Abstract
Objective
We describe the occurrence and course of anterior pituitary dysfunction (PD) after aneurysmal subarachnoid haemorrhage (SAH), and identify clinical determinants for PD in patients with recent SAH.
Methods
We prospectively collected demographic and clinical parameters of consecutive survivors of SAH and measured fasting state endocrine function at baseline, 6 and 14 months. We included dynamic tests for growth-hormone function. We used logistic regression analysis to compare demographic and clinical characteristics of patients with SAH with and without PD.
Results
84 patients with a mean age of 55.8 (±11.9) were included. Thirty-three patients (39%) had PD in one or more axes at baseline, 22 (26%) after 6 months and 6 (7%) after 14 months. Gonadotropin deficiency in 29 (34%) patients and growth hormone deficiency (GHD) in 26 (31%) patients were the most common deficiencies. PD persisted until 14 months in 6 (8%) patients: GHD in 5 (6%) patients and gonadotropin deficiency in 4 (5%). Occurrence of a SAH-related complication was associated with PD at baseline (OR 2.6, CI 2.2 to 3.0). Hydrocephalus was an independent predictor of PD 6 months after SAH (OR 3.3 CI 2.7 to 3.8). PD was associated with a lower score on health-related quality of life at baseline (p=0.06), but not at 6 and 14 months.
Conclusions
Almost 40% of SAH survivors have PD. In a small but substantial proportion of patients GHD or gonadotropin deficiency persists over time. Hydrocephalus is independently associated with PD 6 months after SAH.
Trial registration number
NTR 2085.
Keywords: SUBARACHNOID HAEMORRHAGE, ENDOCRINOLOGY, REHABILITATION, CLINICAL NEUROLOGY
Introduction
Patients with aneurysmal subarachnoid haemorrhage (SAH) with a seemingly good clinical outcome often present with fatigue and experience high levels of psychosocial stress.1 These symptoms may interfere with daily activities and social participation, reducing quality of life in relatively young patients with SAH.2 3 They resemble the symptoms reported by patients with anterior pituitary dysfunction (PD).4 PD has been reported in 0 to 55% of SAH survivors.5–9 The most consistent finding is a high proportion of patients with growth hormone deficiency (GHD).9–11 However, reported incidences vary between studies and the course of PD after SAH is not well known. PD was found to be transient,12 but seemed long-lasting in other studies.13 Increased age, hydrocephalus and vasospasm have been identified as possible predictors for PD after SAH,7 9 however, others could not confirm this.6 13
The conflicting results may be explained by differences in methodology and study population as well as time since onset of SAH.5 7 To evaluate the growth hormone (GH) and adrenocorticotropic releasing hormone (ACTH), most of the studies conducted dynamic tests,9 11 but some did not.6 7 In some studies, patients were selected on the basis of symptoms of fatigue14 or on location of the aneurysm.15
In the Hypopituitarism In Patients with Subarachnoid haemorrhage (HIPS) study. We prospectively assessed the frequency and course of PD over time, using standardised laboratory tests to evaluate neuroendocrine function in a Dutch cohort of aneurysmal SAH survivors. In addition, we searched for clinical risk factors for hypopituitarism after SAH.
Methods
Study design
The HIPS study was a prospective single-centre observational cohort study. It was registered and approved by the IRB at Erasmus MC University Medical Center, and registered in the Dutch trial registry (NTR 2085). All patients gave written informed consent. The inclusion and exclusion of participants are described in detail elsewhere.16 In short, consecutive patients who survived the acute phase of SAH were asked to participate.
Data collection
We prospectively collected baseline characteristics on all patients, including age, medical history, medication use and cardiovascular risk factors. At admission we noted the following neurological parameters: World Federation of Neurological Surgeons (WFNS) grading scale for patients with SAH, Glasgow Coma Scale (GCS), radiological severity of SAH,17 aneurysm location and type of intervention.
