Abstract
Purpose
Previous studies have reported the presence of a circadian rhythm in PERIOD2::LUCIFERASE (PER2::LUC) bioluminescence in mouse photoreceptors, retina, RPE, and cornea. Melatonin (MLT) modulates many physiological functions in the eye and it is believed to be one of the key circadian signals within the eye. The aim of the present study was to investigate the regulation of the PER2::LUC circadian rhythm in mouse cornea and to determine the role played by MLT.
Methods
Corneas were obtained from PER2::LUC mice and cultured to measure bioluminescence rhythmicity in isolated tissue using a Lumicycle or CCD camera. To determine the time-dependent resetting of the corneal circadian clocks in response to MLT or IIK7 (a melatonin type 2 receptor, MT2, agonist) was added to the cultured corneas at different times of the day. We also defined the location of the MT2 receptor within different corneal layers using immunohistochemistry.
Results
A long-lasting bioluminescence rhythm was recorded from cultured PER2::LUC cornea and PER2::LUC signal was localized to the corneal epithelium and endothelium. MLT administration in the early night delayed the cornea rhythm, whereas administration of MLT at late night to early morning advanced the cornea rhythm. Treatment with IIK7 mimicked the MLT phase-shifting effect. Consistent with these results, MT2 immunoreactivity was localized to the corneal epithelium and endothelium.
Conclusions
Our work demonstrates that MLT entrains the PER2::LUC bioluminescence rhythm in the cornea. Our data indicate that the cornea may represent a model to study the molecular mechanisms by which MLT affects the circadian clock.
Keywords: melatonin, cornea, circadian rhythm, entrainment
The circadian clock in the cornea is synchronized to the external light:dark cycle by melatonin.
Circadian rhythms are an ubiquitous feature of living systems. Accumulating evidence suggests that dysfunction of circadian rhythms due to genetic mutations or environmental factors contributes to the development of many diseases.1–4 The retinal circadian clock was the first extra-SCN (suprachiasmatic nucleus) circadian oscillator to be discovered in mammals5,6 and several studies have now demonstrated that many of the physiological, cellular, and molecular rhythms that are present in the retina are under the control of circadian clocks.4 Experimental evidence also indicates that other ocular structures may contain circadian clocks that drive circadian rhythms in the eye. Diurnal fluctuations have been reported in IOP,7,8 photoreceptor disk shedding and phagocytosis,9–13 axial chamber length, choroidal volume, corneal curvature, and cornea thickness.14–22 The changes in axial chamber length, corneal curvature, and thickness are believed to contribute to variations of astigmatism and refractive errors observed at different times of the day.23–25 Further investigations also have shown that a diurnal rhythm in the light- dark cycle is important for normal corneal growth and development,26 and that renewal of the corneal epithelium shows a daily rhythm.27
The PERIOD2::LUCIFERASE (PER2::LUC) knockin mice in which the firefly luciferase gene was fused into the Period 2 (Per2) gene, have been used to study circadian rhythms in many different tissues and/or organs,28 including in the eye. These rhythms are thought to be driven by circadian oscillators endogenous to a variety of structures throughout the eye. For example, endogenous circadian clocks have been identified in mouse photoreceptors,29 retina,29–33 RPE,32 and the cornea.28,34 Circadian clocks in the photoreceptors are directly entrained by changes in light/dark. However, because photoreceptors, and a handful of specialized ganglion cells, are the only cells in the eye capable of detecting light/dark changes, there must be signals emanating from photoreceptors that propagate time-of-day information throughout the eye.
Among the many neurotransmitters present in the retina, melatonin (MLT) is believed to be a key signal that regulates circadian rhythms.35 Melatonin is synthesized by the photoreceptors and by the pineal gland with high levels at night and lower levels during the day.36,37 Retinal MLT is involved in the modulation of many important ocular functions by acting on melatonin receptors (MT1 and MT2) that are present in these structures.38,39 For example, MLT affects the amplitude of the scotopic electroretinogram39–41 and protects RPE cells,42 photoreceptors,41,43 and ganglion cells.41,44
The aim of the present study was to further define the cellular location of corneal PER2::LUC bioluminescence rhythm and to test whether MLT can synchronize the PER2::LUC bioluminescence rhythm.
Materials and Methods
Animals
The PER2::LUC mice28 (C57/Bl6) at approximately 12 weeks of age were used in this study (n = 196). All the procedures were approved by the Institutional Animal Care and Use Committee at Morehouse School of Medicine. Mice were raised at Morehouse School of Medicine in 12 hours light and 12 hours dark with lights on (zeitgeber time [ZT] 0) at 06:00 and lights off (ZT 12) at 18:00 hours. Water and food were available ad libitum. The light was supplied with fluorescent tubes and the light intensity ranged from 200 to 400 lux at cage level.
