Abstract
Objective
Laquinimod is an oral therapeutic agent under investigation for the treatment of Crohn's disease (CD), Huntington's disease, lupus nephritis and multiple sclerosis. This dose escalation study evaluated the safety and efficacy of laquinimod as induction therapy in patients with active moderate–severe CD.
Design
Multicentre, double-blind, sequential-cohort, randomised controlled trial with laquinimod doses of 0.5, 1, 1.5 or 2 mg/day or placebo (n=45 per cohort randomised in a 2:1 ratio) for 8 weeks with 4-week follow-up. Stable concomittant therapies and prior use of anti-tumour necrosis factor agents were permitted. Comprehensive safety assessments were performed and efficacy analyses included the proportions of patients in clinical remission (CD Activity Index (CDAI) <150 and no treatment failure (TF)), and with a clinical response (70 or 100 point CDAI reduction from baseline or remission and no TF).
Results
117 patients received laquinimod and 63 patients received placebo. The overall incidence of adverse events (AEs) in the laquinimod group was similar to the pooled placebo group (86.2%–96.7% vs 82.5%) and most AEs were mild to moderate in severity. Treatment with laquinimod 0.5 mg showed consistent effects on remission (48.3% (CI 31% to 66%) vs 15.9% (CI 9% to 27%)), response 100 (55.2% (CI 37% to 71%) vs 31.7% (CI 22% to 44%)) and response 70 (62.1% (CI 44% to 77%) vs 34.9% (CI 24% to 47%)) versus placebo. Laquinimod 1.0 mg showed less benefit (26.7% remission (CI 14% to 44%) and 53.3% response 70 (CI 36% to 70%)), and no effect was noted on remission/response at higher doses.
Conclusions
Laquinimod was safe and well tolerated, and the effects on remission and response of the 0.5 mg dose suggest a treatment benefit in patients with CD.
Trial registration number
Keywords: CROHN'S DISEASE, PHARMACOTHERAPY, IBD CLINICAL, CLINICAL TRIALS, CLINICAL DECISION MAKING
Significance of this study.
What is already known on this subject?
Crohn's disease (CD) is a chronic IBD without a definitive cure. Patients are faced with a lifetime of recurrent disease flare-ups that are treated with medical and surgical interventions.
Current medications are moderately efficacious for induction and maintenance of clinical remission. However, adverse effects are a problem.
More efficacious therapies with fewer side effects are needed.
What are the new findings?
Laquinimod is a novel oral therapy that reduces pro-inflammatory cytokine activity.
The present study found that laquinimod could be used safely for 8 weeks at doses of 0.5, 1.0, 1.5 and 2.0 mg in patients with CD. The overall incidence of adverse events in the laquinimod dose groups ranged from 86.2% to 96.7%, and was similar to that observed in the placebo treated patients (82.5%).
The exploratory analyses of efficacy suggest that the lower dose of laquinimod (0.5 mg) increased the proportion of patients in clinical remission and the proportion of patients with a clinical response 100 and 70 in comparison with placebo.
How might it impact on clinical practice in the foreseeable future?
Laquinimod is an oral therapy. Based on the results from the current study, laquinimod 0.25 and 0.5 mg is currently in phase III development for the treatment of moderate to severe CD. If found to be effective, laquinimod could provide patients with a novel oral treatment option.
Introduction
Crohn's disease (CD) is a chronic IBD that often begins in adolescence1 and affects between 0.1 and 16 per 100 000 persons worldwide2 with a significant personal, social and economic burden.3–5 The disease can affect any part of the GI tract but is usually located in the terminal ileum and/or colon. Typically, CD has a chronic relapsing remitting course and CD patients are faced with a lifetime of recurrent exacerbations that are treated with medical and surgical interventions, although without definitive cure.
The major therapeutic goals are reduction of acute signs and symptoms, induction and maintenance of remission, and prevention of complications. Therapeutic recommendations depend on the disease location, disease severity, disease complications (fistulas, abscesses, strictures) and previous response to treatment. Various agents, such as sulfasalazine,6 antibiotics,7 8 glucocorticosteroids (GCS),9 thiopurines,10 methotrexate11 and tumour necrosis factor (TNF) antagonists,12 are useful in the induction and/or maintenance of remission. Many of these, however, are only moderately efficacious and are associated with side effects.12–14 In addition, the newer biological agents have a relatively inconvenient parenteral route of administration and are costly.15 These therapeutic challenges mean that over 50% of people with CD require surgery within 10 years16 17 because pharmacological agents can no longer control the disease or complications such as perforation and/or stenosis develop. Therefore, a need exists to develop new efficacious and safe agents for the management of CD.
It is generally believed that CD involves exaggerated T cell immune responses to a subset of commensal enteric bacteria in genetically susceptible individuals, and that environmental factors trigger the onset or periodic reactivation of disease.18 These circumstances result in the production of an excess of pro-inflammatory cytokines.19 Treatment with TNF antagonists such as infliximab, adalimumab or certolizumab induces clinical response and clinical remissions20–22 suggesting that agents that reduce inflammatory cytokines may have therapeutic benefits in patients with CD.
Laquinimod (TV-5600, previously ABR-215062) is a novel synthetic compound with high oral bioavailability, which is being developed as an oral formulation for CD, multiple sclerosis (MS), Huntington's disease and lupus nephritis. Preclinical data in various experimental models of colitis and MS suggest that laquinimod has a direct inhibitory effect on antigen presenting cells and T cells, resulting in downregulation of pro-inflammatory cytokines.23 24 We conducted a randomised trial to assess the safety, tolerability and efficacy of escalating doses of oral laquinimod in patients with CD.
Materials and methods
This phase IIa, randomised, double-blind, placebo-controlled, sequential-cohort, dose-ranging study was designed to evaluate the safety and tolerability of laquinimod in patients with moderate to severe CD. Study participants were recruited from 37 centres in Belgium, France, Israel, Italy, Netherlands, Poland, South Africa, Spain and the UK. Institutional review boards at each study site approved the protocol and all patients provided written informed consent. The trial was conducted in accordance with the Declaration of Helsinki and ethical principles of Good Clinical Practice, according to the International Conference on Harmonisation (ICH) Harmonized Tripartite Guideline. The trial is registered at Clinicaltrials.gov with the identifier NCT00737932.
