Abstract
In type 1 diabetes (T1D) an intense inflammatory response destroys β cells in the pancreas, where insulin is produced and released. A therapy for T1D that reduces the specific autoimmune response in this disease while leaving the remainder of the immune system intact has long been sought. Proinsulin is a major target of adaptive immunity in T1D. We hypothesized that an engineered DNA plasmid encoding proinsulin (BHT-3021) would preserve β cell function in T1D patients through reduction of insulin-specific T cells. We studied 80 subjects over 18 years of age who were diagnosed with T1D within 5 years. Subjects were randomized 2:1 to receive intramuscular injections of BHT-3021 or BHT-placebo, weekly for 12 weeks, and then monitored for safety and immune responses in a blinded fashion. Four dose levels of BHT-3021 were evaluated: 0.3, 1.0, 3.0, and 6.0 mg. C-peptide served as an exploratory measure of efficacy and safety. Islet-specific CD8+ T cell frequencies were assessed with multimers of monomeric human leukocyte antigen class I molecules loaded with peptides containing pancreatic or unrelated antigens. No serious adverse events related to BHT-3021 occurred. C-peptide levels improved relative to placebo at all doses, most notably at 1 mg at 15 weeks (+19.5% BHT-3021 versus −8.8% BHT-placebo, P < 0.026). Proinsulin-reactive CD8+ T cells, but not T cells against unrelated islet or foreign molecules, declined in the BHT-3021 arm (P < 0.006). Thus, we demonstrate that a plasmid encoding proinsulin reduces the frequency of CD8+ T cells reactive to proinsulin while preserving C-peptide over the course of dosing.
INTRODUCTION
One of the hallmarks of type 1 diabetes (T1D) is an inflammatory response that ultimately destroys the β cells of the pancreas, a process termed insulitis. CD8+ T cells directed to various islet antigens including preproinsulin (PPI), glutamic acid decarboxylase (GAD), tyrosine phosphatase–like insulinoma antigen (IA2, also called ICA512), zinc transporter ZnT8, and islet-specific glucose-6-phosphatase catalytic subunit–related protein (IGRP) have been detected in the blood and in the pancreatic islets of individuals with T1D (1–3). Attempts have been made to use antigen-specific therapy to delay T1D, including parenterally and nasally administered insulin (4–6). However, a trial of oral insulin failed to delay T1D, although there was evidence of delay in a subset of patients with high levels of insulin autoantibodies (6, 7). Other clinical trials targeting GAD with alum were unsuccessful in phase 3 in reducing loss of C-peptide—a marker of β cell function—possibly due to the use of an adjuvant that failed to show efficacy in murine models of T1D (8). In contrast, a recent phase 3 trial of a heat shock peptide (DiaPep277) reported successful outcomes for preservation of C-peptide, insulin usage, and HgbA1c (9). These trials involving injection of self-molecules have demonstrated safety, with no serious adverse events reported to date.
One approach that was successful in preclinical experiments in mouse models of T1D was using an engineered DNA vaccine encoding the whole proinsulin molecule, including C-peptide and insulin A and B chains, termed BHT-3021 (10–12). Tolerization to proinsulin prevented and reversed active insulitis in hyperglycemic nonobese diabetic mice, a widely studied mouse model of T1D (12). BHT-3021 is designed to decrease the antigen-specific autoimmune response against proinsulin in T1D. The plasmid was engineered with reduced numbers of proinflammatory hexanucleotide motifs, termed CpG motifs. CpG hexanucleotide sequences activate innate immune responses by binding to Toll-like receptor 9 and other DNA sensors (13). All non-essential CpG sequences were replaced with GpG motifs, which compete with CpG motifs. This antigen-specific plasmid vaccine approach has the theoretical advantage of decreasing the autoimmune response while leaving intact other important, desirable, physiologic roles of the immune system, such as immune regulatory responses against pro-insulin, immune surveillance against tumors, and immune responses against infectious agents.
Adaptive immune responses to islet-associated antigens have been identified in T1D. Pancreatic specimens obtained from T1D patients reveal a lymphocytic infiltrate in the pancreatic islets, composed predominantly of CD8+ T cells, with up-regulated human leukocyte antigen (HLA) class I molecules (1, 14). These findings suggest a key pathophysiologic role for cytotoxic T lymphocytes (CTL) in T1D. CD4+ T cells are also likely involved in the pathogenesis of T1D, further supported by the strong association of susceptibility in T1D with certain HLA class II haplotypes (14). Finally, autoantibodies to pancreatic islet antigens have been found in the overwhelming majority of T1D patients and those at genetic risk for developing the disease. Antibodies to either GAD, IA2, or insulin are present in 95% of prediabetic or new-onset T1D patients; 80% of patients are positive for two or more of these antibodies, and 25% are positive for all three antibodies. Multiple T1D-associated autoantibodies are present rarely in serum of healthy control subjects (3).
Insulin is a primary β cell–specific autoantigen, and insulin auto-antibodies are usually the first to appear in young children with T1D (3, 15). Furthermore, half of the T cells isolated from pancreatic draining lymph nodes of patients with T1D recognize an epitope of the insulin A chain, whereas T cells from healthy subjects that recognize this epitope have not been observed (16). Finally, it has been demonstrated that insulin-reactive T cells from T1D patients exhibit an activated inflammatory T helper 1 (TH1) cell phenotype, whereas insulin-reactive T cells from healthy controls exhibit a protective T regulatory phenotype (17).
Thus, there is a substantial rationale for efforts to reduce the auto-immune response against insulin in individuals with T1D while leaving regulatory responses intact or even enhancing them. The safety of this approach was explored in this clinical trial, with C-peptide as primary clinical endpoint. Antigen-specific modulation was measured using a variety of assays as secondary endpoints. We demonstrate that BHT-3021 is safe and effective at preserving C-peptide during the period of administration and modulates insulin-specific T lymphocytes, but not T cells specific for other antigens.
RESULTS
Baseline characteristics of the intent-to-treat population
Table 1 shows that the baseline characteristics of the intent-to-treat (ITT) population are not significantly different from those randomized to control.
Table 1.