Tissue Culture Preparation and Measurement of Bioluminescence
Mice were anesthetized with CO2 and killed by cervical dislocation. The eyes were removed and the cornea was carefully separated under a dissecting microscope. The cornea was placed on the culture membrane (epithelial layer up) (Millicell-CM, PICM030-50; Millipore, Billerica, MA, USA) in a 35-mm Petri dish with 1.2 mL of 199 medium (Cambrex, Walkersville, MD, USA) containing 0.1 mM D-Luciferin K salt (Molecular Imaging Products, Bend, OR, USA). Dishes were sealed and kept at 37°C. The cultures were prepared under fluorescent tubes between ZT (lights on at ZT0) 8 to 10. The bioluminescence emitting from the cornea was measured with photomultiplier tubes (Lumicycle; Actimetrics, Wilmette, IL, USA). To identify the sources of bioluminescence within the different layers, the cornea was sectioned with a razor blade and placed on a culture membrane in a Petri dish containing 199 medium. The bioluminescence images of a cornea section was obtained by a Zeiss AxioObserver Z1 microscope (Zeiss, Oberkochen, Germany) with an ×10 Fluar objective lens (Zeiss), Marzhauzer scanning stage with LUDL Mac 5000 controller, and a Stanford Photonics (Palo Alto, CA, USA) XR Mega 10Z cooled intensified charge-couple device (CCD) camera in a custom-built light-tight chamber maintained at 37°C (see Evans et al.45 for details). Bioluminescence images were collected every 15 minutes for 3 days. Continuous images of cornea PER2::LUC bioluminescence were converted and analyzed for a quantification of bioluminescence counts by Image-J software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
Mice were killed and corneas were dissected at ZT 9 to ZT 10. Tissues were fixed overnight in 4% paraformaldehyde PBS solution and cryoprotected in a 30% sucrose solution for 48 hours. Tissue was then frozen and embedded in Tissue Tek OCT compound (Sakura, Finetek, Torrance, CA, USA). Then, 10 μm frozen sagittal cornea sections were made and mounted on a positive charged slide glass (VWR Vista Vision Histo Bond; VWR Inc., Radnor, PA, USA). Afterward, the sections were washed in PBS (3 × 10 minutes) and permeabilized with 0.3% Triton X-100 in a blocking buffer containing 1% BSA for 60 minutes at room temperature (RT). Sections were incubated overnight at 4°C with a 1:2000 dilution of 10 mg/mL biotin-conjugated goat anti-firefly luciferase antibody (Abcam, Hartford, CT, USA). To determine the cellular localization of MT2 receptors in the cornea, sections were incubated for 3 hours with a 1:500 dilution of 0.8 mg/mL rabbit anti-MT2 antibody or with the control peptide antigen (Alamone Labs, Jerusalem, Israel). Sections were then incubated with the biotinylated goat anti-rabbit IgG secondary antibody (1:200; Vector Laboratories, Inc., Burlingame, CA, USA). After washing sections with washing solution (PBS with 0.3% Triton X-100), sections were incubated with horseradish peroxidase conjugated with streptavidin (Vectastain Elite ABC kit; Vector Laboratories, Inc.) for 45 minutes at RT. Peroxidase activity was revealed with 3′,3′-diaminobenzidine (DAB) tetrahydrochlorate using a DAB kit (Vector Laboratories, Inc.). Sections were then washed with distilled water and mounted with a mounting medium (VectaMount AQ Mounting Medium; Vector Laboratories, Inc.).
Drug Treatments
Melatonin (Sigma-Aldrich Corp., St. Louis, MO, USA) was first dissolved in ethanol (8 mg/mL). It was then diluted to 100 μM in PBS (100 nM final concentration). The IIK7 (N-butanoyl-2-[2-methoxy-6H-isoindolo(2,1-a)indol-11-yl]ethanamine; Sigma) was dissolved in DMSO 30 mg/mL then diluted with PBS to 1 mM (1 μM final concentration) or 1 μM (1 nM final concentration). These concentrations were selected on the basis on the compound affinity for each of the murine MLT receptors.39 Controls were prepared with the same procedure, but without the active compound. After 3 to 4 days of bioluminescence recording, the culture dishes containing cornea were gently removed from the Lumicycle and 1.2 μL of drug solutions or vehicles were added to the culture dishes. They were then re-sealed, returned to the Lumicycle and placed in the same positions that they were occupying before the treatment. The culture dishes were kept in the Lumicycle until the end of the experiment without a drug washout.
Analysis of Phase-Shifts
Bioluminescence recordings emitted from cornea cultures were detrended by a 24-hour moving average subtraction method and smoothed by a 2-hour moving average. The circadian peak phase was determined as the highest point of the curve picked by Origin (Origin Laboratories, Northampton, MA, USA) software. The amount of phase-shift (in hours) was calculated by comparing the regression lines fitted to the circadian peaks before and after the treatment.32 Phase-shifts in individual cornea were averaged in a 4-hour bin at circadian time (CT) 0 to 4, CT 4 to 8, CT 8 to 12, CT 12 to 16, CT 16 to 20, and CT 20 to 24 and normalized with respective control groups. Two-way ANOVA with post hoc Tukey test was performed to compare the difference between experimental groups and time points.
