–26. doi: 10.1016/j.freeradbiomed.2010.10.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biterova EI, Barycki JJ. Mechanistic details of glutathione biosynthesis revealed by crystal structures of Saccharomyces cerevisiae glutamate cysteine ligase. The Journal of biological chemistry. 2009;284:32700–32708. doi: 10.1074/jbc.M109.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boanca G, Sand A, Barycki JJ. Uncoupling the enzymatic and autoprocessing activities of Helicobacter pylori gamma-glutamyltranspeptidase. The Journal of biological chemistry. 2006;281:19029–19037. doi: 10.1074/jbc.M603381200. [DOI] [PubMed] [Google Scholar]
- Boanca G, Sand A, Okada T, Suzuki H, Kumagai H, Fukuyama K, Barycki JJ. Autoprocessing of Helicobacter pylori gamma-glutamyltranspeptidase leads to the formation of a threonine-threonine catalytic dyad. The Journal of biological chemistry. 2007;282:534–541. doi: 10.1074/jbc.M607694200. [DOI] [PubMed] [Google Scholar]
- Board PG, Moore KA, Smith JE. Purification and properties of gamma-glutamylcyclotransferase from human erythrocytes. The Biochemical journal. 1978;173:427–431. doi: 10.1042/bj1730427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Brown TR, Drummond ML, Barelier S, Crutchfield AS, Dinescu A, Slavens KD, Cundari TR, Anderson ME. Aspartate 458 of human glutathione synthetase is important for cooperativity and active site structure. Biochem Biophys Res Commun. 2011;411:536–542. doi: 10.1016/j.bbrc.2011.06.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature reviews Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Calpena E, Casado M, Martinez-Rubio D, Nascimento A, Colomer J, Gargallo E, Garcia-Cazorla A, Palau F, Artuch R, Espinos C. 5-Oxoprolinuria in Heterozygous Patients for 5-Oxoprolinase (OPLAH) Missense Changes. JIMD reports. 2013;7:123–128. doi: 10.1007/8904_2012_166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, Shi ZZ, Barrios R, Lieberman MW. gamma-glutamyl leukotrienase, a gamma-glutamyl transpeptidase gene family member, is expressed primarily in spleen. J Biol Chem. 1998;273:28277–28285. doi: 10.1074/jbc.273.43.28277. [DOI] [PubMed] [Google Scholar]
- Castonguay R, Lherbet C, Keillor JW. Kinetic studies of rat kidney gamma-glutamyltranspeptidase deacylation reveal a general base-catalyzed mechanism. Biochemistry. 2003;42:11504–11513. doi: 10.1021/bi035064b. [DOI] [PubMed] [Google Scholar]
- Chen X, Schecter RL, Griffith OW, Hayward MA, Alpert LC, Batist G. Characterization of 5-oxo-L-prolinase in normal and tumor tissues of humans and rats: a potential new target for biochemical modulation of glutathione. Clin Cancer Res. 1998;4:131–138. [PubMed] [Google Scholar]
- Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Y, Miller ML, Shen D, Shertzer HG, Stringer KF, Wang B, Schneider SN, Nebert DW, Dalton TP. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology. 2007;45:1118–1128. doi: 10.1002/hep.21635. [DOI] [PubMed] [Google Scholar]
- Chien J, Fan JB, Bell DA, April C, Klotzle B, Ota T, Lingle WL, Gonzalez Bosquet J, Shridhar V, Hartmann LC. Analysis of gene expression in stage I serous tumors identifies critical pathways altered in ovarian cancer. Gynecologic oncology. 2009;114:3–11. doi: 10.1016/j.ygyno.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Currais A, Maher P. Functional consequences of age-dependent changes in glutathione status in the brain. Antioxid Redox Signal. 2013;19:813–822. doi: 10.1089/ars.2012.4996. [DOI] [PubMed] [Google Scholar]
- Dahl N, Pigg M, Ristoff E, Gali R, Carlsson B, Mannervik B, Larsson A, Board P. Missense mutations in the human glutathione synthetase gene result in severe metabolic acidosis, 5-oxoprolinuria, hemolytic anemia and neurological dysfunction. Human molecular genetics. 1997;6:1147–1152. doi: 10.1093/hmg/6.7.1147. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Yang Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem Biophys Res Commun. 2000;279:324–329. doi: 10.1006/bbrc.2000.3930. [DOI] [PubMed] [Google Scholar]
