Abstract
Purpose
Intrinsically photosensitive retinal ganglion cells (ipRGCs) mediate nonimage-forming visual functions such as pupillary constriction and circadian photoentrainment. Optimizing daytime nonimage-forming photostimulation has health benefits. We aimed to enhance ipRGC excitation using flickering instead of steady light.
Methods
Human subjects were tested with a three-dimensional matrix of flickering 463-nm stimuli: three photon counts (13.7, 14.7 and 15.7 log photons cm−2), three duty cycles (12%, 47%, and 93%) and seven flicker frequencies (0.1, 0.25, 0.5, 1, 2, 4, and 7 Hz). Steady-state pupil constrictions were measured.
Results
Among stimuli containing 13.7 log photons cm−2, the one flickering at 2 Hz with a 12% duty cycle evoked the greatest pupil constriction of 48% ± 4%, 71% greater than that evoked by an equal-intensity (12.3 log photons cm−2 s−1) continuous light. This frequency and duty cycle were also best for 14.7 log photons cm−2 stimuli, inducing a 58% ± 4% constriction which was 38% more than that caused by an equal-intensity (13.3 log photons cm−2 s−1) constant light. For 15.7 log photons cm−2 stimuli, the 1-Hz, 47% duty cycle flicker was optimal although it evoked the same constriction as the best 14.7 log photons cm−2 flicker.
Conclusions
Pupillary constriction depends on flicker frequency and duty cycle besides intensity. Among the stimuli tested, the one with the lowest photon count inducing a maximal response is 13.3 log photons cm−2 s−1 flickering at 2 Hz with 12% duty cycle. Our data could guide the design of healthier architectural lighting and better phototherapy devices for treating seasonal affective disorder and jet lag.
Keywords: melanopsin, pupillary light reflex, nonimage-forming vision, phototherapy
We have identified flickering lights that evoke pupillary light reflexes more strongly than steady lights. Our findings could inform the design of healthier architectural lighting, and better phototherapy devices for seasonal affective disorder, nonseasonal depression and jet lag.
The eye mediates both image-forming and nonimage-forming (NIF) visual functions. Whereas image-forming vision enables appreciation of spatial details, NIF vision entails largely subconscious photoresponses including the pupillary light reflex (PLR), circadian photoentrainment, and neuroendocrine regulation. Nonimage-forming photoreception is mediated by not only rod and cone photoreceptors, but also intrinsically photosensitive retinal ganglion cells (ipRGCs), which contain the photopigment melanopsin.1–3 Nonimage-forming vision profoundly influences well-being. For example, daytime NIF photostimulation enhances alertness,4 improves cognitive performance,5 and positively influences mood,6 whereas inadequate or mistimed NIF stimulation can cause sleep disturbances, depression, cognitive impairment, and certain forms of cancer.7
Considering the health impacts of NIF photostimulation, it is beneficial to identify lighting conditions favorable for NIF vision. While extensive research has been done to demonstrate that NIF vision is most sensitive to blue wavelengths,8–15 far less effort has been put into optimizing the temporal distribution of light. Recent reports showed that intermittent light evoked greater NIF responses than continuous light.16–18 Here, we aimed to further enhance NIF responses by finding intermittent stimuli with optimal combinations of intensity, flicker frequency, and duty cycle. We studied the PLR, for two reasons. First, it can be measured quickly, facilitating the testing of many stimulus combinations. Second, the amplitude and time course of the PLR parallel those of ipRGC photoresponses,19 suggesting this behavior can serve as a readout of ipRGC activity so that a stimulus inducing a robust PLR may be inferred to excite ipRGCs potently. Because all NIF responses are driven predominantly by ipRGCs,9,10,20–22 a stimulus that strongly excites ipRGCs is likely effective for all NIF responses, and there is indeed a correlation between the PLR and other NIF photoresponses in humans.23–25 Thus, the findings from this study can probably be extrapolated to other aspects of NIF vision. A possible clinical application is the development of more efficient therapeutic lights for treating depression (both seasonal and nonseasonal), jet lag, and other conditions arising from improper NIF photostimulation.26,27
Materials and Methods
Pupillometry
All procedures were approved by the institutional review board at the University of Michigan, and adhered to tenets of the Declaration of Helsinki. Four females aged 19 to 22 years and five males aged 19 to 37 years served as subjects, after providing informed consent. Five subjects were tested in the experiments shown in Figures 1A and 3 through 5. The other four contributed to the experiment shown in Figure 1C, and one of these generated the data shown in Figure 6. All had normal color vision according to the Ishihara test. The subjects' wake times ranged from 6:00 to 8:30 AM and sleep onset times from 10:00 PM to 12:30 AM. All experiments were performed between 1:30 and 6:30 PM when PLR photosensitivity exhibits insignificant circadian variation.28,29
Figure 1.
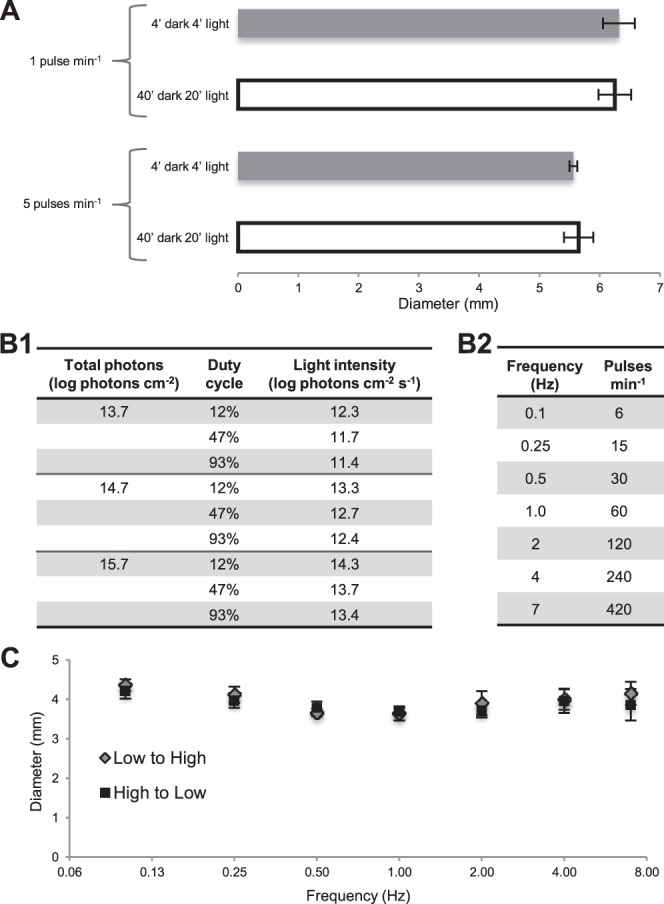
Control experiments and the stimulus matrix. (A) Flickering lights presented for 4 minutes, after 4 minutes of dark adaptation evoked the same steady-state pupil responses as when they were presented for 20 minutes, following 40 minutes of dark adaptation. All stimuli had 12.3 log photons cm−2 s−1 intensity and a 12% duty cycle. Pupil diameters were averaged over the final minute of each trial. Five subjects were tested, with each person contributing two trials to each of the four conditions. (B) This study tested 63 flickering lights varying in 3 parameters: three total photon counts ([1], left column); three duty cycles ([1], middle column); and seven flicker frequencies (2). To maintain a fixed total photon count, intensity was adjusted according to duty cycle ([1], right column). (C) Responses to the seven flicker frequencies were not influenced by the order of presentation. Light pulses of 12.3 log photons cm−2 s−1 with a 12% duty cycle were presented either from the lowest to the highest frequency or from the highest to the lowest, and yielded statistically indistinguishable (P > 0.05) response magnitudes at all frequencies. Four subjects participated in this control, and each was tested with both presentation orders twice.
