Abstract
Flagellar ejection is tightly coupled to the cell cycle in Caulobacter crescentus. The MS ring protein FliF, which anchors the flagellar structure in the inner membrane, is degraded coincident with flagellar release. Previous work showed that removal of 26 amino acids from the C terminus of FliF prevents degradation of the protein and interferes with flagellar assembly. To understand FliF degradation in more detail, we identified the protease responsible for FliF degradation and performed a high-resolution mutational analysis of the C-terminal degradation signal of FliF. Cell cycle-dependent turnover of FliF requires an intact clpA gene, suggesting that the ClpAP protease is required for removal of the MS ring protein. Deletion analysis of the entire C-terminal cytoplasmic portion of FliF C confirmed that the degradation signal was contained in the last 26 amino acids that were identified previously. However, only deletions longer than 20 amino acids led to a stable FliF protein, while shorter deletions dispersed over the entire 26 amino acids critical for turnover had little effect on stability. This indicated that the nature of the degradation signal is not based on a distinct primary amino acid sequence. The addition of charged amino acids to the C-terminal end abolished cell cycle-dependent FliF degradation, implying that a hydrophobic tail feature is important for the degradation of FliF. Consistent with this, ClpA-dependent degradation was restored when a short stretch of hydrophobic amino acids was added to the C terminus of stable FliF mutant forms.
Proteolysis of intracellular proteins serves two important functions in all living organisms: protein quality control by quickly removing misfolded and damaged proteins (13) and regulation of cellular processes by eliciting a critical and irreversible step. A particular protein activity or structure can be specifically removed by proteolysis at the time when it is no longer required or, alternatively, when it becomes harmful to the cell. In both eukaryotic and prokaryotic cells protein degradation is known to be vital for a broad range of biological processes such as cell cycle progression, DNA repair, regulation of gene expression, and cell differentiation (4, 15, 24, 30, 45). In eukaryotic cells the energy-dependent decay of cytoplasmic proteins is mediated mainly by one large multisubunit complex, the 26S proteasome (3). In bacteria at least the following five different ATP-dependent proteases contribute to specific proteolysis in the interior of the cell: ClpAP, ClpXP, HslUV, FtsH, and Lon (14). As a common principle, all of these proteases are composed of a proteolytic domain and an ATPase chaperone domain. Energy-dependent unfolding of the substrate by the chaperone moiety is believed to be the committing and rate-limiting step of proteolysis.
Uncontrolled protein degradation is dangerous for any living cell and must therefore be tightly regulated. But how are proteases able to recognize specific substrates and discriminate these substrates from stable proteins? In eukaryotes, cytoplasmic substrates are specifically modified by polyubiquitination of a side chain lysine. This modification is mediated by multiple ubiquitin ligases and targets the substrates to the 26S proteasome (4). A similar tagging system has not been discovered in prokaryotes so far, indicating that the turnover signal must be contained within the amino acid sequence of protease substrates. A well-documented example is the SsrA peptide tag, which is added to prematurely terminated proteins during translation and targets them for rapid degradation by the ClpXP protease (16).
Although a recent study in which global protein stability in Caulobacter crescentus was analyzed concluded that up to 5% of the protein species are rapidly turned over (19), only relatively few unstable proteins have been identified in bacteria so far, and no common sequence motif responsible for targeting these proteins for degradation has been identified. The search for proteolysis signals is complicated by the fact that different energy-dependent proteases seem to have distinct substrate specificities (14, 16, 20). The only recurring theme is that amino acids essential for the degradation process are often located near the N or C terminus of the unstable protein (14, 15).
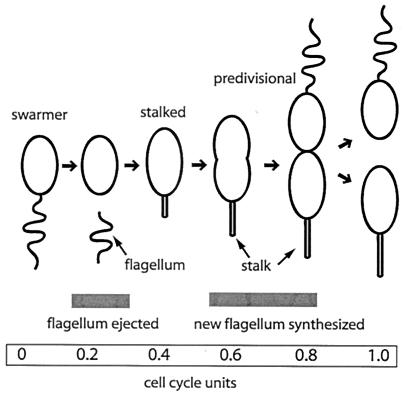
Proteolysis is an essential regulatory component of cell cycle progression in the gram-negative bacterium C. crescentus, and several proteins are specifically degraded during the cell cycle (2, 8, 22, 23, 25, 52). This offers a unique opportunity to analyze modes of substrate recognition by bacterial proteases and their temporal control mechanisms. A prime example is the proteolytic removal of the flagellar MS ring protein FliF, which anchors the flagellum in the cytoplasmic membrane. FliF is degraded when a motile swarmer cell differentiates into a sessile, surface-attached stalked cell (Fig. 1), which coincides with shedding of the flagellum (25). While it is unclear whether FliF degradation is the committing step for flagellar loss during cell differentiation, it has been shown that the C terminus of FliF bears components critical for both flagellar function and FliF destruction (18, 25). Removal of 26 amino acids from the cytoplasmically exposed C terminus completely abolished FliF degradation (25), and nine amino acids close to the C terminus were shown to be required for assembly of the flagellar structure (18).
FIG. 1.
Schematic diagram of the C. crescentus cell cycle. The flagellum is represented by a curved line, and the stalk is represented by a thin outgrowth of the cell envelope. The gray bars indicate the time windows for FliF synthesis and flagellar assembly and for FliF degradation and flagellar ejection during the cell cycle. The corresponding cell cycle time points (in relative cell cycle units) are indicated at the bottom.
To gain insight into cis- and trans-acting factors required for FliF degradation, we carried out a high-resolution mutational analysis of the cytoplasmic C terminus and identified the protease responsible for its degradation. Proteolysis of FliF does not rely on a specific primary amino acid sequence but rather seems to depend on the presence of hydrophobic amino acids in the FliF C terminus. While the introduction of charged residues at the very C terminus completely abolished degradation, addition of short stretches of hydrophobic residues could restore degradation of stable truncated mutant forms of FliF. In vivo experiments suggested that FliF degradation requires the ClpAP protease, which is known to recognize its substrates via signals located at the N or C terminus (16, 46, 49). Finally, electron microscopy analysis failed to demonstrate a clear link between a stable and functional copy of FliF and a failure of flagellar ejection during cell differentiation, suggesting that flagellar release might be triggered by a step preceding FliF degradation.
MATERIALS AND METHODS
Strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli DH10B and S17-1 were used as host strains for molecular cloning experiments and as donor strains for conjugational transfer of plasmids into Caulobacter. E. coli strains were grown at 37°C in Luria-Bertani broth (40) supplemented with kanamycin (50 μg/ml) or tetracycline (12.5 μg/ml) when necessary. C. crescentus strains were grown at 30°C in either PYE complex medium (35) or M2 minimal glucose medium (26) supplemented with kanamycin (5 μg/ml), tetracycline (2.5 μg/ml), or nalidixic acid (20 μg/ml) when necessary. To induce the PxylX promoter in C. crescentus, 0.1% xylose was added to PYE and 0.3% xylose was added to M2 medium.
TABLE 1.