- Daye D, Wellen KE. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Seminars in cell & developmental biology. 2012;23:362–369. doi: 10.1016/j.semcdb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinescu A, Brown TR, Barelier S, Cundari TR, Anderson ME. The role of the glycine triad in human glutathione synthetase. Biochem Biophys Res Commun. 2010;400:511–516. doi: 10.1016/j.bbrc.2010.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinescu A, Cundari TR, Bhansali VS, Luo JL, Anderson ME. Function of conserved residues of human glutathione synthetase: implications for the ATP-grasp enzymes. J Biol Chem. 2004;279:22412–22421. doi: 10.1074/jbc.M401334200. [DOI] [PubMed] [Google Scholar]
- Doulias PT, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Science signaling. 2013;6:rs1. doi: 10.1126/scisignal.2003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Liu X, Hao S, Wang B, Robinson ML, Monnier VM. The LEGSKO mouse: a mouse model of age-related nuclear cataract based on genetic suppression of lens glutathione synthesis. PloS one. 2012;7:e50832. doi: 10.1371/journal.pone.0050832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawaz MV, Topper ME, Firestine SM. The ATP-grasp enzymes. Bioorganic chemistry. 2011;39:185–191. doi: 10.1016/j.bioorg.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Krejsa CM, Pierce RH, White CC, Fausto N, Kavanagh TJ. Caspase-3-Dependent Cleavage of the Glutamate-L-Cysteine Ligase Catalytic Subunit during Apoptotic Cell Death. Am J Pathol. 2002;160:1887–1894. doi: 10.1016/S0002-9440(10)61135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JA, Kansagra P, Kotecki C, Saunders RD, McLellan LI. The modifier subunit of Drosophila glutamate-cysteine ligase regulates catalytic activity by covalent and noncovalent interactions and influences glutathione homeostasis in vivo. J Biol Chem. 2003;278:46369–46377. doi: 10.1074/jbc.M308035200. [DOI] [PubMed] [Google Scholar]
- Frey IM, Rubio-Aliaga I, Siewert A, Sailer D, Drobyshev A, Beckers J, de Angelis MH, Aubert J, Bar Hen A, Fiehn O, Eichinger HM, Daniel H. Profiling at mRNA, protein, and metabolite levels reveals alterations in renal amino acid handling and glutathione metabolism in kidney tissue of Pept2−/− mice. Physiological genomics. 2007;28:301–310. doi: 10.1152/physiolgenomics.00193.2006. [DOI] [PubMed] [Google Scholar]
- Friesen RW, Novak EM, Hasman D, Innis SM. Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J Nutr. 2007;137:2641–2646. doi: 10.1093/jn/137.12.2641. [DOI] [PubMed] [Google Scholar]
- Gamcsik MP, Kasibhatla MS, Teeter SD, Colvin OM. Glutathione levels in human tumors. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 2012;17:671–691. doi: 10.3109/1354750X.2012.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel G, Berger R, Strasak AM, Egle D, Muller-Holzner E, Schmidt S, Rainer J, Presul E, Parson W, Lang S, Jones A, Widschwendter M, Fiegl H. Elevated mRNA expression of CHAC1 splicing variants is associated with poor outcome for breast and ovarian cancer patients. British journal of cancer. 2012;106:189–198. doi: 10.1038/bjc.2011.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos A, Shapiro L. Large conformational changes in the catalytic cycle of glutathione synthase. Structure. 2002;10:1669–1676. doi: 10.1016/s0969-2126(02)00906-1. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Bridges RJ, Meister A. Evidence that the gamma-glutamyl cycle functions in vivo using intracellular glutathione: effects of amino acids and selective inhibition of enzymes. Proc Natl Acad Sci U S A. 1978;75:5405–5408. doi: 10.1073/pnas.75.11.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol. 1999;73:209–267. doi: 10.1002/9780470123195.ch7. xii. [DOI] [PubMed] [Google Scholar]