Results
Circadian Rhythms of Per2::LUC Bioluminescence in Cultured Cornea
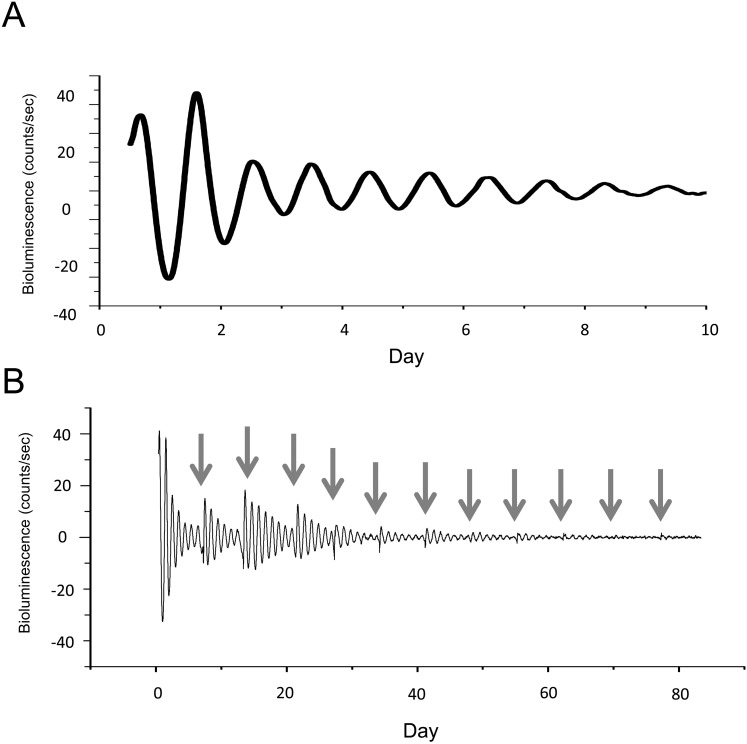
Figure 1A shows a representative measurement of cornea bioluminescence obtained from a PER2::LUC mouse. Similar to results reported in a previous study,28 we could readily detect a robust circadian rhythm in PER2::LUC bioluminescence that could persist for more than 80 days in culture with weekly medium exchanges (Fig. 1B). The average circadian peak phase of PER2::LUC bioluminescence in cornea was ZT 13.13 ± 0.09 hours, just after dark onset, and the average circadian period was 22.83 ± 0.04 hours (mean ± SEM).
Figure 1.
The PER2::LUC bioluminescence rhythms in mouse cornea. Bioluminescence obtained from a cultured PER2::LUC mouse cornea was measured with a Lumicycle for 10 days (A). Rhythmic PER2::LUC bioluminescence was observed for more than 80 days when cultures received weekly medium exchange (B). Arrows indicate the time of medium exchanges.
Localization of the PER2::LUC Bioluminescence Rhythms in Cornea
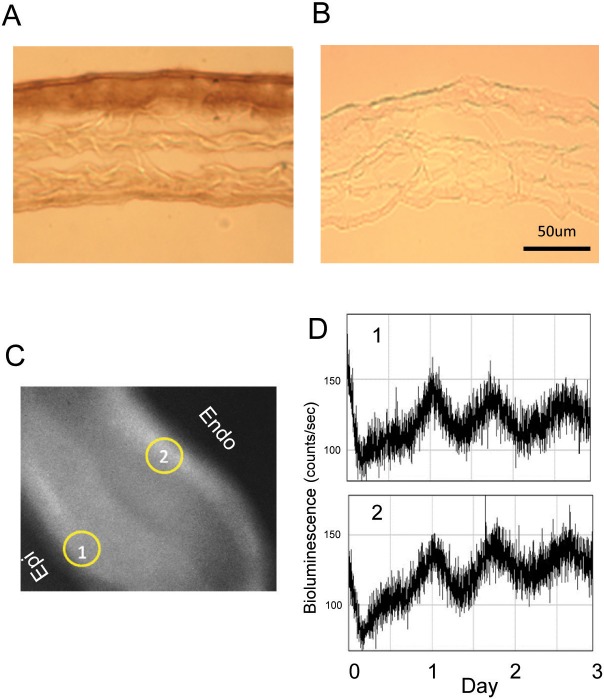
The mouse cornea contains five different layers (epithelium, anterior limiting lamina, stroma, posterior limiting lamina, and endothelium). Using anti-luciferase antibody, we identified strong specific immunoreactivity in corneal epithelium and endothelium (Fig. 2A). No immunoreactivity was detected in controls (Fig. 2B). To validate the result obtained with immunohistochemistry, we investigated the cellular location of the PER2::LUC bioluminescence rhythm in cornea using a CCD camera imaging system. As shown in Figure 2C, PER2::LUC bioluminescence was mostly detected in the epithelium and endothelium layers. The phase and the period of the circadian rhythms in the epithelium and endothelium were similar (Fig. 2D).
Figure 2.
The localization of PER2::LUC to the cornea layers. Cornea obtained from PER2::LUC mice was immunostained with anti-firefly luciferase antibody (A). Specific staining was only observed in epithelium and endothelium layers of cornea (A). Control (i.e., tissue incubated without the primary antibody) did not show any immunoreactivity (B). Images of PER2::LUC bioluminescence obtained with the CCD camera (C). Circadian bioluminescence rhythms in epithelium (D1) and endothelium (D2) were recorded for 3 consecutive days.