Strains used in this study
| Strain | Description | Source, reference, and/or method of construction |
|---|---|---|
| E. coli strains | ||
| DH10B | F−mrcA Δ(mrr hsdRMS mcrBC) φ80dlacZΔM15 Δlac×74 endA1 recA1 deoR Δ(ara, leu) 7697 araD139 galU galK nupG rpsL | Gibco BRL |
| S 17-1 | M294::RP4-2 (Tet::Mu) (Kan::Tn7) | 42 |
| C. crescentus strains | ||
| LS1218 | NA1000 ΔfliF | 25 |
| LS1528 | ΔfliF::fliF (wild-type control for all strains carrying fliF derivatives) | LS1218::pUJ70 (25) |
| LS1707 | ΔfliF Pxyl::fliF | LS1218::pUJ87 (25) |
| NA1000 | Synchronizable holdfast mutant derivative of wild-type strain CB15 | 9 |
| UJ38 | pleD::pBGS18T | NA1000::pUJ163 |
| UJ355 | ΔfliF::fliFΔ1579-1593 (FliFΔ527-531 ΔI) | LS1218::pBG1 |
| UJ356 | ΔfliF::fliFΔ1594-1608 (FliFΔ532-536 ΔII) | LS1218::pBG2 |
| UJ357 | ΔfliF::fliFΔ1609-1623 (FliFΔ537-541 ΔIII) | LS1218::pBG3 |
| UJ358 | ΔfliF::fliFΔ1624-1638 (FliFΔ542-546 ΔIV) | LS1218::pBG4 |
| UJ359 | ΔfliF::fliFΔ1639-1650 (FliFΔ547-550 ΔV) | LS1218::pBG5 |
| UJ360 | ΔfliF::fliFΔ1651-1662 (FliFΔ551-554 ΔVI) | LS1218::pBG6 |
| UJ434 | ΔfliF::fliFΔ1579-1656 (FliFΔ527-552 Δ5) | LS1218::pBG7 |
| UJ553 | ΔfliF::fliFΔ1594-1656 (FliFΔ532-552 Δ6) | LS1218::pBG8 |
| UJ554 | ΔfliF::fliFΔ1609-1656 (FliFΔ537-552 Δ7) | LS1218::pBG9 |
| UJ555 | ΔfliF::fliFΔ1624-1656 (FliFΔ542-552 Δ8) | LS1218::pBG10 |
| UJ556 | ΔfliF::fliFΔ1639-1656 (FliFΔ547-552 Δ9) | LS1218::pBG11 |
| UJ562 | ΔfliF::fliF-M2 (FliFADPDYKDDDK) | LS1218::pUJ26 |
| UJ837 | NA1000 Δ(clpS-clpA)::Ω | This study |
| UJ838 | NA1000 ΔclpA::Ω | This study |
| UJ857 | ΔfliF::fliFΔ1579-1638 (FliFΔ527-546 Δ10) | LS1218::pSG1 |
| UJ858 | ΔfliF::fliFΔ1579-1623 (FliFΔ527-541 Δ11) | LS1218::pSG2 |
| UJ859 | ΔfliF::fliFΔ1579-1608 (FliFΔ527-536 Δ12) | LS1218::pSG3 |
| UJ967 | LS1707 (ΔfliF Pxyl::fliF) ΔclpA::Ω | This study |
| UJ970 | ΔfliF::fliFΔ1594-1638 (FliFΔ532-546 Δ13) | LS1218::pSG4 |
| UJ971 | ΔfliF::fliFΔ1594-1608, 1624-1638 (FliFΔ532-536, 542-546 Δ14) | LS1218::pSG5 |
| UJ972 | ΔfliF::fliF-S1 (FliFP539A) | LS1218::pSG6 |
| UJ973 | ΔfliF::fliF-S2 (FliFE536A K537A H538A D540A, Δ542-552) | LS1218::pSG7 |
| UJ974 | ΔfliF::fliF-S3 (FliFV543D A544R I545E L546K) | LS1218::pSG8 |
| UJ975 | ΔfliF::fliF-S4 (FliFS532R F534K V535D) | LS1218::pSG9 |
| UJ1077 | ΔfliF::fliFΔ1426-1488 (FliFΔ476-496 Δ15) | LS1218::pSG10 |
| UJ1078 | ΔfliF::fliFΔ1489-1536 (FliFΔ497-512 Δ16) | LS1218::pSG11 |
| UJ1079 | ΔfliF::fliFΔ1537-1578 (FliFΔ513-526 Δ17) | LS1218::pSG12 |
| UJ1204 | ΔfliF::fliF-polyR (FliF1-551 RVDR(10)LNS) | LS1218::pBG20 |
| UJ1205 | ΔfliF::fliF-Δ8-polyA (FliFΔ542-552 A(10)) | LS1218::pBG21 |
| UJ1225 | ΔfliF::fliF | LS1218::pBG23 |
| UJ1227 | ΔfliF::fliF-polyA (FliFA(10)) | LS1218::pBG24 |
| UJ1303 | ΔfliF::fliF-Δ5-polyA (FliFΔ527-552 A(10)) | LS1218::pBG28 |
| UJ1304 | fliF::fliF-Δ5-polyA ΔclpA::Ω | UJ838::pBG28 |
| UJ1322 | Pxyl::clpX | NA1000 pMO28 |
| UJ1339 | LS1218 (ΔfliF) ΔclpA::Ω | This study |
| UJ1369 | ΔfliF::fliF-S5 (FliFS553D T554D) | LS1218::pBG33 |
| UJ1370 | ΔfliF::fliF-S6 (FliFS553A T554A) | LS1218::pBG34 |
| UJ1371 | ΔfliF::fliF-S7 (FliFE533A E536A Δ537-552) | LS1218::pBG35 |
| UJ1372 | ΔfliF::fliF-S8 (FliFS532D F534D V535D Δ537-552) | LS1218::pBG36 |
| UJ1373 | ΔfliF::fliF-S9 (FliFK529A R530A Δ532-552) | LS1218::pBG37 |
| UJ1442 | ΔfliF::fliF ΔclpA::Ω | UJ1339::pUJ70 |
| UJ1443 | ΔfliF::fliF-polyA ΔclpA::Ω | UJ1339::pBG24 |
| UJ1444 | ΔfliF::fliF-Δ5-polyA ΔclpA::Ω | UJ1339::pBG28 |
| UJ1445 | ΔfliF::fliF-Δ8-polyA ΔclpA::Ω | UJ1339::pBG21 |
| UJ1446 | ΔfliF::fliF-S2 ΔclpA::Ω | UJ1339::pSG7 |
| UJ1447 | ΔfliF::fliF-S6 ΔclpA::Ω | UJ1339::pBG34 |
| UJ1448 | ΔfliF::fliF-S7 ΔclpA::Ω | UJ1339::pBG35 |
| UJ1449 | ΔfliF::fliF-S9 ΔclpA::Ω | UJ1339::pBG37 |
| UJ1483 | ΔfliF::fliF-Δ7 ΔclpA::Ω | UJ1339::pBG9 |
| UJ1879 | NA1000 ΔclpS | This study |
DNA manipulation techniques.
Standard cloning and PCR protocols were used (7, 40). All PCR products used for cloning were amplified with the high-fidelity polymerase Pfu (Stratagene). The integrity of cloned PCR fragments was confirmed by DNA sequencing using the dideoxy chain termination method (41) with an ABI Prism 310 automatic sequence analyzer (Perkin-Elmer). Plasmids were transferred into E. coli by electroporation and into C. crescentus by conjugation.
Cloning of the clpA gene and construction of ΔclpA and ΔclpS mutants.
The clpA gene from C. crescentus, which is close to the pleD gene on the chromosome (33), was isolated from strain UJ38, which has plasmid pBGS18T integrated at the pleD locus. Genomic DNA from strain UJ38 was digested with HindIII, ligated, and transformed into E. coli DH10B. This resulted in cloning of approximately 9 kb of DNA upstream of the pleD gene, including the clpA locus. The presence of the chromosomal fragment containing the clpA gene was confirmed by restriction analysis, and one positive clone was chosen for further analysis (pMO4). The Δ(clpS-clpA) and ΔclpA mutants were constructed by replacing the clpS-clpA internal PstI fragment and the clpA internal SalI fragment, respectively, with an omega cassette (37). The mutated clpA loci were cloned as ApaI-NheI fragments into the vector pNPTS138, resulting in plasmids pMO36 [Δ(clpS-clpA)] and pMO37 (ΔclpA). Chromosomal replacement was performed by a two-step homologous recombination procedure (23), which gave rise to the Δ(clpS-clpA) strain UJ837 and the ΔclpA strain UJ838. The clpS in-frame deletion was generated by amplifying two 600-bp fragments flanking clpS by using primers Cc2466-F (TCA ACT AGT AAA AGG TCG CCA AGC AGG), clpS-R (TCA AAG CTT CAT CGA CTT GTT CTC TCC), clpS-F (TCA AAG CTT TGC ACC ATG GAA AAG GAC), and clpA-R (TCA GAA TTC TTG AGG TCG ACG CAG TAG). The fragments were digested with SpeI/HindIII and HindIII/EcoRI and subcloned into the vector pNPTS138, resulting in plasmid pDE11. This generated a clpS locus lacking the central 306 bp of the clpS gene (total length, 360 bp). Plasmid pDE11 was then used to replace the wild-type clpS copy on the chromosome with the deletion allele by a two-step homologous recombination procedure (23), giving rise to the ΔclpS mutant strain UJ1879.
