Abstract
Objectives
To study clinical predictors for radiographic progression after 1 year in an early rheumatoid arthritis (RA) trial.
Methods
In the SWEFOT trial population, disease modifying antirheumatic drug (DMARD) naïve RA patients started methotrexate; 3-month responders (DAS28 <3.2) continued (n=147), while non-responders were randomised to addition of sulfasalazine+hydroxychloroquine (n=130) or infliximab (n=128). X-rays were scored by the Sharp-van der Hejde score (SHS) method and radiographic progression was defined as a ≥5 increase after 1 year. Potential baseline predictors of radiographic progression were tested using multivariable logistic regression, adjusted for potential confounders.
Results
79 of 311 patients with available radiographs at baseline and follow-up had radiographic progression. The following baseline parameters were independent predictors of radiographic progression at 1 year: baseline erosions (adjusted OR=2.29, 95% CI 1.24 to 4.24), erythrocyte sedimentation rate (adjusted OR per tertile increase=1.72, 95% CI 1.12 to 2.65) and C-reactive protein (adjusted OR per tertile increase=1.52, 95% CI 1.03 to 2.26). Current smoking was an independent predictor of radiographic progression (adjusted OR=2.17, 95% CI 1.06 to 4.45). These results remained after further adjustment for treatment strategy. Three-dimensional matrix including current smoking status, erosions and C-reactive protein tertiles showed a 12–63% risk gradient from patients carrying none compared with all predictors. Rheumatoid factor (RF)/anti-cyclic citrullinated peptide (anti-CCP) positivity did not significantly predict radiographic progression using SHS increase ≥5 as cut-off. In a secondary exploratory analysis using cut-off >1, both RF and anti-CCP positivity were significant predictors in the unadjusted, but not the adjusted analyses. The other parameters also remained significant using this lower cut-off.
Conclusions
In addition to previously described predictors, we identified smoking as a strong independent risk factor for radiographic progression in early RA.
Trial registration number
Keywords: Early Rheumatoid Arthritis, Outcomes research, Smoking
Introduction
In rheumatoid arthritis (RA), broad evidence supports that treatment strategies focusing on early inflammatory control decrease radiographic progression.1 Nevertheless, a proportion of patients progress, some despite having low disease activity.2 Since accumulation of joint damage over time correlates with decline in both functional capacity and quality of life, it is important to identify those patients at diagnosis who are likely to develop significant radiographic progression.3 4 Indeed, several studies have recently attempted to construct clinically useful risk matrices to predict so-called ‘rapid radiographic progression’ (RRP), corresponding to an increase in Sharp-van der Hejde score (SHS) of ≥5 after 1 year, based on both early RA trials5 6 and cross-sectional cohorts7; their performance has been tested in both early8 and unselected9 RA populations. Among the baseline clinical parameters that have been identified as predictors and included in those matrices are inflammatory markers (erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)), radiographic erosions at baseline, swollen joint counts and auto-antibody status.
However, none of these studies has evaluated whether smoking habits associate with RRP after 1 year, although several studies, especially earlier ones before the era of biological treatment, had indicated that RA patients who smoke develop more radiographic damage.10–13 Furthermore, current smokers have been shown to respond worse clinically to both methotrexate and tumour necrosis factor α (TNFα) inhibitors in early RA.14 15
Here, we examined baseline predictors of radiographic progression in the SWEFOT trial population,16 17 including previously known ones as well as smoking habits.
Methods
Participants in the investigator-initiated, multicentre, randomised SWEFOT trial (n=487) served as our study base; it has been described in detail elsewhere.16 17 Briefly, inclusion criteria were RA according to the 1987 revised American College of Rheumatology (ACR) criteria, age ≥18 years, symptom duration <1 year, 28-joint disease activity score (DAS28) >3.2, no previous disease modifying antirheumatic drug (DMARD) treatment and stable prednisolone dose, if present, for ≥4 weeks before entry and throughout the study of ≤10 mg/day. Patients who achieved DAS28 <3.2 after 3–4 months continued on methotrexate (MTX, N=147), while the other patients were randomised to add either infliximab (N=128) or both sulfasalazine and hydroxychloroquine (N=130).
Anti-cyclic citrullinated peptide (anti-CCP) antibodies were measured with the standard ELISA (Immunoscan-RA Mark2 ELISA test, Euro-Diagnostica, Malmö, Sweden) and rheumatoid factor (RF) was determined by routine methods. Smoking status was defined as current, past or never cigarette smokers; patients were also grouped as current smokers versus non-smokers, pooling past and never smokers in the latter group.