Demographics and baseline characteristics (ITT population).
| 0.3 mg (n = 14) | 1.0 mg (n = 18) | 3.0 mg (n = 14) | 6.0 mg (n = 8) | Placebo (n = 26) | |
|---|---|---|---|---|---|
| Mean age (years) | 29.6 | 31.5 | 31.8 | 27.6 | 29.3 |
| Gender | |||||
| Male | 9 (64.3%) | 10 (55.6%) | 6 (42.9%) | 7 (87.5%) | 18 (69.2%) |
| Female | 5 (35.7%) | 8 (44.4%) | 8 (57.1%) | 1 (12.5%) | 8 (30.8%) |
| Race | |||||
| Caucasian | 10 (71.4%) | 17 (94.4%) | 11 (78.6%) | 8 (100%) | 22 (84.6%) |
| Asian | 1 (7.1%) | 0 | 1 (7.1%) | 0 | 1 (3.8%) |
| Black | 0 | 1 (5.6%) | 1 (7.1%) | 0 | 1 (3.8%) |
| Hispanic | 2 (14.3%) | 0 | 0 | 0 | 1 (3.8%) |
| American Indian or Alaska Native | 0 | 0 | 1 (7.1%) | 0 | 0 |
| Other | 1 (7.1%) | 0 | 0 | 0 | 1 (3.8%) |
| Mean time from diagnosis (months) | 14.0 | 59.7 | 36.9 | 32.2 | 41.1 |
Prespecified efficacy endpoints
C-peptide. C-peptide secretion is considered an important surrogate marker for assessment of pancreatic secretion of insulin (18–20). Area under the curve of C-peptide response (referred to herein as “C-peptide”) to mixed-meal tolerance test (MMTT) is a validated method of assessing endogenous insulin secretion, and subjects with T1D have C-peptide responses to an MMTT at a time when intravenous glucose and glucagon responses were absent (19, 20).
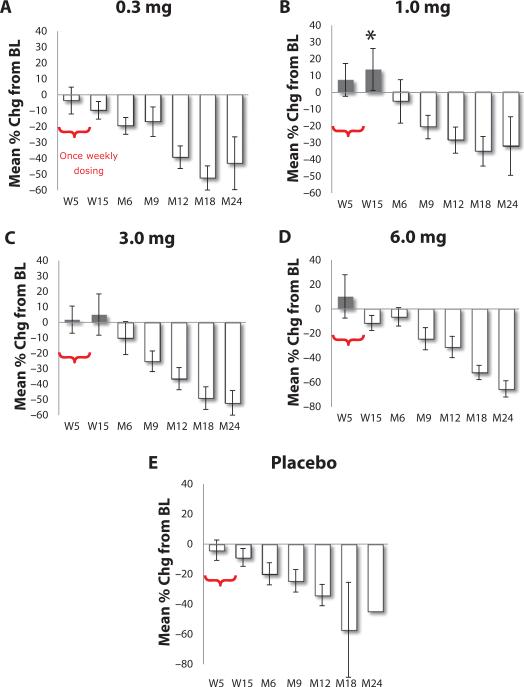
BHT-3021 (Fig. 1) was dosed via the intramuscular route for 12 weeks to individuals with T1D who had residual C-peptide at the time of screening (C-peptide >33 pM) (Fig. 2). Placebo received an equivalent dose of saline. At 15 weeks for 1.0-mg BHT-3021 dose versus BHT-placebo, the percent change from baseline in mean C-peptide was +19.5% (−1.95% lower confidence level, 41.0% upper confidence level) versus −8.8% for placebo (−25.34% lower confidence level, +7.66 upper confidence level; P < 0.026) (Fig. 3 and fig. S1A). Subjects in the 1.0- and 3.0-mg arms had C-peptide levels that were above the screening values at week 15. Figure S1B shows percent change from baseline for C-peptide in scatter plots of all doses and placebo at 15 weeks. In contrast, the placebo group, which started out higher, demonstrated a very steep decrease in C-peptide over the same 6-month period. One potential caveat was a longer mean time from diagnosis for the 1-mg group (59.7 months) compared to placebo (41.1 months), although this difference was not statistically significant (Table 1). These data suggest that BHT-3021 may preserve β cell mass and/or function during the dosing period of 12 weeks and for up to 3 more months (6-month time point) after cessation of dosing. This effect is ultimately lost after discontinuation of therapy. Table S1 shows that treatment with BHT-3021 is not associated with a large reduction in C-peptide.
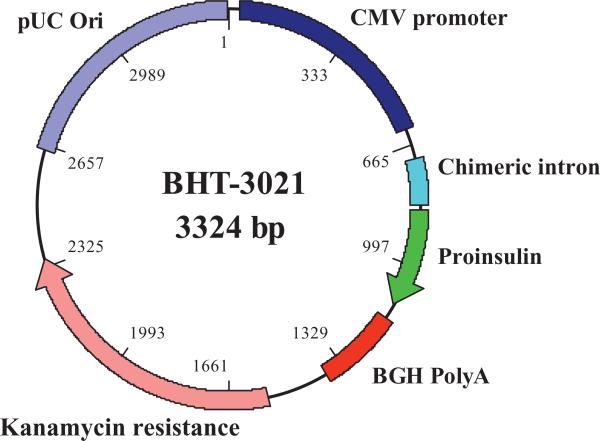
Fig. 1. Structural diagram of BHT-3021.
BHT-3021 is a 3.3-kb bacterial plasmid expression vector containing the coding sequences for human proinsulin (hINS) gene. Important functional and control features of BHT-3021 include the human cytomegalovirus (CMV) immediate-early gene promoter/enhancer, a chimeric intron sequence, the bovine growth hormone gene polyadenylation signal, the kanamycin resistance gene, and the pUC origin of replication for propagation of the vector in Escherichia coli. The backbone of BHT-3021 has been modified to decrease the number of immunostimulatory CpG sequences and substitute immunosuppressive sequences.
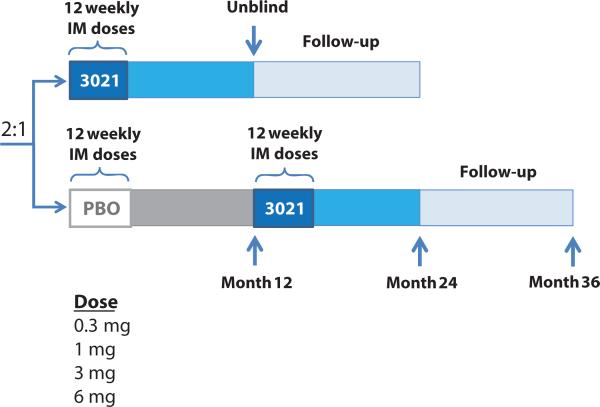
Fig. 2. A schematic of the study trial design.