Patients
Eligible patients were men or women 18–67 years old with a documented diagnosis of CD for at least 3 months prior to screening that was supported by endoscopy or radiology. Patients were classified as having moderate to severe CD defined by a Crohn's Disease Activity Index (CDAI) score of 220–450 and either had C-reactive protein (CRP) concentrations >5 mg/L or documented endoscopic evidence of mucosal ulcerations (presence of at least two ulcers ≥10 mm) within 4 weeks prior to baseline. Key exclusion criteria were a diagnosis of indeterminate colitis; a positive stool culture for enteric pathogens or Clostridium difficile toxin; bowel surgery ≤3 months prior to screening, a planned elective surgery or hospitalisation during the course of the study; clinically significant short bowel syndrome; clinically significant obstructive symptoms; presence of an intra-abdominal abscess or a fistula with clinical or radiological evidence of an associated abscess; ileostomy; colostomy; and serum elevation of alanine transaminase; aspartate transaminase; γ-glutamyl transpeptidase; alkaline phosphatase or direct bilirubin ≥2 times the upper limit of normal at screening. Patients who required parenteral nutrition or those with a history of any malignancy in the year prior to screening (excluding basal cell carcinoma) or a clinically significant or unstable medical or surgical condition were also excluded. Treatment-related exclusion criteria included initiation of oral corticosteroids within 4 weeks or 6-mercaptopurine (6MP), azathioprine or methotrexate within 12 weeks prior to screening; changes in the dose of 6MP, azathioprine or methotrexate within 6 weeks; changes in the dose of 5-aminosalicylic acid (5-ASA) or antibiotics administered for CD within 2 weeks prior to screening; treatment with more than 20 mg/day of prednisolone or budesonide more than 6 mg/day for CD at baseline, or whose corticosteroid dosage regimen was not stable for at least 2 weeks prior to baseline; treatment with cyclosporine, tacrolimus, mycophenolate mofetil or thalidomide within 2 months prior to screening, treatment with natalizumab within 6 months prior to screening, use of amiodarone within 2 years, use of inhibitors of cytochrome P450 (CYP) 3A4 within 2 weeks prior to baseline visit (1 month for fluoxetine), or use of any other investigational drugs within 3 months prior to screening. Patients treated with TNF antagonists within 4 weeks prior to screening were also excluded. The percentage of patients previously treated with TNF antagonists was limited to 60% of patients randomised for each cohort.
Concomitant medications allowed during the study included those used to systemically to treat CD (5-ASA compounds, antibiotics (except for those which inhibit CYP3A4), GCS and immunosuppressants). Other medications allowed included antidiarrhoeal drugs, analgesics, non-steroidal anti-inflammatory drugs and topical preparations. The dose of the allowed medications was to be kept stable throughout the study; dose adjustment of these medications not allowed by the study protocol or the start of a new medication during the study was regarded as a treatment failure (TF).
Design
Laquinimod doses of 0.5, 1.0, 1.5 and 2.0 mg daily were studied sequentially in four distinct escalating cohorts (n=45) in which patients were randomised to laquinimod or placebo in a 2:1 randomisation ratio using an interactive voice/web response system. Evaluation of laquinimod in successive cohorts was dependent upon: (1) randomisation of at least 45 patients for the preceding cohort, (2) closure of screening and randomisation for the preceding cohort and (3) the decision of a safety committee to proceed to the next dose level.
The study consisted of a 1–2-week screening period, an 8-week treatment period and a 4-week follow-up period. Scheduled inclinic visits were conducted at screening, baseline and at weeks 1, 2, 4, 6, 8 and 12 weeks postrandomisation. Treatment with laquinimod/placebo was discontinued at week 8 postrandomisation and a follow-up/study completion visit was conducted at week 12. Patients who discontinued study drug early (prior to week 8) were required to attend a follow-up termination visit within 4 weeks (28 days) of study drug discontinuation.
Study drug
All patients took capsules of oral laquinimod (each containing 0.5 mg) or placebo which were identical in appearance. Participants, care providers and all study personnel were blinded to treatment assignment. Laquinimod plasma concentrations reach steady state following approximately 10–12 days of daily maintenance dosing.25 A loading dose of twice the intended final dose was administered twice daily for the first 2 days with a 12-h interval between the doses. The intent was to decrease the time to steady state plasma concentrations and potentially decrease time to response. Starting on Day 3 and thereafter, the dosing regimen consisted of the intended final dose administered once daily.
Safety assessments
Adverse events (AEs; MedDRA terminology), clinical laboratory tests, vital signs, physical examination and electrocardiogram were evaluated at every visit and during the follow-up period. Any extra-intestinal manifestation potentially related to CD (on the CD complications of the CDAI score or any other extra-intestinal manifestation) whether a new condition or a worsening of a pre-existing condition was recorded as an AE. Worsening of GI signs and symptoms of CD (as captured by the CDAI score) were recorded as an AE only if the outcome was more severe than normally expected in the usual course of the disease.
Efficacy assessments
Definitions of clinical remission and response were based on CDAI scores from baseline to weeks 2, 4, 6, 8 and 12 visits. Patients were given comprehensive instructions for completing the daily CDAI diary score using paper forms. Diaries were completed during the entire period. Patients who prematurely discontinued treatment were also requested to complete diaries daily until a follow-up visit 28 days after treatment discontinuation. TF occurred when either the patient was withdrawn from study because the investigator felt that the patient was not responding to the treatment or the patient underwent surgery for CD; and if the dosage of an allowed medication such as antibiotics or steroids was increased beyond what was allowed per protocol or the patient received rescue therapy with a biological or immunosuppressive drug during the treatment period. Assessment of GI inflammation also included the per cent change in serum CRP and faecal calprotectin concentrations from baseline to weeks 2, 4, 6, 8 and 12 visits.
Statistical analysis
This study was exploratory; therefore, no formal hypothesis testing was planned. A total of 45 patients per cohort (ratio 2:1 for laquinimod and placebo, respectively) was considered adequate to provide an initial estimate of treatment efficacy and dose response. The data for the patients in all of the placebo arms were pooled. The decision to pool placebo patients across cohorts was made prior to unblinding based on analysis of baseline characteristics of the blinded cohorts. Safety and tolerability were analysed for all study patients who received at least one dose of study medication. Baseline characteristics and safety assessments were summarised using descriptive statistics. Tolerability was assessed by the number and percentage of patients who failed to complete the study, and the number of patients who failed to complete the study due to AEs.
The modified intent-to-treat (mITT) analysis set used consisted of all randomised patients excluding observations following TF. This analysis set was chosen in order to minimise bias from including observations collected post-TF and bias from excluding early terminations from the analysis. Values obtained at the time of early termination were carried forward.
The preplanned exploratory efficacy analyses were primarily descriptive: the proportions of patients who were in clinical remission (CDAI <150 and no TF); the proportions of patients who were responders 70 and responders 100 (responded to treatment with a reduction of CDAI score by ≥70 and ≥100 points, or were in remission, and no TF); mean CDAI change from baseline; mean per cent of change from baseline for CRP and faecal calprotectin concentrations. ORs were derived from baseline adjusted logistic regression analyses performed to assess treatment effect at week 8 on: (1) remission; (2) response 100; and (3) response 70. In these analyses, treatment dose was included in the model as a categorical variable, and CDAI score at baseline, prior use of anti-TNF drugs (Yes/No), treatment with oral GCS at baseline (Yes/No) and treatment with immunosuppressive drugs at baseline (Yes/No) were included as covariates.