Figure 3.
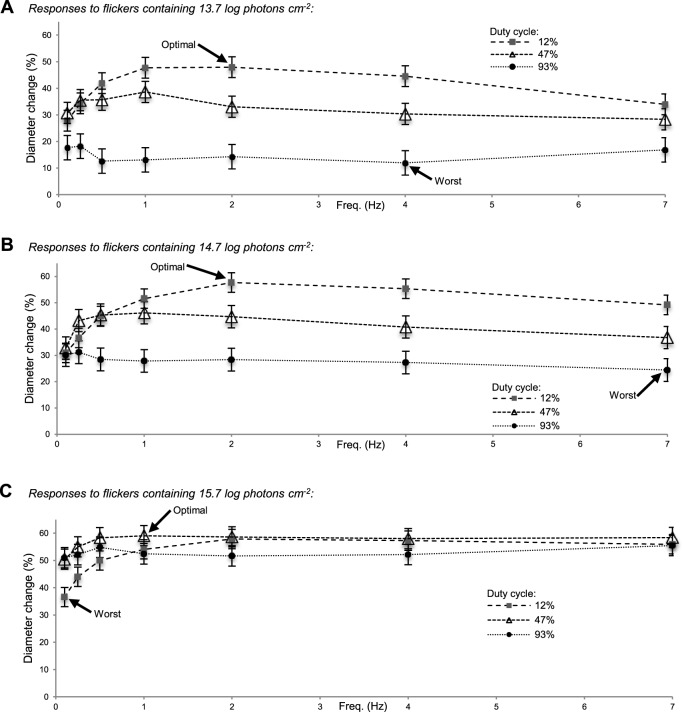
Responses to the 63 flickering stimuli. Averaged final-minute responses to the flickers containing 13.7 log photons cm−2 (A), the ones containing 14.7 log photons cm−2 (B), and those containing 15.7 log photons cm−2 (C), expressed as percent reduction in diameter. Five subjects were tested, with each contributing two trials to all 63 conditions.
Figure 6.
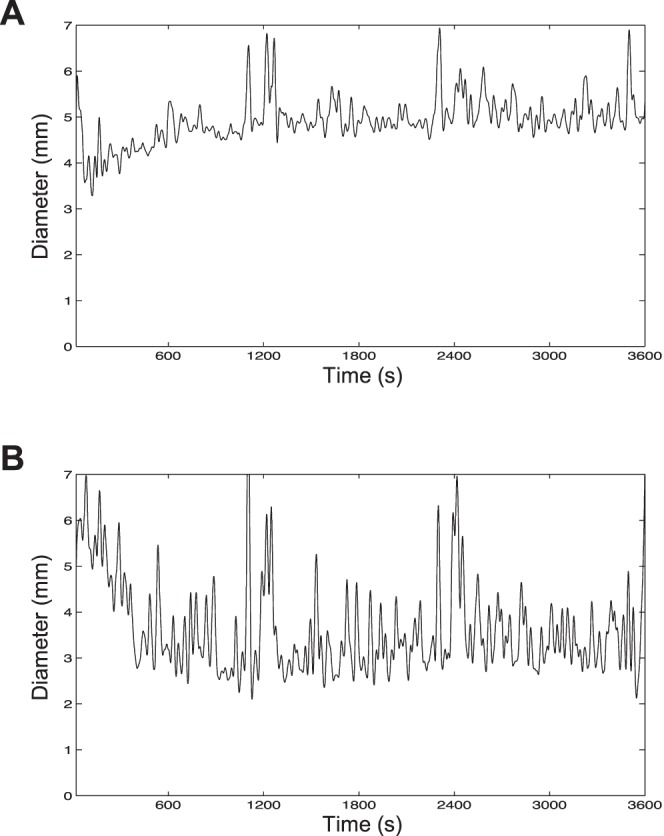
Responses to prolonged photostimulation. After 60 minutes of dark adaptation, a subject was exposed to 60 minutes of either a 12.3 log photons cm−2 s−1 constant light (A) or a 13.3 log photons cm−2 s−1 stimulus flickering at 2 Hz with a 12% duty cycle (B). In both cases, a total of 15.9 log photons cm−2 was delivered per trial. Each trace was generated by averaging three trials, and was filtered using a 4-pole low-pass Butterworth filter with a 0.03-Hz cutoff frequency.
Pupillometry was performed in a darkroom using an infrared pupillometer (A-2000; NeurOptics, Irvine, CA, USA). This instrument's 463-nm blue LED light source was presented to either just the right eye (Figs. 1–5) or both eyes (Fig. 6) at a distance of 6.5 cm, and the left eye's PLR was imaged at 30 Hz; 463 nm is near the optimal wavelength for stimulating the human photoentrainment pathway.9,10 This LED light was rectangular and subtended the subject's visual field 27° vertically and 34° horizontally.
Figure 2.