Effect of Melatonin on the PER2::LUC Bioluminescence Rhythms
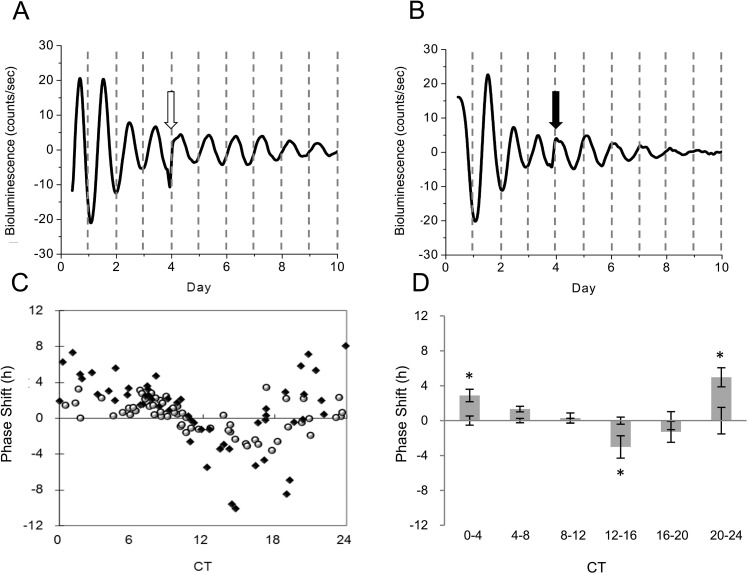
We then generated a phase response curve (PRC) to MLT. On the third day of culture, corneas were treated with 100 nM MLT or a vehicle at several different phases of the circadian cycle and then the bioluminescence was continuously measured for an additional 5 days (Figs. 3A, 3B). The individual phase-shift for each cornea rhythm was plotted to produce the PRC (Fig. 3C). A two-way ANOVA analysis revealed a significant interaction effect between groups and treatment time points (P < 0.001, Fig. 3D). Melatonin significantly phase-delayed cornea rhythms by 3.02 hours when applied at CT 12 to 16 (P < 0.05, Tukey test) and significantly phase-advanced cornea rhythms by 4.97 hours when applied at CT 20 to 24 (P < 0.05, Tukey test) and 2.88 hours at CT 0 to 4 (P < 0.05, Tukey test).
Figure 3.
Phase response curve of PER2::LUC bioluminescence rhythm to MLT administration. Representative examples of PER2::LUC bioluminescence rhythms in response to vehicle (A) and MLT (B). The arrows indicate when the Vehicle (Veh) or MLT was added to the culture dishes respectively. The value of the phase-shift for each individual cornea rhythm was plotted to create a PRC (C). White circles indicate cultures treated with Veh and black squares indicate culture treated with MLT. Data were divided into six bins at 4-hour intervals for statistical analysis (D) (Tukey test, *P < 0.05). n = 5 to 8 for each bin.
Effect of Melatonin Receptor Agonist on PER2::LUC Bioluminescence Rhythms
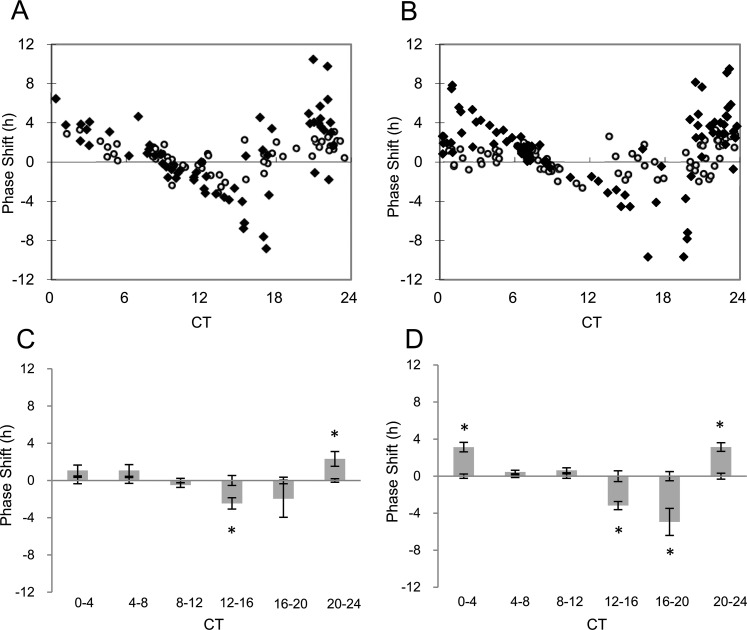
In Figure 4A, we show the PRC of the PER2::LUC bioluminescence rhythm to IIK7, a MT2 receptor agonist, at a concentration of 1 nM (i.e., at a concentration that activates only MT2 receptors39). At this concentration, II7K significantly phase-delayed cornea rhythms by 2.46 hours at CT 12 to 16 (P < 0.05, two-way ANOVA followed by Tukey test; Fig. 4C) and significantly phase-advanced cornea rhythms by 2.32 hours at CT 20 to 24 (P < 0.05, two-way ANOVA followed by Tukey test). We then increased the dosage of IIK7 to 1 μM where both MT1 and MT2 can be activated39 (Fig. 4B). As seen with the lower concentration, administration of 1 μM of IIK7 also phase-delayed cornea rhythms by 3.18 hours at CT 12 to 16 (P < 0.05, two-way ANOVA followed by Tukey test, interaction effect P < 0.001, Fig. 4D), 4.17 hours at CT 16 to 20 (P < 0.05, Tukey test) and phase-advanced cornea rhythms by 3.14 hours at CT 20 to 24 (P < 0.05, Tukey test), 3.13 hours at CT 0 to 4 (P < 0.05, Tukey test). Consistently with the data obtained with IIK7 administration, MT2 immunoreactivity was observed in the corneal epithelium and endothelium (Fig. 5).