Results
Patient disposition and baseline characteristics
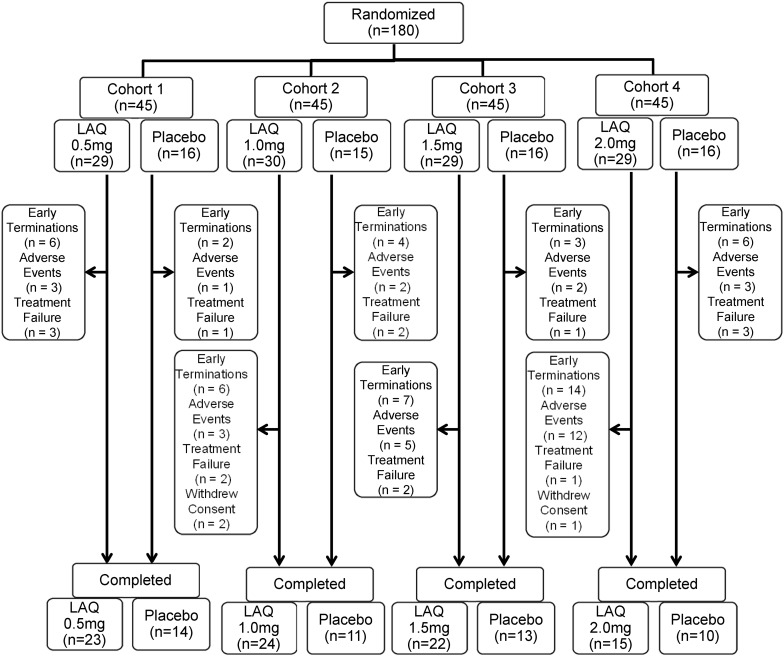
A total of 180 patients randomised to four sequential cohorts were included in the mITT and safety analyses sets. Of these, 63 patients (35.0%) were randomised to receive placebo (across the four cohorts) and 117 patients (65.0%) were randomised to receive 0.5, 1.0, 1.5 or 2.0 mg laquinimod doses (n=29 or 30 patients per dose). The majority of patients in all treatment groups completed 8 weeks of treatment. A higher incidence of early termination occurred in Cohort 4 in both laquinimod 2 mg and placebo arms compared with the other cohorts, but early termination was numerically more common in the laquinimod 2 mg arm (48.3%) than in the placebo arm (37.5%) within this cohort. The most common reason for early terminations in all groups was occurrence of AEs (figure 1).
Figure 1.
Disposition of patients in the sequential cohort dose escalation study of LAQ for Crohn's disease. The number of patients completing the study and with early terminations. For early terminations, the number of patients terminated because of adverse events, treatment failure or withdrew consent is provided. LAQ, Laquinimod.
Treatment cohorts were well balanced with respect to age, time from diagnosis and baseline CDAI scores (table 1). However, there were slight imbalances in (a) the proportion of women in the pooled placebo group versus the laquinimod groups, (b) median baseline CRP concentrations in the 1 and 2 mg laquinimod groups versus other groups and (c) median baseline concentrations of faecal calprotectin in the higher dose (1.5 and 2 mg) laquinimod groups compared with the other groups. Additionally, although the sample sizes are small and thus should be interpreted cautiously, both the placebo and the laquinimod treated patients in the 3rd and 4th cohorts had higher rates of previously undergoing any type of surgical treatments and prior treatment with TNF antagonists than in the placebo and laquinimod treatment patients in Cohorts 1 and 2. There was also a lower occurrence of prior treatment with TNF antagonists within the preceding year in the laquinimod 0.5 and 1.0 mg dose groups compared with the 1.5 and 2.0 mg laquinimod dose groups (27.6% and 16.7% vs 41.4% and 41.4%).
Table 1.
Baseline characteristics
| Pooled placebo | 0.5 mg LAQ |
1.0 mg LAQ |
1.5 mg LAQ |
2.0 mg LAQ (n=29) |
|
|---|---|---|---|---|---|
| N | 63 | 29 | 30 | 29 | 29 |
| Age, years mean (SD) | 35.1 (10.9) | 39.6 (14.8) | 38.2 (11.3) | 40.9 (14.5) | 37.4 (9.9) |
| Female %* | 42.9 | 55.2 | 56.7 | 72.4 | 58.6 |
| Predominately inflammatory disease %† | 54.0 | 75.9 | 66.7 | 41.4 | 41.4 |
| CDAI score mean (SD) | 311.2 (74.5) | 288.3 (56.0) | 291.9 (57.5) | 297.8 (59.1) | 313.2 (58.9) |
| CRP concentration (mg/L) | 21.3 (27.8) | 17.1 (18.3) | 6.7 (12) | 15.1 (22.3) | 6.5 (17.1) |
| Median (IQR) Min–max |
1.8–85.7 | 2.2–127.5 | 1.0–153.6 | 1.0–79.8 | 1.0–153.2 |
| CRP ≥5 mg/L at baseline % | 81 | 86 | 57 | 66 | 45 |
| Faecal calprotectin concentration (µg/g) median (IQR) | 475 (802) | 468 (1434) | 543 (813) | 318 (500) | 270 (422) |
| Min–max | 19.0–6559 | 15.0–6551 | 15.0–5563 | 18.0–8000 | 15.0–3994 |
| Faecal calprotectin concentrations ≥250 µg/g at baseline % | 69.8 | 62.1 | 60 | 51.7 | 37.9 |
| Previous CD operations % | 33.3 | 17.2 | 40.0 | 44.8 | 44.8 |
| Current treatment with either azathioprine, 6-mercaptopurine or methotrexate % | 31.7 | 27.6 | 36.7 | 37.9 | 24.1 |
| Current treatment with 5-aminosalicylates % | 36.5 | 27.6 | 43.3 | 34.5 | 41.4 |
| Current treatment with antibiotics % | 4.8 | 6.9 | 3.3 | 0 | 3.4 |
| Current treatment with steroids % | 27 | 34.5 | 40 | 31 | 17.2 |
| Previous use of anti-TNF in past year % | 42.9 | 27.6 | 16.7 | 41.4 | 41.4 |
*% indicates per cent of patients.
†Montreal definition.
CD, Crohn's disease; CDAI, CD Activity Index; TNF, tumour necrosis factor.
Safety analysis
The median exposure to study drug was similar for all treatment groups (56–57 days). Mean exposure was similar (approximately 50 days) for all groups except for laquinimod 2.0 mg, in which mean exposure was approximately 40 days. This difference was due to the higher rate of early terminations in this group. No patient died during the study. There was no apparent dose–response relationship in the overall incidence of serious adverse events (SAEs), with incidence of 10.3%, 26.7%, 3.4% and 10.3% in the laquinimod 0.5, 1.0, 1.5 and 2.0 mg groups, respectively, compared with 11.1% in the pooled placebo group (see online supplementary table S1). The most common SAE was CD exacerbation which was reported by 2 (6.9%), 3 (10.0%), 1 (3.4%) and 1 (3.4%) of patients in the laquinimod 0.5, 1.0, 1.5 and 2.0 mg groups, respectively, and by 1 (1.6%) of patients in the pooled placebo group. Most other SAEs were reported by single participants.
The overall incidence of AEs was 86.2%, 96.7%, 89.7% and 89.7% for the laquinimod 0.5, 1.0, 1.5 and 2.0 mg doses, respectively, compared with 82.5% for the pooled placebo. No dose–response relationship for the overall incidence of AEs was observed. Most AEs were mild or moderate in severity. The overall incidence of AEs leading to early termination was greater for the laquinimod 2.0 mg group (41.4%) compared with the other laquinimod groups (10.0%–17.2%), and the pooled placebo group (12.7%). The most common AEs leading to discontinuation of the study drug were exacerbation of CD (cause of early termination in 2 (6.9%), 2 (6.7%), 1 (3.4%), 2 (6.9%) and 2 (3.2%) patients). Abdominal pain which has been reported in a previous laquinimod study25 was reported by 1 (3.4%), 0, 1 (3.4%), 2 (6.9%) and 1 (1.6%) patients in laquinimod 0.5, 1.0, 1.5, 2.0 mg groups and pooled placebo group, respectively.
