Abstract
Background
Hyperthermic isolated limb perfusion (HILP) or isolated limb infusion (ILI) are well-accepted regional chemotherapy techniques for in-transit melanoma of extremity. The role and efficacy of repeat regional chemotherapy for recurrence and which salvage procedure is better remains debatable. We aimed to compare toxicities and clinical outcomes by procedure types and the sequence.
Methods
Data from 44 patients, who underwent repeat HILPs or ILIs from 3 institutions beginning 1997 to 2010, were retrospectively reviewed. Regional toxicity using Wieberdink (WBD) grade, systemic toxicity using serum creatine phosphokinase level (CPK), length of hospital stay (LOS), response rates (RR) at 3 months post-procedure, and time to in-field progression (TTP) were analyzed.
Results
Of 44 pts, 45.5% were male, 54.5% female with a median age of 66 years (range, 29–85) at diagnosis. The median follow-up was 21.4 months (range, 4 – 153). Of 70 ILIs and 28 HILPs, following groups were identified: A) ILI→ILI (n=25); B) ILI→HILP (n=10); C) HILP→ILI (n=12); D) HILP→HILP (n=3). The comparison of WBD, CPK, LOS and RR between procedures (HILP vs. ILI), between sequence (initial vs. repeat) and their interactions showed no significant differences statistically. TTP after initial procedure did not differ between HILP and ILI (p=0.08), and no survival difference was seen (p=0.65) when TTP after repeat procedure was compared.
Conclusions
The majority of patients tolerated repeat regional chemotherapy without increased toxicity or LOS; no statistical difference in clinical outcomes were noted when comparing repeat procedures even though repeat HILPs showed higher complete response compared to repeat ILIs.
Keywords: Melanoma, Isolated Limb Infusion, Hyperthermic Isolated Limb Perfusion, Regional chemotherapy
Introduction
Hyperthermic isolated limb perfusion (HILP), first reported in 1958 [1] and isolated limb infusion (ILI) developed in early 1990s [2] have allowed the regional delivery of cytotoxic agents (often Melphalan) to patients with unresectable in-transit metastases confined to extremities from melanoma at 10 fold higher than the systemic dose.[3] For patients with locally advanced melanoma in extremities, the administration of high dose regional chemotherapy via HILP or ILI has proven to be efficacious in locoregional disease control with good overall response rates (ORR).[4] In addition, compared to local treatments, delivery of regional chemotherapy to the affected extremity isolated from the systemic circulation treats the whole area at risk of recurrence by theoretically eradicating clinically occult microscopic disease.[5, 6]
Single institutional complete response (CR) rates usually exceed 50% for HILP using Melphalan alone with ORR approaching 90%.[7, 8] Similar response rates have not been uniformly reported using ILI which seems to have CR rates ranging between 30–38% and ORR between 60–70% with less severe regional and systemic toxicity than HILP. However, the locoregional recurrence rates can range from 22–100% even after CR from HILP and ILI.[7–12] An appropriate management of these patients who develop locoregional recurrence after good initial response to the regional therapy is challenging, and no clear treatment recommendations exist. Although there is currently no consensus on the most appropriate management of these recurrences, a few centers have suggested that favorable results can be achieved utilizing a repeat regional therapy be it planned at the time of the initial procedure or utilized at the time of regional disease progression.[6, 13–16] Thus the treatment approach for this specific group of patients, the decision to continue with the same treatment modality (HILP→HILP, ILI→ILI) or proceed with alternative therapy (HILP→ILI, ILI→HILP), can be vague. Furthermore, whether a difference exists for patients who undergo ILI after prior HILP or HILP after prior ILI in regional toxicity or response rates is uncertain. Given these observations, the goal of this study was to examine our multi-institutional experience in order to develop a more standardized treatment recommendation for patients who recur locoregionally after a regional chemotherapy.
Patients and Methods
After Institutional Review Board approval, a multi-institutional retrospective review from three institutions was performed on patients who underwent at least two regional chemotherapy procedures from 1997 to 2010. Eligible patients included those who underwent a repeat HILP or ILI for extremity melanoma (AJCC stage IIIB or IIIC). Patient variables such as gender, age at diagnosis, and operative variables such as type of procedure, ischemic time, tourniquet time, peak temperature, dose calculation of Melphalan, use of papaverine, number of treatments received were collected. The time to in-field progression (TTP) was defined as the time from procedure to the time of locoregional disease progression. The in-field progression was considered as any recurrent or progressing disease from the primary melanoma site to the level of next nodal basin. Considering the combination of procedures and their sequences, the patients were divided into four groups: group A (ILI→ILI), group B (ILI→HILP), group C (HILP→ILI), or group D (HILP→HILP).