- Hama S, Arata M, Nakamura I, Kasetani T, Itakura S, Tsuchiya H, Yoshiki T, Kogure K. Prevention of tumor growth by needle-free jet injection of anti-C7orf24 siRNA. Cancer Gene Ther. 2012;19:553–557. doi: 10.1038/cgt.2012.31. [DOI] [PubMed] [Google Scholar]
- Hanigan MH, Gallagher BC, Townsend DM, Gabarra V. Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis. 1999;20:553–559. doi: 10.1093/carcin/20.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry. 1993;32:6302–6306. doi: 10.1021/bi00075a026. [DOI] [PubMed] [Google Scholar]
- Harding JJ. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 1970;117:957–960. doi: 10.1042/bj1170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N, Groffen J, Warburton D, Sneddon TP. The human gamma-glutamyltransferase gene family. Human genetics. 2008;123:321–332. doi: 10.1007/s00439-008-0487-7. [DOI] [PubMed] [Google Scholar]
- Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993;268:19675–19680. [PubMed] [Google Scholar]
- Iida T, Kijima H, Urata Y, Goto S, Ihara Y, Oka M, Kohno S, Scanlon KJ, Kondo T. Hammerhead ribozyme against gamma-glutamylcysteine synthetase sensitizes human colonic cancer cells to cisplatin by down-regulating both the glutathione synthesis and the expression of multidrug resistance proteins. Cancer Gene Ther. 2001;8:803–814. doi: 10.1038/sj.cgt.7700371. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Taniguchi N. Gene expression of gamma-glutamyltranspeptidase. Methods Enzymol. 2005;401:408–425. doi: 10.1016/S0076-6879(05)01025-6. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Jackson AA, Persaud C, Meakins TS, Bundy R. Urinary excretion of 5-L-oxoproline (pyroglutamic acid) is increased in normal adults consuming vegetarian or low protein diets. J Nutr. 1996;126:2813–2822. doi: 10.1093/jn/126.11.2813. [DOI] [PubMed] [Google Scholar]
- Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo NE, Ritchie K, Kamarajan P, Miao D, Kapila YL. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer medicine. 2012;1:295–305. doi: 10.1002/cam4.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama S, Iwaki H, Inoue H, Isono T, Yuasa T, Nogawa M, Maekawa T, Ueda M, Kajita Y, Ogawa O, Toguchida J, Yoshiki T. A novel tumor-related protein, C7orf24, identified by proteome differential display of bladder urothelial carcinoma. Proteomics Clin Appl. 2007;1:192–199. doi: 10.1002/prca.200600468. [DOI] [PubMed] [Google Scholar]
- Keillor JW, Castonguay R, Lherbet C. Gamma-glutamyl transpeptidase substrate specificity and catalytic mechanism. Methods Enzymol. 2005;401:449–467. doi: 10.1016/S0076-6879(05)01027-X. [DOI] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lee G, Moon SM, Park MJ, Hong SK, Ahn YH, Kim KR, Paik MJ. Metabolomic screening and star pattern recognition by urinary amino acid profile analysis from bladder cancer patients. Metabolomics. 2010;6:202–206. [Google Scholar]
- Kinlough CL, Poland PA, Bruns JB, Hughey RP. Gamma-glutamyltranspeptidase: disulfide bridges, propeptide cleavage, and activation in the endoplasmic reticulum. Methods Enzymol. 2005;401:426–449. doi: 10.1016/S0076-6879(05)01026-8. [DOI] [PubMed] [Google Scholar]