We recorded SAH-related complications consisting of hydrocephalus (HC), rebleeding, vasospasm, delayed cerebral ischaemia (DCI), intracerebral haematoma (ICH), hypertension (HT) and hyponatraemia. Patients with symptomatic HC were all treated either by external ventricular drainage or external lumbar drainage prior to endocrine evaluation.
Clinical definitions and outcome measures
SAH diagnosis was confirmed by CT of the brain and in cases with negative CT, by lumbar puncture. Presence and location of the aneurysm was determined by CT angiography and/or a digital subtraction angiography.
The methods of endocrine testing and definitions of PD have been described in detail elsewhere.16 In short, at baseline and 6 month after SAH endocrine tests were performed and reviewed by an endocrinologist. Endocrine tests included ghrelin test at baseline and GHRH-arginine test 6 months after SAH, in fasting patients. Fourteen months after SAH, all patients were scheduled for basal hormonal testing. In addition, patients with GHD were scheduled for GHRH-arginine test. All basal serum samples were taken during fasting before 9:00.
ACTH insufficiency was considered if basal cortisol level was <110 nmol/L or if cortisol peak during ghrelin test was <450 nmol/L.18 Criteria for thyroid stimulating hormone (TSH) deficiency were low serum fT4 level (<11 pmol/L) combined with an inadequate TSH levels. Gonadotropin insufficiency was defined when testosterone was ≤10 nmol/L or by inadequate LH levels. In postmenopausal women, LH was regarded as derogatory when below 15 U/L and/or when follicle-stimulating hormone (FSH) was below 35 U/L. In younger women dependent on the stage of cycle and LH, and FSH and E2, gonadal status was assessed. In case oral contraceptives were used, gonadal function could not be assessed. We defined anterior PD as when any of the pituitary axis was considered abnormal.
Health-related quality of life (HRQOL) was assessed using question on life satisfaction modules (QLSM-H), a questionnaire that has been developed to evaluate HRQOL in patients with hypopituitarism, among whom are patients with adult GHD.19
Statistical analysis
Descriptive statistics were used to describe the prevalence of hypopituitarism after SAH. Cardiovascular risk factors prior to SAH, severity of SAH (WFNS, GCS), the presence of hydrocephalus, hypertension, delayed cerebral ischaemia and rebleed were considered as predictors for PD. Independent sample t tests were used for continuous variables and Pearson's χ2 test for categorical variables. Associations of risk factors with PD were expressed as ORs with 95% CI. Those with a significance level of p<0.05 were entered in a multiple logistic regression model using forward selection to determine the independent predictive value of the parameters. We adjusted for potential confounders such as age and sex. All statistical analyses were performed with SPSS V.20.0 (SPSS Inc, Chicago, Illinois, USA).
Results
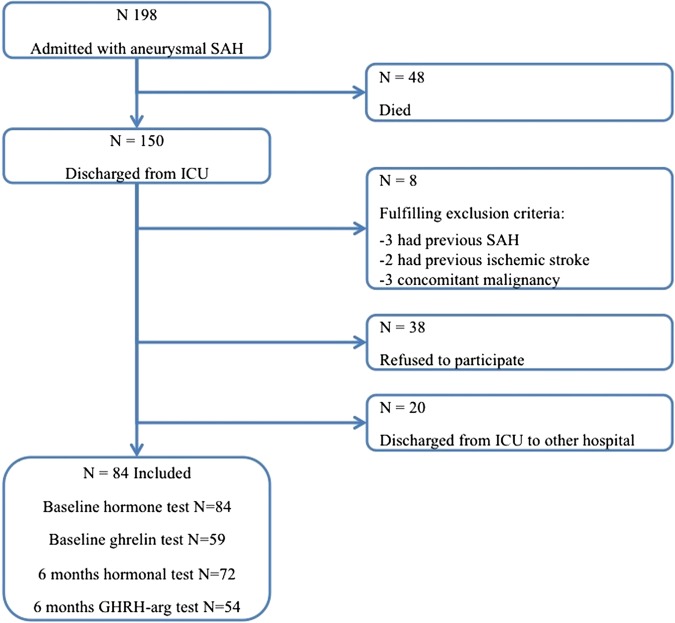
Of the 198 patients with SAH who were admitted to the ICU, 48 (24%) died, 38 (19%) refused to participate, 20 (10%) were discharged to other hospitals before inclusion and 8 were excluded for various other reasons. In total, 84 patients were included in the study (figure 1). There were no significant differences in age (p=0.18), sex (p=0.24), GCS on admission (p=0.81) and duration of hospital stay (p=0.53) between the included and excluded patients who survived the acute phase.