Procedure Techniques: Isolated Limb Infusion (ILI) vs. Hyperthermic Isolated Limb Perfusion (HILP)
The detailed description of ILI technique was previously reported and well documented in the literature.[18, 19] The majority of patients received a dose corrected Melphalan based on the ideal body weight. HILP was performed using a standard technique under general anesthesia previously well-described in the literature. [1, 2, 14]
Toxicities and Outcomes
The regional toxicity was determined by close physical examination of the affected extremity and scored based on Wieberdink (WBD) toxicity grade.[17] Severe regional toxicity was defined as WBD grade ≥ IV. The systemic toxicity was assessed by monitoring peak serum CPK level.[18] The serum CPK level was measured daily, and patients were discharged home after the peak CPK level was documented and showed a decreasing trend towards normal level.
The LOS was defined from the day of procedure to the day of discharge while follow up duration was defined from the day of initial procedure to the day of last clinic visit or date of death. The response rate was determined 3 months post-procedure and recorded as CR, partial response (PR), stable disease (SD) or progression of disease (PD) according to Response Evaluation Criteria in Solid Tumors (RECIST) [18] modified for cutaneous lesions.
Statistical Analysis
Statistical analysis was performed using SAS (version 9.2, SAS Institute Inc., Cary, NC). A two-sided p-value ≤ 0.05 was considered statistically significant. Boxplots were used to visualize the data distribution. For patients who progressed after initial HILP or ILI, TTP was evaluated using two sample Wilcoxon exact test. TTP after repeat procedures was compared using Kaplan-Meier method and log-rank test.
Results
Patient and procedure characteristics
Of the 44 patients in our study, 20 (46%) were male. The median age at the time of initial diagnosis was 66 years (range, 29–85 years). The median follow up was 21.4 months (range, 4–153 months). All patients had in-transit disease or recurrent disease at the time of presentation. The majority of procedures (86%) were performed for lower extremity disease. A total of 98 procedures were performed (28 HILPs and 70 ILIs); 37 patients (84%) had 2 procedures, 4 patients (9%) had 3 procedures, and 3 patients (6.8%) had 4 procedures (Table 1). The overall median duration between initial and repeat procedure was 6.6 months (range, 2–74 months). The mediation duration between initial and repeat procedure for group A (N=25) was 6 months, group B (N=10) 6 months, group C (N=12) 24 months, group D (N=3) 31 months. The ORR for all 98 procedures at 3 months post treatment was 38% (CR 21%, PR 16%).
Table 1.
Demographics and procedure characteristics
| Frequency | % or (range) | |||
|---|---|---|---|---|
| Gender | Male | 20 | 45.5 | |
| Female | 24 | 54.5 | ||
| Age (years) | Median | 66 | (29–85) | |
| Follow-up (months) | 21.4 | (3.7–152.8) | ||
| Extremity | Upper | 6 | 13.6 | |
| Lower | 38 | 86.4 | ||
| Procedures | HILP | 28 | ||
| ILI | 70 | |||
| Repeat × 1 | 37 | 84.1 | ||
| Repeat × 2 | 4 | 9.1 | ||
| Repeat × 3 | 3 | 6.8 | ||
| Median duration between initial and repeat procedure (months) | 6.6 | (1.9–73.7) | ||
| Subgroups | A. ILI → ILI | 25 | ||
| B. ILI → HILP | 10 | |||
| C. HILP → ILI | 12 | |||
| D. HILP → HILP | 3 | |||
| Median TTP after initial procedure (months) | ILI (n=27) | 3 | (1.1–29.5) | |
| HILP (n=4) | 6.4 | (4.2–33) | ||
| Median TTP after repeat procedure (months) | ILI (n=32) | 3 | (1.4–11) | |
| HILP (n=7) | 4.1 | (2.3–18.1) | ||
| Response rate at 3 months for all procedures | CR | 21 | 21.4 | |
| PR | 16 | 16.3 | ||
| SD | 10 | 10.2 | ||
| PD | 37 | 37.7 | ||
| Unknown | 14 | |||
TTP – Time to in-field progression
ILI – Isolated limb infusion
HILP – Hyperthermic isolated limb perfusion
CR – Complete Response
PR – Partial Response
SD – Stable disease
PD – Progression of disease
Pre-operative and intra-operative parameters
Cytotoxic agents were circulated for 60 min during HILP and 30 min during ILI. All patients received the standard dose of Melphalan at the time of procedure. Fifty-four ILIs (77%) were performed with actinomycin D in addition to Melphalan. Sixteen ILIs were also part of a clinical trial using systemic ADH-1 or sorafenib in combination with Melphalan. Papaverine was given during 51% of procedures. The mean ischemic time (ILI only) was 65 minutes (range, 45–110 minutes). The peak temperature was 39°C (range, 37–41°C) for ILIs and was 39.9°C (range, 39–42°C) for HILPs.