The ΔclpA mutation was transduced from strain UJ838 into strains LS1707 (ΔfliF Pxyl::fliF) and LS1218 (ΔfliF) with the help of phage φCR30 (1), generating strains UJ967 and UJ1339, respectively. The transduced clpA mutant strains were verified by their morphological phenotypes and by immunoblot analysis with a polyclonal antibody raised against ClpA at a 1:5,000 dilution (unpublished data). Different fliF alleles were introduced into strain UJ1339 by homologous recombination of the corresponding plasmids, giving rise to the strains listed in Table 1.
Construction of fliF mutant alleles.
The fliF alleles fliF-S1, fliF-S2, fliF-S5, fliF-S6, fliF-S7, fliF-S8, and fliF-S9 were generated by two-step PCR (GeneSOEing) (48) by using pUJ70 as the template, primer #157 (5′-GCC GTC ACC AAC TAC GAG-3′) and the reverse primer (5′-GTC AGC GAC ATC GAC CAG-3′) as flanking primers, and the following mutagenesis primers: #313 (5′-GAA GCA TGC CGA CGA GTC CGT CGC G-3′) and #314 (5′-GAC TCG TCG GCA TGC TTC TCG ACA AAC-3′) for fliF-S1, #315 (5′-TTG TCG CGG CCG CGC CCG CGG AGT CGA CCT GAT GGC TAT G-3′) and #316 (5′-CTC CGC GGG CGC GGC CGC GAC AAA CTC GGA CAC GCG-3′) for fliF-S2, #455 (5′-CTC GTG CAG CCA GTT ACG-3′) and #456 (5′-CGT AAC TGG CTG CAC GAG GAC GAT TGA TGG CTA TGA AGC TCG-3′) for fliF-S5, #455 (see above) and #457 (5′-CGT AAC TGG CTG CAC GAG GCC GCG TGA TGG CTA TGA AGC TCG-3′) for fliF-S6, #458 (5′-GGC GAC AAA CGC GGA CAC GCG CTT GAT CG-3′) and #459 (5′-GTG TCC GCG TTT GTC GCC TCG ACC TGA TGG CTA TGA-3′) for fliF-S7, #460 (5′-CTC GTC ATC CTC ATC CAC GCG CTT GAT CGA CGA-3′) and #461 (5′-GTG GAT GAG GAT GAC GAG TCG ACC TGA TGG CTA TGA-3′) for fliF-S8, and #462 (5′-CAC CGC CGC GAT CGA CGA GGC CTT CAC-3′) and #463 (5′-TCG TCG ATC GCG GCG GTG TCG ACC TGA TGG CTA TGA-3′) for fliF-S9. The fliF-polyA allele was generated by PCR by using primers #157 (see above) and #388 (5′-CGA ATT CAG GCG GCG GCG GCG GCG GCG GCG GCG GCG GCG GTC GAC TCG TGC AGC CA-3′) with plasmid pUJ70 as the template; fliF-Δ8-polyA was generated with primers #157 (see above) and #389 (5′-CGA ATT CAG GCG GCG GCG GCG GCG GCG GCG GCG GCG GCG GTC GAC TCG TCG GGA TG-3′) by using plasmid pBG10 as the template; and fliF-Δ5-polyA was generated with primers #157 (see above) and #400 (5′-CGA ATT CAG GCG GCG GCG GCG GCG GCG GCG GCG GCG GCG GTC GAC GAG GCC TTC AC-3′) by using plasmid pBG7 as the template. The BstEII-EcoRI fragment of the PCR products was then used to replace the equivalent region of pUJ70 to generate the plasmids listed in Table 2. The fliF-polyR allele resulted from a spontaneous frameshift mutation of the fliF-polyA allele that generated the C-terminal FliF sequence RVDRRRRRRRRRRLNS. The plasmids were integrated into the chromosome of strain LS1218 by homologous recombination. The correct site of integration was confirmed by PCR.
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Reference or source |
|---|---|---|
| pBG1 | fliFΔ1579-1593 (ΔI) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG2 | fliFΔ1594-1608 (ΔII) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG3 | fliFΔ1609-1623 (ΔIII) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG4 | fliFΔ1624-1638 (ΔIV) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG5 | fliFΔ1639-1650 (ΔV) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG6 | fliFΔ1651-1662 (ΔVI) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG7 | fliFΔ1579-1656 (Δ5) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG8 | fliFΔ1594-1656 (Δ6) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG9 | fliFΔ1609-1656 (Δ7) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG10 | fliFΔ1624-1656 (Δ8) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG11 | fliFΔ1639-1656 (Δ9) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pBG20 | fliF-polyR in pBGS18T as BamHI-EcoRI fragment | This study |
| pBG21 | fliF-Δ8-polyA in pBGS18T as BamHI-EcoRI fragment | This study |
| pBG24 | fliF-polyA in pBGS18T as BamHI-EcoRI fragment | This study |
| pBG28 | fliF-Δ5-polyA in pBGS18T as BamHI-EcoRI fragment | This study |
| pBG33 | fliF-S5 in pBGS18T as BamHI-EcoRI fragment | This study |
| pBG34 | fliF-S6 in pBGS18T as BamHI-EcoRI fragment | This study |
| pBG35 | fliF-S7 in pBGS18T as BamHI-EcoRI fragment | This study |
| pBG36 | fliF-S8 in pBGS18T as BamHI-EcoRI fragment | This study |
| pBG37 | fliF-S9 in pBGS18T as BamHI-EcoRI fragment | This study |
| pBGS18T | Kanr derivative of pUC18 with oriT (suicide vector in C. crescentus) | M. R. K. Alley |
| pDE11 | Fragments flanking clpS in pNPTS138 | This study |
| pJM22 | Cloning plasmid to generate C-terminal fusion to M2 tag | M. R. K. Alley |
| pMO4 | clpA in pBGS18T as 9-kb HindIII fragment | This study |
| pMO28 | clpX in pBBR1mcs2 as Kpnl-HindIII fragment | This study |
| pMO36 | Δ(clpS-clpA)::Ω (Pstl) in pNPTS138 as Apal-Nhel fragment | This study |
| pMO37 | ΔclpA::Ω (SalI) in pNPTS138 as Apal-Nhel fragment | This study |
| pMR20 | Tetr low-copy-number and broad-host-range vector | C. Mohr and R. Roberts |
| pNPTS138 | Kanr derivative of pLITMUS38 with sacB and oriT | M. R. K. Alley |
| pSG1 | fliFΔ1579-1638 (Δ10) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pSG2 | fliFΔ1579-1623 (Δ11) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pSG3 | fliFΔ1579-1608 (Δ12) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pSG4 | fliFΔ1594-1638 (Δ13) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pSG5 | fliFΔ1594-1608, 1624-1638 (Δ14) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pSG6 | fliF-S1 in pBGS18T as BamHI-EcoRI fragment | This study |
| pSG7 | fliF-S2 in pBGS18T as BamHI-EcoRI fragment | This study |
| pSG8 | fliF-S3 in pBGS18T as BamHI-EcoRI fragment | 18 |
| pSG9 | fliF-S4 in pBGS18T as BamHI-EcoRI fragment | 18 |
| pSG10 | fliFΔ1426-1488 (Δ15) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pSG11 | fliFΔ1489-1536 (Δ16) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pSG12 | fliFΔ1537-1578 (Δ17) in pBGS18T as BamHI-EcoRI fragment | 18 |
| pUJ26 | fliF in pJM22 as Notl-Sall fragment generating fliF-M2 | This study |
| pUJ70 | fliF in pBGS18T as BamHI-EcoRI fragment | 25 |
| pUJ163 | Internal pleD fragment in pBGS18T | This study |
Immunoblot analysis of synchronized cultures of C. crescentus.