Figure 1.
Flowchart of patient inclusion.
The baseline and clinical characteristics are shown in tables 1 and 2. Twenty-eight men (33%) and 56 (67%) women were included. Mean age was 55.7 (±11.9) years. All 84 patients were tested for PD at baseline, 72 (86%) at 6 months and 68 (81%) patients 14 months after SAH. Fifty-nine patients (71%) also had a ghrelin test at baseline and 54 (65%) patients a GHRH-arginine test 6 months after SAH. For the first 10 patients, ghrelin was not available due to logistic problems. Fifteen patients refused dynamic testing.
Table 1.
Baseline characteristics of included patients
| Characteristics | N 84 |
|---|---|
| Age (mean (SD)) | 55.7 (11.9) |
| Male | 28 (33%) |
| History of hypertension | 18 (21%) |
| Body mass index (mean, (SD)) | 25.3 (3.7) |
| History of smoking | 52 (62%) |
| History of hypercholesterolaemia | 15 (18%) |
| History of diabetes mellitus* | 4 (5%) |
*All patients had diabetes mellitus type II.
Table 2.
Clinical characteristics of included patients
| Clinical characteristics | N 84 (%) |
|---|---|
| GCS on admission | |
| 13–15 | 66 (79) |
| 9–12 | 11 (13) |
| 3–8 | 7 (8) |
| WFNS on admission | |
| Grade I | 38 (45) |
| Grade II | 25 (30) |
| Grade III | 3 (4) |
| Grade IV | 11 (13) |
| Grade V | 7 (8) |
| Aneurysm location | |
| Anterior Circulation | 49 (58) |
| Posterior Circulation | 35 (42) |
| Intervention | |
| Coiling | 66 (79) |
| Clipping | 17 (20) |
| None | 1 (1) |
| Complications | |
| None | 43 (51) |
| Hydrocephalus | 31 (37) |
| Rebleed | 2 (2) |
| Delayed cerebral ischaemia | 8 (10) |
| Intraparenchymal haematoma | 15 (18) |
| Intraventricular haematoma | 56 (67) |
| Vasospasm | 7 (8) |
GCS, Glasgow Coma Scale.
During follow-up, five patients died of cardiovascular disease consisting of myocardial infarction and cardiac dysrhythmia. In addition, cerebral infarction occurred in 3 (4%) and myocardial infarction occurred in two patients (2%). None of these patients had PD prior to the cardiovascular event. One of the patients with GHD developed prostate carcinoma.
Frequency and course of hypopituitarism
Mean time to baseline evaluation was 32 days. Twenty-nine (34%) patients had gonadotropin insufficiency, 26 (31%) possible GHD, 1 (1%) TSH deficiency and 1 (1%) ACTH insufficiency. All female patients (n=19) with gonadotropin deficiency were postmenopausal (mean age 58±10 years). Thirty-seven (44%) patients were deficient on one or more of the endocrine axes at initial evaluation. In 26 (32%) patients only a single axis was deficient and in 11 (13%) two axes were deficient. GHD combined with gonadotropin deficiency was present in all but one patient who had combined TSH and gonadotropin deficiency.
