Summary
Quantitative assessment of immunogen-specific T cell responses may provide a meaningful surrogate marker of functional immunity in patients following haemopoietic stem cell transplantation (HSCT). We developed a flowcytometric assay to quantify antigen-specific T cell immunity to influenza-A and studied the T cell response to influenza vaccination in five children, 3–21 months post-HSCT. All patients showed an increase in influenza-A-specific CD4+ immunity following vaccination while none had a detectable IgG response to the vaccine. This assay proved sufficiently sensitive to evaluate changes in T cell memory in immunocompromised individuals and could be used to better characterize post-HSCT immune reconstitution.
Keywords: BMT, T cells, vaccines, clinical studies, flow cytometry
Impaired immunity to infectious organisms contributes to the poor outcome after haemopoietic stem cell transplantation (HSCT) (Ochs et al, 1995; Chakrabarti et al, 2002; Martelli & Reisner, 2002; Veys et al, 2003). Vaccination has been used to improve immune function but the optimal vaccination strategy has not been identified and little attention has been directed at assessing the T cell response to vaccines (Avigan et al, 2001).
We describe here a sensitive technique for quantifying and characterizing antigen-specific T cells that was used to measure T cell immunity to influenza A in normal adults and children, and influenza vaccination response in paediatric HSCT patients.
Study design and patients
Patients, donors and vaccination
The response to influenza vaccination was studied in five paediatric HSCT patients (Table I) who had received a single, 0.5 ml intramuscular injection of Fluzone-R influenza virus vaccine (Aventis-Pasteur; 2003–2004). Blood samples were collected before and 6 weeks post-vaccination. Peripheral blood mononuclear cells (PBMC) from control healthy adult and paediatric donors were obtained from the Dana–Farber Cancer Institute (DFCI) and Children's Hospital respectively. The Institutional Review Board of the Children's Hospital or DFCI approved all clinical studies.
Table I.
Patient characteristics.
| UPN | Age (years) |
Type of transplant |
Months since transplant |
Immunosuppression | WBC (×109/1) |
Platelets (×109/1) |
Absolute CD4 (×109/1) |
Absolute CD8 (×109/1) |
Mono (% of WBC) |
mDC (×109/1) |
pDC (×109/1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 5 | Auto | 3.3 | No | 3.25 | 200 | 0.077 | 0.045 | 6 | 0.040 | 0.0023 |
| 02 | 8 | MSD | 4 | FK506 | 3.73 | 190 | 0.129 | 0.032 | 8 | 0.061 | 0.0039 |
| 03 | 13 | URD | 14.8 | No | 5.11 | 230 | 0.720 | 0.313 | 6 | 0.047 | 0.0024 |
| 04 | 13 | MSD | 21.4 | FK506 | 6.04 | 163 | 0.736 | 1.361 | 5 | 0.042 | 0.0027 |
| 05 | 14 | MSD | 21.6 | MMF | 5.47 | 348 | 0.732 | 0.748 | 4 | 0.017 | 0.0014 |
WBC, white blood cell count; mono, monocytes; pDC, plasmacytoid dendritic cells (Lin−/HLA-DR+/CD11c−/CD123+); mDC, monocytoid dendritic cells (Lin−/HLA-DR+/CD11c+/CD123−); URD, unrelated donor; auto, autologous donor; MSD, matched sibling donor; FK506, tacrolimus; MMF, mycophenolate mofetil.
Reagents and flow cytometry
Antibodies were from Immunotech/Beckman-Coulter (Miami, FL, USA) and carboxyfluorescein diacetate, succinimidyl ester (CFSE) from Molecular Probes (Eugene, OR, USA). Ficolled PBMC were stained with antibodies and/or tetramers as described (Day et al, 2003). DR4 tetramers were loaded with the HA 306–318 peptide (HA306). Five-colour flow cytometry/sorting was performed using FC500 or Epics Altra cytometers (Beckman-Coulter, Miami, FL, USA).
CFSE assay
Ficolled PBMC were stained with CFSE (0.2 μM) for 5 min at 37°C and plated in 96-well round-bottomed plates at 2 × 105 cells/well in Roswell Park Memorial Institute (RPMI) medium (supplemented 10% v/v human AB serum, l-glutamine and HEPES), together with influenza-A antigen (irradiated lysates of a cell line infected with influenza A Texas 1/77 strain, H3N2 serotype), cytomegalovirus (CMV) antigen (from Microbix, Toronto, Canada) or tetanus toxoid (Calbiochem, San Diego, CA, USA). Cells were incubated at 37°C for 7 d, before being washed and stained with antibodies and/or tetramers as described (Day et al, 2003). Proliferation was detected by loss of CFSE fluorescence (Mannering et al, 2003). Staining with HLA-DR provided a second parameter of activation. Replicate experiments (n = 8) yielded a mean inter-experiment co-efficient of variation of 12.6% for CD8+ and 14.7% for CD4+ proliferation (data not shown). Sorted CFSE-labelled CD4 cells were cultured at a ratio of 10:1 with autologous plastic-adherent monocytes with or without antigen.