X-rays of hands and feet were obtained at baseline and after 1 year, and analysed by the modified SHS method. Radiographic progression was defined as an increase in total SHS of ≥5 after 1 year, as previously suggested,6 8 which corresponds to the minimal clinically important progression,18 and has also been referred to as RRP. In a secondary exploratory analyses, we tested a lower cut-off of SHS >1 after 1 year.
For this study, 311 patients from the SWEFOT trial were included, based mostly on availability of full sets of radiographs at baseline and 1-year follow-up. Their baseline characteristics (table 1) were representative for the whole study population, and did not differ from the subgroup included in the multivariable analyses which had complete data including smoking status.
Table 1.
Baseline characteristics of SWEFOT patients with radiographic data
| All* (n=311) | In model† (n=269) | |
|---|---|---|
| Male, n (%) | 87 ((28%) | 75 (28%) |
| Age, years, median (IQR) | 57 (46–64) | 57 (46–63) |
| Cigarette smoking status | ||
| Current smokers, n (%) | 65 (24%) | 64 (24%) |
| Past smokers, n (%) | 104 (37%) | 100 (37%) |
| Never smokers, n (%) | 106 (39%) | 105 (39%) |
| Symptom duration, months, median (IQR) | 5 (4–8) | 6 (4–9) |
| RF positive, n (%) | 208 (67%) | 179 (67%) |
| Anti-CCP antibody positive, n (%) | 180 (61%) | 156 (62%) |
| RF and anti-CCP positive, n (%) | 146 (50%) | 125 (49%) |
| Sharp van der Hejde score, median (IQR) | 2 (0–6) | 2 (0–6) |
| Joint space narrowing score, median (IQR) | 0 (0–3) | 0 (0–3) |
| Erosion score, median (IQR) | 0 (0–2) | 0 (0–2) |
| Erosions, n (%) | 128 (41%) | 114 (42%) |
| Concurrent prednisolone, n (%) | 42 (14%) | 35 (13%) |
| Concurrent NSAIDs, n (%) | 192 (62%) | 171 (64%) |
| HAQ score, median (IQR) | 1.1 (0.8–1.6) | 1.1 (0.9–1.6) |
| DAS28, median (IQR) | 5.8 (5.0–6.4) | 5.8 (5.0–6.4) |
| 28-Swollen joint count, median (IQR) | 10 (6–14) | 10 (6–14) |
| 28-Tender joint count, median (IQR) | 8 (5–13) | 8 (5–13) |
| C-reactive protein, median (IQR) | 19 (9–46) | 19 (9–47) |
| Erythrocyte sedimentation rate, median (IQR) | 34 (22–54) | 35 (22–54) |
| VAS–patient global health, median (IQR) | 59 (40–74) | 59 (41–74) |
| VAS–pain, median (IQR) | 59 (42–73) | 60 (44–73) |
| Shared epitope, n (%) | 208 (74%) | 177 (73%) |
*In the whole group/multivariable model, missing numbers were as follows: RF, n=2/2; anti-CCP, n=16/14; smoking status, n=36/0; shared epitope, n=28/25; HAQ score, n=7/0; VAS scales, n=3/0; symptom duration, n=1/0.
†Multivariable model shown in table 2. There were no significant differences compared to the whole group.
Anti-CCP, anti-cyclic citrullinated peptide; DAS28, 28-joint disease activity score; HAQ, Health Assessment Questionnaire; NSAIDs, non-steroidal anti-inflammatory drugs; RF, rheumatoid factor; VAS, visual analog scale.
Statistical methods
The primary outcome measure was radiographic progression (increase in total SHS of ≥5 after 1 year), and the association between each baseline variable and the risk of radiographic progression after 1 year (yes/no) was calculated using logistic regression. The results were expressed as ORs with 95% CIs. First, unadjusted analyses were performed for baseline variables, chosen and categorised based on previous reports5 6 8 and clinical relevance (see table 2). For the unadjusted analyses, correlations between baseline variables were assessed by Spearman correlation. As expected, high co-linearity was observed for health assessment questionnaire (HAQ) score and DAS28 and its components, so only HAQ was kept in the model, and excluded when DAS28 and its components, respectively, were analysed. Finally, we further adjusted for treatment strategy by adding a three group variable to the model indicating whether the patient continued MTX or which arm he/she was randomised to.
Table 2.