Regional toxicity by WBD toxicity grade
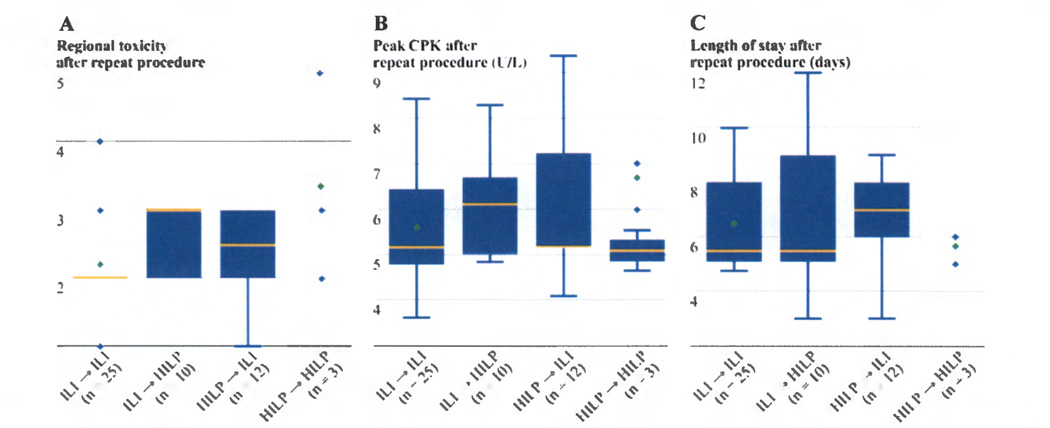
The most common regional toxicity experienced was WBD grade II after ILI and WBD grade III after HILP. For HILPs performed, 11% of patients had WBD grade IV toxicity, and 1 patient (3.5%) had WBD grade V toxicity requiring an amputation. Of the 70 ILIs performed, only 1 patient (<1%) had WBD grade IV toxicity and no patient undergoing ILI had a grade V toxicity. Even though it appears that HILPs are associated with an increased incidence of severe regional toxicity (WBD grade ≥ IV) compared to ILIs, no statistical difference was detected (p=0.08) likely due to the small sample size of the groups. Similarly, there was no difference in regional toxicity between initial and repeat procedure (p=0.25) or among different sequence of procedures (p=0.09). (Figure 1A)
Figure 1.
a) The regional toxicity measured by Wieberdink toxicity grade after repeat procedures showed no statistical difference among 4 subgroups (p=0.09). b) No significant difference was detected for the peak creatine phosphokinase (U/L) (natural log transformed) after repeat procedures (p=0.71). c) No significant difference was observed for the length of stay after repeat procedures (p=0.71).
[Boxplots were used to visualize the distribution for groups A to C. The box in the boxplot has lines at the lower quartile (25%), median (50%), and upper quartile values (75%) while the red circle marks the mean value. Whiskers extend from each end of the box to the most extreme values within 1.5 times the interquartile range from the ends of the box. The data with values beyond the ends of the whiskers, displayed with black circles, are potential outliers. For group D (N=3), due to small sample size, the data points (shown as triangles) were displayed for clarity.]