Figure 4.
Phase response curve of PER2::LUC bioluminescence rhythm to IIK7 administration: 1 nM of IIK7 induced a significant phase-shift at CT 12 to 16 and CT 20 to 24 (A); 1 μM of IIK7 also induced significant phase-shift at CT 0 to 4 and CT 12 to 24 (B). White circles indicate cultures treated with Veh and black squares indicate culture treated with IIK7. Data were divided to six bins at 4-hour intervals for statistical analysis ([C] for 1 nM and [D] for 1 μM) (Tukey test, *P < 0.05). n = 7 to 17 for each bin.
Figure 5.
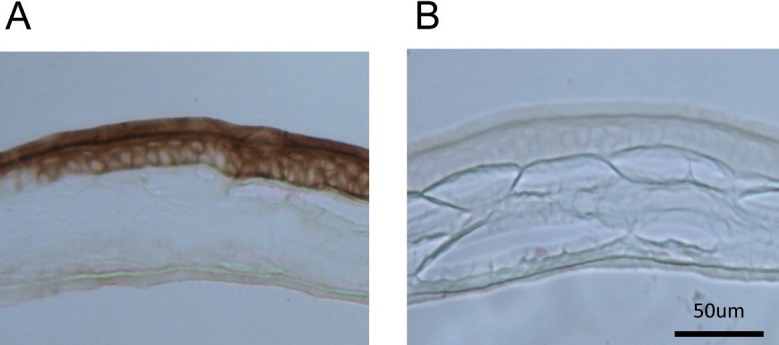
Immunoreactivity of MT2 receptor in the cornea. Immunoreactivity of MT2 was localized to the corneal epithelium and endothelium (A). Control (i.e., tissue incubated with the control peptide antigen) did not show any immunoreactivity (B).
Discussion
In this study, we investigated the PER2::LUC bioluminescence circadian rhythm in the cornea and we localized PER2::LUC expression to the epithelial and endothelial layers of the mouse cornea. We also demonstrated that MLT can phase-shift this circadian rhythm, possibly by acting on MT2 receptors. Consistent with what has been previously shown,28 we observed a robust and persistent (up to 80 days) PER2::LUC bioluminescence rhythm with a phase and period similar to what has been reported previously in the same animal model.28 The mouse cornea is composed of epithelium, anterior limiting lamina, stroma, posterior limiting lamina, and endothelium layers.46 Although previous studies have reported bioluminescence circadian rhythms in this tissue,28,34 the localization of the PER2::LUC bioluminescence was still unknown. Our study indicates that the corneal epithelium and the endothelium contain a circadian clock driving the circadian rhythm in this tissue (Fig. 2) and MLT can entrain these circadian rhythms (Fig. 3).
As previously mentioned, accumulating experimental evidence indicates that circadian rhythms control important physiological functions of corneal epithelium and endothelium. The renewal of the corneal epithelium has been shown to exhibit circadian regulation,27 and the mitotic rate and proliferation of corneal epithelial cells has been shown to have circadian variation.47,48 Furthermore, it has been reported that mitotic rhythm can be phase-shifted by MLT.49 These circadian variations affect the process of corneal epithelial wound healing50 and influence the action of drugs on the corneal epithelium.51,52 Similarly, it has been reported that IIK7 enhanced wound healing in rabbit epithelium, and that the positive effect of MLT administration on wound healing was blocked by DH97, a selective MT2 antagonist, thus suggesting MT2 signaling as important for corneal wound healing in rabbit.53 Although a previous study has suggested that corneal mitotic activity is not driven by MLT, because such a rhythm persists after the photoreceptors were destroyed,54 it is worthwhile to mention that MLT of pineal origin could still have affected and driven corneal mitotic rhythm.
Our data indicate that activation of the MT2 receptor can induce a phase-shift of corneal PER2::LUC bioluminescence rhythms (Fig. 4A). Although a higher concentration of IIK7 seems to increase the amplitude of the phase-shifts (Figs. 4C, 4D), a careful inspection of the individual data points (Figs. 4A, 4B) does not support this conclusion. Hence, we believe that the corneal PER2::LUC bioluminescence rhythm is phase-shifted predominantly by activation of MT2 receptors. The observation that MLT or IIK7 can still produce a phase-shift (advance) in the early subjective morning (Figs. 3, 4) when MLT levels are low indicates that the action of MLT on the regulation of circadian rhythms may vary among different tissues.