- Krejsa CM, Franklin CC, White CC, Ledbetter JA, Schieven GL, Kavanagh TJ. Rapid activation of glutamate cysteine ligase following oxidative stress. J Biol Chem. 2010;285:16116–16124. doi: 10.1074/jbc.M110.116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bachhawat AK. OXP1/YKL215c encodes an ATP-dependent 5-oxoprolinase in Saccharomyces cerevisiae: functional characterization, domain structure and identification of actin-like ATP-binding motifs in eukaryotic 5-oxoprolinases. FEMS yeast research. 2010;10:394–401. doi: 10.1111/j.1567-1364.2010.00619.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Tikoo S, Maity S, Sengupta S, Sengupta S, Kaur A, Bachhawat AK. Mammalian proapoptotic factor ChaC1 and its homologues function as gamma-glutamyl cyclotransferases acting specifically on glutathione. EMBO Rep. 2012;13:1095–1101. doi: 10.1038/embor.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BK, Penrose JF, Freeman GJ, Austen KF. Expression cloning of a cDNA for human leukotriene C4 synthase, an integral membrane protein conjugating reduced glutathione to leukotriene A4. Proc Natl Acad Sci U S A. 1994;91:7663–7667. doi: 10.1073/pnas.91.16.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, Zimmerman LJ, Liebler DC, Slebos RJ, Lorkiewicz PK, Higashi RM, Fan TW, Dang CV. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell metabolism. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MW, Wiseman AL, Shi ZZ, Carter BZ, Barrios R, Ou CN, Chevez-Barrios P, Wang Y, Habib GM, Goodman JC, Huang SL, Lebovitz RM, Matzuk MM. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:7923–7926. doi: 10.1073/pnas.93.15.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Lim J, Lawson GW, Nakamura BN, Ortiz L, Hur JA, Kavanagh TJ, Luderer U. Glutathione-deficient mice have increased sensitivity to transplacental benzo[a]pyrene-induced premature ovarian failure and ovarian tumorigenesis. Cancer Res. 2013;73:908–917. doi: 10.1158/0008-5472.CAN-12-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nature reviews. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, Sasaki AT, Anastasiou D, Mullarky E, Vokes NI, Sasaki M, Beroukhim R, Stephanopoulos G, Ligon AH, Meyerson M, Richardson AL, Chin L, Wagner G, Asara JM, Brugge JS, Cantley LC, Vander Heiden MG. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Kuhlenkamp J, Garcia-Ruiz C, Kaplowitz N. Hormone-mediated down-regulation of hepatic glutathione synthesis in the rat. J Clin Invest. 1991;88:260–269. doi: 10.1172/JCI115286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiological reviews. 2012;92:1515–1542. doi: 10.1152/physrev.00047.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Huang CS, Babaoglu K, Anderson ME. Novel kinetics of mammalian glutathione synthetase: characterization of gamma-glutamyl substrate cooperative binding. Biochem Biophys Res Commun. 2000;275:577–581. doi: 10.1006/bbrc.2000.3337. [DOI] [PubMed] [Google Scholar]
- Mannervik B. Five decades with glutathione and the GSTome. J Biol Chem. 2012;287:6072–6083. doi: 10.1074/jbc.X112.342675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Maeda S, Watanabe A, Sano Y, Aiuchi T, Nakajo S, Itabe H, Nakaya K. A novel 21-kDa cytochrome c-releasing factor is generated upon treatment of human leukemia U937 cells with geranylgeraniol. Biochem Biophys Res Commun. 2006;346:454–460. doi: 10.1016/j.bbrc.2006.05.161. [DOI] [PubMed] [Google Scholar]
- Mayatepek E. 5-Oxoprolinuria in patients with and without defects in the gamma-glutamyl cycle. Eur J Pediatr. 1999;158:221–225. doi: 10.1007/s004310051054. [DOI] [PubMed] [Google Scholar]
- Meakins TS, Persaud C, Jackson AA. Dietary supplementation with L-methionine impairs the utilization of urea-nitrogen and increases 5-L-oxoprolinuria in normal women consuming a low protein diet. J Nutr. 1998;128:720–727. doi: 10.1093/jn/128.4.720. [DOI] [PubMed] [Google Scholar]