C. crescentus cells were grown in M2 minimal glucose medium and synchronized by density gradient centrifugation (44). Isolated swarmer cells were released into fresh minimal medium at an optical density at 660 nm of 0.3. Samples were removed for immunoblot analysis at 20-min intervals and resuspended in sodium dodecyl sulfate sample buffer (25). Cell cycle progression was monitored by light microscopy. Total protein, corresponding to equal volumes of culture, was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoblotting was performed as described previously (1). The anti-FliF antibody (25) was used at a 1:10,000 dilution. A polyclonal goat anti-rabbit immunoglobulin G antibody (Gibco BRL) coupled to horseradish peroxidase was used as the secondary antibody at a 1:10,000 dilution and was detected by chemoluminescence (Renaissance; NEN) on X-ray film (Curix; AGFA). For cells containing stable FliF derivatives correct cell cycle progression was confirmed by determining the oscillating levels of CtrA by immunoblotting by using an anti-CtrA antibody (38) at a 1:10,000 dilution.
Microscopy techniques.
Cell morphology was observed by light microscopy with an Olympus AX70 microscope. Pictures were taken with a charge-coupled device camera (Hamamatsu C4742-95). Flagellar assembly and structure were investigated by electron microscopy. Cells growing exponentially in minimal medium were harvested, concentrated 10-fold, and fixed with negative stain as described by Aldridge and Jenal (1). Pictures were taken with a Philips 401 electron microscope.
RESULTS
Cell cycle-dependent degradation of FliF requires the clpA ATPase.
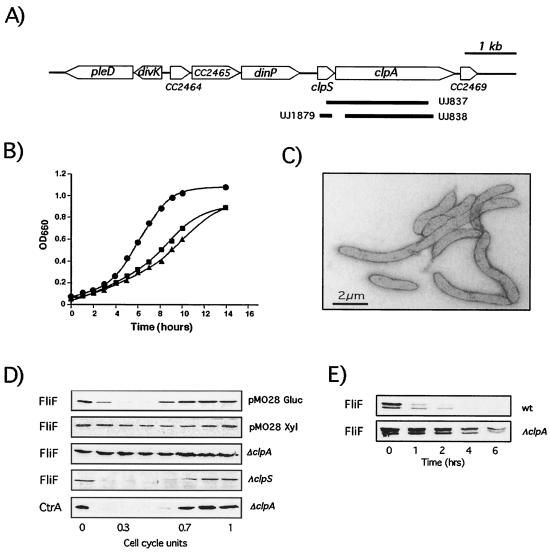
The Clp protease is composed of the ClpP peptidase complex flanked by two hexameric ATPase rings (17, 29). In most gram-negative bacteria two alternative ATPase subunits, ClpA and ClpX, can assemble with the ClpP peptidase (36). It was found previously that C. crescentus cells contain about 1,600 ClpP complexes, 800 ClpX rings, and 300 to 400 ClpA hexamers, indicating that Clp ATPases and ClpP peptidase are present in the cell at similar concentrations (34; Østerås and Jenal, unpublished observations). In agreement with this, we found that cells containing clpA at a high copy number stabilized CtrA, a known ClpXP substrate (23), while cells containing clpX at a high copy number were not able to efficiently degrade the FliF motor protein during the swarmer cell-to-stalked cell differentiation (Fig. 2D). This suggested that when there is an oversupply of one of the two Clp ATPases, the specific Clp protease activity mediated by the other ATPase is inhibited and that the ClpAP protease might be involved in FliF turnover. To verify this, the C. crescentus clpA locus was cloned as described in Materials and Methods, and clpA mutants were generated by replacing the chromosomal copy of clpA or of clpA and the upstream clpS gene with an interposon (Fig. 2A). Unlike a clpX mutant (23), both mutant strains (UJ837 and UJ838) remained viable, but they showed a moderate growth and cell division defect (Fig. 2B and C).
FIG. 2.
Characterization C. crescentus ΔclpS, ΔclpA, and Δ(clpS-clpA) mutant strains. (A) Chromosomal clpS-clpA locus. The arrows indicate annotated genes and their directions. The pleD gene codes for a response regulator involved in polar differentiation (1), dinP codes for DNA damage-inducible protein P, and the CC2464, CC2465, and CC2469 genes code for hypothetical proteins (33). The bars below the clpS and clpA genes indicate the fragments deleted in mutant strains UJ837, UJ838, and UJ1879. (B) Growth of ΔclpA and Δ(clpS-clpA) mutant strains. Symbols: •, NA1000 (wild type); ▴, UJ837 [Δ(clpS-clpA)]; ▪, UJ838 (ΔclpA). OD660, optical density at 660 nm. (C) Electron micrograph of cells of mutant strain UJ838 lacking clpA. (D) Concentration of FliF during the cell cycle determined for the following strains by immunoblot analysis using anti-FliF antibodies: NA1000 containing plasmid pMO28 (expressing clpX from the xylose-inducible promoter, PxylX [32]) grown in the presence of glucose (pMO28 Gluc) or xylose (pMO28 Xyl), UJ838 (ΔclpA), and UJ1879 (ΔclpS). The bottom panel shows the concentration of CtrA during the cell cycle of strain UJ838 (ΔclpA). Cell cycle progression (in relative cell cycle units) is indicated below the panels. (E) Stability of FliF in isogeneic clpA+ (LS1707) and clpA strains (UJ967). In both strains, the only copy of fliF is expressed from the Pxyl promoter (32). Cells were shifted from a medium containing xylose (fliF induced) to a medium with glucose (fliF repressed), and the time after the shift (in hours) is indicated. After the block of FliF synthesis, the FliF concentration was analyzed for equal numbers of cells by using an anti-FliF antibody. Note that repression of fliF expression had no effect on growth of the cells. The additional FliF band corresponds to a breakdown product of full-length FliF, observed when fliF is expressed from Pxyl (32).
Swarmer cells of the clpA mutant strains progressed normally through the cell cycle (data not shown). Also, CtrA turnover in the absence of ClpA was indistinguishable from wild-type CtrA turnover (Fig. 2D) (8). In contrast, the FliF protein was completely stabilized in strains lacking ClpA and was present at high levels throughout the cell cycle (Fig. 2D). To confirm that this was caused by stabilization of the FliF protein rather than a change in the expression pattern, we constructed a wild-type strain and a ΔclpA mutant strain with the only copy of the fliF gene under control of the xylose promoter. The activity of the C. crescentus Pxyl promoter can be rapidly repressed by shifting cells from a medium containing xylose to a medium lacking the inducer (32). After repression of fliF transcription, the FliF protein level was monitored in both wild-type and clpA mutant backgrounds by immunoblot analysis (Fig. 2E). While FliF was removed rapidly by proteolysis after its synthesis had been stalled in wild-type cells, stabilization of FliF was observed in the clpA mutant strain. This strongly suggested that the ClpA protein is required for FliF degradation in vivo and that the ClpAP protease is responsible for removal of the MS ring structure during C. crescentus cell differentiation. Cell cycle-dependent FliF degradation was not affected in a strain lacking only the clpS gene (Fig. 2D), indicating that the accessory protein ClpS is not involved in FliF turnover.
FliF turnover signal resides at the C-terminal end.