The frequency and number of affected axes are depicted in table 3. Twenty-two (26.2%) patients had PD 6 months after SAH, which consisted of gonadotropin insufficiency (20%), GHD (9.5%) and TSH deficiency (1.2%). All female patients with gonadotropin deficiency were postmenopausal. Four patients had two axes affected consisting of GHD and gonadotropin deficiency in all cases.
Table 3.
Occurrence of pituitary deficiency
| Hormone deficiencies | Baseline | 6 months’ follow-up | 14 months’ follow-up | |||
|---|---|---|---|---|---|---|
| N (%) | m/f ratio | N (%) | m/f ratio | N (%) | m/f ratio | |
| Any deficiency | 37 (44%) | 10/27 | 22 (26%) | 10/12 | 6 (7%) | 5/1 |
| GH deficiency | 26 (31%) | 7/19 | 8 (10%) | 7/1* | 5 (6%) | 5/0* |
| Gonadotropin deficiency | 29 (34%) | 10/19 | 17 (20%) | 5/12 | 4 (5%) | 3/1 |
| ACTH deficiency | 1 (1%) | 0/1 | – | – | – | – |
| TSH deficiency | 1 (1%) | 0/1 | – | – | – | – |
Number (%) of deficient pituitary axes and male/female ratio of deficient patients at baseline, 6 and 14 months after SAH.
*p<0.001; OR:17.8 after 6 months.
ACTH, adrenocorticotropic releasing hormone; GH, growth hormone; SAH, subarachnoid haemorrhage; TSH, thyroid stimulating hormone.
Of the 8 patients with GHD at 6 months, two refused retesting at 14 months. In one patient, GH function had recovered, testing negative for GHRH-arginine test. PD was present in 6 (7.2%) patients. Five male patients (84%) had GHD of whom 3 (60%) also had gonadotropin deficiency. One postmenopausal female patient had isolated gonadotropin deficiency. In all other patients, pituitary functions were normal.
Clinical determinants for PD
Table 4 shows the clinical and radiological characteristics of the SAH patients in relation to the presence or absence of endocrine dysfunction at baseline and after 6 months’ follow-up.
Table 4.
Potential risk factors for PD at baseline and 6 months after SAH
| Clinical characteristics | Baseline | 6 months’ follow-up | ||
|---|---|---|---|---|
| With PD N=37 |
Without PD N=47 |
With PD N=22 |
Without PD N=62 |
|
| Age; mean (SD) | 57.5 (11.9) | 54.3 (11.8) | 52.9 (12.0) | 56.7 (11.8) |
| Male | 10 (27%) | 18 (38%) | 10 (45%) | 18 (29%) |
| Days in hospital; mean (SD) | 18.4 (8.1) | 18.0 (10.9) | 19.7 (9.1) | 17.7 (9.9) |
| History of smoking | 23 (62%) | 29 (62%) | 14 (64%) | 38 (61%) |
| History of hypertension | 11 (30%) | 7 (15%) | 6 (27%) | 12 (19%) |
| History of DM | 0 | 4 (9%) | 0 | 4 (6%) |
| History of hypercholesterolaemia | 8 (22%) | 7 (15%) | 2 (9%) | 13 (21%) |
| BMI; mean (SD) | 25.5 (4.1) | 25.1 (3.4) | 25.2 (3.6) | 25.3 (3.8) |
| GCS on admission | * | |||
| 13–15 | 27 (73%) | 39 (83%) | 14 (64%) | 52 (84%) |
| 9–12 | 5 (13.5%) | 6 (13%) | 4 (18%) | 7 (11%) |
| 3–8 | 5 (13.5%) | 2 (4%) | 4 (18%) | 3 (5%) |
| WFNS on admission | * | |||
| Grade I | 14 (38%) | 24 (51%) | 8 (36%) | 31 (50%) |
| Grade II | 11 (30%) | 14 (30%) | 5 (23%) | 20 (32%) |
| Grade III | 2 (5%) | 1 (2%) | 1 (5%) | 2 (3%) |
| Grade IV | 5 (13.5%) | 6 (13%) | 4 (18%) | 7 (11%) |
| Grade V | 5 (13.5%) | 2 (4%) | 4 (18%) | 3 (5%) |