Predictors of rapid radiographic progression (RRP, SHS score increase ≥5) at 1 year follow-up of the SWEFOT trial*
| All patients | Patients in multivariable model | ||
|---|---|---|---|
| Unadjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)† | |
| Number | 311 | 269 | 269 |
| Number with RRP | 79 | 72 | 72 |
| Demographic | |||
| Male | 1.61 (0.93 to 2.78) | 1.86 (1.05 to 3.32) | 1.77 (0.96 to 3.24) |
| Age (per 10 years increase) | 1.01 (0.84 to 1.22) | 1.01 (0.83 to 1.23) | 0.94 (0.76 to 1.16) |
| Symptom duration (per month←) | 1.06 (0.98 to 1.14) | 1.03 (0.95 to 1.12) | 1.02 (0.93 to 1.11) |
| Smoking habits | |||
| Current vs never smokers | 2.33 (1.20 to 4.51) | 2.49 (1.28 to 4.85) | 2.25 (1.12 to 4.54) |
| Past vs never smokers | 0.73 (0.38 to 1.42) | 0.75 (0.38 to 1.47) | 0.75 (0.38 to 1.48) |
| Current smokers vs non- smokers | 2.70 (1.50 to 4.87) | 2.85 (1.57 to 5.16) | 2.67 (1.44 to 4.95) |
| Baseline disease parameters‡ | |||
| RF positive | 1.44 (0.81 to 2.54) | 1.36 (0.75 to 2.46) | 1.05 (0.55 to 1.98) |
| Anti-CCP antibody positive | 1.46 (0.84 to 2.53) | 1.35 (0.76 to 2.39) | 1.00 (0.54 to 1.87) |
| RF and/or anti-CCP positive§ | 1.32 (0.94 to 1.86) | 1.27 (0.89 to 1.82) | 1.04 (0.71 to 1.54) |
| Erosions | 2.38 (1.41 to 4.00) | 2.43 (1.40 to 4.22) | 2.28 (1.28 to 4.07) |
| Concurrent prednisolone use | 1.21 (0.58 to 2.49) | 1.30 (0.60 to 2.81) | 1.21 (0.53 to 2.77) |
| DAS28 (per unit increase) | 1.36 (1.06 to 1.74) | 1.30 (1.00 to 1.69) | 1.37 (1.04 to 1.81) |
| Swollen joint count (<10, 10–17, >17) | 0.98 (0.63 to 1.51) | 1.14 (0.72 to 1.81) | 1.32 (0.81 to 2.15) |
| Tender joint count (per 10 increase) | 1.02 (0.66 to 1.56) | 0.96 (0.60 to 1.52) | 1.08 (0.67 to 1.76) |
| CRP (<10, 10–35 vs >35 mg/dL) | 1.66 (1.18 to 2.34) | 1.68 (1.18 to 2.41) | 1.52 (1.03 to 2.24) |
| ESR (<21, 21–50, >50 mm/h) | 1.59 (1.09 to 2.30) | 1.68 (1.13 to 2.49) | 1.67 (1.10 to 2.53) |
| VAS–global health (per 10 increase) | 1.12 (1.00 to 1.26) | 1.09 (0.97 to 1.22) | 1.09 (0.96 to 1.23) |
| HAQ (per unit increase) | 1.40 (0.93 to 2.08) | 1.17 (0.77 to 1.78) | 1.12 (0.71 to 1.76) |
| HLA-DRB1 shared epitope positive | 1.57 (0.81 to 3.02) | 1.41 (0.72 to 2.77) | 1.12 (0.55 to 2.28) |
Abbreviations and numbers of missing as in table 1.
*Association between each parameter and the odds of having a rapid RRP after 1 year (yes/no), calculated by logistic regression and expressed as ORs with 95% CIs. ORs for dichotomised variables are calculated for being positive, and for continuous variables per unit (DAS28, HAQ) or per 10 increase (age, ESR, VAS–global health), while CRP was grouped as in previous reports (<10, 10–35 vs >53 mg/L), and symptom duration per month increase.
†Adjusted for gender, symptom duration, current smoking, baseline erosions and HAQ.
‡As high co-linearity was observed for anti-CCP and RF status, and also HAQ score and DAS28 and its components, only anti-CCP status and HAQ were kept in the model, and excluded when RF status or DAS28 and its components, respectively, were analysed.
§Three group comparison, double positive (2), single positive (1), no positive (0).
Anti-CCP, anti-cyclic citrullinated peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; RF, rheumatoid factor; SHS, Sharp–van der Heijde.
Using the parameters showing the strongest association with radiographic progression, three-parameter risk matrices were created, showing the actual proportion of patients with radiographic progression in each box and the total number of patients in that box.
Statistical analysis was performed using SAS V.9.1. All tests were two-sided and the significance level was set at 0.05.