- Meister A. On the enzymology of amino acid transport. Science. 1973;180:33–39. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- Meister A. The gamma-glutamyl cycle. Diseases associated with specific enzyme deficiencies. Ann Intern Med. 1974;81:247–253. doi: 10.7326/0003-4819-81-2-247. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annual review of biochemistry. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Metges CC, Yu YM, Cai W, Lu XM, Wong S, Regan MM, Ajami A, Young VR. Oxoproline kinetics and oxoproline urinary excretion during glycine- or sulfur amino acid-free diets in humans. Am J Physiol Endocrinol Metab. 2000;278:E868–876. doi: 10.1152/ajpendo.2000.278.5.E868. [DOI] [PubMed] [Google Scholar]
- Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- Mungrue IN, Pagnon J, Kohannim O, Gargalovic PS, Lusis AJ. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J Immunol. 2009;182:466–476. doi: 10.4049/jimmunol.182.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjo S, Asada K, Yamashita S, Nakajima T, Nakazawa K, Maekita T, Ichinose M, Sugiyama T, Ushijima T. Identification of gastric cancer risk markers that are informative in individuals with past H. pylori infection. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2012;15:382–388. doi: 10.1007/s10120-011-0126-1. [DOI] [PubMed] [Google Scholar]
- Naumov VA, Generozov EV, Zaharjevskaya NB, Matushkina DS, Larin AK, Chernyshov SV, Alekseev MV, Shelygin YA, Govorun VM. Genome-scale analysis of DNA methylation in colorectal cancer using Infinium HumanMethylation450 BeadChips. Epigenetics: official journal of the DNA Methylation Society. 2013;8:921–934. doi: 10.4161/epi.25577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AJ, Coggan M, Board PG. Identification and characterization of gamma-glutamylamine cyclotransferase, an enzyme responsible for gamma-glutamyl-epsilon-lysine catabolism. The Journal of biological chemistry. 2010;285:9642–9648. doi: 10.1074/jbc.M109.082099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AJ, Yamada T, Liu D, Coggan M, Clark AG, Board PG. The identification and structural characterization of C7orf24 as gamma-glutamyl cyclotransferase. An essential enzyme in the gamma-glutamyl cycle. The Journal of biological chemistry. 2008;283:22031–22042. doi: 10.1074/jbc.M803623200. [DOI] [PubMed] [Google Scholar]
- Ochi T. Hydrogen peroxide increases the activity of gamma-glutamylcysteine synthetase in cultured Chinese hamster V79 cells. Arch Toxicol. 1995;70:96–103. doi: 10.1007/BF02733669. [DOI] [PubMed] [Google Scholar]
- Ochi T. Menadione causes increases in the level of glutathione and in the activity of gamma-glutamylcysteine synthetase in cultured Chinese hamster V79 cells. Toxicology. 1996;112:45–55. doi: 10.1016/0300-483x(96)03348-3. [DOI] [PubMed] [Google Scholar]
- Ohkama-Ohtsu N, Oikawa A, Zhao P, Xiang C, Saito K, Oliver DJ. A gamma-glutamyl transpeptidase-independent pathway of glutathione catabolism to glutamate via 5-oxoproline in Arabidopsis. Plant physiology. 2008;148:1603–1613. doi: 10.1104/pp.108.125716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Hattori A, Ueda M, Kageyama S, Yoshiki T, Kakeya H. Multiple NF-Y-binding CCAAT boxes are essential for transcriptional regulation of the human C7orf24 gene, a novel tumor-associated gene. Febs J. 2011;278:4088–4099. doi: 10.1111/j.1742-4658.2011.08314.x. [DOI] [PubMed] [Google Scholar]
- Oinonen C, Rouvinen J. Structural comparison of Ntn-hydrolases. Protein Sci. 2000;9:2329–2337. doi: 10.1110/ps.9.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Suzuki H, Wada K, Kumagai H, Fukuyama K. Crystal structures of gamma-glutamyltranspeptidase from Escherichia coli, a key enzyme in glutathione metabolism, and its reaction intermediate. Proc Natl Acad Sci U S A. 2006;103:6471–6476. doi: 10.1073/pnas.0511020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Suzuki H, Wada K, Kumagai H, Fukuyama K. Crystal structure of the gamma-glutamyltranspeptidase precursor protein from Escherichia coli - Structural changes autocatalytic processing and implications for the maturation mechanism. J Biol Chem. 2007;282:2433–2439. doi: 10.1074/jbc.M607490200. [DOI] [PubMed] [Google Scholar]