Headache was the most common AE in all treatment groups (table 2). Headaches were reported by 7 (24.1%), 8 (26.7%), 7 (24.1%), 13 (44.8%) and 13 (20.6%) of patients in the laquinimod 0.5, 1, 1.5, 2.0 mg groups and pooled placebo respectively with approximately half assessed by the investigator as possibly related to treatment. The incidence of headache was higher in the laquinimod 2.0 mg group (44.8%) compared with other groups. Three patients (one patient in the 1.0 mg group and two patients in the 2.0 mg group) discontinued due to headache.
Table 2.
Common adverse events in laquinimod dose groups and pooled placebo group
| Preferred term n (%) | Pooled placebo (n=63) | LAQ 0.5 mg (n=29) | LAQ 1.0 mg (n=30) | LAQ 1.5 mg (n=29) | LAQ 2.0 mg (n=29) |
|---|---|---|---|---|---|
| Headache | 13 (20.6) | 7 (24.1) | 8 (26.7) | 7 (24.1) | 13 (44.8) |
| Abdominal pain | 8 (12.7) | 5 (17.2) | 4 (13.3) | 7 (24.1) | 7 (24.1) |
| Nausea | 4 (6.3) | 1 (3.4) | 6 (20) | 2 (6.9) | 6 (20.7) |
| Vomiting | 5 (7.9) | 5 (17.2) | 6 (20) | 1 (3.4) | 6 (20.7) |
| Abdominal pain upper | 3 (4.8) | 1 (3.4) | 0 (0) | 2 (6.9) | 5 (17.2) |
| Myalgia | 1 (1.6) | 0 (0) | 1 (3.3) | 0 (0) | 5 (17.2) |
| Pyrexia | 8 (12.7) | 7 (24.1) | 1 (3.3) | 6 (20.7) | 4 (13.8) |
| Crohn's disease* | 7 (11.1) | 2 (6.9) | 5 (16.7) | 5 (17.2) | 4 (13.8) |
| Diarrhoea | 2 (3.2) | 6 (20.7) | 1 (3.3) | 0 (0) | 3 (10.3) |
| Asthenia | 3 (4.8) | 1 (3.4) | 2 (6.7) | 3 (10.3) | 2 (6.9) |
| Back pain | 5 (7.9) | 3 (10.3) | 3 (10.0) | 4 (13.8) | 1 (3.4) |
| Dizziness | 1 (1.6) | 1 (3.4) | 1 (3.3) | 3 (10.3) | 1 (3.4) |
| Tachycardia | 0 (0) | 0 (0) | 0 (0) | 3 (10.3) | 1 (3.4) |
| Cough | 2 (3.2) | 2 (6.9) | 3 (10.0) | 2 (6.9) | 1 (3.4) |
| Abdominal distension | 0 (0) | 3 (10.3) | 0 (0) | 1 (3.4) | 1 (3.4) |
| Alanine aminotransferase increased | 1 (1.6) | 0 (0) | 3 (10.0) | 0 (0) | 1 (3.4) |
| Rectal haemorrhage | 0 (0) | 0 (0) | 1 (3.3) | 3 (10.3) | 0 (0) |
| Fatigue | 2 (3.2) | 2 (6.9) | 0 (0) | 3 (10.3) | 0 (0) |
*Exacerbation of Crohn's disease.
There were no consistent trends in change from baseline for any biochemical or haematological parameter in any of the dose groups or the pooled placebo group. Mean changes from baseline for pancreatic amylase were consistently greater for all laquinimod dose groups compared with pooled placebo group. Changes to 2×upper limit of normal (ULN) P-amylase levels anytime during the study occurred for 2 (7.4%), 2 (7.4%), 3 (10.3%), 6 (21.4%) and 0 patients in the laquinimod 0.5, 1.0, 1.5, 2.0 mg groups and pooled placebo, respectively.
Efficacy analysis
Clinical remission and clinical responses 100 and 70
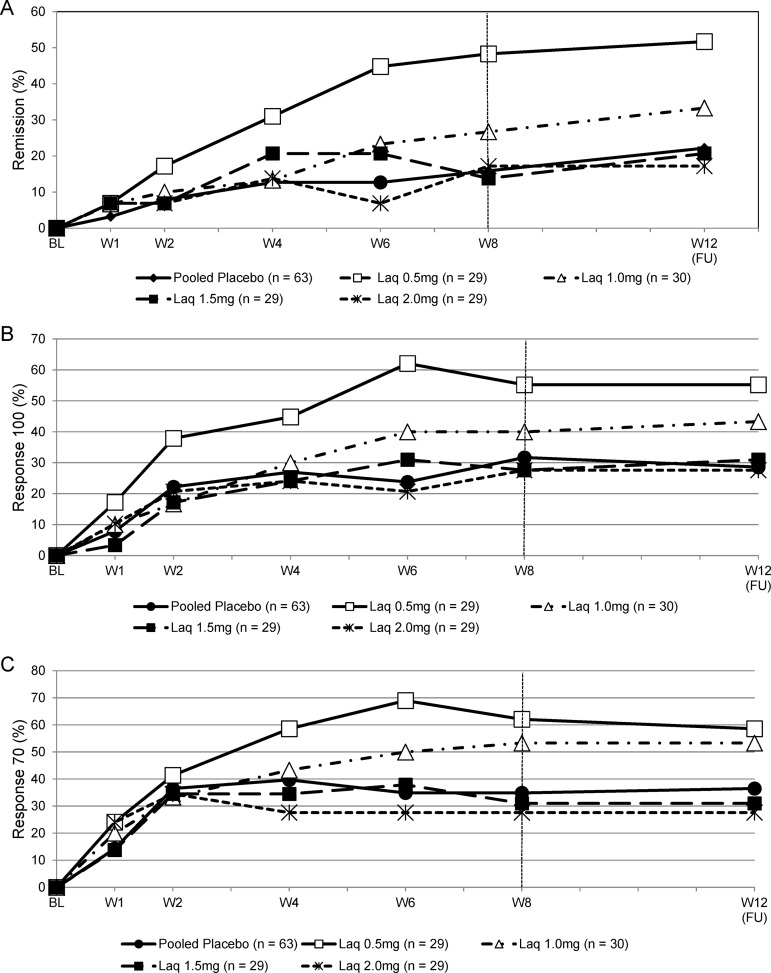
The proportion of patients in clinical remission, CDAI 100 and 70 responders are provided in table 3 and plotted as a function of visits for pooled placebo and each laquinimod dose in figure 2A–C. At week 8, the proportion of patients in clinical remission was higher for the laquinimod 0.5 mg (48.3%) and 1 mg (26.7%) groups compared with the laquinimod 1.5 and 2 mg groups (13.85% and 17.2%) and the pooled placebo group (15.9%). The differences in proportions of responders and (95% CI) for each laquinimod dose (0.5, 1, 1.5 and 2 mg) compared with the pooled placebo were: 32.4% (12.1% to 52.7%), 10.8% (−7.4% to 29.0%), −2.1% (−17.5% to 13.4%) and 1.4% (−15.1% to 17.8%).
Table 3.