This study was approved by the regional ethical committees of the participating clinics and registered at http://www.clinicaltrials.gov (NCT00764725). All patients were provided with oral and written information prior to inclusion, and consented to participate by signing the informed consent document.
Results
The baseline characteristics for the 311 patients with radiographic data at baseline and 1 year follow-up are shown in table 1; 79 had radiographic progression.
The association between each baseline parameter and radiographic progression was tested using unadjusted logistic regression (table 2). Significant associations were observed for current smoking, baseline erosions, DAS28 and its inflammatory components (CRP, ESR), while no significant associations were observed for the previously reported predictors swollen joint count and auto-antibody status (RF, anti-CCP antibodies). These significant associations remained when tested in a multivariable model (table 2). Of the 269 patients in the multivariable model, 80 remained on methotrexate monotherapy, while the others were randomised to add infliximab (n=94) or to triple therapy (n=95). Further adjustment for treatment strategy did not modify the observed associations between the significant predictors and radiographic progression: current smokers versus non-smokers (OR=2.78, 95% CI 1.48 to 5.19), erosions (OR=2.21, 95% CI 1.24 to 3.97), CRP (<10, 10–35, >35 mg/L, OR=1.49, 95% CI 0.98 to 2.25) and ESR (<21, 21–50, >50 mm/h, OR=1.62, 95% CI 1.03 to 2.53). Since auto-antibody status was, in contrast to some previous publications, not found to be a significant predictor of radiographic progression using the cut-off of SHS score increase ≥5, we made a secondary exploratory analysis to see whether it was a predictor using a lower cut-off threshold (SHS score increase >1). As shown in table 3, both positivity for RF (OR=1.75, 95% CI 1.07 to 2.86) and anti-CCP (OR=1.64, 95% CI 1.02 to 2.64) or both (OR=1.50, 95% CI 1.12 to 2.02) were then significant predictors in the unadjusted analyses, although only a trend in the multivariable model. The other parameters also remained significant using this lower cut-off (current smoking habits, baseline erosions, DAS28, CRP and ESR).
Table 3.
Predictors of radiographic progression at 1 year follow-up of the SWEFOT trial using SHS score increase >1 as cut-off *
| All patients | Patients in multivariate model | ||
|---|---|---|---|
| Unadjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)† | |
| Number | 311 | 269 | 269 |
| Number with SvdH score increase >1 | 136 | 118 | 118 |
| Demographic | |||
| Male | 1.38 (0.84 to 2.26) | 1.58 (0.92 to 2.70) | 1.44 (0.82 to 2.53) |
| Age (per 10 years increase) | 1.17 (0.99 to 1.38) | 1.18 (0.99 to 1.41) | 1.12 (0.93 to 1.36) |
| Symptom duration (per month←) | 1.05 (0.98 to 1.13) | 1.03 (0.96 to 1.11) | 1.01 (0.93 to 1.09) |
| Smoking habits | |||
| Current vs never smokers | 2.20 (1.17 to 4.12) | 2.34 (1.24 to 4.42) | 2.31 (1.18 to 4.54) |
| Past vs never smokers | 0.84 (0.48 to 1.47) | 0.88 (0.50 to 1.55) | 0.92 (0.52 to 1.65) |
| Current smokers vs non-smokers | 2.39 (1.35 to 4.22) | 2.49 (1.40 to 4.42) | 2.42 (1.33 to 4.42) |
| Baseline disease parameters‡ | |||
| RF positive | 1.75 (1.07 to 2.86) | 1.83 (1.08 to 3.10) | 1.59 (0.90 to 2.79) |
| Anti-CCP antibody positive | 1.64 (1.02 to 2.64) | 1.80 (1.07 to 3.01) | 1.38 (0.79 to 2.39) |
| RF and/or anti-CCP positive§ | 1.50 (1.12 to 2.02) | 1.60 (1.16 to 2.22) | 1.38 (0.98 to 1.95) |
| Erosions | 2.39 (1.50 to 3.80) | 2.72 (1.65 to 4.49) | 2.60 (1.55 to 4.38) |
| Concurrent prednisolone use | 1.07 (0.56 to 2.06) | 1.09 (0.53 to 2.22) | 1.08 (0.50 to 2.33) |
| DAS28 (per unit increase) | 1.13 (0.91 to 1.40) | 1.06 (0.84 to 1.34) | 1.10 (0.86 to 1.41) |
| Swollen joint count (<10, 10–17, >17) | 0.96 (0.66 to 1.41) | 0.94 (0.62 to 1.43) | 1.06 (0.68 to 1.64) |
| Tender joint count (per 10 increase) | 0.83 (0.57 to 1.22) | 0.75 (0.50 to 1.14) | 0.81 (0.52 to 1.26) |
| CRP (<10, 10–35 vs >35 mg/dL) | 1.43 (1.07 to 1.92) | 1.42 (1.04 to 1.94) | 1.30 (0.92 to 1.82) |
| ESR (<21, 21–50, >50 mm/h) | 1.65 (1.29 to 2.28) | 1.64 (1.15 to 2.33) | 1.63 (1.12 to 2.37) |
| VAS–global health (per 10 increase) | 1.03 (0.94 to 1.14) | 1.01 (0.91 to 1.11) | 1.00 (0.90 to 1.11) |
| HAQ (per unit increase) | 1.12 (0.79 to 1.60) | 1.00 (0.68 to 1.45) | 0.93 (0.62 to 1.40) |
| HLA-DRB1 shared epitope positive | 1.47 (0.85 to 2.53) | 1.32 (0.74 to 2.36) | 1.07 (0.58 to 1.98) |
*Association between each parameter and the odds of having a rapid radiographic progression after 1 year (yes/no), calculated by logistic regression and expressed as ORs with 95% CIs. ORs for dichotomised variables are calculated for being positive, and for continuous variables per unit (DAS28, HAQ) or per 10 increase (age, VAS–global health), while CRP and ESR was grouped as in previous reports which corresponded to tertiles, and symptom duration per month increase.