Eighty subjects were enrolled in the study. Four dose levels of BHT-3021 were evaluated: 0.3, 1.0, 3.0, and 6.0 mg. After completion of the dose-finding phase of the study (dose escalation phase), additional subjects were enrolled to expand select dose cohorts to obtain additional safety and efficacy data (expansion phase). The dose escalation portion of the study enrolled subjects sequentially into the 1-mg and then the 3-mg cohorts (randomized active/placebo, 2:1); the cohorts for the 0.3- and 6-mg dose levels were then enrolled concurrently. After the dose escalation enrollment was complete, subjects were randomized (active/placebo, 2:1) into the expansion cohort to receive BHT-3021 (at doses of 0.3, 1, or 3 mg) or BHT-placebo. IM, intramuscular.
Fig. 3. Mean percent change in C-peptide from baseline.
C-peptide was assessed as a measure of β cell function during the 12 weekly doses and thereafter. C-peptide measured as described in Materials and Methods (18–20). n = 14 for 0.3-mg dose; n = 15 for 1.0-mg dose; n = 13 for 3.0-mg dose; n = 8 for 6.0-mg dose; n = 23 for placebo. The mean percent change from baseline (BL) ± confidence interval is displayed. W refers to week after initiation of 12 weekly doses at time zero, whereas M refers to month after initiation of 12 weekly doses at time zero.
Mean HbA1c, insulin requirements, and blood glucose levels
HbA1c allows a measure of the changes in glucose homeostasis, over a long segment, because it reflects the glycosylation of hemoglobin, and thus reflects the status of plasma glucose, with the predominant contribution from plasma glucose over the past month. Generally, levels of HbA1c above 53 mmol/mol (7.0%) are considered diabetic, with standards varying depending on the organization who is deciding the guideline. Figure S2A displays the mean HbA1c by treatment group for the MMTT population. Differences in baseline HbA1c among the groups were noted, reflecting varying levels of glycemic control at entry. The mean HbA1c was relatively stable at entry and at 15 weeks, and then increased after cessation of dosing at month 6 in all groups, although the differences were not statistically significant. Notably, there was a decrease in monitoring with fewer study visits beyond week 15. Figure S2B displays the mean total insulin usage by treatment group. Total insulin usage was stable for the treatment groups for the initial 6 to 9 months of the study and then increased subsequently. Mean insulin usage for the 1-mg dose fell during the period of dosing. The overall increase in insulin usage was concurrent with higher HbA1c. In particular, over the duration of study drug dosing, insulin usage was stable when compared to baseline in each of the treatment groups, although the differences were not statistically significant from placebo.
Immunological studies
Enumeration of antigen-specific CD8+ T cells during therapy
A prespecified immunological study was designed to quantify the changes in islet-specific CD8+ T cells before and after treatment with BHT-3021. All patients were typed for HLA. Sixty-four patients had HLA class I types for which multimers were available. Twenty-one of the 64 patients had too few cells at baseline to allow comparison over time. Two patient samples were not collected. Therefore, a total of 41 of the 80 patients were evaluated at baseline and at least one time point after treatment. We used the combinatorial quantum dot (Qdot) technique (21) to simultaneously detect CD8+ T cells specific for nine different β cell–derived antigens, and a cadre of viral epitopes, to measure responses to non-islet antigens (21).
We analyzed delta (stimulated) C-peptide in relation to changes in CD8 islet autoreactivity from baseline in patients treated with active drug compared to placebo, for each of the epitopes tested, and for HLA-A2, HLA-A3, and HLA-B7. We then distinguished epitopes present in the BHT DNA vaccine (that is, proinsulin, but not the leader peptide in PPI) from other islet autoantigens [GAD, IA2, PPI leader sequence, islet amyloid polypeptide (IAPP), and IGRP]. Finally, we accounted for one insulin epitope (insulin B10-18), which is also present in injected insulin, which was used for insulin replacement therapy in all patients in the study (22). Because it is known that immune responses to injected insulin may develop after initiation of insulin therapy, insulin replacement may act as confounder regarding changes induced by BHT-3021. Therefore, CD8+ T cell responses to this epitope were separated from the two other epitopes present in BHT-3021. Finally, we distinguished no change in T cell response (δ = 0) in cases where there was no response detectable at any time reliably, from those where the frequencies were the same at t = 0 and 15 weeks.
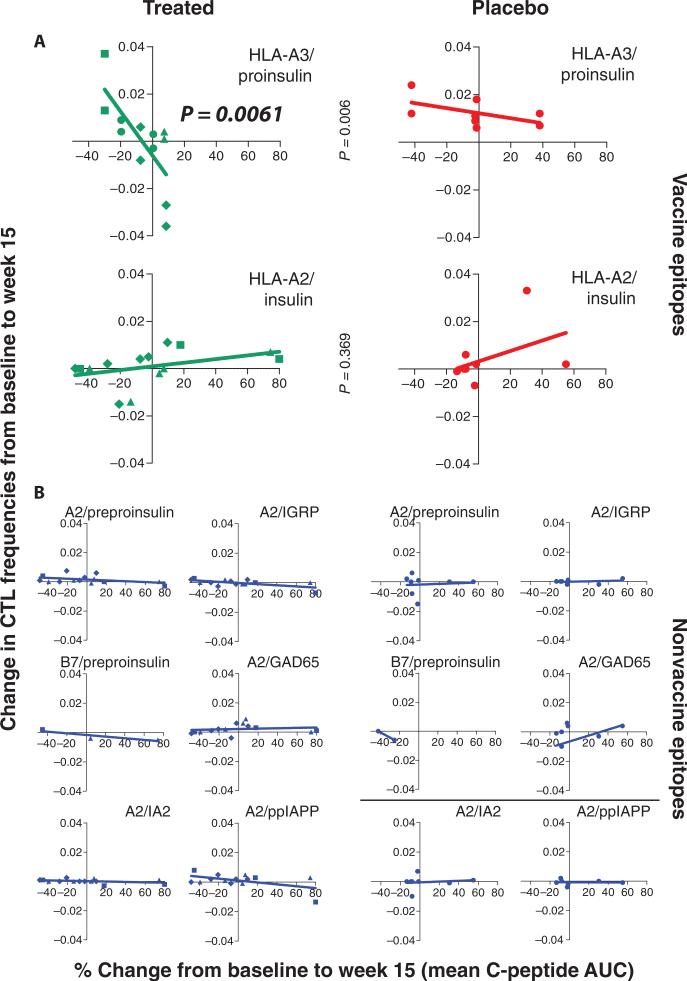
When the change in the frequency of CD8 lymphocytes to pro-insulin was compared with the percent change in the mean C-peptide, there was a negative correlation for proinsulin, but not for insulin or other β cell antigens including preproinsulin, IA2, IGRP, GAD65, or prepro-IAPP (ppIAPP) (Fig. 4; P = 0.006 for HLA-A3 proinsulin, treated versus placebo, using linear regression analysis, n = 12 and 8, respectively; P > 0.05 for all other epitopes). These results indicate that BHT-3021 induced antigen-specific reductions in CD8 cells reactive to proinsulin, but not to other antigens, and that the magnitude of the reduction was inversely correlated with the improvement in C-peptide.