Influenza-A enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) for influenza-A IgG and IgM were performed using kits from IBL-America (Minneapolis, MN, USA) according to the manufacturer's instructions.
Statistical analysis
Pre-post-vaccine comparisons were done using a Wilcoxon signed rank test. Statistical significance was set at the α = 0.05 level.
Results and discussion
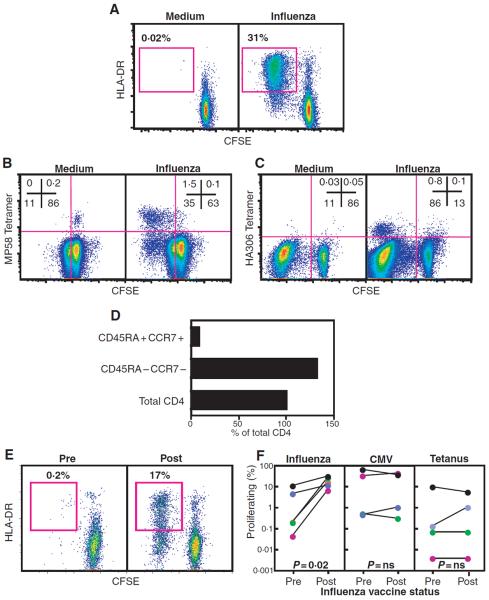
CFSE dim (i.e. proliferating)/activated T cells could be readily detected in PBMC from healthy adult (n = 4) and paediatric (n = 19) donors stimulated with influenza-A antigen (representative donor shown in Fig 1A). Both CD4+ (median 4.3%, range 0–32.5%) and CD8+ (median 2.14%, range 0–31.2%) T cell proliferation was detected in all donors but one, and in children as young as 6 months of age (data not shown), consistent with the high prevalence of influenza-A exposure. There was no change in T cell response with age (Rs = 0.14, P = 0.56), suggesting that the frequencies of T cells that are specific for influenza A are comparable in children and adults. Simultaneous staining with major histocompatibility complex MHC I- (Fig 1B) and MHC II-tetramers (Fig 1C) confirmed that the CFSE-dim/active population contained T cells that were specific for at least two immunodominant CD8+ and CD4+ epitopes, and that protein antigens contained in the viral lysates were processed and presented to epitope-specific T cells. While virtually all tetramer+ cells had a CFSE-dim/activated phenotype after stimulation, they comprised a minority of the total proliferating/activated population (Fig 1B and C), consistent with the fact that these two epitopes represent only a fraction of all epitopes eliciting a T cell response (Danke & Kwok, 2003). Although it is likely that the CFSE-dim/active population also contained non-antigen-specific `by-stander' T cells, the tetramer analysis suggested that the assay sensitively detected influenza-A–specific T cells.
Fig 1.
Evaluating T cell immunity to influenza-A in healthy donors and HSCT patients. (A) CFSE-labelled PBMC from a representative healthy donor cultured in the absence (left) or presence (right) of influenza-A antigen (7 μg/ml) for 7 d. Plots are gated on CD8+ cells, and percentages refer to frequency of proliferating/activated T cells detected by loss of CFSE fluorescence and gain of HLA-DR expression. (B) CFSE-labelled PBMC from an HLA-A*0201 donor cultured as in A and stained with HLA-A*0201-MP58 tetramer. Plots are gated on CD8 and numbers refer to the percentage of CD8+ cells in the quadrants shown. (C) CFSE-labelled PBMC from an HLA-DR*0401 donor cultured in the absence (left) or presence (right) of influenza-A antigen (7 μg/ml) for 7 d. Interleukin 2 (IL2) (5 U/ml) was added on day 3 of culture. Cells were stained with HLA-DR*0401-HA306 tetramer. Plots are gated on CD4 and numbers refer to the percentage of CD4+ cells in the quadrants shown. (D) CD4+ cells from an influenza-A responding healthy donor were sorted based on expression of the markers shown and cultured with autologous monocytes pulsed with influenza-A antigen. Proliferation is expressed as a percentage of the maximum proliferation seen in the total CD4+ population. Proliferation of sorted populations with unpulsed monocytes was <0.1%. (E) CFSE-labelled PBMC from UPN 01 cultured with influenza-A before (left) or after (right) influenza vaccination. Plots are gated on CD4+ lymphocytes. (F) PBMC from patients obtained pre- or post-influenza vaccination were CFSE-labelled and cultured with influenza-A (left), CMV (middle) or tetanus (right) antigen for 7 d. Graphs depict the percentage of CD4+ cells that were CFSE dim and HLA-DR+ following stimulation with antigen (values for UPN 01 are shown as red circles; UPN 02, green; UPN 03, blue; UPN 04, purple; UPN 05, black). Proliferation in control conditions was <0.3% and subtracted from the corresponding value for antigen-specific proliferation.
To address whether the antigen-specific T cell proliferation originated from the memory T cell pool, we separated memory and naïve CD4+ cells prior to antigen-stimulation. Cells responsive to antigen had an antigen-experienced (CD45RA−/CCR7−), but not naïve (CD45RA+/CCR7+), phenotype (Fig 1D), confirming that the assay detected pre-existing influenza-A–specific T cell memory, and that antigen-stimulation did not have a generalized mitogenic effect (Seder & Ahmed, 2003).