Melatonin receptor immunoreactivity has been localized to the corneal epithelium and endothelium in many species.53,55–59 Our results indicate that, in the mouse, MT2 receptors are expressed in the corneal epithelium and endothelium (Fig. 5) and MLT acts directly on the cells expressing PER2::LUC bioluminescence.
The action of MLT and its associated receptors on the regulation of circadian rhythms is well established (see Dubocovich et al.,60 Tosini et al.61). Indeed MLT, via MT2, receptors can phase-shift electrical activity in the SCN62,63 and locomotor activity.63,64 Our new data further support this hypothesis. We have recently reported that disruption of MLT signaling affects clock gene expression in photoreceptors, and may affect the expression of these genes in the inner retina and/or ganglion cell layer.65 These data together with our new results indicate that the action of MLT on the circadian clock is cell specific.
Previous studies have shown that in the retina, MLT is synthetized by photoreceptors36 and it is believed that retinal MLT is rapidly metabolized in the retina.66 Although we believe that the MLT synthetized by the photoreceptors is responsible for the entrainment of the corneal circadian rhythms, we cannot exclude that MLT of pineal origin (i.e., circulating MLT) may also reach the corneal tissue and entrain the corneal circadian clock. Indeed, several studies have shown that administration of exogenous MLT via different routes (intraperitoneal injection or via drinking water) can reach the eye and can alter retinal physiology.39,41,67
In conclusion, our study demonstrates that the cornea is a robust model to study circadian rhythms and that the epithelium and endothelium layers of the cornea contain circadian clocks. These circadian rhythms may be entrained by MLT via MT2 receptor signaling. Our study also indicates that corneal epithelial and endothelial cells may represent a new tool to study the molecular mechanisms by which MLT can entrain the circadian clock.
Acknowledgments
We thank Jason DeBruyne, PhD, for critical reading of the manuscript.
Supported by National Institutes of Health Grants EY022216 and EY020821 (GT); SC1GM112567 (AJD); and by 5U54NS083932, S21MD000101, G12-RR03034, and U54-NS083932 to Morehouse School of Medicine.
Disclosure: K. Baba, None; A.J. Davidson, None; G. Tosini, None
References
- 1. Musiek ES,, Lim MM,, Yang G,, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013; 123: 5389–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans JA,, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 2013; 119: 283–323. [DOI] [PubMed] [Google Scholar]
- 3. Dibner C,, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. 2015; 277: 513–527. [DOI] [PubMed] [Google Scholar]
- 4. McMahon DG,, Iuvone PM,, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res. 2014; 39: 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tosini G,, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996; 272: 419–421. [DOI] [PubMed] [Google Scholar]
- 6. Tosini G,, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998; 789: 221–228. [DOI] [PubMed] [Google Scholar]
- 7. Aihara M,, Lindsey JD,, Weinreb RN. Twenty-four-hour pattern of mouse intraocular pressure. Exp Eye Res. 2003; 77: 681–686. [DOI] [PubMed] [Google Scholar]
- 8. Alcantara-Contreras S,, Baba K,, Tosini G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cells death in the mouse. Neurosci Lett. 2011; 494: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976; 194: 1071–1074. [DOI] [PubMed] [Google Scholar]
- 10. Besharse JC,, Hollyfield JG. Turnover of mouse photoreceptor outer segments in constant light and darkness. Invest Ophthalmol Vis Sci. 1979; 18: 1019–1024. [PubMed] [Google Scholar]
- 11. Teirstein PS,, Goldman AI,, O'Brien PJ. Evidence for both local and central regulation of rat rod outer segment disc shedding. Invest Ophthalmol Vis Sci. 1980; 19: 1268–1273. [PubMed] [Google Scholar]
- 12. Terman JS,, Remé CE,, Terman M. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993; 605: 256–264. [DOI] [PubMed] [Google Scholar]
- 13. Grace MS,, Wang LM,, Pickard GE,, Besharse JC,, Menaker M. The tau mutation shortens the period of rhythmic photoreceptor outer segment disk shedding in the hamster. Brain Res. 1996; 735: 93–100. [DOI] [PubMed] [Google Scholar]
- 14. Mapstone R,, Clark CV. Diurnal variation in the dimensions of the anterior chamber. Arch Ophthalmol. 1985; 103: 1485–1486. [DOI] [PubMed] [Google Scholar]
- 15. Nickla DL,, Wildsoet C,, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998; 66: 163–181. [DOI] [PubMed] [Google Scholar]
- 16. Read SA,, Collins MJ,, Iskander DR. Diurnal variation of axial length intraocular pressure, and anterior eye biometrics. Invest Ophthalmol Vis Sci. 2008; 49: 2911–2918. [DOI] [PubMed] [Google Scholar]