Systemic toxicity by serum CPK level
The serum CPK was measured daily and used to monitor systemic toxicity. The median peak CPK was 214 U/L (range, 37–11674 U/L) for ILI and was 616 U/L (range, 129–3945 U/L) for HILP (p=0.56). There was no significant difference in peak CPK between initial and repeat procedure (p=0.98) or among different sequence of procedures (p=0.71). (Figure 1B)
Length of hospital stay
The median LOS after ILI was 7 days and was 6 days for HILP (p=0.23). There was no significant difference in LOS among four subgroups (ILI→ILI, ILI→HILP, HILP→HILP, HILP→ILI) (p=0.69). When the LOS was evaluated in terms of interaction between the procedure type and sequence, these variables did not influence the patient’s LOS after repeat procedures (p=0.71). (Figure 1C)
Overall response rates
The ORR for ILI (n=70) was 33% (17% CR and 16% PR), but the ORR for HILP (n=28) was higher at 49.9% (32% CR and 18% PR). When comparing the CR rates and ORR between ILI and HILP, no significant difference was noted for either CR or ORR (p=0.17 and p=0.12, respectively). When CR rates were compared based on initial vs. repeat procedure or interaction between procedure type and its sequence, no statistical difference was noted (p=0.43 and p=0.64, respectively). Similarly, when ORR was compared based on initial versus repeat procedure, or between procedure type and sequence, there was no significant difference (p=0.82 and p=0.70, respectively). The response rates appeared to be equivalent for both ILIs and HILPs when compared as a group or when performed as either initial or repeat procedure.
Time to in-field progression
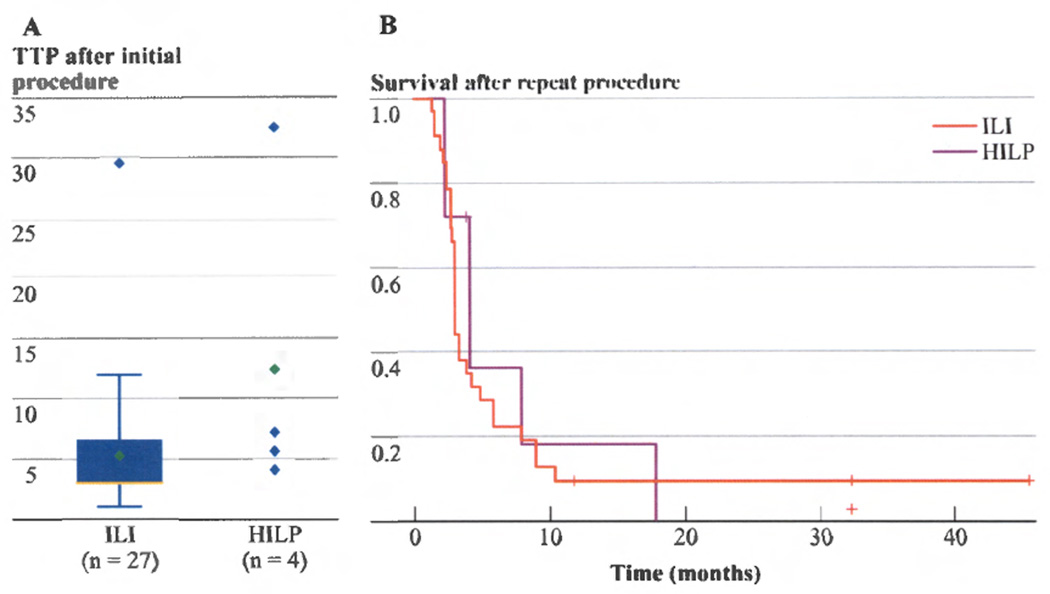
The median time to locoregional progression after initial ILI was 3 months compared to 6.4 months after initial HILP (p=0.08).(Figure 2A) After repeat ILI or HILP, the median TTP was 3 months versus 4.1 months, respectively. When comparing overall outcome after repeat ILI or HILP, the shorter TTP after ILI did not result in a significant survival difference as there was no statistical difference in survival between groups on log rank analysis (p=0.65).(Figure 2B)
Figure 2.
a) After initial procedures, no significant difference in time to in-field progression (TTP) between ILI and HILP was noted (p = 0.08). b) After repeat procedures, no sufficient survival impact from TTP difference between ILI and HILP was noted (p = 0.65).