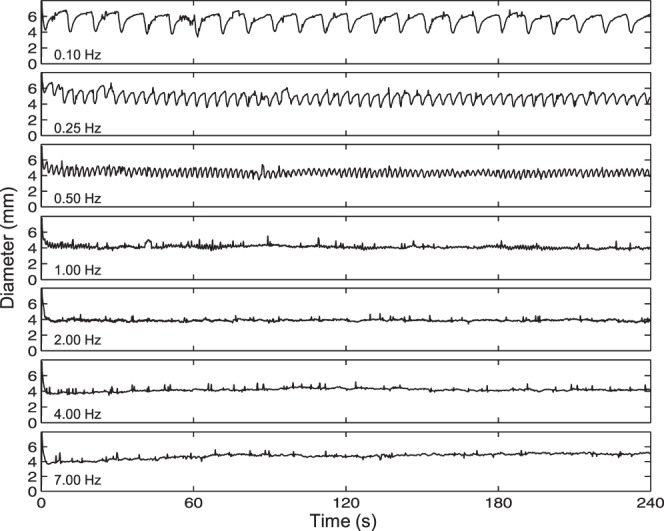
Example recordings. One subject's responses to the seven-frequency family of flickering lights with a 12% duty cycle and a photon count of 13.7 log photons cm−2. All responses were filtered using a 4-pole, low-pass Butterworth filter with a cutoff frequency of 3 Hz.
Figure 4.
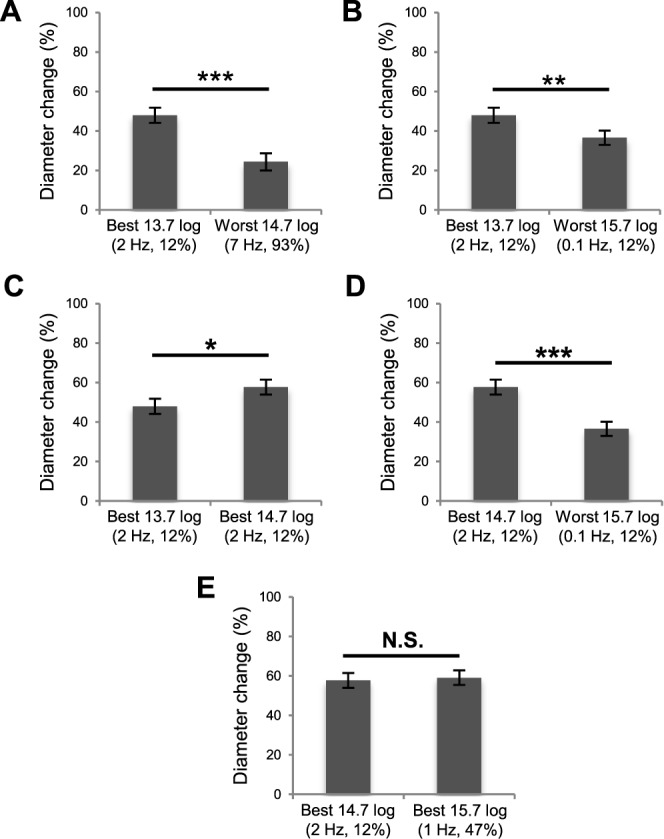
Statistical comparisons across different photon counts. (A) The greatest response evoked by a 13.7 log photons cm−2 flicker was larger than the smallest response induced by a 14.7 log photons cm−2 flicker. (B) The best 13.7 log response was larger than the weakest response evoked by a 15.7 log photons cm−2 stimulus. (C) The best 13.7 log response was smaller than the best 14.7 log response. (D) The best 14.7 log response was larger than the weakest 15.7 log response. (E) The best 14.7 log response was not significantly different from the best 15.7 log response (P = 0.7156). * P < 0.05. ** P < 0.01. *** P < 0.001.
Figure 5.
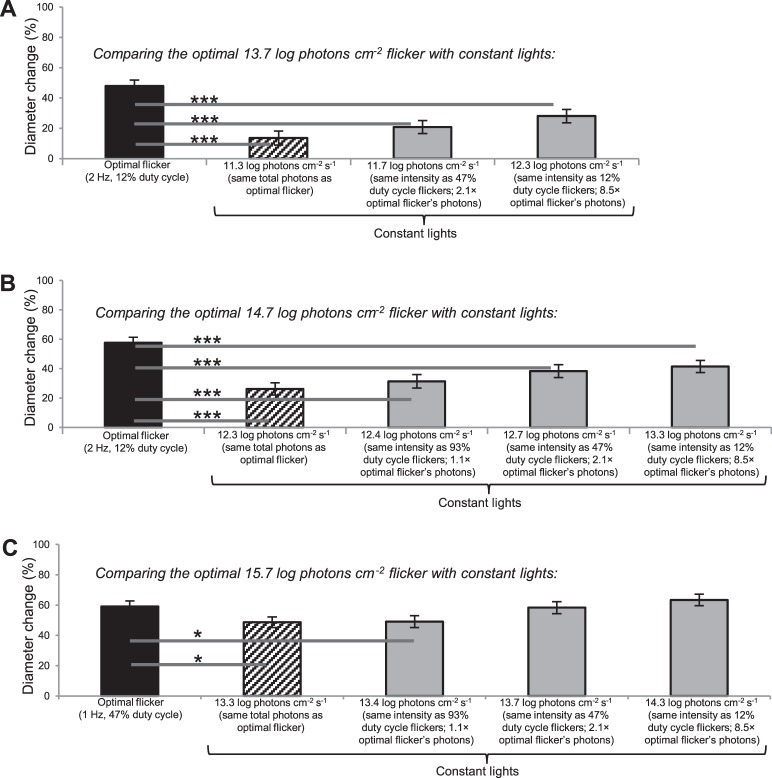
Comparisons with responses to constant lights. The optimal flicker responses shown in Fig. 3 are replotted here (black columns) and compared with responses to constant lights that have either the same photon counts as the optimal flickers (hashed columns) or the same intensities as the various flicker duty cycles (gray columns). * P < 0.05. *** P < 0.001. Five subjects participated, each contributing two trials to all 14 conditions.