- Orlowski M, Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970;67:1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M, Meister A. Partial reactions catalyzed by -glutamylcysteine synthetase and evidence for an activated glutamate intermediate. J Biol Chem. 1971;246:7095–7105. [PubMed] [Google Scholar]
- Orlowski M, Richman PG, Meister A. Isolation and properties of gamma-L-glutamylcyclotransferase from human brain. Biochemistry. 1969;8:1048–1055. doi: 10.1021/bi00831a036. [DOI] [PubMed] [Google Scholar]
- Palekar AG, Tate SS, Meister A. Formation of 5-oxoproline from glutathione in erythrocytes by the gamma-glutamyltranspeptidase-cyclotransferase pathway. Proc Natl Acad Sci U S A. 1974;71:293–297. doi: 10.1073/pnas.71.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud C, Forrester T, Jackson AA. Urinary excretion of 5-L-oxoproline (pyroglutamic acid) is increased during recovery from severe childhood malnutrition and responds to supplemental glycine. J Nutr. 1996;126:2823–2830. doi: 10.1093/jn/126.11.2823. [DOI] [PubMed] [Google Scholar]
- Pompella A, Corti A, Paolicchi A, Giommarelli C, Zunino F. Gamma-glutamyltransferase, redox regulation and cancer drug resistance. Current opinion in pharmacology. 2007;7:360–366. doi: 10.1016/j.coph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Quintana-Cabrera R, Bolanos JP. Glutathione and gamma-glutamylcysteine in the antioxidant and survival functions of mitochondria. Biochemical Society transactions. 2013;41:106–110. doi: 10.1042/BST20120252. [DOI] [PubMed] [Google Scholar]
- Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975;250:1422–1426. [PubMed] [Google Scholar]
- Ristoff E, Larsson A. Inborn errors in the metabolism of glutathione. Orphanet journal of rare diseases. 2007;2:16. doi: 10.1186/1750-1172-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem. 2002;277:44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, Makino N, Sugiyama F, Yagami K, Moriguchi T, Takahashi S, Bannai S. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280:37423–37429. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- Seelig GF, Simondsen RP, Meister A. Reversible dissociation of gamma-glutamylcysteine synthetase into two subunits. J Biol Chem. 1984;259:9345–9347. [PubMed] [Google Scholar]
- Sekura R, Meister A. Glutathione turnover in the kidney; considerations relating to the gamma-glutamyl cycle and the transport of amino acids. Proc Natl Acad Sci U S A. 1974;71:2969–2972. doi: 10.1073/pnas.71.8.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZZ, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, Danney CM, Matzuk MM, Lieberman MW. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci U S A. 2000;97:5101–5106. doi: 10.1073/pnas.97.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama K, Wachino J, Arakawa Y, Saidijam M, Rutherford NG, Henderson PJF. Metabolism of glutamine and glutathione via gamma-glutamyltranspeptidase and glutamate transport in Helicobacter pylori: possible significance in the pathophysiology of the organism. Mol Microbiol. 2007;64:396–406. doi: 10.1111/j.1365-2958.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- Siitonen T, Alaruikka P, Mantymaa P, Savolainen ER, Kavanagh TJ, Krejsa CM, Franklin CC, Kinnula V, Koistinen P. Protection of acute myeloblastic leukemia cells against apoptotic cell death by high glutathione and gamma-glutamylcysteine synthetase levels during etoposide-induced oxidative stress. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 1999;10:1361–1367. doi: 10.1023/a:1008382912096. [DOI] [PubMed] [Google Scholar]