The degradation defect of the FliF-Δ5 derivative, which lacked 26 amino acids at the immediate C terminus (Fig. 3A), was originally observed with a plasmid-borne copy of the mutant allele (25). To rule out the possibility that the degradation defect was due to a plasmid copy number effect, the fliF-Δ5 allele was inserted into the chromosome of the fliF null mutant strain LS1218 at the fliF locus. FliF was stabilized in the resulting strain, UJ434, confirming the results obtained earlier (Fig. 3B). All fliF mutant alleles described below were present as single copies in the chromosomal fliF locus (see Materials and Methods). To further confine the FliF turnover signal, additional deletions covering the entire C-terminal cytoplasmic portion were introduced. Removal of 22 amino acids immediately following the second transmembrane domain of FliF (Δ1) (Fig. 3A) had no effect on protein stability (25). Similarly, FliF mutant proteins FliF-Δ15, FliF-Δ16, and FliF-Δ17, containing successive deletions of 21, 16, and 14 amino acids, were degraded normally during the cell cycle (Fig. 3), confirming that the FliF turnover signal is contained within the last 26 amino acids of the protein.
FIG. 3.
Behavior of FliF derivatives containing long deletions in the cytoplasmic C-terminal domain during the cell cycle. (A) The positions of the in-frame deletions Δ1, Δ15, Δ16, Δ17, Δ5, ΔI, ΔII, ΔIII, ΔIV, ΔV, and ΔVI in the cytoplasmic C-terminal domain of FliF are indicated by lines below the amino acid sequence. The Δ1 and Δ5 deletions have been described previously (25). The second transmembrane domain (TM2) is indicated as proposed by Jenal and Shapiro (25). The shaded sequences have been predicted to form α-helical secondary structures (18). The last two α-helices are referred to as helix 1 and helix 2, and the area between them is referred to as the loop region. The region corresponding to helix 2 is critical for flagellar assembly (18). The numbers beside the amino acid sequence indicate positions in the FliF protein. We took into account the finding that the experimentally verified start codon of FliF is 18 codons upstream of the start codon predicted in the GenBank entry (25). (B) Immunoblots showing the concentrations of wild-type FliF (FliF-wt) and FliF mutant proteins Δ15, Δ16, Δ17, and Δ5 during the cell cycle. The cell cycle progression (in relative cell cycle units) is indicated. For flagellar release a plus sign indicates that flagella were assembled but could not be detected at stalk tips, as determined by electron microscopy. NA., not applicable (no flagella were assembled).
A secondary structure prediction program proposed that two α-helices are connected by a short loop in the 42 C-terminal amino acids of FliF (Fig. 3) (18). These secondary structure elements have been shown to overlap two regions in the FliF C terminus required for flagellar assembly and function (18). The position of the predicted α-helices is used in Fig. 3, 4, and 5 as relative coordinates of the mutations described. Replacement of the proline residue in the middle of the loop region did not alter FliF stability, suggesting that the presumptive turn region is not critical for recognition of FliF by its cognate protease (S1) (Fig. 4).
FIG.4.
Mutational analysis of the FliF C terminus and definition of its requirements for cell cycle-dependent turnover. A schematic diagram of the deletion, substitution, and insertion mutations created in the last 28 amino acids of the FliF C terminus is shown on the left. Immunoblots displaying the concentrations of the corresponding mutant proteins during the cell cycle are shown on the right. The designations of the FliF mutant copies are indicated on the left. The shaded areas in the diagrams indicate the regions that have been predicted to form α-helical secondary structures in the C terminus of FliF (18). The consequences of the mutational alterations for flagellar release are indicated on the right. A plus sign indicates that flagella were assembled but could not be detected at stalk tips, as determined by electron microscopy. NA., not applicable (no flagella were assembled). The flagellar release data were partially adopted from reference 18. The cell cycle progression (in relative cell cycle units) is indicated below the panels. The levels of FliF derivatives shown are quantified and summarized in Fig. S6 in the supplemental material.
FliF turnover signal does not reside in a specific primary amino acid sequence.
To accurately define the extent and nature of the degradation signal at the FliF C terminus, mutant derivatives with short successive deletions of four or five amino acids (ΔI to ΔVI) (Fig. 3A and 4) dispersed over the entire Δ5 region were constructed and analyzed. Surprisingly, each of these FliF derivatives was degraded normally during the cell cycle (Fig. 4), implying that the turnover signal was redundant in nature with regard to the primary amino acid sequence.
FliF mutants with longer deletions in the last 28 amino acids that were progressively shortened towards either end of the Δ5 region were analyzed next (Δ6 to Δ12) (Fig. 4). While removal of 21 amino acids from the C terminus (Δ6) clearly interfered with FliF degradation, shorter deletions from this end did not affect proteolysis. The exception was the FliF-Δ8 mutant protein, which was stabilized even though it contained more sequence than the unstable version FliF-Δ7 (Fig. 4). When the FliF derivatives Δ12, Δ11, and Δ10, which carried deletions whose sizes progressively increased from the upstream end of the Δ5 region and which extended 10, 15, and 20 amino acids into the critical region for turnover (Fig. 4), were analyzed only the longest deletion (Δ10) was found to interfere with degradation (Fig. 4). Loss of the first 10 or 15 amino acids of this region (Δ12 and Δ11) had no effect on FliF degradation. Together, the results suggested that the essential determinants for turnover could be localized in the center of the Δ5 region. To test this hypothesis, we investigated cell cycle-dependent turnover of the FliF mutant proteins FiF-Δ13 and FliF-Δ14, which lacked different pieces of the Δ5 middle region (Fig. 4). Surprisingly, both FliF derivatives were turned over normally during the cell cycle. Together, these experiments excluded the possibility that the degradation signal at the C-terminal end of FliF is contained within a specific amino acid sequence motif.
Charged amino acids at the C-terminal end of FliF abolish cell cycle-dependent turnover.
It has been reported that several unstable bacterial proteins have hydrophobic amino acids at or close to the C terminus (6, 8, 12, 27, 39, 47). To test if the net charge at the C terminus affects FliF degradation, the last two amino acids of FliF (serine-threonine) were replaced with either two aspartate residues or two alanine residues (FliF-S5 and FliF-S6) (Fig. 4). While the addition of two alanine residues at the C terminus had no effect on protein turnover, substitution with aspartate residues stabilized FliF considerably. Similarly, longer tags with charged amino acids, like the M2 epitope (ADPDYKDDDK), or addition of 10 arginine residues (RRRRRRRRRRLNS) strongly interfered with proteolysis. In contrast, addition of 10 alanine residues to the FliF C terminus (FliF-polyA) did not result in stabilization of the motor protein (Fig. 4). Rather, the immunoblot signal for this FliF derivative was weaker than that of wild-type FliF, indicating that addition of a long hydrophobic tail progressively destabilized FliF (see below). To test if a polyalanine tail was sufficient to trigger FliF degradation, a tag consisting of 10 alanine residues was added to the stable derivatives FliF-Δ8 and FliF-Δ5. In both cases (Δ8-polyA and Δ5-polyA), addition of the tag resulted in destabilization (Fig. 4). This indicated that charged amino acids at the FliF C terminus inhibited degradation, while exposed nonpolar hydrophobic amino acids were able to promote FliF turnover.
In light of these findings, the contradictory results obtained with FliF mutant versions having deletions of various lengths at various positions (Δ6 to Δ9) might be attributed to the exposure of charged or nonpolar regions at the newly formed C termini. For example, mutant FliF-Δ8 has five charged amino acids within the eight C-terminal residues of its sequence and was stable despite the fact that a slightly shorter version, FliF-Δ7, was degraded normally (Fig. 4). To test if the high density of charged amino acids exposed at the C terminus of FliF-Δ8 was responsible for the lack of cell cycle-dependent degradation, four of the five charged amino acids were replaced by alanine residues. The concentration of the resulting FliF mutant protein, FliF-S2, fluctuated normally throughout the cell cycle, indicating that increased hydrophobicity or removal of charged residues at the C terminus was able to restore the wild-type degradation pattern (Fig. 4).