| Location of aneurysm | ||||
| Anterior circulation | 17 (46%) | 31 (66%) | 12 (55%) | 37 (60%) |
| Posterior circulation | 20 (54%) | 16 (34%) | 10 (45%) | 25 (40%) |
| SAH treatment | ||||
| Coiling | 30 (81%) | 36 (77%) | 19 (86%) | 47 (76%) |
| Clipping | 6 (19%) | 11 (23%) | 2 (9%) | 15 (24%) |
| Complications | ‡ | |||
| Any | 23 (62%) | 18 (38%) | 14 (64%) | 27 (44%) |
| Hydrocephalus | 18 (49%) | 13 (28%) | 13 (59%)† | 18 (29%) |
| Delayed Cerebral Ischaemia | 7 (19%) | 7 (15%) | 3 (14%) | 11 (18%) |
| Rebleed | 1 (3%) | 1 (2%) | 0 | 2 (3%) |
| ICH | 5 (13.5%) | 10 (21%) | 3 (14%) | 12 (19%) |
| IVH | 26 (70%) | 30 (64%) | 13 (59%) | 43 (69%) |
| Hypertension after SAH | 20 (54%) | 29 (62%) | 14 (64%)* | 22 (35%) |
*p<0.05.
†OR 3.3 (95% CI 2.7 to 3.8).
‡OR 2.6 (95% CI 2.2 to 3.0).
BMI, body mass index; GCS, Glasgow Coma Scale; ICH, intraparenchymal haematoma; IVH, intraventricular haematoma; PD, pituitary dysfunction; SAH, subarachnoid haemorrhage. WFNS, World Federation of Neurological Surgeons grading scale for patients with SAH.
In univariable analysis, hydrocephalus (OR 3.3, 95% CI 1.2 to 8.9), GCS <13 on admission (OR 2.79, 95% CI 1.0 to 8.9), WFNS >2 on admission (OR 2.9, 95% CI 1.1 to 8.3) and hypertension after SAH (OR3.2, 95% CI 1.2 to 8.7) were associated with the occurrence of PD 6 months after SAH. In a multivariable analysis adjusting for age, gender and hypertension, patients with any SAH-related complication had a significantly higher chance of having PD at baseline (OR 2.6, 95% CI 2.2 to 3.0). Hydrocephalus remained independently associated with hypopituitarism at 6 months (OR 3.3, 95% CI 2.7 to 3.8). GHD at 6 month after SAH was associated with male gender (OR 17.8, 95% CI 2.0 to 58.9). This association persisted after retesting 14 months after SAH. We found no association between aneurysm location and pituitary function. Other non-significant findings are added in online supplementary table S4.
We found a trend (p=0.06) for lower score on the QLSM-H at baseline in patients with PD compared with patients without PD, but this trend was not present any more after 6 or 14 months.
Discussion
In this prospective observational study, 39% of survivors of aneurysmal SAH were deficient in one or more pituitary axes at baseline. PD did not occur during follow-up in patients with normal pituitary function at baseline. PD persisted for 6 months in 26% of patients and for at least 14 months in 7%. Gonadotropin and GH were most often affected. Patients were prone to have PD in the early stage after SAH if they had developed any of the well-known complications related to SAH. Hydrocephalus was associated with PD 6 months after SAH and male sex was associated with persistent GHD.
The prevalence of patients with hypopituitarism after SAH exceeds the prevalence in the normal population.20 Together with the decrease in PD over time, and the association of PD to SAH-related complications and the severity of SAH symptoms, this supports the evidence for a causal relation between SAH and hypopituitarism.