As noted earlier, one potential application of this study is to inform the design of better phototherapeutic devices. Phototherapy typically entails viewing a light for 30 minutes to 2 hours,30 but considering the large number of stimuli to be tested (see below), presenting each for such duration would be impractical. Thus, we started with a control experiment to ascertain whether flicker-induced PLRs would reach steady state within several minutes, and whether several minutes of prior dark adaptation would suffice. Two protocols were compared: 4-minute dark adaptation followed by 4-minute flickering light, versus 40-minute dark adaptation followed by 20 minutes of the same stimulus. We tested two frequencies, 1 and 5 pulses min−1, both with a 12% duty cycle (i.e., the stimulus was lit 12% of the time per flicker cycle), and measured the PLR by averaging pupil diameter over the final minute of stimulation. These protocols yielded similar (P > 0.05) final-minute PLRs for both frequencies (Fig. 1A), demonstrating the shorter protocol accurately assessed steady-state responses.
Using the 4-minute dark/4-minute light protocol, we tested a three-dimensional matrix of 63 flickering stimuli: 3 total photon counts × 3 duty cycles × 7 flicker frequencies (Fig. 1B, Supplementary Fig. S1). Each subject was tested by all 63 stimuli, with every stimulus tested twice on 2 separate days. Each person participated in one 56-minute session per day in which 7 flicker frequencies (Fig. 1B2) of the same duty cycle and photon count were presented, and the person was always tested at about the same time of day. To determine whether the order of presenting the seven frequencies might influence response amplitude, they were presented in either increasing or decreasing order in another control experiment. They yielded nearly identical results (Fig. 1C), indicating no ordering effect. For consistency, we tested the 7 frequencies in increasing order in all experiments.
Data Analysis and Statistics
In Figures 3 through 5, results are expressed as the mean percent change in pupil diameter during the final minute of the 4-minute stimulus presentation:
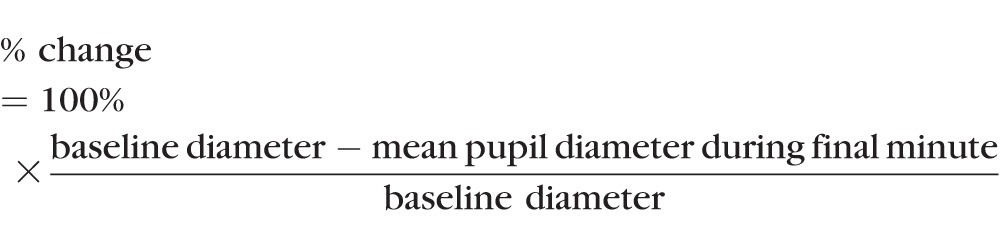 |
In this equation, baseline diameter refers to the pupil diameter under fully dark-adapted conditions, which presumably allow spiking activity of ipRGCs to completely recover from prior photostimulation. The fully dark-adapted diameter was used because we wanted the percent-change calculation to reflect, as well as possible, the absolute firing rate of ipRGCs. Because the 4-minute prestimulus dark adaptation was insufficient for photoreceptors to fully dark adapt, each subject's baseline pupil diameter was measured in a separate experiment where pupil diameter was measured after 1-hour dark adaptation. Every subject was measured on 4 separate days and the measurements agreed closely (Supplementary Fig. S2), indicating the baseline pupil diameter was remarkably constant. The average of the four measurements was used in all calculations based on that subject's data.
In the experiments shown in Figures 3 through 5, statistical comparisons employed a linear mixed model31,32 that accounted for repeated measures within subjects (see Supplementary Material). Elsewhere, the paired, two-tailed Student's t-test was used. Differences were considered significant if P < 0.05. All error estimates are SEM unless stated otherwise.
Electrophysiological Recording From Mouse ipRGCs
All procedures in this experiment were approved by the University Committee on Use and Care of Animals, adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were described in detail previously.33 Briefly, retinas were isolated from dark-adapted opn4Cre/+;GFP, mice whose ipRGCs were labeled with green fluorescent protein (GFP). Retinas were superfused by 32°C Ames' medium. GFP-labeled ipRGCs were visualized using a multiphoton laser and whole-cell-recorded using a K+-based intracellular solution. Cells with dendrites stratifying exclusively in the OFF sublamina of the inner plexiform layer were identified as M1 cells, whereas those with sparse, ON-stratifying dendrites were M2. The stimuli were full-field lights produced by the blue channel (peak emission ∼440 nm) of an OLED microdisplay.
Results
Pupillary Responses to Flickering Stimuli
Figure 2 shows one subject's single-trial responses to the 7-frequency series with 13.7 log photons cm−2 and a 12% duty cycle. The response to the 0.1-Hz flicker clearly tracked the individual flashes, with pupil diameter dropping to ∼4 mm at the peak of each flash response and relaxing to ∼6 mm just before the next pulse. During the steady state of the response to the 0.25-Hz flicker, minimum pupil diameter was again ∼4 mm, but postpulse dilation reached only ∼5.5 mm. The 0.5-Hz response still showed tracking. During the steady state, peak constriction again reduced pupil diameter to ∼4 mm, but peak dilation was even less than for the 0.25-Hz response, under ∼5 mm. The 1-Hz response was remarkably flat with diameter staying around 4 mm, although small oscillations suggesting pulse tracking were seen intermittently. The 2-Hz response was also very stable with pupil diameter averaging just under 4 mm, but tracking was absent. For the 4-Hz response, pupil diameter dipped below 4 mm during the first half minute and then relaxed slightly over time, stabilizing at just over 4 mm. In the 7-Hz response, pupil diameter also dropped slightly below 4 mm early on, but then showed a more pronounced dilation, with steady-state diameter at nearly 5 mm. Thus, among these 7 stimuli, 2 Hz was optimal because it caused the greatest steady-state constriction.
Figure 3 summarizes the results from all subjects for all 63 flickering stimuli, showing averaged final-minute percent reductions in pupil diameter. For the 13.7 log photons cm−2 stimuli (Fig. 3A), the 2-Hz flicker with 12% duty cycle induced the greatest diameter change (48% ± 4%) while the 4-Hz, 93% duty cycle flicker reduced pupil diameter the least (12% ± 5%). All the flickers containing 14.7 log photons cm−2 (Fig. 3B) induced greater pupil constrictions than their 13.7 log photons cm−2 counterparts, with the 2-Hz, 12% duty cycle stimulus being optimal (58% ± 4% diameter change) and the 7-Hz, 93% duty cycle one being the weakest (24% ± 4% diameter change). The 15.7 log photons cm−2 flickers induced even larger responses (Fig. 3C). For this photon count, the 1-Hz, 47% duty cycle flicker was the most potent (59% ± 4% diameter change), although several other stimuli were almost as effective, producing remarkably flat response versus frequency curves suggestive of response saturation. The least effective 15.7 log photons cm−2 flicker (0.1 Hz, 12% duty cycle) evoked a 37% ± 4% diameter change.