Fig. 4. Antigen-specific CD8+ T cells were enumerated with Qdot multimer technology using class I HLA multimers loaded with various antigens (21–24).
Antigen and HLA haplotype are shown in each panel. CTL frequencies are defined as percentage of antigen-specific CD8+ T cells. Changes in CTL from baseline to week 15 are shown on the y axis, and percent change in C-peptide from baseline to week 15 is shown on the x axis. Changes in CTL were calculated by subtracting the baseline values from values at week 15. (A and B) Analysis was performed on all treated (0.3 mg: diamonds, 1 mg: triangles, 3 mg: squares, 6 mg: circles) and placebo patients positive for HLA-A2, HLA-A3, and/or HLA-B7 (A) and for control antigens (B). Statistics were performed with linear regression analysis. AUC, area under the curve.
Analysis of the frequencies of virus-specific CD8+ T cells over time showed no differences between treated subjects and placebo. CTL frequencies against vaccine epitopes significantly increased in placebo over time compared to treated patients (P = 0.003 using one-tailed Mann-Whitney test at week 15; n = 16 for placebo and n = 30 for treated; see fig. S3A). For proinsulin HLA-A3–specific CD8+ T cells, differences in placebo versus treated were most pronounced at week 15, with differences waning after cessation of therapy (n = 8 for placebo and n = 16 for treated; P = 0.0142 using Mann-Whitney test at week 15; fig. S3B).
Treatment arms were evenly distributed for the criterion of baseline CD8 islet autoreactivity, ruling out the possibility that the changes in T cell response to BHT-3021 at 15 weeks were due to selective imbalance seen at time 0 (fig. S4).
Enzyme-linked immunospot analysis of cytokine production in CD4 T cells specific for insulin B9-23 and other islet cell antigens
We chose to measure interferon-γ (IFN-γ) and interleukin-10 (IL-10), because IFN-γ is the major TH1 cytokine and IL-10 is a key cytokine produced by regulatory T cells. There was no consistent change in IL-10 immune responses to any islet epitopes including those contained in BHT-3021 as well as those unrelated to BHT-3021 (fig. S5A), and no change in IFN-γ responses to the immunodominant insulin epitope (fig. S5B), at 15 weeks. There were insufficient data available for correlation of enzyme-linked immunospot (ELISpot) analysis and CD8 multimer analysis for the same patient at matching time points.
Autoantibodies to pancreatic antigens
Autoantibodies to pancreatic antigens were measured at baseline and week 15 (3 weeks after the final BHT-3021 administration) in all subjects for which samples were available. In general, there were few changes in antibody status at week 15 such that individuals who were positive at baseline for a specific antibody maintained positivity at week 15, and, conversely, if they were negative at baseline, they remained negative at week 15 (table S2). A few exceptions existed, specifically a single placebo subject who converted from negative to positive for GAD65, and four subjects (two active and two placebo) converted from negative to positive for insulin antibodies (IAs). No subjects converted from negative to positive for IA2.
Because the plasmid DNA BHT-3021 encodes the proinsulin protein, changes in the immune response to insulin were of particular interest. To determine whether the change in IA status in these four subjects correlated with any clinical outcomes, the changes in C-peptide at week 5, week 15, and month 6 for the subjects converting from negative to positive for IA confirmed that there were no consistent C-peptide changes that correlated with the induction of IAs. The subject with the largest induction of IA had the best preservation of C-peptide over time. We conclude that the induction of IA did not correlate with an undesirable precipitous decline in C-peptide in these subjects.
Safety
Treatment-emergent adverse events
The independent Data and Safety Monitoring Board (DSMB) determined that there were no treatment-related adverse events that appeared to be related to the study drug. Detailed description of all treatment-emergent adverse events (TEAEs) is presented in table S3. Summary statistics consisted of numbers and percentages of subjects for categorical measures and means, medians, SDs, and minimum and maximum values for continuous measures as calculated with version 9.1.3 of the SAS statistical software package for the calculation of all summaries, listings, graphs, and statistical analyses of adverse events.
TEAEs were reported for 12 (85.7%) of 14 subjects treated with 0.3 mg of BHT-3021, 18 (100%) of 18 subjects treated with 1.0 mg, 11 (78.6%) of 14 subjects treated with 3.0 mg, 7 (87.5%) of 8 subjects treated with 6.0 mg, and 25 (96.2%) of 26 subjects treated with BHT-placebo. Grade 3 or higher TEAEs were reported for 4 (28.6%) of 14 subjects treated with 1.0 mg of BHT-3021, 2 (28.6%) of 14 subjects treated with 3.0 mg, 3 (37.5%) of 8 subjects treated with 6.0 mg, and 4 (15.4%) of 26 subjects treated with BHT-placebo. The various types of TEAEs, none related to the study drug, are summarized in table S3. TEAEs considered to be possibly related to study drug were reported for 5 (35.7%) of 14 subjects treated with 0.3 mg of BHT-3021, 6 (33.3%) of 18 subjects treated with 1.0 mg, 6 (42.9%) of 14 subjects treated with 3.0 mg, 4 (50.0%) of 8 subjects treated with 6.0 mg, and 6 (23.1%) of 26 subjects treated with BHT-placebo. Most of these events were noted by the investigator to be grade 1; a few were grade 2 events. Serious TEAEs were reported for 1 (7.1%) of 14 subjects treated with 0.3 mg of BHT-3021, 1 (5.6%) of 18 subjects treated with 1.0 mg, and 4 (15.4%) of 26 subjects treated with BHT-placebo; none of these events was considered to be related to study drug.