Proportions of patients in remission, responders 70 and responders 100 at week 8
| Pooled placebo | 0.5 mg LAQ |
1.0 mg LAQ |
1.5 mg LAQ |
2.0 mg LAQ |
|
|---|---|---|---|---|---|
| N | 63 | 29 | 30 | 29 | 29 |
| Clinical remission | |||||
| n (%) | 10 (15.9) | 14 (48.3) | 8 (26.7) | 4 (13.8) | 5 (17.2) |
| 95% CI | (8.9 to 26.8) | (31.4 to 65.6) | (14.2 to 44.4) | (5.5 to 30.6) | (7.6 to 34.5) |
| Response 100 | |||||
| n (%) | 20 (31.7%) | 16 (55.2%) | 12 (40.0%) | 8 (27.6%) | 8 (27.6%) |
| 95% CI | (21.6 to 44.0) | (37.5 to 71.6) | (24.6 to 57.7) | (14.7 to 45.7) | (14.7 to 45.7) |
| Response 70 | |||||
| n (%) | 22 (34.9) | 18 (62.1) | 16 (53.3) | 9 (31.0) | 8 (27.6) |
| 95% CI | (24.3 to 47.2) | (44.0 to 77.3) | (36.1 to 69.8) | (17.3 to 49.2) | (14.7 to 45.7) |
Figure 2.
The proportion of patients in the pooled placebo and laquinimod dose groups who showed (A) remission (B) response 100 and (C) response 70 using the Crohn's Disease Activity Index over time.
The proportions of CDAI 100 and 70 responders showed similar trends to those observed for the remission rates (figure 2B, C). At week 8, the proportion of patients who responded using either definition was higher for the laquinimod 0.5 and 1.0 mg groups compared with the higher laquinimod doses (1.5 and 2.0 mg) groups and the pooled placebo group.
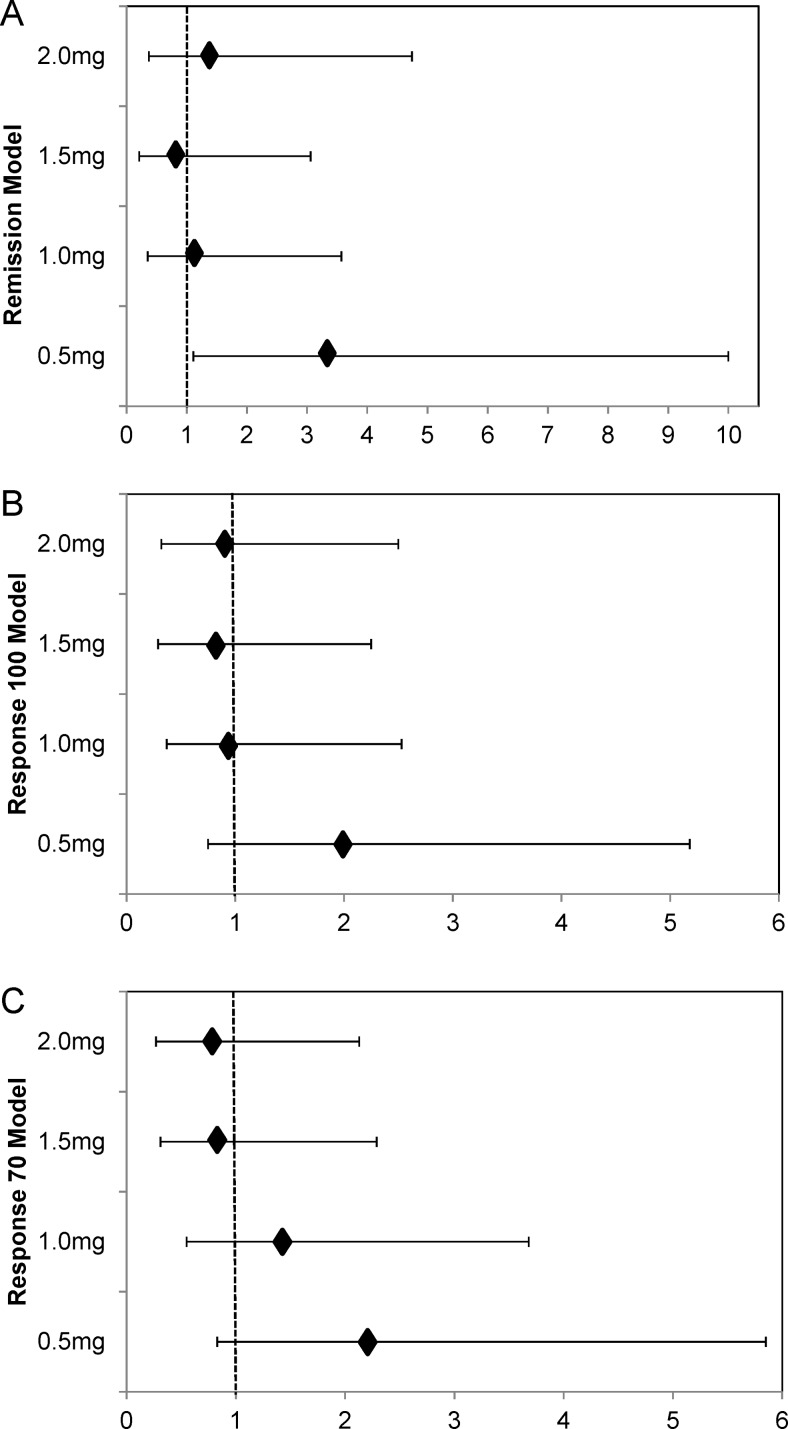
The results of the logistic regression analysis were consistent with a beneficial effect of laquinimod (figure 3A–C). Following adjustment for baseline covariates, the OR (95% CI) for clinical remission in the laquinimod compared with placebo was 3.3 (1.11 to 10.0) for 0.5 mg laquinimod, 1.1 (0.35 to 3.57) for 1.0 mg, 0.79 (0.21 to 3.06) for 1.5 mg and 1.32 (0.37 to 4.74) for 2.0 mg. The odd ratios (95% CI) of response 100 were 1.97 (0.75 to 5.18), 0.96 (0.37 to 2.53), 0.81 (0.29 to 2.25) and 0.90 (0.32 to 2.50) for the 0.5, 1.0, 1.5 and 2.0 mg dose groups compared with pooled placebo and the ORs (95% CI) for response 70 were 2.2 (0.83 to 5.85), 1.43 (0.55 to 3.68), 0.84 (0.31 to 2.29) and 0.76 (0.27 to 2.13) for the laquinimod 0.5, 1, 1.5 and 2.0 mg groups compared with pooled placebo.
Figure 3.
The proportion of patients in the pooled placebo and laquinimod dose groups who showed (A) Remission (B) Response 100 and (C) Response 70 using the CDAI over time.
Post hoc analyses on the correlations among CDAI scale item, abdominal pain and the incidence of abdominal pain as an AE
A post hoc analysis was conducted to determine whether the occurrence of abdominal pain as an AE affected the CDAI scale item of abdominal pain. There was no difference in the range of scores for abdominal pain between patients who did or did not report an AE of abdominal pain. The mean and median abdominal scores in the CDAI were higher for those who reported abdominal pain as an AE (56.9 and 65) versus those did no report abdominal pain as an AE (42.7 and 35).
Time to remission/response
As illustrated in figure 2A, patients in the laquinimod 0.5 and 1 mg groups entered clinical remission earlier than those in the pooled placebo group. No consistent difference was observed between the laquinimod 1.5 and 2.0 mg treatment groups compared with the pooled placebo. The 0.5 and 1.0 mg doses both showed an effect based on the time to response for both CDAI response 100 and 70 to pooled placebo (figure 2B, C). Kaplan–Meier survival curves confirmed that subjects in both the 0.5 and 1.0 mg dose groups achieved remission and response earlier than the pooled placebo group.