In summary, responses to stimuli with identical photon counts but different frequencies and/or duty cycles could vary in amplitude up to fourfold. In Figure 4, we compared responses to stimuli with different photon counts. The response to the best 13.7 log flicker was nearly twice the response to the worst 14.7 log flicker (Fig. 4A). Remarkably, this 13.7 log response was also significantly greater than the response to the worst 15.7 log flicker despite containing 100-fold fewer photons (Fig. 4B), although it was smaller than the best 14.7 log response (Fig. 4C). The best 14.7 log response was 57% larger than the worst 15.7 log response (Fig. 4D), but was statistically comparable to the best 15.7 log response (Fig. 4E).
Comparisons With Pupillary Responses to Constant Light
We next compared the most effective flickers (labeled “optimal” in Fig. 3) with constant lights that had either the same photon counts as these flickers, or the same intensities as flickers with various duty cycles. Both the optimal 13.7 log and 14.7 log photons cm−2 flickers induced significantly greater responses than all the constant lights compared, even though these constant lights had up to 8.5-fold more photons (Figs. 5A, 5B). While the optimal 15.7 log photons cm−2 flicker was significantly more potent than a constant light with an equal number of photons and another with ∼10% more photons, it evoked statistically similar response amplitudes as the two highest-intensity constant lights (Fig. 5C).
Prolonged Photostimulation Following Prolonged Dark Adaptation
As mentioned in the Introduction, a potential application of this study is phototherapy. In all the experiments discussed so far, each stimulus was presented to just one eye for 4 minutes, after 4 minutes of dark adaptation. However, phototherapy sessions typically last for much longer and are often performed shortly after waking in the morning from a dark-adapted state, and light is delivered to both eyes. In the final PLR experiment, we simulated a phototherapy session by illuminating both eyes, and increasing the duration of both dark adaptation and photostimulation to 1 hour. We compared the best 14.7 log flicker (13.3 log photons cm−2 s−1, 2-Hz, 12% duty cycle) with an equal–photon count constant light (12.3 log photons cm−2 s−1). Recordings averaged from three trials by one subject are shown in Figure 6. The first several minutes of these recordings should be disregarded because presenting the lights after prolonged dark adaptation caused them to appear uncomfortably bright, resulting in squinting and frequent eye blinks. After the initial discomfort, however, the eyes remained wide open and blinked minimally. Both stimuli induced remarkably stable responses beyond the initial ∼10 minutes. The constant light induced a steady-state pupil diameter of ∼5 mm (Fig. 6A), whereas the response to the flicker stabilized at ∼3 mm (Fig. 6B). The two responses were significantly different (P < 0.001) during the final 10 minutes, inducing 22% ± 4% versus 48% ± 7% (SD) diameter reduction.
Both M1- and M2-Type Mouse ipRGCs Prefer Flickering Light
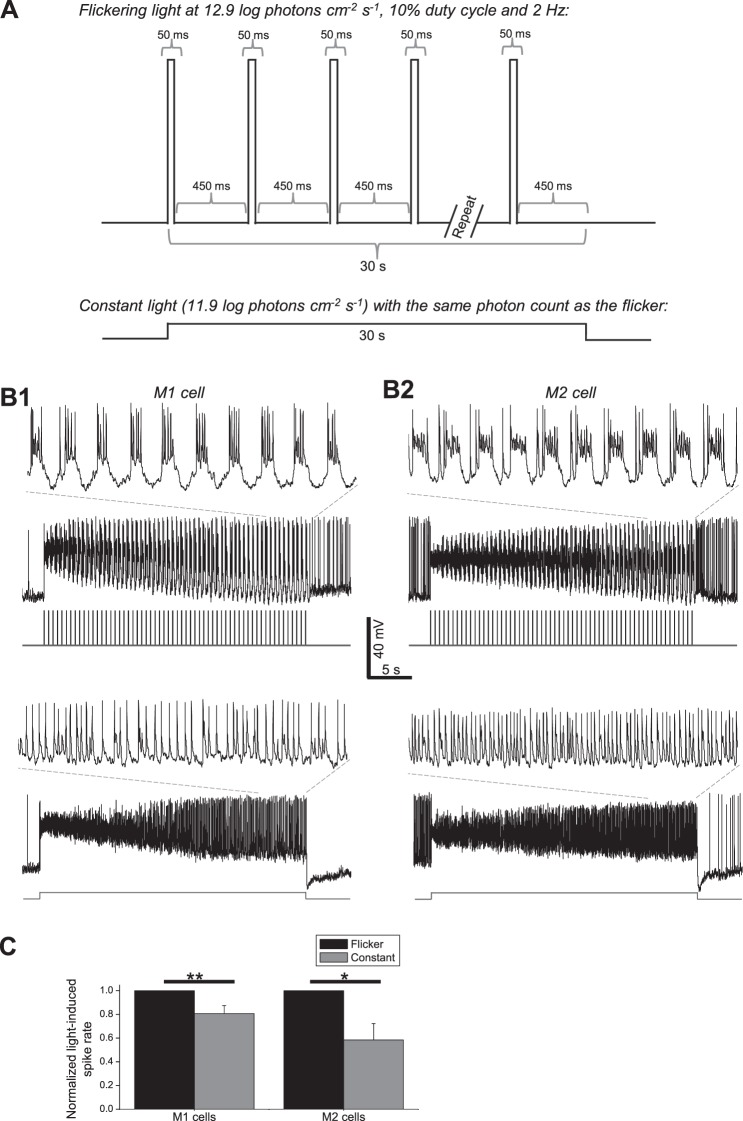
The above human PLR data may be relevant to other ipRGC-mediated NIF visual responses. Although rodents possess five functionally diverse types of ipRGCs (M1–M5),33–38 only two types are known to exist in primates, one ON-stratifying and the other OFF-stratifying.39–44 They showed similar responses to a 2-Hz flicker and to longer light steps, and both innervate the olivary pretectal nucleus which drives the PLR.39,45 But it is unknown whether both types prefer flickering light, and whether other NIF visual nuclei are likewise innervated by both.43 If the two cell types have different preferences for flickering versus constant light and if these other nuclei receive input from just one type, then findings based on the PLR might not be applicable to all other NIF visual responses. To provide a preliminary answer to this question, we whole-cell recorded from mouse M1 and M2 cells, which are thought to be homologous to the OFF- and ON-stratifying primate ipRGCs, respectively.41,42 A flickering light and an equal–photon count constant light were presented, and the former evoked significantly larger spiking responses in both ipRGC types (Fig. 7). Assuming that M1 and M2 cells are true homologues of the OFF- and ON-stratifying ipRGCs and that these are the only types of primate ipRGCs, this preliminary result suggests that flickering light is likely more effective than steady light for inducing not only the PLR but also other NIF photoresponses in humans. Indeed, intermittent light phase-shifts human circadian rhythms more efficiently than continuous light.46–48
Figure 7.