†Adjusted for gender symptom duration, current smoking, baseline erosions and HAQ.
‡As high co-linearity was observed for anti-CCP and RF status, and also HAQ score and DAS28 and its components, only anti-CCP status and HAQ were kept in the model, and excluded when RF status or DAS28 and its components, respectively, were analysed.
§Three group comparison, double positive (2), single positive (1), no positive (0).
Abbreviations and numbers of missing as in table 1.
Anti-CCP, anti-cyclic citrullinated peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; RF, rheumatoid factor; SHS, Sharp–van der Heijde.
Based on this, a three-dimensional matrix was constructed including current smoking status, baseline erosions and CRP (figure 1). A step-up gradient was observed, where 63% of the patients carrying all the predictors had developed radiographic progression after 1 year, in comparison to only 12% of patients without these three baseline predictors. A similar gradient was observed within each of the treatment subgroups (figure 2), and in men and women (figure 3).
Figure 1.
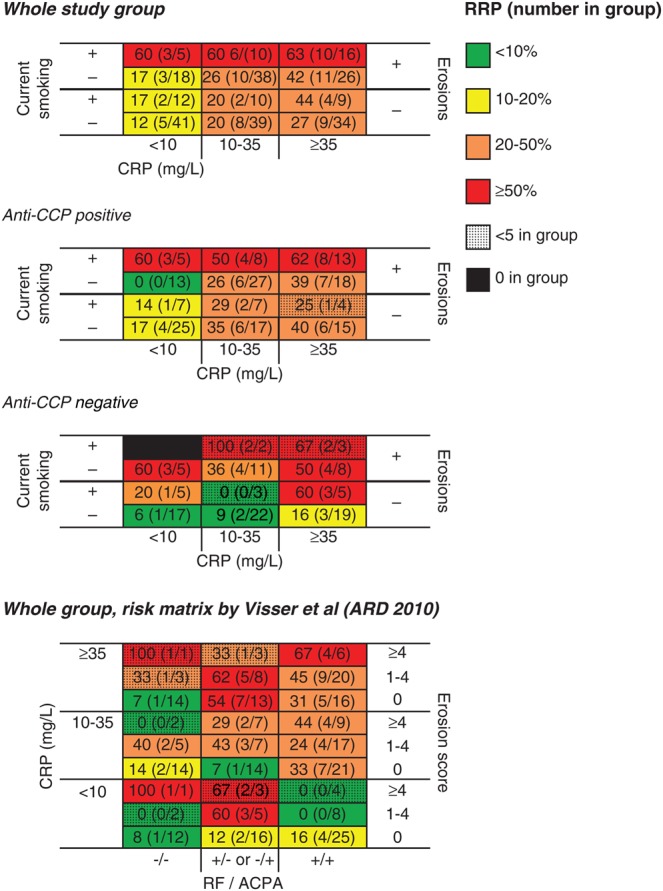
Risk matrices showing the proportion of SWEFOT patients who have developed radiographic progression (% and number with increase in Sharp–van der Hejde score ≥5 divided by total number in group) after 1 year, sub-grouped by baseline parameters identified using multivariable logistic regression analysis in the whole group and stratified by anti-cyclic citrullinated peptide (anti-CCP) status. Finally, we tested a previously reported matrix by Visser et al.5 CRP, C-reactive protein; RF, rheumatoid factor; RRP, rapid radiographic progression.