Proportion of TFs
The rate of TFs was higher for the laquinimod 2.0 mg group, 15/29 patients (51.7%), compared with the 0.5, 1.0 and 1.5 mg groups, 6/29 (20.7%), 6/30 (20.0%) and 7/29 (24.1%) patients, respectively, and the pooled placebo group, 17/63 patients (27.0%). Only three patients who completed the study without early termination had TFs due to medications: 2/63 patients (3.2%) in the pooled placebo group due to antibiotics and 1/29 patients (3.4%) in the laquinimod 2.0 mg group due to GCS.
Per cent change from baseline in serum CRP and faecal calprotectin concentrations
Considerable intrapatient variability was observed for both serum CRP and faecal calprotectin concentrations. At baseline, the serum CRP concentrations in patients ranged from 1.0 mg/L to above 153 mg/L. At week 8, mean (±SD) per cent change in CRP from baseline for the laquinimod 0.5 mg group was −15.0% (±76%) compared with pooled placebo group 19.9% (±101%); mean (±SD) changes in CRP concentrations from baseline for the other doses were 98.9% (±249%) for 1.0 mg, 30.2% (±115%) for 1.5 mg and 233.5% (±405%) for 2.0 mg. In a post hoc analysis, for patients with abnormal CRP concentrations at baseline (CRP ≥5 mg/L), a greater proportion had a shift from abnormal to normal concentrations of CRP at week 8 for the laquinimod 0.5 mg group compared with the pooled placebo (36.0% vs 5.9%). No consistent effect on CRP normalisation was shown for the other doses.
The range of faecal calprotectin concentrations at baseline across patients varied widely from <20 µg/g to >8000 µg/g (table 1). There was no consistent laquinimod treatment effect on the mean per cent change from baseline in faecal calprotectin concentration. At week 8, the mean (±SD) per cent changes from baseline for the laquinimod doses were 0.5 mg 25.5% (±132%), 1 mg 0.2% (±92%), 1.5 mg 13.9% (±109%) and 2.0 mg 44.3% (±199%) compared with 91% (±389%) in the pooled placebo group. For post hoc analyses, participants were classified as to whether they had faecal calprotectin concentrations ≥250 µg/g at baseline; the percentage of patients ranged from 69.8% of pooled placebo group to 37.9% of patients on laquinimod 2.0 mg. Analysis of this subgroup of patients revealed that a greater percentage in the all laquinimod treated groups showed at least a 50% reduction and faecal calprotectin concentrations <250 µg/g at week 8 compared with the pooled placebo group (26.7%–38.9% vs 13.6%, respectively).
Discussion
The results of this first study of laquinimod in active CD suggest that doses of 0.5 and 1.0 mg, administered for 8 weeks, had a favourable safety profile. Headache was the most common AE observed and the overall rate of AEs in the laquinimod dose groups was similar to that observed in the pooled placebo group. The incidence of abdominal pain as an AE at 0.5 and 1 mg/day, which has been previously reported for laquinimod at a dose of 0.6 mg,26 was comparable with that in the pooled placebo group and lower than in the 1.5 and 2.0 mg groups. For patients in the 0.5 mg group, no elevation of liver enzymes was seen; elevations occurred for one placebo patient, three patients in the laquinimod 1.0 mg dose and one patient in 2.0 mg dose. Placebo and laquinimod treated patients in the 4th cohort discontinued therapy more frequently than patients in other cohorts, and discontinuation occurred more frequently in the 2.0 mg arm compared with the placebo arm (48.3% vs 37.5%).
While the study was not powered to assess efficacy, exploratory analyses suggested that laquinimod at 0.5 mg had a consistent therapeutic effect across all outcome measures. In all, 48% of patients in the laquinimod 0.5 mg group were in remission at the end of 8 weeks compared with 15.9% in the pooled placebo group. Similarly, 62% and 55% of patients in the 0.5 mg dose group demonstrated CDAI 70 and 100 responses respectively, compared with 35% and 32% of patients on placebo. The benefit of laquinimod 0.5 mg was already detectable by week 2 and remained consistent throughout the eight treatment weeks and the 4 weeks of follow-up. Although the 1.0 mg dose showed a trend towards a therapeutic effect for induction of clinical remission, the results were less robust than those observed with the 0.5 mg dose. Laquinimod at doses of 1.5 and 2.0 mg showed no apparent benefit compared with the pooled placebo group for either remission or response.
Several aspects of the study need to be considered in evaluating the finding of potential therapeutic effect at 0.5 mg/day laquinimod and a lack of effect for the higher doses of 1.5 and 2.0 mg/day. This trial was conducted with a sequential cohort design for the purposes of investigating the safety of laquinimod doses higher than doses previously studied for other indications at the time of this study's initiation. The higher doses in this study allowed a range of exposure to laquinimod while ensuring patient safety. Due to the inherent nature of the dose escalation design of the trial, the patient characteristics varied slightly across the cohorts. Patients who participated in the last two cohorts appear to have had a higher disease activity than the patients in the first two cohorts, as reflected by the higher proportions of patients with previous surgery and/or exposure to TNF antagonists. Second, remission and response are measured using the CDAI scale which has abdominal pain as one of main components. Patients receiving laquinimod doses of 1.5 and 2.0 mg had twice as many reports of abdominal pain. A post hoc analysis evaluating the relationship between the CDAI item of abdominal pain and AE reports of abdominal pain did not find a difference in the distribution of scores between patients who did or did not report abdominal pain as an AE, but AE reporting of ‘abdominal pain’ in CD trials is usually quite inconsistent. A third possible reason that the patients in the lower dose of laquinimod, 0.5 mg dosing group, did better compared with placebo and the other dose groups is because there was a relatively higher percentage of patients in 0.5 mg laquinimod cohort with inflammatory disease (76%) which may explain that laquinimod was more effective in this very group.
Indeed, in previous studies, laquinimod has been shown to have an immune regulatory effect, mediated by the NFκB pathway on dendritic cells, which leads to a diminished production of pro-inflammatory cytokines (TNF-α, IFN-γ, interleukin (IL)-13 and IL-17) and chemokines27 28 and inhibition of leucocyte migration in the central nervous system (CNS).24 29 In CD, GI inflammation is believed to be caused by an activated innate/macrophage immune response to NFκB activation involving increased cytokines and chemokines and other inflammatory molecules, and an acquired immune response involving T and B cells and a loss of tolerance to enteric commensal bacteria.19 Thus, laquinimod's proposed mechanism of action may make it ideally suited to reduce the GI inflammation. Results in the experimental autoimmune encephalitis model of MS and in patients with MS, another chronic and destructive inflammatory disease, have been encouraging and suggest that laquinimod could act as an anti-inflammatory agent without impairing the organism's ability to mount a humoral response (personal communication, MDS Pharma Services France, 2007 Final Report).
Two objective measures of GI inflammation were used in the present study, serum CRP and faecal calprotectin. Because of the large intrapatient variability (mainly of calprotectin) observed in this study, it was difficult to determine laquinimod's effect on these measures. There was an entry requirement into the study of having a serum CRP >5 mg/L or documented endoscopic evidence of mucosal ulcerations but there were no entry requirements at the level of faecal calprotectin concentrations. A post hoc analysis in the present study evaluating changes in faecal calprotectin levels of patients with faecal calprotectin concentrations ≥250 µg/g at baseline showed that there were reductions in faecal calprotectin levels at 8 weeks regardless of laquinimod dose. A cut-off of 250 μg/g has been suggested to correspond with the presence of significant ulcerations.30 These results suggest that laquinimod may have an inhibitory effect on GI inflammation which needs to be further evaluated.