Discontinuations
Two subjects treated with 3.0 mg of BHT-3021 were discontinued from study drug treatment because of TEAEs that investigators could not be certain were unrelated to study drug. One subject reported a grade 2 headache, and one subject developed grade 1 vaginal candidiasis. Upon completion of the trial and review of data on all patients, there was no statistical association of these particular adverse events, or any others, to study drug. There were no deaths in the study. We conclude that BHT-3021 met its primary endpoint for safety, with no substantial toxicities noted.
DISCUSSION
There is no approved immunotherapy for the treatment of T1D. The mainstay of treatment is insulin replacement, a lifesaving breakthrough that was discovered more than 90 years ago. A therapeutic agent that targets the primary pathogenesis of the disease has long been sought.
A major autoimmune response in T1D is directed to insulin (1–3, 5, 6). Here, we have attempted to modulate, in an antigen-specific manner, the adaptive immune response to proinsulin with an engineered DNA vaccine encoding proinsulin. The vaccine is engineered to reduce the immunogenicity of the encoded proinsulin by substituting CpG hexameric motifs, which stimulate the innate immune response, with GpG hexameric nucleotide sequences, known to modulate innate immunity (13). Here, we show that this approach modulated C-peptide, with an actual rise in this marker of β cell function during the dosing period at two doses. We also demonstrate that as the C-peptide increases, there is a deletion of CD8+ T cells reactive to proinsulin, but there is no effect on other antigen-specific T cell responses. This is a firm indication that antigen-specific modulation has occurred.
There was no increase in adverse events or in serious adverse events associated with BHT-3021 (table S3). This is a particularly important outcome because T1D is more commonly observed in children and young adults in whom BHT-3021 will ultimately need to be tested.
We assessed C-peptide to ascertain whether this vaccine might impact the levels of C-peptide. We observed significant improvement in C-peptide during the dosing period. The 1-mg dose was most effective compared to placebo (P < 0.026) (Fig. 3). Treatment with 1.0 and 3.0 mg of BHT-3021 led to C-peptide levels that were above the screening values at week 15. Thus, these data provide evidence of preservation of C-peptide during the dosing period, an effect that was lost when subjects were no longer exposed to the antigen-encoding vaccine. This result is surprising and unexpected because the trial was not powered to measure efficacy outcomes and because the trial was performed in adults with disease duration up to 5 years and proportionately lower β cell mass and perhaps more end-stage immune responses than those observed in recent-onset diabetic subjects.
HgbA1c was well controlled during dosing of the DNA plasmid compared to placebo (fig. S2A). The mean HbA1c was relatively stable initially through 15 weeks of treatment with BHT-3021 and then increased at month 6 in all groups. Insulin usage appeared relatively stable overall when compared to baseline in each of the treatment groups (fig. S2B). The data from week 104 are not statistically significant (n = 3). Neither the HbA1c nor the insulin usage data were significantly different from control at any dose.
CD8+ T cells are critical in the pathogenesis of T1D (1–3, 5, 6). CD8+ T cells specific for proinsulin, other islet cell antigens, and viral antigens were assessed with HLA class I multimers, a technology that allows for enumeration of the frequency of antigen-specific T cells with flow cytometry (21, 23). We demonstrate antigen-specific reduction in CD8 cells reactive to proinsulin, but not to other antigens, and that the magnitude of the reduction was inversely correlated with the improvement in C-peptide (Fig. 4).
CD8+ T cells specific for proinsulin have been detected in the islets of patients with T1D using the same HLA monomers used in our studies (1). Reduction in the frequency of such CD8+ T cells in this study correlated with increases in C-peptide during the period of dosing (Fig. 4). We speculate that proinsulin-specific CD8+ T cells are either deleted by apoptosis because they receive signals through their cognate T cell receptors in the absence of costimulatory signals provided by antigen-presenting cells or actively suppressed by regulatory T cells and sequestered from the pancreatic islets and from the peripheral circulation where we attempted to detect them.
The particular HLA types and epitopes used in the analysis with multimers are relevant to the pathophysiology of T1D. A recent study using tetramers, rather than the Qdot multimers used in the current paper, but with the same HLA molecules and islet epitopes as the ones used in the current experiments for BHT-3021, detected similar CD8+ T cells in peripheral blood, which are also seen in the inflamed pancreas of the same patient with T1D (24). Thus, these peripheral CD8+ T cells found in the circulation are known to locate in the inflamed islets (24). Another recent investigation revealed that CD8+ T cells cloned from peripheral blood and reactive against one of the epitopes in the multimer study used in this paper were pathogenic (25). These CD8 clones caused insulitis and β cell destruction when injected into humanized (HLA-A2 transgenic) mice, demonstrating diabetogenicity of these particular circulating islet autoreactive human CD8+ T cells detected in our assay in this clinical trial (25). These T cells under investigation in this clinical trial may thus have real pathogenic relevance to T1D (24, 25).
Limitations of this study include the fixed dosing regimen and limited dosing period. It is possible that other dosing regimens will provide more robust benefit in the initial dosing period and/or in the maintenance of tolerance in extended dosing. In addition, because of the limited dosing period, it is unclear whether long-term benefit in T1D can be achieved. Follow-on studies are needed to assess the activity of alternative dosing regimens in both the initiation and maintenance of antigen-specific tolerance as well as to assess the durability of the effect of continued dosing. The Qdot assay used in Fig. 4 could be used to optimize dose and frequency as we strive to attain depletion of CD8+ T cells to proinsulin while maintaining stability of other antigen-specific T cells.
Together, the preservation of C-peptide during the period of dosing of BHT-3021, along with the immunological studies with major histocompatibility complex class I multimers, indicates that BHT-3021 induces antigen-specific modulation of the immune response to proinsulin, but not to other antigens. A long sought-after goal of therapy in autoimmune disease aims to reduce or abolish the unwanted autoimmune responses that contribute to pathology. There is strong evidence that immunity to insulin, a primary β cell–specific antigen, is one of the fundamental aspects underlying the pathophysiology of T1D. The results of this 12-week trial with an engineered DNA plasmid encoding proinsulin indicate that there is antigen-specific suppression of immunity to proinsulin during the period of dosing. Longer trials with BHT-3021 are warranted, given the reduction in immunity to proinsulin and the favorable safety profile.