- Slavens KD, Brown TR, Barakat KA, Cundari TR, Anderson ME. Valine 44 and valine 45 of human glutathione synthetase are key for subunit stability and negative cooperativity. Biochem Biophys Res Commun. 2011;410:597–601. doi: 10.1016/j.bbrc.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoke JE, Bloch K. Studies on the mechanism of action of glutathione synthetase. J Biol Chem. 1955;213:825–835. [PubMed] [Google Scholar]
- Sousa Silva M, Gomes RA, Ferreira AE, Ponces Freire A, Cordeiro C. The glyoxalase pathway: the first hundred years… and beyond. Biochem J. 2013;453:1–15. doi: 10.1042/BJ20121743. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nature reviews. Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke O, Marstein S, Jellum E, Lie SO. Accumulation of pyroglutamic acid (5-oxoproline) in homocystinuria. Scand J Clin Lab Invest. 1982;42:361–369. [PubMed] [Google Scholar]
- Strasak AM, Pfeiffer RM, Klenk J, Hilbe W, Oberaigner W, Gregory M, Concin H, Diem G, Pfeiffer KP, Ruttmann E, Ulmer H, Vorarlberg Health, M., Promotion Program Study, G Prospective study of the association of gamma-glutamyltransferase with cancer incidence in women. Int J Cancer. 2008;123:1902–1906. doi: 10.1002/ijc.23714. [DOI] [PubMed] [Google Scholar]
- Sullivan LB, Martinez-Garcia E, Nguyen H, Mullen AR, Dufour E, Sudarshan S, Licht JD, Deberardinis RJ, Chandel NS. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Molecular cell. 2013;51:236–248. doi: 10.1016/j.molcel.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WM, Huang ZZ, Lu SC. Regulation of gamma-glutamylcysteine synthetase by protein phosphorylation. Biochem J. 1996;320(Pt 1):321–328. doi: 10.1042/bj3200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kumagai H. Autocatalytic processing of gamma-glutamyltranspeptidase. J Biol Chem. 2002;277:43536–43543. doi: 10.1074/jbc.M207680200. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y, Sugano S, Sato E, Nagao T, Yokote K, Tatsuno I, Prives C. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci U S A. 2010;107:7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura K, Kawachi H, Eishi Y, Kitagaki K, Negi M, Kobayashi M, Uchida K, Inoue J, Inazawa J, Kawano T, Board PG. gamma-Glutamylcyclotransferase as a novel immunohistochemical biomarker for the malignancy of esophageal squamous tumors. Hum Pathol. 2014;45:331–341. doi: 10.1016/j.humpath.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Tang F, Xie C, Huang D, Wu Y, Zeng M, Yi L, Wang Y, Mei W, Cao Y, Sun L. Novel potential markers of nasopharyngeal carcinoma for diagnosis and therapy. Clinical biochemistry. 2011;44:711–718. doi: 10.1016/j.clinbiochem.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Tattoli I, Sorbara MT, Vuckovic D, Ling A, Soares F, Carneiro LA, Yang C, Emili A, Philpott DJ, Girardin SE. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell host & microbe. 2012;11:563–575. doi: 10.1016/j.chom.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Thompson GA, Meister A. Utilization of L-cystine by the gamma-glutamyl transpeptidase-gamma-glutamyl cyclotransferase pathway. Proc Natl Acad Sci U S A. 1975;72:1985–1988. doi: 10.1073/pnas.72.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxidative medicine and cellular longevity. 2013;2013:972913. doi: 10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z, Anders MW. Identification of an important cysteine residue in human glutamate-cysteine ligase catalytic subunit by site-directed mutagenesis. Biochem J. 1998;336(Pt 3):675–680. doi: 10.1042/bj3360675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uejima D, Nishijo K, Kajita Y, Ishibe T, Aoyama T, Kageyama S, Iwaki H, Nakamura T, Iida H, Yoshiki T, Toguchida J. Involvement of cancer biomarker C7orf24 in the growth of human osteosarcoma. Anticancer Res. 2011;31:1297–1305. [PubMed] [Google Scholar]