Subgroup analysis
We further investigated whether having an ILI as the initial procedure prior to repeat ILI or HILP produced a significant difference in clinical outcome measured by LOS, toxicity, and response rates. When comparing these variables between group A (ILI→ILI) and group B (ILI→HILP) (Table 2), no significant difference in LOS and regional or systemic toxicity were noted between the two groups. However, patients in group B who underwent an initial ILI followed by HILP for recurrence showed an improved response rate with 70% ORR and 50% CR rate compared to patients in group A who underwent a repeat ILI with 40% ORR and 24% CR rate although the difference was not significant (p=0.11). Also, patients in group D, who underwent initial and repeat HILP, obtained 100% ORR with 66% CR rate after the repeat procedure. Although we were unable to fully evaluate, other than using descriptive statistics, the impact of having HILP as the initial procedure followed by a repeat ILI (group C) due to the excessive amount of missing variables, a repeat ILI after an initial HILP produced the lowest response rate in our study. While admittedly these results represent a small number of highly selected patients and were not statistically significant, there did appear to be an identifiable trend observed for patients in group B (ILI→HILP) and group D (HILP→HILP) in that repeat HILP after initial ILI or initial HILP was associated with a better response rate than repeat ILI. One must keep in mind the small sample size, different duration of response, type of treatment, and heterogeneity of therapy when comparing these groups as major limitations of the study and due to the small groups there is a lack of power to detect differences.
Table 2.
Subgroup analysis for toxicities and response rates
| A. ILI→ILI | B. ILI→HILP | C. HILP→ILI | D. HILP→HILP | |||||
|---|---|---|---|---|---|---|---|---|
| N | 25 | 10 | 12 | 3 | ||||
| Initial | Repeat | Initial | Repeat | Initial | Repeat | Initial | Repeat | |
| Median Length of Stay (days) | 7 | 6 | 6.5 | 6 | 6 | 7 | 6 | 6 |
| Median Wieberdink Toxicity Grade | 2 | 2 | 2 | 3 | 3 | 2.5 | 3 | 3 |
| Median Peak Creatine Phosphokinase U/L (range) | 214 (39–4830) | 179 (37–4548) | 197 (42–8705) | 456.5 (129–3945) | 760 (673–927) | 180 (60–11674) | N/A | 1122 (399–1191) |
| Overall Response Rate % | 40 | 40 | 30 | 70 | N/A | 18 | 100 | 100 |
| Partial Response Rate % | 24 | 16 | 10 | 20 | N/A | 0 | 33 | 33 |
| Complete Response Rate % | 16 | 24 | 20 | 50 | N/A | 18 | 66 | 66 |
Discussion
Even though many exciting discoveries have recently been made in the field of melanoma, increasing the armamentarium for adjuvant treatment options such as pegylated interferon-α2b, vemurafenib, ipilimumab and combination therapies,[19] the regional chemotherapy still remains an attractive option for extensive in-transit or bulky recurrent melanoma not amenable to complete surgical excisions as well as for patients who refuse or are not candidates for systemic therapy. However, despite the fact that the regional chemotherapy for advanced melanoma confined to the extremities is well-accepted, the treatment approach has not been standardized as the recent review by Beasley et al pointed out.[20] In the current study, we reviewed the clinical outcomes from different sequence of repeat regional therapies to evaluate an optimal treatment approach for patients who present with recurrence after the initial regional therapy.