MATERIALS AND METHODS
Plasmid construction
BHT-3021 is a 3.3-kb bacterial plasmid expression vector containing the coding sequences for the hINS gene. Important functional and control features of BHT-3021 were engineered into the final construct, including the human CMV immediate-early gene promoter/enhancer, a chimeric intron sequence, the bovine growth hormone gene polyadenylation signal, the kanamycin resistance gene, and the pUC origin of replication for propagation of the vector in E. coli. The backbone of BHT-3021 was modified to decrease the number of immunostimulatory CpG sequences. All nonessential CpG motifs were then substituted with immunomodulatory sequences, known as GpG sequences (13). Figure 1 shows the main structural features of BHT-3021. BHT-3021 was formulated in phosphate-buffered saline (PBS) containing 0.9 mM Ca2+ as a sterile solution for intramuscular injection at a concentration of 2.0 mg DNA/ml. Placebo patients received PBS.
Enrollment and recruitment
The study was performed with informed consent from all subjects and under protocols that were approved by the Institutional Review Boards at each institution. Before initiating the clinical trial, an Investigational New Drug Application was submitted to and accepted by the U.S. Food and Drug Administration and approval from the National Institutes of Health Recombinant DNA Advisory Committee was obtained. A total of 144 subjects were screened for the study. Eighty subjects (48 in the dose escalation cohorts and 32 subjects in the expansion cohort) were randomized. Inclusion criteria were as follows: (i) diagnosis of type 1a diabetes mellitus based on American Diabetes Association criteria; (ii) between 18 and 40 years of age; (iii) within 5 years of diagnosis of T1D; (iv) detectable fasting C-peptide; (v) C-peptide increase during MMTT with a minimal stimulated value of ≥0.2 pmol/ml; (vi) presence of antibodies to at least one of the following antigens: insulin, GAD65, or IA2 (if IA-positive only, determination must have been completed within 2 weeks of insulin initiation); (vii) agreement to intensive management of diabetes with an HbA1c goal of <7.0%; (viii) if female, subjects must have been (a) surgically sterile and (b) postmenopausal or, (c) if of reproductive potential, subjects must have been willing to use medically acceptable birth control (for example, female hormonal contraception, barrier methods, or sterilization) until 3 months after completion of any treatment period; (ix) if male and of reproductive potential, subjects must have been willing to use medically acceptable birth control until 3 months after completion of any treatment period, unless the female partner was postmenopausal or surgically sterile; (x) serum creatinine ≤1.5 × upper limit of normal (ULN); (xi) aspartate aminotransferase <2 × ULN; and (xii) white blood cells (WBCs) ≥3 × 109/liter; platelets ≥100 × 109/liter; and hemoglobin ≥10.0 g/dl. Exclusion criteria were as follows: (i) unable or unwilling to comply with the requirements of the study protocol; (ii) body mass index >30 kg/m2; (iii) unstable blood sugar control, defined as one or more episodes of serious hypoglycemia (hypoglycemia that required the assistance of another person) within the 30 days before enrollment; (iv) previous immune therapy for T1D; (v) administration of an experimental agent for T1D at any time, or use of an experimental device for T1D within 30 days before screening, unless approved by the Medical Monitor; (vi) history of any organ transplant, including islet cell transplant; (vii) active autoimmune or immune deficiency disorder other than T1D (such as sarcoidosis and rheumatoid arthritis), unless approved by the Medical Monitor; (viii) 24-hour urinary albumin excretion >300 mg at screening; (ix) uncontrolled or untreated retinopathy at screening; (x) serum bilirubin > ULN, except those subjects whose abnormal values were attributed to any stable, benign condition (such as Gilbert's syndrome); (xi) thyroid-stimulating hormone outside the normal range at screening, except those subjects on stable doses of thyroid hormone replacement therapy; (xii) known HIV positivity or evidence of high-risk behavior; (xiii) active hepatitis B or active hepatitis C infection; and (xiv) pregnant or lactating women.
Trial design
The overall study design is shown in Fig. 2. Subjects were screened for eligibility within 6 weeks before randomization. Subjects were randomized to BHT-3021 or BHT-placebo (PBS vehicle) in a 2:1 ratio and entered the blinded treatment period when BHT-3021 or BHT-placebo was administered intramuscularly weekly for 12 weeks (weeks 0 to 11). Four weeks after the last dose of BHT-3021 or BHT-placebo (week 15), subjects underwent a complete evaluation for safety, β cell function, and anti-insulin responses. Subjects were monitored for safety and immune response in a blinded fashion until 12 months after the first dose of BHT-3021 or BHT-placebo (the blinded evaluation period). Each subject's treatment assignment was then unblinded. Subjects who received BHT-3021 entered a 12-month long-term follow-up period, during which they were monitored for delayed adverse events, pancreatic function, and immune response. Subjects who received BHT-placebo were eligible for crossover to receive 12 weeks of treatment with BHT-3021 in an open-label manner.
Eighty subjects were enrolled in the study. Four dose levels of BHT-3021 were evaluated: 0.3, 1.0, 3.0, and 6.0 mg. An initial nine subjects were enrolled into an open-label cohort. After completion of the dose-finding phase of the study (dose escalation phase), additional subjects were enrolled to expand one or more dose cohorts to obtain additional safety and efficacy data (expansion phase).
Clinical primary and secondary endpoints
The primary objective was to evaluate the safety of BHT-3021 given as weekly injections over 12 weeks. The secondary objectives were to evaluate the effect of BHT-3021 on antibody and T cell responses to diabetes-related antigens (insulin, GAD65, and IA2), to describe changes in pancreatic β cell function after treatment with BHT-3021, and to describe changes in insulin requirements and blood glucose levels after treatment with BHT-3021.
Primary endpoints
The safety parameters assessed in the study were adverse events and serious adverse events, physical examinations, vital signs, clinical laboratory testing (hematology, chemistry, urinalysis), ophthalmologic examination, 12-lead electrocardiography, 24-hour urine protein, stimulated C-peptide levels, pregnancy testing, and glucose measures (nighttime and self-monitored blood glucose).
Secondary endpoints
C-peptide was used as both an exploratory efficacy measure and a safety measure to ensure that no marked decline in pancreatic function was observed with treatment with BHT-3021. Markers of metabolic control included HbA1c and fasting plasma glucose. Total daily insulin dose was assessed at baseline and during the study. The pharmacodynamic parameters assessed in the study were (i) immune response to pancreatic antigens, as measured by antibodies to insulin, GAD65, and IA2, as well as T cell responses to pancreatic antigens, and (ii) blood markers of immune activation.