- Van Der Werf P, Griffith OW, Meister A. 5-Oxo-L-prolinase (L-pyroglutamate hydrolase). Purification and catalytic properties. J Biol Chem. 1975;250:6686–6692. [PubMed] [Google Scholar]
- Van der Werf P, Orlowski M, Meister A. Enzymatic conversion of 5-oxo-L-proline (L-pyrrolidone carboxylate) to L-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the -glutamyl cycle. Proc Natl Acad Sci U S A. 1971;68:2982–2985. doi: 10.1073/pnas.68.12.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf P, Stephani RA, Meister A. Accumulation of 5-oxoproline in mouse tissues after inhibition of 5-oxoprolinase and administration of amino acids: evidence for function of the gamma-glutamyl cycle. Proc Natl Acad Sci U S A. 1974;71:1026–1029. doi: 10.1073/pnas.71.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50:335–356. [PubMed] [Google Scholar]
- Watanabe T, Abe K, Ishikawa H, Iijima Y. Bovine 5-oxo-L-prolinase: simple assay method, purification, cDNA cloning, and detection of mRNA in the coronary artery. Biological & pharmaceutical bulletin. 2004;27:288–294. doi: 10.1248/bpb.27.288. [DOI] [PubMed] [Google Scholar]
- Wellner VP, Sekura R, Meister A, Larsson A. Glutathione synthetase deficiency, an inborn error of metabolism involving the gamma-glutamyl cycle in patients with 5-oxoprolinuria (pyroglutamic aciduria) Proc Natl Acad Sci U S A. 1974;71:2505–2509. doi: 10.1073/pnas.71.6.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch DJ, Creely DP, Hauser SD, Mathis KJ, Krivi GG, Isakson PC. Molecular cloning and expression of human leukotriene-C4 synthase. Proc Natl Acad Sci U S A. 1994;91:9745–9749. doi: 10.1073/pnas.91.21.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MB, Chen Y, Wickham S, Heroux A, Cahill K, Hanigan MH, Mooers BH. Novel insights into eukaryotic gamma-glutamyltranspeptidase 1 from the crystal structure of the glutamate-bound human enzyme. J Biol Chem. 2013;288:31902–31913. doi: 10.1074/jbc.M113.498139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JM, Meister A. New substrates of 5-oxo-L-prolinase. The Journal of biological chemistry. 1982;257:12039–12042. [PubMed] [Google Scholar]
- Willis MN, Liu Y, Biterova EI, Simpson MA, Kim H, Lee J, Barycki JJ. Enzymatic defects underlying hereditary glutamate cysteine ligase deficiency are mitigated by association of the catalytic and regulatory subunits. Biochemistry. 2011;50:6508–6517. doi: 10.1021/bi200708w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A, Njalsson R, Carlsson K, Elgadi A, Rozell B, Abraham L, Ercal N, Shi ZZ, Lieberman MW, Larsson A, Norgren S. Glutathione is essential for early embryogenesis–analysis of a glutathione synthetase knockout mouse. Biochem Biophys Res Commun. 2011;412:121–126. doi: 10.1016/j.bbrc.2011.07.056. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem. 2002;277:49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Soberman RJ, Lewis RA, Austen KF. Isolation and characterization of leukotriene C4 synthetase of rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1985;82:8399–8403. doi: 10.1073/pnas.82.24.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Braun A, Bauman Z, Olteanu H, Madzelan P, Banerjee R. Expression profiling of homocysteine junction enzymes in the NCI60 panel of human cancer cell lines. Cancer Res. 2005;65:1554–1560. doi: 10.1158/0008-5472.CAN-04-1554. [DOI] [PubMed] [Google Scholar]
- Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, Lu W, Burger JA, Croce CM, Plunkett W, Keating MJ, Huang P. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nature cell biology. 2012;14:276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]