Gonadotropin and/or GHD occurred more frequently than ACTH or TSH deficiency, which is in accordance with other studies.5 9 21 Since it is the anterior lobe of the pituitary gland that contains predominantly thyrotrope, somatotrope and gonadotrope cells,22 this observation suggests that this lobe is more vulnerable to damage secondary to SAH. This might be due to differences in the position of the pituitary parts in the skull or to differences in blood supply. Blood is supplied to the anterior lobe through long pituitary portal vessels that transcend from above the diaphragma sellae, whereas the blood supply to the remaining part of the pituitary gland is derived form middle and inferior pituitary arteries.4
In the majority of patients with PD, pituitary function recovered during follow-up, while in a small proportion of patients PD persisted. This has been described in similar studies,9 12 and is in accordance with studies that found only low numbers of patients with PD long after SAH.21 23 Recent findings suggest that the adult pituitary gland is capable of regeneration after injury,24 which might explain the recovery of pituitary function in our patients. The low proportion of patients with persistent PD described in our and other recent studies differs strongly from that of the older studies.5 6 11 This is probably explained by methodological differences between the studies. In some studies the tests used for detecting PD were not validated.11 In other studies, GHRH-arginine test outcomes were not adjusted for body mass index (BMI),10 13 and because obesity is a true confounder of the GHRH-arginine test and other dynamic tests, this strongly reduces the accuracy of the GH response.18 25 26 In our study, we used two different dynamic tests to establish GHD, and BMI related cut-off points were used in accordance with recent recommendations.21 27 Furthermore, we retested patients with GHD 14 months after SAH to explore whether GHD was a permanent or a transient phenomenon.
We found an independent association between hydrocephalus and persistent PD. Few studies have evaluated possible clinical determinants of hypopituitarism after SAH. Vasospasm and hydrocephalus were identified as risk factors for PD in one retrospective study with a non-consecutive series of patients without using dynamic tests.7 Other studies reported an association between female gender and corticotrophin deficiency11 and between older age and GHD in the acute phase after SAH.9 We observed that PD or GHD was predominantly present in men, which is in line with a recent study.21 We did not find an association of PD and the location of the aneurysm, which is in line with the results of a recent published study.15 However, numbers were small.
Hydrocephalus might play an important role in altering the pituitary function by increasing the intracranial pressure and altering anatomic configuration, which might lead to damage of the hypothalamic-pituitary complex. We also observed that severity of SAH, expressed as worse GCS and worse WFNS-scores, were associated with hypopituitarism in univariable analysis. Even though these determinants were not significant in multivariable analysis (possibly due to the low number of cases with PD in our study), this adds to the evidence that the pituitary gland is damaged by SAH and its complications. Not many studies describe the association of long-term symptoms as HRQOL with the occurrence of PD. In our study, a strong overall association was not present.
Whether patients with SAH should be screened for PD is under debate, because clinical significance is not clear. However, there are various other reasons besides the possible association with long-term symptoms to consider early neuroendocrine evaluation of patients with SAH. Adrenal insufficiency might be life-threatening in the early stressful period after SAH and recent findings suggest that early PD has a negative effect on outcome after SAH.28 Although we found adrenal insufficiency in only 1 (2%) patient, in other studies the proportion of patients with adrenal insufficiency was higher, up to 40%.9 11
Early screening for PD might be beneficial in the long-term after SAH. Symptoms of GHD consist of fatigue, low energy level, and decline in cognitive function such as memory and planning,4 symptoms often found after SAH.3 29 These symptoms might hamper the rehabilitation process. A few studies show a decrease in performance functions and life satisfaction in patients with PD after SAH,30 but others do not support this.21 23
Our study has several limitations. Not all of our patients had a dynamic test to evaluate GHD. This may have led to an underestimation of the number of patients with PD. Furthermore, two patients with GHD refused re-evaluation after 14 months. The follow-up period was limited and the possibility of recovery of pituitary function beyond this 14-month period cannot be ruled out.