Responses of mouse ipRGCs to flickering versus constant lights. Whole-cell current-clamp recordings were made from 11 mouse ipRGCs (seven M1 cells and four M2 cells) under conditions that preserved synaptic input. (A) Two stimuli were presented to each cell, in random order: a 12.9 log photons cm−2 s−1 light flickering at 2 Hz with a 10% duty cycle, and a steady 11.9 log photons cm−2 s−1 light with the same photon count as the flicker. All stimuli were full-field 440-nm light. (B) Representative responses from an M1 cell (1) and M2 cell (2). The insets show magnified views of the final 5 seconds of the responses. (C) Averaged data from all cells, illustrating that both ipRGC types displayed larger steady-state spiking responses to the flicker. All mouse ipRGCs spike spontaneously in the dark.33 Thus, to quantify the light-induced spiking increase, spike rate was averaged during the 5 seconds before light onset to calculate the spontaneous spike rate and during the last 5 seconds of photostimulation to calculate the steady-state spike rate, and the former rate was subtracted from the latter rate. In the histogram, each cell's flicker-induced spike rate increase was normalized to 1. * P < 0.05. ** P < 0.01.
Discussion
Flicker Responses of ipRGCs and the Nonimage-Forming Visual System
Intrinsically photosensitive retinal ganglion cells respond to light both directly using melanopsin and indirectly through input from rods and cones,39 and the PLR similarly consists of melanopsin- and rod/cone-driven components.19,49 The different intensity thresholds of rod input, cone input and melanopsin confer ipRGCs with a dynamic range spanning at least 9 log units.39 Rod/cone input and melanopsin also play nonredundant roles in the temporal domain: ipRGCs and the PLR require rod/cone input to track fast irradiance changes but use melanopsin for prolonged integration.17,50 Melanopsin's response to a brief flash starts slowly and terminates even slower, requiring tens of seconds to return to the baseline.51 This slow decay provides a window of temporal summation—that is, a second pulse presented during this time induces a response superimposed on the first response, so that the second response peaks higher than the first. The degree of such paired-pulse facilitation may be expected to increase as the interpulse interval decreases, but another phenomenon must also be taken into account, namely, adaptation. All photoreceptors exhibit light adaptation, meaning they become less sensitive during illumination. After lights off, photoreceptors undergo dark adaptation to regain photosensitivity over time.52,53 Thus, for a flickering stimulus, a decrease in interpulse interval tends to facilitate temporal summation but reduce the extent of dark adaptation. The flicker frequency that strikes the best balance between these opposing effects presumably corresponds to the optimal frequency. Here, we have found this frequency to be around 1 to 2 Hz for most intensities and duty cycles. Two previous studies also showed that sinusoidal waves evoked PLRs most effectively within this frequency range54,55; however, different duty cycles were not explored, and the intensities tested were probably insufficient to activate melanopsin significantly.
Adaptation may also explain why flickering lights induce stronger PLRs than steady lights. During prolonged illumination, ipRGCs lose sensitivity over time and drive a PLR whose amplitude decreases progressively. In comparison, when the stimulus contains a train of short pulses, each pulse desensitizes the photoreceptors only briefly, after which they are allowed to partially recover sensitivity through dark adaptation. Gooley and colleagues17 reported such steady-versus-flicker difference for the PLR using low-intensity, cone-selective green light. In the current study, we observed a similar difference using blue light that effectively stimulated melanopsin in addition to the classical photoreceptors.19,56–58 Differences in the duration of light versus dark adaptation could also partly explain our observation that, for nonsaturating intensities, the 12% duty cycle usually evoked greater PLRs than the longer duty cycles (Figs. 3A, 3B). The duration of each pulse in the 12% duty cycle flicker was about 1/4 and 1/8 of that for the 47% and 93% flickers, respectively, thereby desensitizing photoreceptors the least while allowing the most dark adaptation between pulses.
Potential Strategies for Further Optimization
Our most effective 14.7 log photons cm−2 stimulus reduced steady-state pupil diameter by ∼60% and increasing light intensity 10-fold did not cause greater constriction, suggesting response saturation. This diameter could reflect the equilibrium point where any increase in ipRGC spiking would be negated by the resultant decrease in the amount of light entering the eye. However, our stimuli were generated using a relatively small light source and illuminated only one eye. Stimulating both eyes using wider-field lights can reduce pupil diameter as much as ∼75%,59 more than the ∼60% achieved here. Thus, an obvious way to enhance the efficacy of our stimuli is to present them to both eyes through a Ganzfeld system so that the entire visual field is illuminated.60
Additional improvement in efficacy could be achieved by using flickers with shorter duty cycles than the ones we tested. Trains of 2-ms flashes have been shown to phase-shift circadian rhythms far more than prolonged lights with comparable photon counts.48,61,62 If presented as a 2-Hz flicker, these 2-ms pulses would correspond to a duty cycle of 0.4%. Due to hardware limitation, we were unable to test such a short duty cycle.
Finally, the bistable properties of melanopsin could be exploited to further enhance NIF responses to intermittent light. Melanopsin exists in two photosensitive states with different spectral sensitivities.63 In the excitable state, melanopsin is most sensitive to short-wavelength blue light and photon absorption activates the photopigment. Once excited, melanopsin becomes more sensitive to longer wavelengths, and the absorption of a second photon reverses melanopsin to its excitable state. Under certain conditions, NIF photoresponses can be enhanced when the excitation light is preceded by a long-wavelength light, presumably because the latter increases the number of melanopsin molecules ready for photoexcitation.64,65 It would be of interest to test whether pre-exposure to red light enhances the effectiveness of intermittent light.