- 17. Kikkawa Y. Diurnal variation in corneal thickness. Exp Eye Res. 1973; 15: 1–9. [DOI] [PubMed] [Google Scholar]
- 18. Scheving LE,, Scheving LA,, Tsai TH,, Pauly JE. Effect of fasting on circadian rhythmicity in deoxyribonucleic acid synthesis of several murine tissues. J Nutr. 1984; 114: 2160–2166. [DOI] [PubMed] [Google Scholar]
- 19. Harper CL,, Boulton ME,, Bennett D,, et al. Diurnal variations in human corneal thickness. Br J Ophthalmol. 1996; 80: 1068–1072. Erratum in: Br J Ophthalmol. 1997;81:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kida T,, Liu JH,, Weinreb RN. Effect of 24-hour corneal biomechanical changes on intraocular pressure measurement. Invest Ophthalmol Vis Sci. 2006; 47: 4422–4426. [DOI] [PubMed] [Google Scholar]
- 21. Kida T,, Liu JH,, Weinreb RN. Effects of aging on corneal biomechanical properties and their impact on 24-hour measurement of intraocular pressure. Am J Ophthalmol. 2008; 146: 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Read SA,, Collins MJ. Diurnal variation of corneal shape and thickness. Optom Vis Sci. 2009; 86: 170–180. [DOI] [PubMed] [Google Scholar]
- 23. Read SA,, Collins MJ,, Carney LG. The diurnal variation of corneal topography and aberrations. Cornea. 2005; 24: 678–687. [DOI] [PubMed] [Google Scholar]
- 24. Campbell MC,, Bunghardt K,, Kisilak ML,, Irving EL. Diurnal rhythms of spherical refractive error, optical axial length, and power in the chick. Invest Ophthalmol Vis Sci. 2012; 53: 6245–6253. [DOI] [PubMed] [Google Scholar]
- 25. Chakraborty R,, Read SA,, Collins MJ. Diurnal variations in ocular aberrations of human eyes. Curr Eye Res. 2014; 39: 271–281. [DOI] [PubMed] [Google Scholar]
- 26. Stone RA,, Pardue MT,, Iuvone PM,, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013; 114: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doughty MJ. Morphometric analysis of the surface cells of rabbit corneal epithelium by scanning electron microscopy. Am J Anat. 1990; 189: 316–328. [DOI] [PubMed] [Google Scholar]
- 28. Yoo SH,, Yamazaki S,, Lowrey PL,, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004; 101: 5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jaeger C,, Sandu C,, Malan A,, Mellac K,, Hicks D,, Felder-Schmittbuhl MP. Circadian organization of the rodent retina involves strongly coupled layer-specific oscillators. FASEB J. 2015; 29: 1493–1504. [DOI] [PubMed] [Google Scholar]
- 30. Ruan GX,, Zhang DQ,, Zhou T,, Yamazaki S,, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci U S A. 2006; 103: 9703–9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruan GX,, Allen GC,, Yamazaki S,, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008; 6: e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baba K,, Sengupta A,, Tosini M,, Contreras-Alcantara S,, Tosini G. Circadian regulation of the PERIOD 2::LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis. 2010; 16: 2605–2611. [PMC free article] [PubMed] [Google Scholar]
- 33. Buhr ED,, Van Gelder RN. Local photic entrainment of the retinal circadian oscillator in the absence of rods, cones, and melanopsin. Proc Natl Acad Sci U S A. 2014; 111: 8625–8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pezuk P,, Mohawk JA,, Yoshikawa T,, Sellix MT,, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms. 2010; 25: 432–441. [DOI] [PubMed] [Google Scholar]
- 35. Tosini G,, Baba K,, Hwang CK,, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res. 2012; 103: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu C,, Fukuhara C,, Wessel JH,, III, Iuvone PM,, Tosini G. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004; 315: 197–201. [DOI] [PubMed] [Google Scholar]
- 37. Tosini G,, Davidson AJ,, Fukuhara C,, Kasamatsu M,, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007; 21: 3866–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wiechmann AF,, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008; 27: 137–160. [DOI] [PubMed] [Google Scholar]
- 39. Baba K,, Benleulmi-Chaachoua A,, Journé AS,, et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal. 2013; 6:ra89. [DOI] [PMC free article] [PubMed]
- 40. Rufiange M,, Dumont M,, Lachapelle P. Correlating retinal function with melatonin secretion in subjects with an early or late circadian phase. Invest Ophthalmol Vis Sci. 2002; 43: 2491–2499. [PubMed] [Google Scholar]
- 41. Baba K,, Pozdeyev N,, Mazzoni F,, et al. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009; 106: 15043–15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liang FQ,, Green L,, Wang C,, Alssadi R,, Godley BF. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp Eye Res. 2004; 78: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 43. Liang FQ,, Aleman TS, ZaixinY, Cideciyan AV, Jacobson SG, Bennett J. Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport. 2001; 12: 1011–1014. [DOI] [PubMed] [Google Scholar]