Another limitation is patient selection. Twenty patients were transferred to another hospital for treatment before inclusion was fulfilled. Furthermore, we have chosen to include patients who survived the acute phase of SAH. Our selection was based on the idea that we wanted to investigate the course of hypopituitarism in the long-term survivors. Our results cannot, therefore, be generalised to all patients with acute SAH.
In conclusion, PD is an actual complication in SAH survivors. Hydrocephalus after SAH is an independent clinical predictor of long-term hypopituitarism after SAH.
Supplementary Material
Acknowledgments
The authors thank J van Eck from the department of endocrinology for performing dynamic tests.
Footnotes
Contributors: LK was involved in design of the study, data collection, interpretation of the data and writing the manuscript. KB took part in design of the study, data collection and revising the manuscript. MHH-K was involved in interpretation of the data and revising the manuscript. EMS was involved in design, data collection and revising the manuscript. HJGvdB-E, AJvdL and DWJD took part in revising the manuscript. SJCMMN, GMR and FvK were involved in conceptualisation and design of the study, interpretation of the data and revising the manuscript.
Funding: This study was financially supported by an unrestricted grant from Pfizer and by the Dutch Brain Foundation.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: The IRB at Erasmus MC University Medical Center.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hop JW, Rinkel GJ, Algra A, et al. Quality of life in patients and partners after aneurysmal subarachnoid hemorrhage. Stroke 1998;29:798–804. [DOI] [PubMed] [Google Scholar]
- 2.Berry E. Post-traumatic stress disorder after subarachnoid haemorrhage. Br J Clin Psychol 1998;37(Pt 3):365–7. [DOI] [PubMed] [Google Scholar]
- 3.Visser-Meily JM, Rhebergen ML, Rinkel GJ, et al. Long-term health-related quality of life after aneurysmal subarachnoid hemorrhage: relationship with psychological symptoms and personality characteristics. Stroke 2009;40:1526–9. [DOI] [PubMed] [Google Scholar]
- 4.Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, et al. Hypopituitarism. Lancet 2007;369:1461–70. [DOI] [PubMed] [Google Scholar]
- 5.Aimaretti G, Ambrosio MR, Di Somma C, et al. Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin Endocrinol (Oxf) 2004;61:320–6. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulou I, Kouyialis AT, Tzanella M, et al. High incidence of neuroendocrine dysfunction in long-term survivors of aneurysmal subarachnoid hemorrhage. Stroke 2004;35:2884–9. [DOI] [PubMed] [Google Scholar]
- 7.Jovanovic V, Pekic S, Stojanovic M, et al. Neuroendocrine dysfunction in patients recovering from subarachnoid hemorrhage. Hormones (Athens) 2010;9:235–44. [DOI] [PubMed] [Google Scholar]
- 8.Kreitschmann-Andermahr I, Hoff C, Niggemeier S, et al. Pituitary deficiency following aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2003;74:1133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanriverdi F, Dagli AT, Karaca Z, et al. High risk of pituitary dysfunction due to aneurysmal subarachnoid haemorrhage: a prospective investigation of anterior pituitary function in the acute phase and 12 months after the event. Clin Endocrinol 2007;67:931–7. [DOI] [PubMed] [Google Scholar]
- 10.Aimaretti G, Ambrosio MR, Di Somma C, et al. Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin Endocrinol 2004;61:320–6. [DOI] [PubMed] [Google Scholar]
- 11.Kreitschmann-Andermahr I, Hoff C, Saller B, et al. Prevalence of pituitary deficiency in patients after aneurysmal subarachnoid hemorrhage. J Clin Endocrinol Metab 2004;89:4986–92. [DOI] [PubMed] [Google Scholar]