Figure 2.
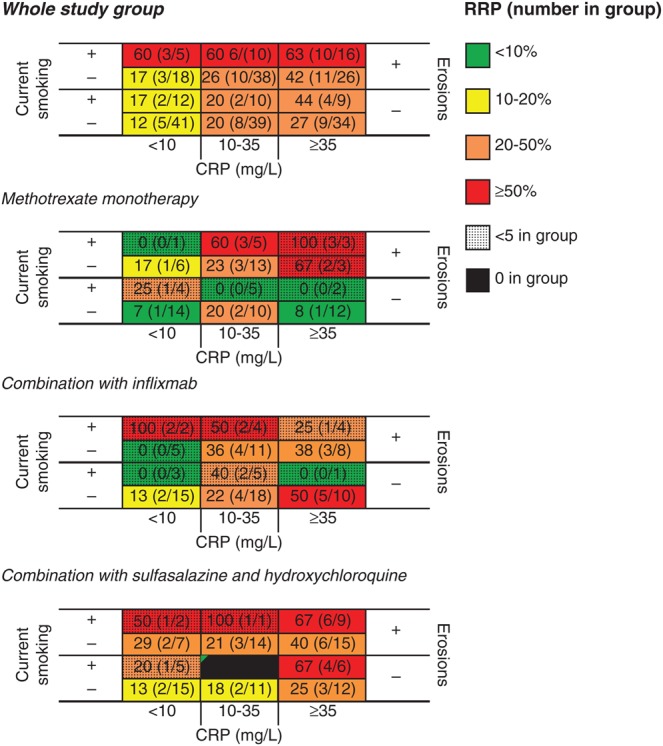
Risk matrices showing the proportion of SWEFOT patients who have developed radiographic progression (% and number with increase in Sharp–van der Hejde score ≥5 divided by total number in group) after 1 year in all early rheumatoid arthritis patients and stratified by treatment month 3–12. CRP, C-reactive protein; RRP, rapid radiographic progression.
Figure 3.
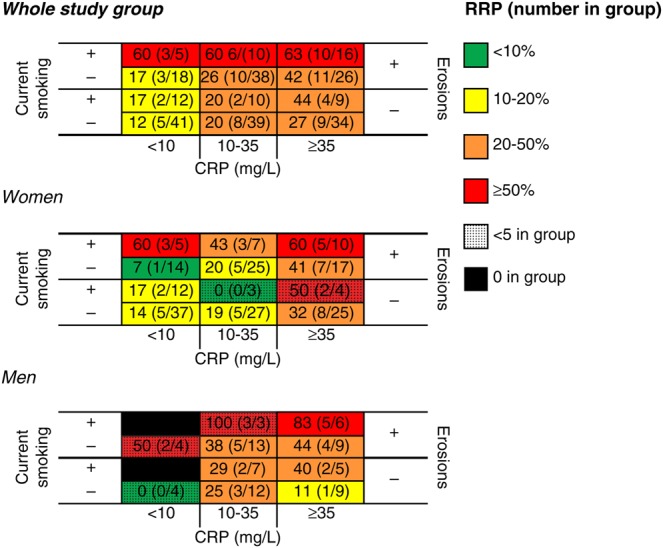
Risk matrices showing the proportion of SWEFOT patients who have developed radiographic progression (% and number with increase in Sharp–van der Hejde score ≥5 divided by total number in group) after 1 year in all early rheumatoid arthritis patients and stratified by sex. CRP, C-reactive protein; RRP, rapid radiographic progression.
A risk matrix reported by Visser et al5 from another early RA trial population, also including erosions (divided by number) and CRP levels as well as auto-antibody status, was also tested (see figure 1). Furthermore, we made separate analyses for anti-CCP positive and anti-CCP negative patients, showing the lowest proportion with radiographic progression in anti-CCP negative patients lacking all baseline predictors.