Laquinimod is under development by Teva Pharmaceuticals for a variety of therapeutic indications including MS, Huntington's disease, lupus nephritis and CD. As the most consistent therapeutic effects were seen with the 0.5 mg laquinimod dose, the phase III clinical development programme for CD will focus on assessing the efficacy of daily oral doses of 0.5 mg, along with a lower dose, 0.25 mg, on the induction and maintenance of clinical remission in patients with moderate to severe CD. Laquinimod may eventually offer an attractive oral therapeutic option for patients with moderate to severe CD.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in this study, the Safety Committee for their safety assessments during the study, Pippa Loupe, PhD, of Research and Development, Teva Pharmaceuticals (Overland Park KS USA) and Anita Chadha-Patel, PhD, of ACP Clinical Communications (Watford, UK) who assisted in the development of this manuscript.
Footnotes
Collaborators: Laquinimod for Crohn's Disease Study Investigators Belgium: Geert D'Haens Severine Vermeire, Filip Baert; France: Jean Frederic Colombel, Jean-Louis Dupas, Yoram Bouhnik, Bruno Bonaz, Xavier Hebuterne, Matthieu Allez; Israel: Eran Israeli, Gerald Fraser, Ori Segol, Sigal Fishman, Adi Lahat, Ehud Melzer; Italy: Francesco Pallone, Massimo Campieri, Antonio Gasbarrini, Anna Kohn, Maurizo Vecchi; The Netherlands: Cyriel Ponsioen, Janneke Van der Woude; Poland: Grazyna Rydzewska, Tomasz Mach, Leszek Paradowski; South Africa: John Philip Wright, Gillian Ann Watermeyer, Keith Edward Pettengell, Adam Dawood Mahomed, Maarten Jeroen Prins, Nazimuddin Aboo; Spain: Julian Panes Diaz, Carlos Taxonera, Javier Gisbert, Joaquin Hinojosa, Fernand Gomollon Garcia, Valle Garcia Sanchez; UK: Miles Parkes, Chris Probert, Keith Leiper, Chuka Nwokolo, John Mayberry, Stuart Bloom.
Contributors: GD’H, WJS, JFC, PR and BGF were involved in the study protocol design, study implementation, data interpretation and manuscript writing. KB and AH as clinical trial leaders monitored data collection, interpreted study findings and assisted with manuscript writing. HB and AS wrote the statistical analysis plan, monitored data collection, analysed the study results and assisted with manuscript writing. PL and AC-P assisted with writing of the manuscript. The Safety Committee conducted safety assessments throughout the study. The Laquinimod for Crohn's disease Investigators managed each of the study sites across 37 centres in Belgium, France, Israel, Italy, Netherlands, Poland, South Africa, Spain and the UK.
Funding: This study was sponsored by Teva Pharmaceuticals, Netanya, Israel.
Competing interests: GD'H reports grants and personal fees from Teva Pharmaceuticals, during the conduct of the study, grants and personal fees from Abvie, grants and personal fees from Jansen, grants and personal fees from MSD, grants and personal fees from Centocor, grants from Takeda, grants from GivenImaging, grants and personal fees from GSK, personal fees from Pfizer, personal fees from UCB, personal fees from Takeda, personal fees from Millenium, personal fees from Boerhinger Ingelheim, personal fees from Elan, personal fees from Ferring, personal fees from Dr. Falk Pharma, personal fees from Shire, personal fees from Cosma, personal fees from AstraZeneca, personal fees from Vifor, personal fees from Tillotts, personal fees from Otsuka, personal fees from Photopill, personal fees from GivenImaging, personal fees from PDL, personal fees from Amgen, personal fees from AM Pharma, personal fees from Galapagos, personal fees from Versant, personal fees from Novonordisk, personal fees from Norgine, personal fees from Giuliani, outside the submitted work. WJS has received consulting fees from Abbott, ActoGeniX NV, AGI Therapeutics, Alba Therapeutics, Albireo, Alfa Wasserman, Amgen, AM-Pharma BV, Anaphore, Astellas, Athersys, Atlantic Healthcare, Aptalis, BioBalance, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Celek Pharmaceuticals, Cellerix SL, Cerimon Pharmaceuticals, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cytokine Pharmasciences, Eagle Pharmaceuticals, EnGene, Eli Lilly, Enteromedics, Exagen Diagnostics, Ferring Pharmaceuticals, Flexio Therapeutics, Funxional Therapeutics, Genzyme, Gilead Sciences, Given Imaging, GlaxoSmithKline, Human Genome Sciences, Ironwood Pharmaceuticals, KaloBios Pharmaceuticals, Lexicon Pharmaceuticals, Lycera, Meda Pharmaceuticals, Merck Research Laboratories, Merck Serono, Millennium Pharmaceuticals, Nisshin Kyorin Pharmaceuticals, Novo Nordisk, NPS Pharmaceuticals, Optimer Pharmaceuticals, Orexigen Therapeutics, PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProtAb, Purgenesis Technologies, Relypsa, Roche, Salient Pharmaceuticals, Salix Pharmaceuticals, Santarus, Schering Plough, Shire Pharmaceuticals, Sigmoid Pharma, Sirtris Pharmaceuticals, SLA Pharma UK, Targacept, Teva Pharmaceuticals, Therakos, Tilliotts Pharma AG, TxCell SA, UCB Pharma, Viamet Pharmaceuticals, Vascular Biogenics, Warner Chilcott UK and Wyeth; research grants from Abbott, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Janssen, Millennium Pharmaceuticals, Novartis, Pfizer, Procter and Gamble, Shire Pharmaceuticals and UCB Pharma; payments for lectures/speakers bureaux from Abbott, Bristol-Myers Squibb and Janssen; and holds stock/stock options in Enteromedics. JFC reports grants and personal fees from Teva Pharmaceuticals, during the conduct of the study; grants and personal fees from Abbott Labs, grants from Ferring Pharmaceuticals, grants and personal fees from Merck Sharp & Dohme, personal fees from Bristol-Myers Squibb, personal fees from Celgene, personal fees from Genetech, personal fees from Giuliani S.p.A, personal fees from Hospira, personal fees from Takeda, personal fees from Nestle, personal fees from Pfizer, personal fees from Receptos, personal fees from Schering-Plough, personal fees from UCB, personal fees from Sanofi-Aventis, outside the submitted work. PR reports grants from Teva Pharmaceuticals during the conduct of the study; grants and personal fees from Centocor, grants and personal fees from Merck, grants and personal fees from UCB, grants and personal fees from Abbott Labs, personal fees from Millenium Pharmaceuticals, personal fees from Genentech, personal fees from Neovacs, personal fees from Merck Serono, personal fees from Bristol-Myers Squibb, personal fees from Robarts Clinical Trials, personal fees from Tillotts Pharma, personal fees from Pfizer, personal fees from Dr. Falk Pharma, outside the submitted work. KB reports personal fees from Teva Pharmaceuticals during the conduct of the study. HB reports personal fees from Teva Pharmaceuticals during the conduct of the study. AH reports personal fees from Teva Pharmaceuticals during the conduct of the study; personal fees from Chisma Pharmaceuticals outside the submitted work. AS reports personal fees from Teva Pharmaceuticals during the conduct of the study. BGF reports grants from Teva Pharmaceuticals, during the conduct of the study; grants and personal fees from AbbVie/Abbott, personal fees from Actogenix, personal fees from Albireo, grants and personal fees from Amgen, grants and personal fees from Astra Zeneca, personal fees from Avaxia Biologics, personal fees from Axcan, personal fees from Baxter Healthcare, personal fees from Boehringer-Ingelheim, grants and personal fees from Bristol-Myers Squibb, personal fees from Calypso Biotech, personal fees from Celgene, personal fees from Elan/Biogen, personal fees from EnGene, personal fees from Ferring, grants and personal fees from Genentech/Roche, personal fees from GiCare, personal fees from Gilead, personal fees from Given Imaging, personal fees from GSK, personal fees from Ironwood, grants and personal fees from Janssen/JnJ, personal fees from Kyowa Kakko Kirin Co, personal fees from Lexicon, personal fees from Lilly, personal fees from Merck, personal fees from Millennium, personal fees from Nektar, personal fees from Novo Nordisk, personal fees from Prometheus Therapeutics, grants and personal fees from Pfizer, personal fees from Receptos, personal fees from Salix, grants from Santarus, grants from Sanofi, personal fees from Shire, personal fees from Sigmoid, personal fees from Synergy, personal fees from Takeda, personal fees from Teva, grants and personal fees from Tillotts, grants and personal fees from UCB, personal fees from Vertex, personal fees from Warner-Chilcott, personal fees from Wyeth, personal fees from Zealand, personal fees from Zyngenia, outside the submitted work.