- 12.Klose M, Brennum J, Poulsgaard L, et al. Hypopituitarism is uncommon after aneurysmal subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2010;73:95–101. [DOI] [PubMed] [Google Scholar]
- 13.Aimaretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitarism: a prospective 12-month study. J Clin Endocrinol Metab 2005;90:6085–92. [DOI] [PubMed] [Google Scholar]
- 14.Brandt L, Säveland H, Valdemarsson S, et al. Fatigue after aneurysmal subarachnoid hemorrhage evaluated by pituitary function and 3D-CBF. Acta Neurol Scand 2004;109:91–6. [DOI] [PubMed] [Google Scholar]
- 15.Dutta P, Mukherjee KK, Chaudhary PK, et al. Pituitary dysfunction in survivors of spontaneous subarachnoid hemorrhage of anterior communicating artery and middle cerebral artery aneurysms: a comparative study. Neurology India 2012;60:390–4. [DOI] [PubMed] [Google Scholar]
- 16.Blijdorp K, Khajeh L, Ribbers GM, et al. Diagnostic value of a ghrelin test for the diagnosis of GH deficiency after subarachnoid hemorrhage. Eur J Endocrinol 2013;169:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Gijn J, Hijdra A, Wijdicks EF, et al. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg 1985;63:355–62. [DOI] [PubMed] [Google Scholar]
- 18.Gasco V, Beccuti G, Baldini C, et al. Acylated ghrelin as a provocative test for the diagnosis of GH deficiency in adults. Eur J Endocrinol 2012;168:23–30. [DOI] [PubMed] [Google Scholar]
- 19.Herschbach P, Henrich G, Strasburger CJ, et al. Development and psychometric properties of a disease-specific quality of life questionnaire for adult patients with growth hormone deficiency. Eur J Endocrinol 2001;145:255–65. [DOI] [PubMed] [Google Scholar]
- 20.Regal M, Páramo C, Sierra SM, et al. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf) 2001;55:735–40. [DOI] [PubMed] [Google Scholar]
- 21.Gardner CJ, Javadpour M, Stoneley C, et al. Low prevalence of hypopituitarism after subarachnoid haemorrhage using confirmatory testing and with BMI-specific GH cut-off levels. Eur J Endocrinol 2013;168:473–81. [DOI] [PubMed] [Google Scholar]
- 22.Kioussi C, Carriere C, Rosenfeld MG. A model for the development of the hypothalamic-pituitary axis: transcribing the hypophysis. Mech Dev 1999;81:23–35. [DOI] [PubMed] [Google Scholar]
- 23.Lammert A, Bode H, Hammes HP, et al. Neuro-endocrine and neuropsychological outcome after aneurysmal subarachnoid hemorrhage (aSAH): a prospective cohort study. Exp Clin Endocrinol Diabetes 2011;119:111–16. [DOI] [PubMed] [Google Scholar]
- 24.Fu Q, Gremeaux L, Luque RM, et al. The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology 2012;153:3224–35. [DOI] [PubMed] [Google Scholar]
- 25.Biller BM, Samuels MH, Zagar A, et al. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab 2002;87:2067–79. [DOI] [PubMed] [Google Scholar]
- 26.Ghigo E, Aimaretti G, Corneli G. Diagnosis of adult GH deficiency. Growth Horm IGF 2008;18:1–16. [DOI] [PubMed] [Google Scholar]
- 27.Popovic V. Approach to testing growth hormone (GH) secretion in obese subjects. J Clin Endocrinol Metab 2013;98:1789–96. [DOI] [PubMed] [Google Scholar]
- 28.Aimaretti G, Corneli G, Rovere S, et al. Insulin-like growth factor I levels and the diagnosis of adult growth hormone deficiency. Horm Res, 2004;62(Suppl 1):26–33. [DOI] [PubMed] [Google Scholar]
- 29.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 2010;41:e519–36. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan L, Roberts B, Bushnik T, et al. The impact of hypopituitarism on function and performance in subjects with recent history of traumatic brain injury and aneurysmal subarachnoid haemorrhage. Brain Inj 2009;23:639–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.