Potential Applications
As explained in the Results section, the mouse ipRGC recordings suggest that our human PLR data are likely relevant to other NIF visual functions. Thus, these data could inform the design of healthier architectural lighting technologies that promote daytime NIF vision. Another possible application is phototherapy of seasonal affective disorder, non-seasonal depression, and jet lag. All commercially available phototherapy devices emit constant light. Even with intense light, phototherapy typically requires up to 2 hours per session.30 The discovery that intermittent light induces larger NIF responses than does steady light suggests that intermittent light could enhance the efficiency and/or efficacy of phototherapy. We have determined the best combination of flicker frequency and duty cycle at three light levels. These optimal stimuli induced PLRs with minimal time-dependent decay (Figs. 2, 6), suggesting very sustained, nearly nonadapting spiking in ipRGCs. Assuming the efficacy of phototherapy is proportional to the total number of ipRGC spikes generated per therapy session, such flickers could shorten each session and/or reduce the light intensity required. In this study, the lowest photon count light causing maximal steady-state pupil constriction was the 2-Hz, 13.3 log photons cm−2 s−1 flicker with 12% duty cycle. This has the same photon count as a 12.3 log photons cm−2 s−1 constant light which is roughly 3 to 4 log units less intense than the 10,000 lux light boxes commonly used in phototherapy. Prolonged exposure to intense light could damage retinal photoreceptors.66 By reducing intensity, flickering lights could make phototherapy safer.
Supplementary Material
Acknowledgments
We thank Kerby Shedden at the University of Michigan Center for Statistical Consultation and Research for assistance with statistical analysis; and Adetayo Abdulrazak, Christopher Breuler, Sania Cheema, Benjamin Li, Nenita Maganti, Karun Nair, and Sureel Shah for help with data collection.
Supported by NIH Grants EY013934 (GVV) and EY007003 (Vision Research Core Grant).
Disclosure: G.V. Vartanian, None; X. Zhao, None; K.Y. Wong, None
References
- 1. Panda S,, Provencio I,, Tu DC,, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003; 301: 525–527. [DOI] [PubMed] [Google Scholar]
- 2. Hattar S,, Lucas RJ,, Mrosovsky N,, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003; 424: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berson DM,, Dunn FA,, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002; 295: 1070–1073. [DOI] [PubMed] [Google Scholar]
- 4. Cajochen C,, Munch M,, Kobialka S,, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005; 90: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 5. Vandewalle G,, Collignon O,, Hull JT,, et al. Blue light stimulates cognitive brain activity in visually blind individuals. J Cogn Neurosci. 2013; 25: 2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stephenson KM,, Schroder CM,, Bertschy G,, Bourgin P. Complex interaction of circadian and non-circadian effects of light on mood: shedding new light on an old story. Sleep Med Rev. 2012; 16: 445–454. [DOI] [PubMed] [Google Scholar]
- 7. Turner PL,, Van Someren EJ,, Mainster MA. The role of environmental light in sleep and health: effects of ocular aging and cataract surgery. Sleep Med Rev. 2010; 14: 269–280. [DOI] [PubMed] [Google Scholar]
- 8. Brainard GC,, Hanifin JP,, Warfield B,, et al. Short-wavelength enrichment of polychromatic light enhances human melatonin suppression potency. J Pineal Res. 2015; 58: 352–361. [DOI] [PubMed] [Google Scholar]
- 9. Brainard GC,, Hanifin JP,, Greeson JM,, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001; 21: 6405–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thapan K,, Arendt J,, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod non-cone photoreceptor system in humans. J Physiol. 2001; 535: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glickman G,, Byrne B,, Pineda C,, Hauck WW,, Brainard GC. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol Psychiatry. 2006; 59: 502–507. [DOI] [PubMed] [Google Scholar]
- 12. Figueiro MG,, Bierman A,, Rea MS. Retinal mechanisms determine the subadditive response to polychromatic light by the human circadian system. Neurosci Lett. 2008; 438: 242–245. [DOI] [PubMed] [Google Scholar]
- 13. Revell VL,, Barrett DC,, Schlangen LJ,, Skene DJ. Predicting human nocturnal nonvisual responses to monochromatic and polychromatic light with a melanopsin photosensitivity function. Chronobiol Int. 2010; 27: 1762–1777. [DOI] [PubMed] [Google Scholar]
- 14. Viola AU,, James LM,, Schlangen LJ,, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008; 34: 297–306. [DOI] [PubMed] [Google Scholar]
- 15. Meesters Y,, Dekker V,, Schlangen LJ,, Bos EH,, Ruiter MJ. Low-intensity blue-enriched white light (750 lux) and standard bright light (10000 lux) are equally effective in treating SAD. A randomized controlled study. BMC Psychiatry. 2011; 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lall GS,, Revell VL,, Momiji H,, et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010; 66: 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gooley JJ,, Ho Mien I,, St Hilaire MA,, et al. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. 2012; 32: 14242–14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ho Mien I,, Chua EC,, Lau P,, et al. Effects of exposure to intermittent versus continuous red light on human circadian rhythms, melatonin suppression, and pupillary constriction. PLoS One. 2014; 9: e96532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gamlin PD,, McDougal DH,, Pokorny J,, Smith VC,, Yau KW,, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007; 47: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guler AD,, Ecker JL,, Lall GS,, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008; 453: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goz D,, Studholme K,, Lappi DA,, Rollag MD,, Provencio I,, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS One. 2008; 3: e3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hatori M,, Le H,, Vollmers C,, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One. 2008; 3: e2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Figueiro MG,, Bullough JD,, Parsons RH,, Rea MS. Preliminary evidence for a change in spectral sensitivity of the circadian system at night. J Circadian Rhythms. 2005; 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roecklein K,, Wong P,, Ernecoff N,, et al. The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res. 2013; 210: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee SI,, Hida A,, Kitamura S,, Mishima K,, Higuchi S. Association between the melanopsin gene polymorphism OPN4*Ile394Thr and sleep/wake timing in Japanese university students. J Physiol Anthropol. 2014; 33: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenthal NE,, Sack DA,, Gillin JC,, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984; 41: 72–80. [DOI] [PubMed] [Google Scholar]