- 44. Belforte NA,, Moreno MC,, de Zavalía N,, et al. Melatonin: a novel neuroprotectant for the treatment of glaucoma. J Pineal Res. 2010; 48: 353–364. [DOI] [PubMed] [Google Scholar]
- 45. Evans JA,, Leise TL,, Castanon-Cervantes O,, Davidson AJ. Intrinsic regulation of spatiotemporal organization within the suprachiasmatic nucleus. PLoS One. 2011; 6: e15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith RS. Systematic Evaluation of the Mouse Eye: Anatomy Pathology, and Biomethods. Boca Raton, FL: CRC Press; 2002: 3–24. [Google Scholar]
- 47. Sandvig KU,, Haaskjold E,, Refsum SB. Time dependency in the regenerative response to injury of the rat corneal epithelium. Chronobiol Int. 1994; 11: 173–199. [DOI] [PubMed] [Google Scholar]
- 48. Oishi T,, Murakami N. Effects of duration and intensity of illumination on several parameters of the chick eye. Comp Biochem Physiol A Comp Physiol. 1985; 81: 319–323. [DOI] [PubMed] [Google Scholar]
- 49. Sasaki M,, Masuda A,, Oishi T. Circadian rhythms of corneal mitotic rate retinal melatonin and immunoreactive visual pigments, and the effects of melatonin on the rhythms in the Japanese quail. J Comp Physiol A. 1995; 176: 465–471. [DOI] [PubMed] [Google Scholar]
- 50. Buffa A,, Rizzi E,, Falconi M,, et al. Bromodeoxyuridine incorporation in corneal epithelium: an immunocytochemical study in rats. Boll Soc Ital Biol Sper. 1993; 69: 767–773. [PubMed] [Google Scholar]
- 51. Burns ER,, Scheving LE. Isoproterenol-induced phase shifts in circadian rhythm of mitosis in murine corneal epithelium. J Cell Biol. 1973; 56: 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cardoso SS,, Sowell JG. Control of cell division in the cornea of rats. Interaction between isoproterenol and dexamethasone. Proc Soc Exp Biol Med. 1974; 147: 309–313. [DOI] [PubMed] [Google Scholar]
- 53. Crooke A,, Guzman-Aranguez A,, Mediero A,, et al. Effect of melatonin and analogues on corneal wound healing: involvement of Mt2 melatonin receptor. Curr Eye Res. 2015; 40: 56–65. [DOI] [PubMed] [Google Scholar]
- 54. Oishi T,, Mohri Y,, Kaneko T,, et al. Retinal melatonin is not involved in corneal mitotic rhythms in the Japanese quail: effects of formoguanamine hydrochloride and eye-lid suture. J Pineal Res. 1996; 21: 149–154. [DOI] [PubMed] [Google Scholar]
- 55. Meyer P,, Pache M,, Loeffler KU,, et al. Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002; 86: 1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wiechmann AF,, Rada JA,, Sundberg JP. Melatonin receptor expression in the cornea and sclera. Exp Eye Res. 2003; 77: 219–225. [DOI] [PubMed] [Google Scholar]
- 57. Wiechmann AF,, Udin SB, Summers Rada JA. Localization of Mel1b melatonin receptor-like immunoreactivity in ocular tissues of Xenopus laevis. Exp Eye Res. 2004; 79: 585–594. [DOI] [PubMed] [Google Scholar]
- 58. Rada JA,, Wiechmann AF. Melatonin receptors in chick ocular tissues: implications for a role of melatonin in ocular growth regulation. Invest Ophthalmol Vis Sci. 2006; 47: 25–33. [DOI] [PubMed] [Google Scholar]
- 59. Wiechmann AF,, Hollaway LR,, Rada JA. Melatonin receptor expression in Xenopus laevis surface corneal epithelium: diurnal rhythm of lateral membrane localization. Mol Vis. 2009; 15: 2384–2403. [PMC free article] [PubMed] [Google Scholar]
- 60. Dubocovich ML,, Delagrange P,, Krause DN,, Sugden D,, Cardinali DP,, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010; 62: 343–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tosini G,, Owino S,, Guillaume JL,, Jockers R. Understanding melatonin receptor pharmacology: latest insights from mouse models, and their relevance to human disease. Bioessays. 2014; 36: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jin X,, von Gall C,, Pieschl RL,, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003; 23: 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rawashdeh O,, Hudson RL,, Stepien I,, Dubocovich ML. Circadian periods of sensitivity for ramelteon on the onset of running-wheel activity and the peak of suprachiasmatic nucleus neuronal firing rhythms in C3H/HeN mice. Chronobiol Int. 2011; 28: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dubocovich ML,, Yun K,, Al-Ghoul WM,, Benloucif S,, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998; 12: 1211–1220. [DOI] [PubMed] [Google Scholar]
- 65. Hiragaki S,, Baba K,, Coulson E,, Kunst S,, Spessert R,, Tosini G. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS One. 2014; 9: e106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cahill GM,, Besharse JC. Retinal melatonin is metabolized within the eye of Xenopus laevis. Proc Natl Acad Sci U S A. 1989; 86: 1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Grace MS,, Chiba A,, Menaker M. Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis Neurosci. 1999; 16: 909–918. [DOI] [PubMed] [Google Scholar]