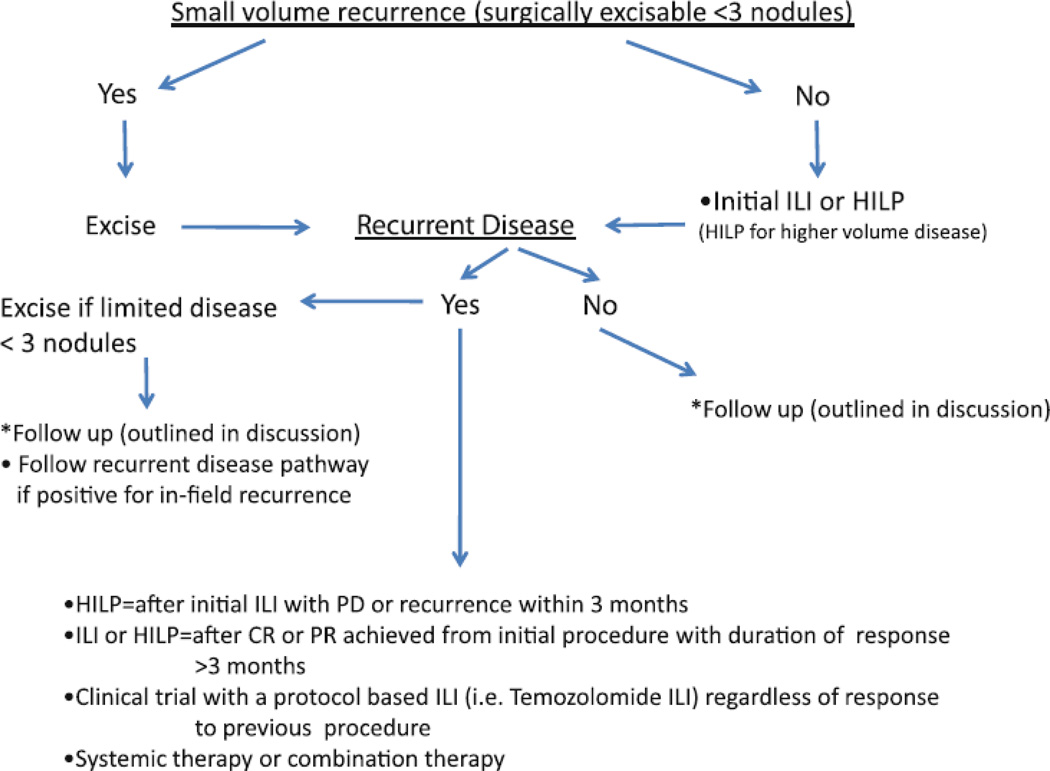
An algorithm using ILI for the initial treatment approach to in-transit disease or local recurrence was previously proposed based on a multi-institutional experience.[21] In this paper, we focus on approach to patients with locoregional recurrence or progression after initial regional chemotherapies (ILI or HILP) (Figure 3). Our proposed approach to the initial and recurrent regional in-transit disease is as follows. As our data demonstrates no significant difference in toxicity, LOS and response rates between HILP and ILI, it is reasonable to offer an ILI as the initial regional chemotherapy of choice based on its less complex and less invasive nature when compared to HILP. For a patient who presents with a high volume disease or with a nodal disease, HILP may be considered as the initial procedure of choice since a nodal dissection is part of the exposure for the root of the extremity vasculature for HILP.
Figure 3.
Treatment algorithm for recurrent extremity melanoma after regional chemotherapy
When a patient presents with small volume recurrences, which are amenable to complete surgical excision after the initial regional therapy, resection should be performed to render the patient disease-free. When a patient presents with unresectable recurrence after the initial procedure, the repeat procedure should be determined according to the pattern of recurrence, duration of response and the type of initial procedure. For example, if the patient continues to have a progression of disease or recurrence within 3 months from an initial ILI, the patient should undergo HILP as the next procedure rather than a repeat ILI since HILP does appear to offer a higher response rate (group B) as seen in table 2. For a patient who had HILP as the initial procedure, a repeat regional therapy with an ILI is unlikely to improve the response rate as this rapid progression of disease after HILP probably suggests aggressive tumor biology. Of the 4 subgroups we evaluated, the patients who underwent a repeat ILI after a failed HILP showed lowest ORR of 18%. These patients may benefit from a clinical trial with a protocol based ILI using an alternative intra-arterial chemotherapeutic agent such as temozolomide (TMZ) or systemic chemotherapy or combination of the two. However, if a patient had a CR or significant PR after the initial ILI or HILP with good duration of response (at least greater than 3 months before recurrence or progression), then it may be reasonable to consider either a repeat ILI or HILP or a protocol based ILI. If a patient recurs after the repeat regional therapy, the patient can follow the recurrent disease pathway in the proposed algorithm seen in figure 3. Clinical trials for patients with unresectable melanoma of extremity (direct intralesional injection, systemic therapies, protocol based regional therapies) should also be considered early in the management of the patient with in-transit melanoma. Our current follow up schedule for these patients (after ILI or HILP regardless of initial or repeat) includes every 3 months clinic visits with a physical exam and a whole body PET or CT for the first 2 years, then every 4 months with scans for year 3. We recommend every 6 months follow up with scans for year 4 and 5. Once the patient is beyond 5 years out from the last recurrence, a yearly physical exam and whole body imaging is suggested.