Patient consent: Obtained.
Ethics approval: Institutional review boards at each study site.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: on behalf of the Laquinimod for Crohn's Disease Investigators, Filip Baert, Jean-Louis Dupas, Yoram Bouhnik, Bruno Bonaz, Xavier Hebuterne, Matthieu Allez, Eran Israeli, Gerald Fraser, Ori Segol, Sigal Fishman, Adi Lahat, Ehud Melzer, Francesco Pallone, Massimo Campieri, Antonio Gasbarrini, Anna Kohn, Maurizo Vecchi, Cyriel Ponsioen, Janneke Van der Woude, Grazyna Rydzewska, Tomasz Mach, Leszek Paradowski, John Philip Wright, Gillian Ann Watermeyer, Keith Edward Pettengell, Adam Dawood Mahomed, Maarten Jeroen Prins, Nazimuddin Aboo, Julian Panes Diaz, Carlos Taxonera, Javier Gisbert, Joaquin Hinojosa, Fernand Gomollon Garcia, Valle Garcia Sanchez, Miles Parkes, Chris Probert, Keith Leiper, Chuka Nwokolo, John Mayberry, and Stuart Bloom
References
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007;5:1424–9. [DOI] [PubMed] [Google Scholar]
- 2.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol 2006;12:6102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen RD. The quality of life in patients with Crohn's disease. Aliment Pharmacol Ther 2002;16:1603–9. [DOI] [PubMed] [Google Scholar]
- 4.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology 2008;135:1907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odes S, Vardi H, Friger M, et al. Cost analysis and cost determinants in a European inflammatory bowel disease inception cohort with 10 years of follow-up evaluation. Gastroenterology 2006;131:719–28. [DOI] [PubMed] [Google Scholar]
- 6.Das KM. Pharmacotherapy of inflammatory bowel disease. Part 1. Sulfasalazine. Postgrad Med 1983;74:141–8, 150–1. [DOI] [PubMed] [Google Scholar]
- 7.Frank MS, Brandt LJ, Bernstein LH. Pharmacotherapy of inflammatory bowel disease. Part 2. Metronidazole. Postgrad Med 1983;74:155–7, 160. [DOI] [PubMed] [Google Scholar]
- 8.Pineton de Chambrun GP, Torres J, Darfeuille-Michaud A, et al. The role of anti(myco)bacterial interventions in the management of IBD: is there evidence at all? Dig Dis 2012;30:358–67. [DOI] [PubMed] [Google Scholar]
- 9.Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011;106:590–9. [DOI] [PubMed] [Google Scholar]
- 10.Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2010;(6):CD000545. [DOI] [PubMed] [Google Scholar]
- 11.Girardin M, Manz M, Manser C, et al. First-line therapies in inflammatory bowel disease. Digestion 2012;86(Suppl 1):6–10. [DOI] [PubMed] [Google Scholar]
- 12.Thomson AB, Gupta M, Freeman HJ. Use of the tumor necrosis factor-blockers for Crohn's disease. World J Gastroenterol 2012;18:4823–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchner AM, Blonski W, Lichtenstein GR. Update on the management of Crohn's disease. Curr Gastroenterol Rep 2011;13:465–74. [DOI] [PubMed] [Google Scholar]
- 14.Katz S. “Mind the Gap”: an unmet need for new therapy in IBD. J Clin Gastroenterol 2007;41:799–809. [DOI] [PubMed] [Google Scholar]
- 15.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology 2009;136:1182–97. [DOI] [PubMed] [Google Scholar]
- 16.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 17.Loftus EV, Jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther 2002;16:51–60. [DOI] [PubMed] [Google Scholar]
- 18.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006;3:390–407. [DOI] [PubMed] [Google Scholar]
- 19.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 2002;15:79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Targan SR, Hanauer SB, Van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 21.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 22.Rutgeerts P, D'Haens G, Targan SR, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology 1999;117:761–9. [DOI] [PubMed] [Google Scholar]
- 23.Bruck W, Wegner C. Insight into the mechanism of laquinimod action. J Neurol Sci 2011;306:173–9. [DOI] [PubMed] [Google Scholar]
- 24.Yang JS, Xu LY, Xiao BG, et al. Laquinimod (ABR-215062) suppresses the development of experimental autoimmune encephalomyelitis, modulates the Th1/Th2 balance and induces the Th3 cytokine TGF-beta in Lewis rats. J Neuroimmunol 2004;156:3–9. [DOI] [PubMed] [Google Scholar]
- 25.D'Haens GR, Sandborn W, Rutgeerts P, et al. Pharmacokinetics of laquinimod in patients with active moderate to severe Crohn's disease. Accepted for presentation at ECCO 2014. 21 February 2014.
- 26.Comi G, Jeffery D, Kappos L, et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med 2012;366:1000–9. [DOI] [PubMed] [Google Scholar]
- 27.Gurevich M, Gritzman T, Orbach R, et al. Laquinimod suppress antigen presentation in relapsing-remitting multiple sclerosis:in vitro high-throughput gene expression study. J Neuroimmunol 2010;221:87–94. [DOI] [PubMed] [Google Scholar]
- 28.Jolivel V, Luessi F, Masri J, et al. Modulation of dendritic cell properties by laquinimod as a mechanism for modulating multiple sclerosis. Brain 2013;136:1048–66. [DOI] [PubMed] [Google Scholar]
- 29.Wegner C, Stadelmann C, Pfortner R, et al. Laquinimod interferes with migratory capacity of T cells and reduces IL-17 levels, inflammatory demyelination and acute axonal damage in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol 2010;227:133–43. [DOI] [PubMed] [Google Scholar]
- 30.D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.