Discussion
In this standard care-based trial on early DMARD naïve RA patients, we confirmed that baseline radiographic erosions and levels of inflammatory markers predict the risk of radiographic progression after 1 year. Furthermore, current smoking habits turned out to be a strong independent predictor of radiographic progression, a finding perhaps not so surprising, since several older studies have previously reported an association between cigarette smoking and a more severe RA.10–15 Recently de Rooy et al19 performed a meta-analysis of six RA cohorts using radiographs from different follow-up time-points and found significantly more severe joint damage over time in smokers, although only significant in those from BARFOT and Leiden when analysed separately. However, smoking habits have not been included in any of the recent studies on risk matrix of radiographic progression, often referred to as RRP. When we constructed a risk matrix including the strongest predictors—smoking status, erosions and CRP tertiles—a step-up gradient was observed from 12% up to 63% risk of radiographic progression in patient with no as compared with all risk factors. Current smokers with baseline erosions had ≥60% risk of radiographic progression, irrespective of grade of inflammation. Furthermore, a previously reported early RA trial-based risk matrix,5 including autoantibody status instead of smoking status, performed reasonably well in our trial population. Given that, and the fact that anti-CCP positive RA is regarded as an aetiologically distinct and more severe disease subset, we made separate matrices for anti-CCP positive and negative disease. Current smokers with erosive disease had a high risk of radiographic progression in both subsets, while the lowest risk was observed for non-smokers with low CRP levels. Swollen joint count has been included in two of the previously reported matrices,6 7 but no association was observed in our study group. Consequently, these matrices did not perform well. Replacing CRP with ESR showed similar results.
The strength of this study is that it is conducted within a clinical trial which was performed in an unselected early RA population and reflects common standard care, namely that patients receive methotrexate at diagnosis, and those who did not achieve a low disease activity after 3–4 months received either an addition of infliximab, or sulfasalazine together with hydroxychloroquine. The findings for the independent predictors remained after further adjustment for treatment strategy. Thus, the matrix may help to identify at baseline those patients at risk of radiographic progression, irrespective of which treatment is chosen on clinical grounds. However, the separate risk matrices for each treatment arm should be interpreted with caution due to the limited number within smaller subgroups, although these show an overall similar pattern to that in the whole group. Further, the non-significant associations observed for anti-CCP status and shared epitope positivity (see crude ORs in table 2) might have reached significance if the statistical power had been larger. Since anti-CCP or RF positivity has been found to associate significantly in other early RA cohorts, we performed an exploratory analysis using a lower cut-off threshold of SHS increase >1: both auto-antibodies were then significant predictors in the unadjusted analyses. Nevertheless, current smoking remained a strong predictor.
The study group was too small to perform a full multivariable model, so we critically selected the variables to be included as potential confounders based on previous reports and clinical relevance. Based on the current knowledge about the pathogenesis of RA, smoking induces citrullination and may thus be regarded as a mediator of anti-CCP positivity. Therefore, we made stratified analyses, subgrouping RA into the two aetiologically distinct subsets of anti-CCP positive and negative disease, and evaluating whether an effect modification was present. However, anti-CCP positivity can also be an outcome of smoking, and thus a potential collider. Given that, and the fact that anti-CCP positivity did not significantly associate with radiographic progression in our study population (using the primary definition of SHS score increase ≥5, although significant in an exploratory analysis using >1 as a cut-off), we did not include it in the set of adjustment variables and considered it only as a potential effect modifier, by showing the stratified results in the risk matrices, as described above.
One subgroup showed somewhat contra-intuitive findings. The proportion of anti-CCP positive non-smokers with low CRP and baseline erosions that progressed was lower (0%) than in the group with no baseline erosions (17%). The treatment in these two subgroups did not differ (even numbers randomised to either treatment) and we have no clear explanation but would like to interpret this finding with caution, since the proportion with progression in both subgroups was relatively low (0% and 17%) and only one other subgroup had risk below 20%.
One potential limitation is that data on smoking intensity (pack-years) was not available. However, a previous study did not find any association with outcome when pack-years were evaluated in the context of the actual smoking status (current, past, never smoker),14 indicating that the actual smoking habits have most impact. Furthermore, our study was not designed to elucidate the mechanisms through which smoking affects radiographic progression. There is limited evidence available on this and mechanistic studies are required. Our study confirmed the broad evidence that there is a higher proportion of RF/anti-CCP positivity in current than in non-current smokers (double positive 61% vs 46%, single positive 29% vs 29%, double negative 10% vs 25%, p=0.01).
Taken together, our findings confirm and extend previous reports on predictors for radiographic progression in early RA and highlight the risk associated with smoking. The identified predictors are easily accessible and objective clinical variables, and showed clinically meaningful differences when tested in risk matrixes. Being based on a trial that reflects standard care in Sweden, the external validity of this risk matrix should be high, and now awaits validation in other early RA populations with available data on smoking status.
Acknowledgments
We wish to thank all the participating clinicians in the SWEFOT study group, nurses and patients who made the original trial possible.
Footnotes
Contributors: All co-authors contributed to the conception and design of the SWEFOT trial. SS and RvV designed the current study. SS performed the analysis and interpreted the data together with RvV, KF, PG, HR and SE. SS drafted the article and all co-authors revised it critically for important intellectual content and approved the final version.