Antibodies to pancreatic antigens
Radioimmunoassays were performed on baseline samples to determine the initial immune response to insulin. Analysis at subsequent time points was used to evaluate any change in the response that may have resulted from BHT-3021 treatment. Analysis of reactivity to GAD65 and IA2 was also measured as an overall indication of autoimmune responses to islet antigens. Antibodies to GAD65, IA2, and insulin were measured at screening and were part of the entry criteria. Methods for detecting T1D-associated antibodies have been described previously (3, 26).
Qdot HLA-peptide multimers for measurement of frequency of antigen-specific CD8+ T cells
Multimeric HLA-A2–peptide complexes were prepared as previously described (21). Briefly, recombinant HLA-A2 and human β2-microglobulin were solubilized in urea and injected together with each synthetic peptide into a refolding buffer consisting of 100 mM tris (pH 8.0), 400 mM arginine, 2 mM EDTA, 5 mM reduced glutathione, and 0.5 mM oxidized glutathione. Refolded complexes were biotinylated by incubation for 2 hours at 30°C with BirA enzyme (Avidity). The biotinylated complexes were purified by gel filtration on a Superdex 75 column (Amersham Pharmacia Biotech). Multimeric HLA-peptide complexes were produced by addition of streptavidin-conjugated Qdots (21) (Invitrogen) to achieve a 1:20 streptavidin-Qdot/biotinylated HLA class I ratio. Qdot-585, Qdot-605, Qdot-655, Qdot-705, and Qdot-800 were used. Samples from HLA-A2/A3/B7– positive subjects were stained with a mixture containing nine diabetes-associated epitopes, an HLA-A2 epitope expressed in HLA-A2, and a mixture of viral antigens (table S4).
Cell staining with Qdot-labeled multimeric complexes
Peripheral blood mononuclear cells (PBMCs) (2 × 106) were stained simultaneously with all Qdot-labeled multimers (0.1 μg of each specific multimer) in 60 μl of PBS supplemented with 0.5% bovine serum albumin (BSA) and incubated for 15 min at 37°C. Subsequently, 10 μl of allophycocyanin-labeled anti-CD8 (stock 1:10) and 10 μl of fluorescein isothiocyanate–labeled anti-CD4, anti-CD14, anti-CD16, anti-CD20, and anti-CD40 antibodies (Becton Dickinson) were added for 30 min at 4°C. After the cells were washed twice, they were resuspended in PBS/0.5% BSA containing 7-aminoactinomycin D (eBioscience) to exclude dead cells and analyzed with the LSR II (Becton Dickinson).
Data analysis and statistical methods
Patients with HLA class I type A2, A3, or B7 were stained with the corresponding multimers. Data were reported as the percentage of CD8+ T cells that were specific for (or bound to) each multimer. Changes in antigen-specific T cell percentages were calculated by subtracting the baseline values from each subsequent time point.
Analysis of islet-specific immune response was performed by evaluating BHT-3021–specific responses separately from responses not specific to this agent. For example, for each patient, the vaccine-specific changes were calculated for each appropriate multimer [insulin B10-18, PPI(76–84), and PPI(79–88)]. The evaluation of islet-specific nonvaccine responses was calculated for the peptides PPI(15–24), PPI(4–13), GAD65, IA2, IGRP, and ppIAPP. In this case, a single patient could have as many as six different data points. For islet epitopes in the Qdot combinatorial method, specifically, the coefficient of variation was determined at 10.8% (HLA-A2 peptide), 34.9% (virus mix), 15.9% (insulin B10-18), 0.0% (IA2), 0.0% (IGRP), 6.3% (PPI), 4.5% (GAD65), and 6.9% (ppIAPP) (21).
Changes in CTL were calculated by subtracting the baseline values from values at week 15. Analysis was performed on all treated (all doses) and placebo patients positive for HLA-A2, HLA-A3, and/or HLA-B7. Statistics were performed with linear regression analysis.
Enzyme-linked immunospot
ELISpots were performed on the first 48 patients enrolled in the dose escalation phase of the study. ELISpots were performed at the Barbara Davis Center for Childhood Diabetes (Aurora, CO). ELISpot data from these 48 patients are not presented because of low signal-to-noise ratio. The final 32 patients were included in the expansion phase, and ELISpots were performed on these individuals at the Contract Research Organization (CRO) Cellular Technology Ltd. PBMCs from patients from Australia/New Zealand were prepared at the CRO Cancer Trials Australia, Melbourne. PBMCs from U.S. patients were prepared at Cellular Technology Ltd. Frozen PBMCs were shipped in bulk to Cellular Technology Ltd. where the assays were performed. IL-10 and IFN-γ antigen–specific immune responses were evaluated. The autoimmune response to insulin and GAD65 was measured as an indication of the ongoing autoimmune response to islet antigens. The immune response to a panel of viral peptides was used to monitor irrelevant CD8 (not T1D-associated) immune responses. The immune response to mosquito antigen was used to monitor antigen-specific, but not diabetes-related, CD4 T cell responses.
Crossover phase
Subjects who received BHT-placebo were eligible for crossover to receive 12 weeks of treatment with BHT-3021 in an open-label manner. The dose of BHT-3021 during the open-label crossover period was the “best dose” based on evaluation of available safety, immune response, and efficacy data. The best dose was defined as that dose or doses already administered in the clinical trial that the DSMB found to have an acceptable safety profile, and which the Sponsor determined at the time of crossover to present the best balance of safety, biological activity (immune response), and/or efficacy. More than one dose could have been designated as a best dose, as long as all doses presented comparable safety and efficacy profiles. Crossover subjects were fully evaluated at the end of the dosing period (week 15), after which they entered the open-label evaluation period that lasted until 12 months after the first dose of BHT-3021. Finally, the subjects were entered in the 12-month long-term follow-up period.
Supplementary Material
Acknowledgments
Funding: This work was supported by Bayhill Therapeutics.
All four founders have issued U.S. and European patents on aspects of the work described in the paper (granted patents: US 7,811,813; 7,579,328; US 7,544,669; AU 2002362019; CN 02827318.4; NZ 533294; EP 1,931,390; AU 20329440; EP 1,569,696; NZ 540,276; IL168715; JP 4750419).
Footnotes
ClinicalTrials.gov registration number: NCT00453375.
Author contributions: B.O.R., N.S., P.A.G., J.R.F.A., L.C.H., G.S.E., L.Y., M.L., W.A.H., J.B.B., M.v.H., J.Q., R.S.K., W.H.R., P.J.U., H.G., and L.S. planned and oversaw the clinical trial, analyzed and interpreted the data sets, and contributed to writing and editing of the manuscript. The BHT-3021 Investigators provided clinical and scientific input, recruited patients and conducted the trial, and reviewed the manuscript.