For these patients who present with the advanced melanoma (in-transit disease) confined to the extremity (stage IIIb or IIIc), studies have shown that the burden of disease and status of the regional nodal basin are reliable predictors of response rates to the regional therapy and overall survival. On the other hand, a good response from regional chemotherapies does not impact either the disease free survival or the overall survival.[22–24] Thus, it is important to explore different treatment options and alternatives to Melphalan to improve survival of these patients in addition to improving quality of life. Realizing the importance of alternative therapeutic options, active trials and research are being conducted to augment anti-tumor effect delivered via regional chemotherapy. For example, the animal study with ILI using TMZ showed encouraging tumor responses when compared to systemic TMZ and ILI with Melphalan.[25] The efficacy and safety of TMZ as an alternative to Melphalan is currently being examined in a phase I multi-institutional trial. The idea of combination therapy utilizing both the regional delivery of high dose therapeutic agent and a systemic agent targeting a specific molecular pathway to enhance regional anti-tumor effect is also being explored. Some of our patients were enrolled in the research investigating the role of ADH-1 and sorafenib as systemic agents to increase the effect of Melphalan. In the phase I trial, the systemic ADH-1 used in conjunction with Melphalan via ILI produced a CR rate of 50% with no dose-limiting toxicities.[26] Even though the phase II trial recently published showed CR rate of 38% with the TTP of 4.6 months without significant difference compared to standard ILI,[27] it is exciting to see different treatment options being explored with systemic targeting agents to improve drug sensitivity to tumors. Furthermore, the potential role of ipilimumab, a recently approved immunomodulator, in the adjuvant setting is also being investigated in phase III trial for stage III patients (EORTC 18071), and it may play a significant role in our patient population in the future.
Conclusion
A repeat regional chemotherapy is a practical option for a patient with recurrence after an initial regional therapy. Even though our study was not sufficiently powered to show the statistical difference in response rates for different subgroups analyzed, HILP may be more suitable as a repeat procedure especially if the response to the initial ILI was poor. We can also conclude that for a patient, whose response to the initial HILP is poor, an alternative treatment method such as systemic therapy, trial based local injections, or repeat regional therapy with non-traditional agent on protocol should be considered rather than the standard Melphalan based therapy. The patient should be counseled on risks, benefits and alternatives to each procedure. Ultimately, choosing the most appropriate method of treatment needs to be individualized in a multidisciplinary setting exploiting all available options that include looking at repeat perfusions, systemic therapies and clinical trials taking into account all the current advances in the armamentarium in melanoma treatment.
Footnotes
Presented in part at the Society of Surgical Oncology 64th Annual Cancer Symposium in San Antonio, TX
No financial support was needed to conduct this study and all authors state that they have no conflicts of interest relevant to our paper.
References
- 1.Creech O, Jr, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148:616–632. doi: 10.1097/00000658-195810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson JF, Lai DT, Ingvar C, Kam PC. Maximizing efficacy and minimizing toxicity in isolated limb perfusion for melanoma. Melanoma Res. 1994;4(Suppl 1):45–50. [PubMed] [Google Scholar]
- 3.Muchmore JH, Wanebo HJ. Regional chemotherapy: overview. Surg Oncol Clin N Am. 2008;17:709–730. vii. doi: 10.1016/j.soc.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AM, de Wilt JH, ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003;4:429–437. doi: 10.1016/s1470-2045(03)01141-0. [DOI] [PubMed] [Google Scholar]
- 5.Noorda EM, Takkenberg B, Vrouenraets BC, et al. Isolated limb perfusion prolongs the limb recurrence-free interval after several episodes of excisional surgery for locoregional recurrent melanoma. Ann Surg Oncol. 2004;11:491–499. doi: 10.1245/ASO.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 6.Feldman AL, Alexander HR, Jr, Bartlett DL, et al. Management of extremity recurrences after complete responses to isolated limb perfusion in patients with melanoma. Ann Surg Oncol. 1999;6:562–567. doi: 10.1007/s10434-999-0562-x. [DOI] [PubMed] [Google Scholar]