Similarly, when three hydrophobic amino acids close to the C terminus of the unstable, slightly shorter derivative FliF-Δ7 were replaced with charged residues, the resulting mutant derivative (FliF-S8) was stabilized (Fig. 4). In contrast, changing the two charged amino acids closest to the C terminus of FliF-Δ7 to hydrophobic residues (FliF-S7) did not impair degradation (Fig. 4). Finally, when two charged amino acid residues exposed at the C terminus of the stable FliF-Δ6 protein were replaced by alanine residues, degradation of the resulting FliF-S9 derivative was partially restored (Fig. 4), again indicating that as few as two prominently exposed charged residues can result in almost complete stabilization of the FliF protein.
These findings are consistent with the hypothesis that hydrophobicity represents at least part of the C-terminal degradation signal of FliF. However, neither replacement of several hydrophobic amino acids with charged residues in two stretches of the FliF C terminus (FliF-S3 and FliF-S4) nor deletion of these amino acids (FliF-ΔII and FliF-ΔIV) altered the stability of the MS ring protein (Fig. 4). Thus, the position relative to the C terminus and possibly the three-dimensional arrangement of the hydrophobic residues in the FliF tail region might be critical for the recognition by the proteolytic system responsible for FliF turnover.
A striking feature of FliF mutant derivatives with long hydrophobic tails was a weak immunoblot signal that indicated decreased stability. We reasoned that an extended stretch of hydrophobic amino acids could have altered the specificity of FliF for its protease. To test this, we analyzed the stability of several of these FliF derivatives in a clpA mutant background (Fig. 5A). Both wild-type FliF and the unstable mutant derivative FliF-Δ7 were stabilized in the absence of ClpA (Fig. 5A), arguing that truncated versions of FliF can still be targeted into the correct proteolytic pathway. Similarly, mutant FliF proteins with short artificial hydrophobic tails (FliF-S2, FliF-S6, and FliF-S9) were stabilized in the clpA mutant strain (Fig. 5A). In contrast, proteolytic turnover of FliF proteins with longer stretches of nonpolar amino acids (FliF-polyA, FliFΔ5-polyA, FliFΔ8-polyA, and FliF-S7) was not dependent on ClpA (Fig. 5A). This suggested that the degree of hydrophobicity at the C terminus is used as a sorting signal to channel unstable proteins into the correct proteolytic pathway. Polyalanine derivatives of FliF remained unstable in mutants lacking one of the alternative ATP-dependent proteases (ClpXP, Lon, and FtsH) (data not shown), suggesting that proteins with extended hydrophobic tails might be recognized and removed by several proteases simultaneously.
FIG.5.
Behavior of FliF derivatives with altered C termini during the cell cycle in the absence of ClpA. (A) The designations of the FliF mutant copies are indicated on the left. The schematic diagrams specify the mutations in the FliF C terminus (see Fig. 4). The immunoblots on the right display the concentrations of the corresponding FliF mutant proteins during the cell cycle for cells lacking clpA. The cell cycle progression (in relative cell cycle units) is indicated below the panels. The levels of FliF derivatives shown are quantified and summarized in Fig. S7 in the supplemental material. (B) Immunoblot displaying the cellular concentrations of wild-type FliF (wt) and a selection of FliF mutant proteins in asynchronous cultures. The arrow indicates the position of the FliF proteins. (C) Pulse-chase analysis of the stability of wild-type FliF and a selection of FliF mutant derivatives. Asynchronous cultures expressing the corresponding wild-type and mutant alleles were pulse-labeled and chased for increasing amounts of time as described previously (25) The FliF protein was immunoprecipitated from lysed cell extracts. Protein levels were determined quantitatively from autoradiographs with a PhosphorImager, and the values were plotted as percentages of the highest value.
To confirm that addition of polyalanine tails indeed altered the stability of FliF, immunoblot and pulse-chase experiments were carried out. While the level and stability of FliF with a polyarginine tail were increased compared to the level and stability of wild-type FliF, the cellular levels and stabilities of polyalanine forms were considerably reduced (Fig. 5B and C). In contrast, the FliF-S2 mutant form, which required ClpA for cell cycle-dependent turnover, also showed wild-type-like stability (Fig. 5C).
The MS ring acts both as the platform for flagellar assembly and as a membrane anchor for the flagellar structure. FliF degradation temporally coincides with ejection of the flagellum during C. crescentus cell differentiation. This has led to the hypothesis that timed destruction of FliF might trigger flagellar ejection (25). Because mutational uncoupling of flagellar assembly and FliF degradation has not been possible so far (18), this assumption could not be tested. However, full-length FliF molecules with short charged peptide tags (FliF-S5, FliF-M2, and FliF-polyR) not only were stabilized but also retained their normal function in flagellar assembly and motor performance (data not shown). When cells expressing these stable and functional forms of FliF were analyzed by electron microscopy, no flagellar structures were found attached to the tip of the stalk of stalked or predivisional cells (data not shown). Similarly, only a small minority of cells lacking the clpA gene retained the polar flagellar structure during cell differentiation. This indicated that ejection of the flagellum was normal in these cells and that FliF degradation is not a specific prerequisite for flagellar release during the cell cycle.
DISCUSSION
Specific proteolysis is an integral part of development of prokaryotic and eukaryotic cells and is often used to irreversibly shift the balance of regulatory networks or to remodel complex macromolecular structures. Here we tried to address the following two important questions of this posttranslational regulatory mechanism. How are proteins, which are bound for degradation, specifically recognized by their cognate proteases, and how are complex subcellular structures efficiently remodeled or removed by degradation of one or several of their key components? The data presented here outline a detailed analysis of the degradation signal of a membrane-integral bacterial protein, which serves as an assembly platform and anchor of the flagellar motor. Our results indicate that the FliF motor protein is specifically degraded during the C. crescentus cell cycle by the ClpAP protease and that the turnover signal resides in the immediate C terminus of FliF. The signal is not based on a distinct primary amino acid sequence but rather seems to depend on the precise distribution of hydrophobic and charged amino acids. Importantly, protease specificity seems to be determined by the number of amino acids with aliphatic side chains exposed at or close to the C terminus of the FliF membrane protein. While the C terminus is clearly required for specific degradation of FliF, our experiments failed to confirm that FliF turnover is the rate-limiting step for flagellar ejection.
ClpA is essential for FliF degradation.
We present evidence that the ClpA ATPase is strictly required for FliF degradation in vivo, which implies that the soluble ClpAP protease is responsible for the degradation of the membrane-integral FliF protein during C. crescentus cell differentiation. However, in the absence of in vitro degradation data, one has to consider the possibility that ClpA or ClpAP could also be required indirectly for FliF turnover (e.g., by triggering the synthesis or activation of an unknown factor, which in turn is required for FliF destruction). Alternatively, ClpA could be involved in a serial activation cascade of two or more proteases, similar to the action of caspases during apoptosis (51). In support of a direct role for ClpAP in FliF cell cycle turnover, it was found previously that none of the other ATP-dependent proteases present in C. crescentus, including the membrane-bound FtsH protease (10), ClpXP (23), Lon, and HslUV (U. Jenal and M. R. K. Alley, unpublished results), are involved in cell cycle-dependent degradation of FliF.
Nature of the FliF degradation signal.
In order to define the interaction between the ClpAP protease and the FliF substrate, we embarked on a detailed mutational analysis of the FliF C terminus. Our data strongly implied that the degradation signal for FliF is contained within the very C-terminal 28 amino acids of the protein, but extensive mutational analysis of this part of FliF failed to identify a specific primary amino acid sequence responsible for turnover. Short deletions covering the last 28 amino acids as well as amino acid substitutions in this region, all resulted in FliF proteins with a normal cell cycle-dependent degradation pattern. Only larger deletions covering at least 20 of the last 28 amino acids (Δ6 and Δ10) (Fig. 4) resulted in stable proteins. This suggested that the signals for ClpA recognition contained in this part of the protein must be redundant in nature or that the FliF C terminus could contain multiple ClpA binding sites that functionally overlap. Alternatively, since the folding stability of protein ends is important for the degradation mechanism of N- or C-terminally tagged substrates (31), part of the information of the degradation signal could be hidden in a particular three-dimensional structure of the FliF C terminus.