We next used the assay to assess Influenza-A–specific T cell immunity before and after influenza vaccination in five paediatric HSCT recipients (representative patient shown in Fig 1E). Prior to vaccination, the CD4 proliferation to influenza-A was a mean of 3.3% (range 0.04–11%) (Fig 1F). Following vaccination, CD4 proliferation increased in all patients (to mean 19.0%, range 6.9–31.8%, P = 0.02). This increase was specific as the proliferation to control antigens (CMV and tetanus) was unchanged (Fig 1F). Influenza-A CD8+ proliferation also increased in three of five patients but was not statistically significant for the group (data not shown), consistent with the limited efficacy of soluble vaccine antigens in inducing CD8+ T cell response (Haining et al, 2004).
All patients had detectable influenza-A−specific IgG levels prior to vaccination, reflecting pre-existing or passively transferred immunity and no significant change in IgG levels was detected following vaccination (P = 0.26; data not shown). IgM levels remained negative in four of five patients after vaccination (data not shown). Only one patient had an IgM response; this patient also had the highest influenza-A–specific CD4 proliferation before and after immunization (11% pre and 32% post). Our data suggest that cellular response may be a more sensitive measure of vaccine-elicited immunity than antibody levels in immunocompromised individuals, and offer a method of characterizing the relationship between T and B cell response to vaccination.
We demonstrated the use of a sensitive and precise assay for measuring T cell response to whole antigens that does not require knowledge of immunogenic epitopes or their human leucocyte antigen (HLA)-restriction, and is sufficiently sensitive to detect changes in antigen-specific T cell frequency following influenza vaccination in HSCT patients. This functional read-out of antigen-specific T cells can define T cell subsets capable of responding, and will enable detailed examination of T cell immunity to pathogens and vaccines in HSCT patients (Danke & Kwok, 2003). This in turn may improve the assessment of new strategies to accelerate post-HSCT immune reconstitution and facilitate the study of T cell memory in humans.
Acknowledgements
The authors are indebted to the patients and families who took part in this study, and to the pharmacy and nursing staff of the Jimmy Fund Clinic at the Dana-Farber Cancer Institute who care for them. Dr Ian Thornley's thoughtful criticism is greatly appreciated. This study was supported by grant nos 5K08HL72750 (W.N.H.) and 1P01CA100265 (L.M.N., K.W.W., and E.C.G).
References
- Avigan D, Pirofski LA, Lazarus HM. Vaccination against infectious disease following hematopoietic stem cell transplantation. Biological Blood Marrow Transplantation. 2001;7:171–183. doi: 10.1053/bbmt.2001.v7.pm11302551. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O'Gorman P, Chakraverty R, Marshall T, Osman H, Mahendra P, Craddock C, Waldmann H, Hale G, Fegan CD, Yong K, Goldstone AH, Linch DC, Milligan DW. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–4363. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- Danke NA, Kwok WW. HLA class II-restricted CD4+ T cell responses directed against influenza viral antigens postinfluenza vaccination. Journal of Immunology. 2003;171:3163–3169. doi: 10.4049/jimmunol.171.6.3163. [DOI] [PubMed] [Google Scholar]
- Day CL, Seth NP, Lucas M, Appel H, Gauthier L, Lauer GM, Robbins GK, Szczepiorkowski ZM, Casson DR, Chung RT, Bell S, Harcourt G, Walker BD, Klenerman P, Wucherpfennig KW. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. Journal of Clinical Investigation. 2003;112:831–842. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining WN, Anderson DG, Little SR, von Berwelt-Baildon MS, Cardoso AA, Alves P, Kosmatopoulos K, Nadler LM, Langer R, Kohane DS. pH-triggered microparticles for peptide vaccination. Journal of Immunology. 2004;173:2578–2585. doi: 10.4049/jimmunol.173.4.2578. [DOI] [PubMed] [Google Scholar]
- Mannering SI, Morris JS, Jensen KP, Purcell AW, Honeyman MC, van Endert PM, Harrison LC. A sensitive method for detecting proliferation of rare autoantigen-specific human T cells. Journal of Immunological Methods. 2003;283:173–183. doi: 10.1016/j.jim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Martelli MF, Reisner Y. Haploidentical`megadose'CD34+cell transplants for patients with acute leukemia. Leukemia. 2002;16:404–405. doi: 10.1038/sj.leu.2402382. [DOI] [PubMed] [Google Scholar]
- Ochs L, Shu XO, Miller J, Enright H, Wagner J, Filipovich A, Miller W, Weisdorf D. Late infections after allogeneic bone marrow transplantations: comparison of incidence in related and unrelated donor transplant recipients. Blood. 1995;86:3979–3986. [PubMed] [Google Scholar]
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nature Immunology. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- Veys P, Amrolia P, Rao K. The role of haploidentical stem cell transplantation in the management of children with haematological disorders. British Journal of Haematology. 2003;123:193–206. doi: 10.1046/j.1365-2141.2003.04655.x. [DOI] [PubMed] [Google Scholar]