- 7.Klaase JM, Kroon BB, van Geel AN, et al. Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion with melphalan. Surgery. 1994;115:39–45. [PubMed] [Google Scholar]
- 8.Thompson JF, Hunt JA, Shannon KF, Kam PC. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch Surg. 1997;132:903–907. doi: 10.1001/archsurg.1997.01430320105017. [DOI] [PubMed] [Google Scholar]
- 9.Storm FK, Morton DL. Value of therapeutic hyperthermic limb perfusion in advanced recurrent melanoma of the lower extremity. Am J Surg. 1985;150:32–35. doi: 10.1016/0002-9610(85)90006-6. [DOI] [PubMed] [Google Scholar]
- 10.Hansson JA, Simert G, Vang J. The effect of regional perfusion treatment on recurrent melanoma of the extremities. Acta Chir Scand. 1977;143:33–39. [PubMed] [Google Scholar]
- 11.Di Filippo F, Calabro A, Giannarelli D, et al. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer. 1989;63:2551–2561. doi: 10.1002/1097-0142(19890615)63:12<2551::aid-cncr2820631233>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Lejeune FJ, Lienard D, Leyvraz S, Mirimanoff RO. Regional therapy of melanoma. Eur J Cancer. 1993;29A:606–612. doi: 10.1016/s0959-8049(05)80163-7. [DOI] [PubMed] [Google Scholar]
- 13.Grunhagen DJ, van Etten B, Brunstein F, et al. Efficacy of repeat isolated limb perfusions with tumor necrosis factor alpha and melphalan for multiple in-transit metastases in patients with prior isolated limb perfusion failure. Ann Surg Oncol. 2005;12:609–615. doi: 10.1245/ASO.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett DL, Ma G, Alexander HR, et al. Isolated limb reperfusion with tumor necrosis factor and melphalan in patients with extremity melanoma after failure of isolated limb perfusion with chemotherapeutics. Cancer. 1997;80:2084–2090. doi: 10.1002/(sici)1097-0142(19971201)80:11<2084::aid-cncr7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Klop WM, Vrouenraets BC, van Geel BN, et al. Repeat isolated limb perfusion with melphalan for recurrent melanoma of the limbs. J Am Coll Surg. 1996;182:467–472. [PubMed] [Google Scholar]
- 16.Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer. 2009;115:1932–1940. doi: 10.1002/cncr.24220. [DOI] [PubMed] [Google Scholar]
- 17.Wieberdink J, Benckhuysen C, Braat RP, et al. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–910. doi: 10.1016/0277-5379(82)90235-8. [DOI] [PubMed] [Google Scholar]
- 18.Gehan EA, Tefft MC. Will there be resistance to the RECIST (Response Evaluation Criteria in Solid Tumors)? J Natl Cancer Inst. 2000;92:179–181. doi: 10.1093/jnci/92.3.179. [DOI] [PubMed] [Google Scholar]
- 19.Eggermont AM, Robert C. New drugs in melanoma: It's a whole new world. Eur J Cancer. 2011;47:2150–2157. doi: 10.1016/j.ejca.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 20.Beasley GM, Tyler DS. Standardizing regional therapy: developing a consensus on optimal utilization of regional chemotherapy treatments in melanoma. Ann Surg Oncol. 2011;18:1814–1818. doi: 10.1245/s10434-011-1656-9. [DOI] [PubMed] [Google Scholar]
- 21.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–715. doi: 10.1016/j.jamcollsurg.2008.12.019. discussion 715-707. [DOI] [PubMed] [Google Scholar]
- 22.Lindner P, Thompson JF, De Wilt JH, et al. Double isolated limb infusion with cytotoxic agents for recurrent and metastatic limb melanoma. Eur J Surg Oncol. 2004;30:433–439. doi: 10.1016/j.ejso.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Klaase JM, Kroon BB, van Geel AN, et al. A retrospective comparative study evaluating the results of a single-perfusion versus double-perfusion schedule with melphalan in patients with recurrent melanoma of the lower limb. Cancer. 1993;71:2990–2994. doi: 10.1002/1097-0142(19930515)71:10<2990::aid-cncr2820711017>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15:3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 25.Ueno T, Ko SH, Grubbs E, et al. Temozolomide is a novel regional infusion agent for the treatment of advanced extremity melanoma. Am J Surg. 2004;188:532–537. doi: 10.1016/j.amjsurg.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Beasley GM, McMahon N, Sanders G, et al. A phase 1 study of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with locally advanced in-transit malignant melanoma. Cancer. 2009;115:4766–4774. doi: 10.1002/cncr.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beasley GM, Riboh JC, Augustine CK, et al. Prospective multicenter phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with advanced extremity melanoma. J Clin Oncol. 2011;29:1210–1215. doi: 10.1200/JCO.2010.32.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]