Competing interests: The current study received no specific funding, but the first author (SS) has a research position (ALF clinical post-doc position) funded by the Stockholm county and the Karolinska Institute, and for KF this work was supported by grants from the Swedish Rheumatism Association, the Thelma Zoégas foundation in Helsingborg, and Stiftelsen för Rörelsehindrade i Skåne.
Ethics approval: Regional ethical committees of the participating clinics.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.van Nies JA, Krabben A, Schoones JW, et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis 2014;73:861–70. [DOI] [PubMed] [Google Scholar]
- 2.Rezaei H, Saevarsdottir S, Forslind K, et al. In early rheumatoid arthritis, patients with a good initial response to methotrexate have excellent 2-year clinical outcomes, but radiological progression is not fully prevented: data from the methotrexate responders population in the SWEFOT trial. Ann Rheum Dis 2012;71:186–91. [DOI] [PubMed] [Google Scholar]
- 3.Clarke AE, St-Pierre Y, Joseph L, et al. Radiographic damage in rheumatoid arthritis correlates with functional disability but not direct medical costs. J Rheumatol 2001;28:2416–24. [PubMed] [Google Scholar]
- 4.Welsing PM, van Gestel AM, Swinkels HL, et al. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum 2001;44:2009–17. [DOI] [PubMed] [Google Scholar]
- 5.Visser K, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis 2010;69:1333–7. [DOI] [PubMed] [Google Scholar]
- 6.Vastesaeger N, Xu S, Aletaha D, et al. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1114–21. [DOI] [PubMed] [Google Scholar]
- 7.Fautrel B, Granger B, Combe B, et al. Matrix to predict rapid radiographic progression of early rheumatoid arthritis patients from the community treated with methotrexate or leflunomide: results from the ESPOIR cohort. Arthritis Res Ther 2012;14:R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durnez A, Vanderschueren G, Lateur L, et al. Effectiveness of initial treatment allocation based on expert opinion for prevention of rapid radiographic progression in daily practice of an early RA cohort. Ann Rheum Dis 2011;70:634–7. [DOI] [PubMed] [Google Scholar]
- 9.Lillegraven S, Paynter N, Prince FH, et al. Performance of matrix-based risk models for rapid radiographic progression in a cohort of patients with established rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyhall-Wahlin BM, Petersson IF, Nilsson JA, et al. High disease activity disability burden and smoking predict severe extra-articular manifestations in early rheumatoid arthritis. Rheumatology (Oxford) 2009;48:416–20. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe F. The effect of smoking on clinical, laboratory, and radiographic status in rheumatoid arthritis. J Rheumatol 2000;27:630–7. [PubMed] [Google Scholar]
- 12.Harrison BJ, Silman AJ, Wiles NJ, et al. The association of cigarette smoking with disease outcome in patients with early inflammatory polyarthritis. Arthritis Rheum 2001;44:323–30. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos NG, Alamanos Y, Voulgari PV, et al. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients? Clin Exp Rheumatol 2005;23:861–6. [PubMed] [Google Scholar]
- 14.Saevarsdottir S, Wedren S, Seddighzadeh M, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the Epidemiological Investigation of Rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum 2011;63:26–36. [DOI] [PubMed] [Google Scholar]
- 15.Saevarsdottir S, Wallin H, Seddighzadeh M, et al. Predictors of response to methotrexate in early DMARD naive rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis 2011;70:469–75. [DOI] [PubMed] [Google Scholar]
- 16.van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 2009;374:459–66. [DOI] [PubMed] [Google Scholar]
- 17.van Vollenhoven RF, Geborek P, Forslind K, et al. Conventional combination treatment versus biological treatment in methotrexate-refractory early rheumatoid arthritis: 2 year follow-up of the randomised, non-blinded, parallel-group Swefot trial. Lancet 2012;379:1712–20. [DOI] [PubMed] [Google Scholar]
- 18.Bruynesteyn K, van der Heijde D, Boers M, et al. Determination of the minimal clinically important difference in rheumatoid arthritis joint damage of the Sharp/van der Heijde and Larsen/Scott scoring methods by clinical experts and comparison with the smallest detectable difference. Arthritis Rheum 2002;46:913–20. [DOI] [PubMed] [Google Scholar]
- 19.de Rooy DPC, van Nies JAB, Kapetanovic MC, et al. Smoking as a risk factor for the radiological severity of rheumatoid arthritis: a study on six cohorts. Ann Rheum Dis 2014;73:1384–7. [DOI] [PubMed] [Google Scholar]