While hydrophobic regions seemed to be specifically required for FliF turnover, the addition of amino acids with charged side chains to the C terminus clearly interfered with degradation. Replacement of the last two amino acids of the FliF wild-type sequence with two negatively charged residues stabilized the protein, while introduction of two alanine residues at the same site did not interfere with ClpA-dependent degradation of FliF. Similarly, addition of extended charged tails to wild-type FliF completely stabilized the protein. Also, the deletion derivative Δ8, which lacked only 10 amino acids, was stable, while the shorter Δ7 mutant was degraded normally. This unexpected result can also be explained by the high density of charged amino acids left at the newly created C terminus of the Δ8 mutant protein. Replacing the four charged amino acids located at the C terminus of this mutant form with alanine residues restored wild type-like stability and ClpA-dependent degradation during the cell cycle. In contrast, the addition of charges but not the introduction of aliphatic side chains stabilized the normally degraded mutant Δ7. Together, these findings strongly indicated that strategically positioned amino acids with aliphatic side chains were able to promote ClpA-dependent degradation of FliF, while charged amino acids at or close to the C terminus had the opposite effect.
Nonpolar amino acids at either protein end have been shown to be critical turnover determinants for several known ClpA substrates (21, 46, 49). The best-understood example for ClpA recognition and ClpAP degradation is the SsrA tag, which is added to the C terminus of truncated proteins in a process called transtranslation (28). In E. coli SsrA-tagged proteins are rapidly degraded by several proteases, including ClpAP and ClpXP (16, 20), and the information for recognition of the tagged proteins lies entirely within the 11-amino-acid tag. Both ClpX and ClpA recognize aliphatic side chains of the SsrA tag. While the ClpX protein binds to the last three amino acids of the tag, ClpA recognizes three alanine residues and a leucine residue in the first half of the tag (11). ClpA binding studies and in vitro degradation assays with SsrA-tagged substrates have led to the proposal that ClpA might recognize short clusters of aliphatic residues with variations in spacing (11). This is consistent with our findings for the C. crescentus FliF motor protein, whose degradation also seems to rely on at least two contiguous short stretches of nonpolar amino acids at the C-terminal end. An additional parallel between ClpA-dependent degradation of FliF and SsrA-tagged proteins lies in the observation that the addition of charged amino acids to the very C terminus blocks protein degradation, even though in both cases the residues at the very end of the substrate proteins do not seem to be required for specific ClpA recognition (11, 16, 28).
Amino acids with aliphatic side chains are critical for recognition of substrate proteins by both ClpA and ClpX. Interestingly, addition of extended stretches of nonpolar amino acids to FliF leads to a relaxed protease specificity. Because the mutant derivatives also showed a clear decrease in stability compared to wild-type FliF, it is likely that they are subject to continuous and uncontrolled degradation. The observation that FliF derivatives with a polyalanine tail were degraded in all single protease mutant strains tested, including clpA, clpX, lon, and ftsH mutants, argued that long hydrophobic tails can target proteins to multiple ATP-dependent proteases simultaneously. Similarly, proteins containing an SsrA tag, which is also composed of mostly nonpolar amino acids, are recognized by multiple proteases (16, 20, 28).
FliF degradation and flagellar ejection.
We postulated previously that the timed destruction of the MS ring protein could be the initial step leading to flagellar release. This idea was supported by the observation that the C. crescentus MS ring, in contrast to the ring structure from Salmonella, is very sensitive to trypsin treatment (M. Kanbe, Y. Umino, S. I. Aizawa, and U. Jenal, unpublished data), indicating that it is a relatively fragile structure. To determine if the flagellar structure was ejected, stalked cells were analyzed by electron microscopy for the existence of a flagellum at the tip of the stalk. The rationale behind this analysis was that a failure to eject the flagellum during the swarmer-to-stalked cell transition would produce a stalked pole occupied by a flagellar structure, a phenotype that has been described for several mutants lacking regulatory components of pole development (5, 43, 50). However, no flagella were observed at the stalk tips of cells expressing stable but functional FliF mutant forms, indicating that degradation of FliF is not a strict requirement for flagellar ejection. Similarly, cells lacking the clpA gene also did not retain flagella at the stalk tips, even though the FliF protein was completely stabilized under these conditions. Since FliF degradation is independent of other structural components of the flagellum (1), flagellar ejection and FliF turnover might still be initiated by a common preceding step. For example, the rate-limiting step of flagellar ejection during swarmer-to-stalked cell differentiation could be the disassembly of the MS ring. Perturbation of the structure in the inner membrane would lead to flagellar ejection and allow FliF to be degraded by the ClpAP protease. The addition of charged amino acids at the C-terminal end of FliF inhibits degradation but does not interfere with MS ring disassembly and the loss of the axial part of the flagellum. Alternatively, it is possible that specific removal of another component of the flagellar base precedes FliF degradation and triggers flagellar ejection. In this case, FliF degradation could be a direct consequence of the loss of an interaction partner. A preceding event leading to FliF degradation could also explain temporal control of FliF degradation in light of the fact that ClpA levels do not fluctuate during the cell cycle.
Supplementary Material
Acknowledgments
We thank G. Lesage for his help in making constructs and S. I. Aizawa for helpful discussions and critical reading of the manuscript.