- 27. Tuunainen A,, Kripke DF,, Endo T. Light therapy for non-seasonal depression. Cochrane Database Syst Rev. 2004; CD004050. [DOI] [PMC free article] [PubMed]
- 28. Munch M,, Leon L,, Crippa SV,, Kawasaki A. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Invest Ophthalmol Vis Sci. 2012; 53: 4546–4555. [DOI] [PubMed] [Google Scholar]
- 29. Zele AJ,, Feigl B,, Smith SS,, Markwell EL. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One. 2011; 6: e17860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Golden RN,, Gaynes BN,, Ekstrom RD,, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005; 162: 656–662. [DOI] [PubMed] [Google Scholar]
- 31. West BT,, Welch KB,, Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. London: Chapman & Hall/CRC; 2006. [Google Scholar]
- 32. Gelman A,, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 33. Zhao X,, Stafford BK,, Godin AL,, King WM,, Wong KY. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J Physiol. 2014; 592: 1619–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ecker JL,, Dumitrescu ON,, Wong KY,, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010; 67: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen SK,, Badea TC,, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011; 476: 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu C,, Hill DD,, Wong KY. Intrinsic physiological properties of the five types of mouse ganglion-cell photoreceptors. J Neurophysiol. 2013; 109: 1876–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmidt TM,, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009; 29: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmidt TM,, Kofuji P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J Neurosci. 2010; 30: 16262–16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dacey DM,, Liao HW,, Peterson BB,, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005; 433: 749–754. [DOI] [PubMed] [Google Scholar]
- 40. Jusuf PR,, Lee SC,, Hannibal J,, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci. 2007; 26: 2906–2921. [DOI] [PubMed] [Google Scholar]
- 41. Grunert U,, Jusuf PR,, Lee SC,, Nguyen DT. Bipolar input to melanopsin containing ganglion cells in primate retina. Vis Neurosci. 2011; 28: 39–50. [DOI] [PubMed] [Google Scholar]
- 42. Neumann S,, Haverkamp S,, Auferkorte ON. Intrinsically photosensitive ganglion cells of the primate retina express distinct combinations of inhibitory neurotransmitter receptors. Neuroscience. 2011; 199: 24–31. [DOI] [PubMed] [Google Scholar]
- 43. Hannibal J,, Kankipati L,, Strang CE,, Peterson BB,, Dacey D,, Gamlin PD. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J Comp Neurol. 2014; 522: 2231–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reifler AN,, Chervenak AP,, Dolikian ME,, et al. The rat retina has five types of ganglion-cell photoreceptors. Exp Eye Res. 2015; 130: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dacey DM,, Peterson BB,, Robinson FR,, Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003; 37: 15–27. [DOI] [PubMed] [Google Scholar]
- 46. Gronfier C,, Wright KP,, Jr,, Kronauer RE,, Jewett ME,, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004; 287: E174–E181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rimmer DW,, Boivin DB,, Shanahan TL,, Kronauer RE,, Duffy JF,, Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol Regul Integr Comp Physiol. 2000; 279: R1574–R1579. [DOI] [PubMed] [Google Scholar]
- 48. Zeitzer JM,, Ruby NF,, Fisicaro RA,, Heller HC. Response of the human circadian system to millisecond flashes of light. PLoS One. 2011; 6: e22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kardon R,, Anderson SC,, Damarjian TG,, Grace EM,, Stone E,, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009; 116: 1564–1573. [DOI] [PubMed] [Google Scholar]
- 50. Wong KY,, Dunn FA,, Graham DM,, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007; 582: 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Do MT,, Kang SH,, Xue T,, et al. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009; 457: 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fain GL,, Matthews HR,, Cornwall MC,, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiological reviews. 2001; 81: 117–151. [DOI] [PubMed] [Google Scholar]
- 53. Wong KY,, Dunn FA,, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005; 48: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 54. Clarke RJ,, Zhang H,, Gamlin PD. Characteristics of the pupillary light reflex in the alert rhesus monkey. J Neurophysiol. 2003; 89: 3179–3189. [DOI] [PubMed] [Google Scholar]
- 55. Varju D. Pupillary reactions to sinusoidal light intensity changes (the effect of relative phase conditions on the medium pupillary enlargement in binocular modulation of the retinal light intensity [in German]. Kybernetik. 1964; 2: 124–127. [DOI] [PubMed] [Google Scholar]
- 56. Gooley JJ,, Rajaratnam SM,, Brainard GC,, Kronauer RE,, Czeisler CA,, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010; 2:31ra33. [DOI] [PMC free article] [PubMed]
- 57. McDougal DH,, Gamlin PD. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010; 50: 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park JC,, Moura AL,, Raza AS,, Rhee DW,, Kardon RH,, Hood DC. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci. 2011; 52: 6624–6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Loewenfeld IE. The Pupil: Anatomy Physiology, and Clinical Applications. 2nd ed. Oxford: Butterworth-Heinemann; 1999. [Google Scholar]
- 60. Lei S,, Goltz HC,, Chandrakumar M,, Wong AM. Full-field chromatic pupillometry for the assessment of the post-illumination pupil response driven by melanopsin-containing retinal ganglion cells. Invest Ophthalmol Vis Sci. 2014; 55: 4496–4503. [DOI] [PubMed] [Google Scholar]
- 61. Vidal L,, Morin LP. Absence of normal photic integration in the circadian visual system: response to millisecond light flashes. J Neurosci. 2007; 27: 3375–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van Den Pol AN,, Cao V,, Heller HC. Circadian system of mice integrates brief light stimuli. Am J Physiol. 1998; 275: R654–R657. [DOI] [PubMed] [Google Scholar]
- 63. Koyanagi M,, Kubokawa K,, Tsukamoto H,, Shichida Y,, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005; 15: 1065–1069. [DOI] [PubMed] [Google Scholar]
- 64. Mure LS,, Cornut PL,, Rieux C,, et al. Melanopsin bistability: a fly's eye technology in the human retina. PLoS One. 2009; 4: e5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mure LS,, Rieux C,, Hattar S,, Cooper HM. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms. 2007; 22: 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hunter JJ,, Morgan JI,, Merigan WH,, Sliney DH,, Sparrow JR,, Williams DR. The susceptibility of the retina to photochemical damage from visible light. Prog Retin Eye Res. 2012; 31: 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.