This work was supported by Swiss National Science Foundation fellowship 31-59050.99 to U.J.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aldridge, P., and U. Jenal. 1999. Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Mol. Microbiol. 32:379-391. [DOI] [PubMed] [Google Scholar]
- 2.Alley, M. R. K., J. R. Maddock, and L. Shapiro. 1993. Requirement of the carboxyl terminus of the bacterial chemoreceptor for its targeted proteolysis. Science 259:1754-1757. [DOI] [PubMed] [Google Scholar]
- 3.Baumeister, W., J. Walz, F. Zuhl, and E. Seemuller. 1998. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92:367-380. [DOI] [PubMed] [Google Scholar]
- 4.Ciechanover, A., A. Orian, and A. L. Schwartz. 2000. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 22:442-451. [DOI] [PubMed] [Google Scholar]
- 5.Crymes, W. B., Jr., D. Zhang, and B. Ely. 1999. Regulation of podJ expression during the Caulobacter crescentus cell cycle. J. Bacteriol. 181:3967-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Defenbaugh, D. A., and H. Nakai. 2003. A context-dependent ClpX recognition determinant located at the C terminus of phage Mu repressor. J. Biol. Chem. 278:52333-52339. [DOI] [PubMed] [Google Scholar]
- 7.Dieffenbach, C. W., and G. S. Dveksler. 1995. PCR primer: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 8.Domian, I. J., K. C. Quon, and L. Shapiro. 1997. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell 90:415-424. [DOI] [PubMed] [Google Scholar]
- 9.Evinger, M., and N. Agabian. 1977. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol. 132:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer, B., G. Rummel, P. Aldridge, and U. Jenal. 2002. The FtsH protease is involved in development, stress response, and heat shock control in Caulobacter crescentus. Mol. Microbiol. 44:461-478. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. M., I. Levchenko, M. Seidel, S. H. Wickner, R. T. Sauer, and T. A. Baker. 2001. Overlapping recognition determinants within the ssrA degradation tag allow modulation of proteolysis. Proc. Natl. Acad. Sci. USA 98:10584-10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn, J. M., S. B. Neher, Y. I. Kim, R. T. Sauer, and T. A. Baker. 2003. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell 11:671-683. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg, A. L., and A. C. St John. 1976. Intracellular protein degradation in mammalian and bacterial cells: part 2. Annu. Rev. Biochem. 45:747-803. [DOI] [PubMed] [Google Scholar]
- 14.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman, S. 1999. Regulation by proteolysis: developmental switches. Curr. Opin. Microbiol. 2:142-147. [DOI] [PubMed] [Google Scholar]
- 16.Gottesman, S., E. Roche, Y. Zhou, and R. T. Sauer. 1998. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 12:1338-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimaud, R., M. Kessel, F. Beuron, A. C. Steven, and M. R. Maurizi. 1998. Enzymatic and structural similarities between the Escherichia coli ATP-dependent proteases, ClpXP and ClpAP. J. Biol. Chem. 273:12476-12481. [DOI] [PubMed] [Google Scholar]
- 18.Grünenfelder, B., S. Gehrig, and U. Jenal. 2003. Role of the cytoplasmic C terminus of the FliF motor protein in flagellar assembly and rotation. J. Bacteriol. 185:1624-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grünenfelder, B., G. Rummel, J. Vohradsky, D. Röder, H. Langen, and U. Jenal. 2001. Proteomic analysis of the bacterial cell cycle. Proc. Natl. Acad. Sci. USA 98:4681-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman, C., D. Thevenet, P. Bouloc, G. C. Walker, and R. D'Ari. 1998. Degradation of carboxy-terminal-tagged cytoplasmic proteins by the Escherichia coli protease HflB (FtsH). Genes Dev. 12:1348-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoskins, J. R., S. Y. Kim, and S. Wickner. 2000. Substrate recognition by the ClpA chaperone component of ClpAP protease. J. Biol. Chem. 275:35361-35367. [DOI] [PubMed] [Google Scholar]
- 22.Jenal, U. 2000. Signal transduction mechanisms in Caulobacter crescentus development and cell cycle control. FEMS Microbiol. Rev. 24:177-191. [DOI] [PubMed] [Google Scholar]
- 23.Jenal, U., and T. Fuchs. 1998. An essential protease involved in bacterial cell-cycle control. EMBO J. 17:5658-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenal, U., and R. Hengge-Aronis. 2003. Regulation by proteolysis in bacterial cells. Curr. Opin. Microbiol. 6:163-172. [DOI] [PubMed] [Google Scholar]
- 25.Jenal, U., and L. Shapiro. 1996. Cell cycle-controlled proteolysis of a flagellar motor protein that is asymmetrically distributed in the Caulobacter predivisional cell. EMBO J. 15:2393-2406. [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, R. C., and B. Ely. 1977. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics 86:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keiler, K. C., L. Shapiro, and K. P. Williams. 2000. tmRNAs that encode proteolysis-inducing tags are found in all known bacterial genomes: a two-piece tmRNA functions in Caulobacter. Proc. Natl. Acad. Sci. USA 97:7778-7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keiler, K. C., P. R. Waller, and R. T. Sauer. 1996. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science 271:990-993. [DOI] [PubMed] [Google Scholar]
- 29.Kessel, M., M. R. Maurizi, B. Kim, E. Kocsis, B. L. Trus, S. K. Singh, and A. C. Steven. 1995. Homology in structural organization between E. coli ClpAP protease and the eukaryotic 26 S proteasome. J. Mol. Biol. 250:587-594. [DOI] [PubMed] [Google Scholar]
- 30.King, R. W., R. J. Deshaies, J. M. Peters, and M. W. Kirschner. 1996. How proteolysis drives the cell cycle. Science 274:1652-1659. [DOI] [PubMed] [Google Scholar]
- 31.Lee, C., M. P. Schwartz, S. Prakash, M. Iwakura, and A. Matouschek. 2001. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol. Cell 7:627-637. [DOI] [PubMed] [Google Scholar]
- 32.Meisenzahl, A. C., L. Shapiro, and U. Jenal. 1997. Isolation and characterization of a xylose-dependent promoter from Caulobacter crescentus. J. Bacteriol. 179:592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nierman, W. C., T. V. Feldblyum, M. T. Laub, I. T. Paulsen, K. E. Nelson, J. Eisen, J. F. Heidelberg, M. R. Alley, N. Ohta, J. R. Maddock, I. Potocka, W. C. Nelson, A. Newton, C. Stephens, N. D. Phadke, B. Ely, R. T. DeBoy, R. J. Dodson, A. S. Durkin, M. L. Gwinn, D. H. Haft, J. F. Kolonay, J. Smit, M. B. Craven, H. Khouri, J. Shetty, K. Berry, T. Utterback, K. Tran, A. Wolf, J. Vamathevan, M. Ermolaeva, O. White, S. L. Salzberg, J. C. Venter, L. Shapiro, and C. M. Fraser. 2001. Complete genome sequence of Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 98:4136-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osteras, M., A. Stotz, S. Schmid Nuoffer, and U. Jenal. 1999. Identification and transcriptional control of the genes encoding the Caulobacter crescentus ClpXP protease. J. Bacteriol. 181:3039-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poindexter, J. S. 1964. Biological properties and classification of the Caulobacter group. Bacteriol. Rev. 28:231-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porankiewicz, J., J. Wang, and A. K. Clarke. 1999. New insights into the ATP-dependent Clp protease: Escherichia coli and beyond. Mol. Microbiol. 32:449-458. [DOI] [PubMed] [Google Scholar]
- 37.Prentki, H., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 38.Quon, K. C., G. T. Marczynski, and L. Shapiro. 1996. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell 84:83-93. [DOI] [PubMed] [Google Scholar]
- 39.Roche, E. D., and R. T. Sauer. 1999. SsrA-mediated peptide tagging caused by rare codons and tRNA scarcity. EMBO J. 18:4579-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon, R., U. Prieffer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-790. [Google Scholar]
- 43.Sommer, J. M., and A. Newton. 1989. Turning off flagellum rotation requires the pleiotropic gene pleD: pleA, pleC, and pleD define two morphogenic pathways in Caulobacter crescentus. J. Bacteriol. 171:392-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephens, C. M., and L. Shapiro. 1993. An unusual promoter controls cell-cycle regulation and dependence on DNA replication of the Caulobacter fliLM early flagellar operon. Mol. Microbiol. 9:1169-1179. [DOI] [PubMed] [Google Scholar]
- 45.Tinker-Kulberg, R. L., and D. O. Morgan. 1999. Pds1 and Esp1 control both anaphase and mitotic exit in normal cells and after DNA damage. Genes Dev. 13:1936-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobias, J. W., T. E. Shrader, G. Rocap, and A. Varshavsky. 1991. The N-end rule in bacteria. Science 254:1374-1377. [DOI] [PubMed] [Google Scholar]
- 47.Tsai, J. W., and M. R. Alley. 2001. Proteolysis of the Caulobacter McpA chemoreceptor is cell cycle regulated by a ClpX-dependent pathway. J. Bacteriol. 183:5001-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallejo, A. N., R. J. Pogulis, and L. R. Pease. 1994. In vitro synthesis of novel genes: mutagenesis and recombination by PCR. PCR Methods Appl. 4:123-130. [DOI] [PubMed] [Google Scholar]
- 49.Wang, L., M. Elliott, and T. Elliott. 1999. Conditional stability of the HemA protein (glutamyl-tRNA reductase) regulates heme biosynthesis in Salmonella typhimurium. J. Bacteriol. 181:1211-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, S. P., P. L. Sharma, P. V. Schoenlein, and B. Ely. 1993. A histidine protein kinase is involved in polar organelle development in Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 90:630-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolf, B. B., and D. R. Green. 2002. Apoptosis: letting slip the dogs of war. Curr. Biol. 12:177-179. [DOI] [PubMed] [Google Scholar]
- 52.Wright, R., C. Stephens, G. Zweiger, L. Shapiro, and M. R. Alley. 1996. Caulobacter Lon protease has a critical role in cell-cycle control of DNA methylation. Genes Dev. 10:1532-1542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.