Competing interests: L.S., W.H.R., H.G., and P.J.U. founded Bayhill Therapeutics in 2002. L.S., W.H.R., and P.J.U. received consulting money from Bayhill. H.G. was formerly a full-time employee at Bayhill. Bayhill Therapeutics was dissolved in 2013, and its assets acquired by Tolerion Inc., a company founded by L.S., W.H.R., P.J.U., and H.G. in 2013. L.S., W.H.R., P.J.U., and H.G. own equity in Tolerion Inc.
Citation: B. O. Roep, N. Solvason, P. A. Gottlieb, J. R. F. Abreu, L. C. Harrison, G. S. Eisenbarth, L. Yu, M. Leviten, W. A. Hagopian, J. B. Buse, M. von Herrath, J. Quan, R. S. King, W. H. Robinson, P. J. Utz, H. Garren, The BHT-3021 Investigators, L. Steinman, Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci. Transl. Med. 5, 191ra82 (2013).
REFERENCES AND NOTES
- 1.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, Roep BO, von Herrath MG. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO. Autoreactive CD8 T cells associated with β cell destruction in type 1 diabetes. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verge CF, Stenger D, Bonifacio E, Colman PG, Pilcher C, Bingley PJ, Eisenbarth GS. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes. 1998;47:1857–1866. doi: 10.2337/diabetes.47.12.1857. [DOI] [PubMed] [Google Scholar]
- 4.Kupila A, Sipilä JP, Keskinen T, Simell M, Knip K, Pulkki O. Simell, Intranasally administered insulin intended for prevention of type 1 diabetes—A safety study in healthy adults. Diabetes Metab. Res. Rev. 2003;19:415–420. doi: 10.1002/dmrr.397. [DOI] [PubMed] [Google Scholar]
- 5.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial—Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 6.Skyler JS, Weinstock RS, Raskin P, Yale JF, Barrett E, Gerich JE, Gerstein HC, Inhaled Insulin Phase III Type 1 Diabetes Study Group Use of inhaled insulin in a basal/bolus insulin regimen in type 1 diabetic subjects: A 6-month, randomized, comparative trial. Diabetes Care. 2005;28:1630–1635. doi: 10.2337/diacare.28.7.1630. [DOI] [PubMed] [Google Scholar]
- 7.Mamchak AA, Manenkova Y, Leconet W, Zheng Y, Chan JR, Stokes CL, Shoda LK, von Herrath M, Bresson D. Preexisting autoantibodies predict efficacy of oral insulin to cure autoimmune diabetes in combination with anti-CD3. Diabetes. 2012;61:1490–1499. doi: 10.2337/db11-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludvigsson J, Krisky D, Casas R, Battelino T, Castaño L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ, Veeze HJ, Palmer J, Samuelsson U, Elding Larsson H, Åman J, Kärdell G, Neiderud Helsingborg J, Lundström G, Albinsson E, Carlsson A, Nordvall M, Fors H, Arvidsson CG, Edvardson S, Hanås R, Larsson K, Rathsman B, Forsgren H, Desaix H, Forsander G, Nilsson NÖ, Åkesson CG, Keskinen P, Veijola R, Talvitie T, Raile K, Kapellen T, Burger W, Neu A, Engelsberger I, Heidtmann B, Bechtold S, Leslie D, Chiarelli F, Cicognani A, Chiumello G, Cerutti F, Zuccotti GV, Gomez Gila A, Rica I, Barrio R, Clemente M, López Garcia MJ, Rodriguez M, Gonzalez I, Lopez JP, Oyarzabal M, Reeser HM, Nuboer R, Stouthart P, Bratina N, Bratanic N, de Kerdanet M, Weill J, Ser N, Barat P, Bertrand AM, Carel JC, Reynaud R, Coutant R, Baron S. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 2012;366:433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 9. [28 April 2013]; http://www.andromedabio.com/page.php?pageID=80.
- 10.Bresson D, von Herrath M. Humanizing animal models: A key to autoimmune diabetes treatment. Sci. Transl. Med. 2011;3:68ps4. doi: 10.1126/scitranslmed.3002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coon B, An LL, Whitton JL, von Herrath MG. DNA immunization to prevent auto-immune diabetes. J. Clin. Invest. 1999;104:189–194. doi: 10.1172/JCI7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solvason N, Lou YP, Peters W, Evans E, Martinez J, Ramirez U, Ocampo A, Yun R, Ahmad S, Liu E, Yu L, Eisenbarth G, Leviten M, Steinman L, Garren H. Improved efficacy of a tolerizing DNA vaccine for reversal of hyperglycemia through enhancement of gene expression and localization to intracellular sites. J. Immunol. 2008;181:8298–8307. doi: 10.4049/jimmunol.181.12.8298. [DOI] [PubMed] [Google Scholar]
- 13.Ho PP, Fontoura P, Ruiz PJ, Steinman L, Garren H. An immunomodulatory GpG oligo-nucleotide for the treatment of autoimmunity via the innate and adaptive immune systems. J. Immunol. 2003;171:4920–4926. doi: 10.4049/jimmunol.171.9.4920. [DOI] [PubMed] [Google Scholar]
- 14.Roep BO. The role of T-cells in the pathogenesis of type 1 diabetes: From cause to cure. Diabetologia. 2003;46:305–321. doi: 10.1007/s00125-003-1089-5. [DOI] [PubMed] [Google Scholar]
- 15.Williams AJ, Norcross AJ, Dix RJ, Gillespie KM, Gale EA, Bingley PJ. The prevalence of insulin autoantibodies at the onset of type 1 diabetes is higher in males than females during adolescence. Diabetologia. 2003;46:1354–1356. doi: 10.1007/s00125-003-1197-2. [DOI] [PubMed] [Google Scholar]
- 16.Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, Hering BJ, Hafler DA. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224–228. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 17.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J. Clin. Invest. 2004;113:451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve β-cell function: Report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 19.Greenbaum CJ, Harrison LC. Immunology of Diabetes Society, Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes. 2003;52:1059–1065. doi: 10.2337/diabetes.52.5.1059. [DOI] [PubMed] [Google Scholar]
- 20.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, Battelino T, Haastert B, Ludvigsson J, Pozzilli P, Lachin JM, Kolb H. Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group, Mixed-meal tolerance test versus glucagon stimulation test for the assessment of β-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31:1966–1971. doi: 10.2337/dc07-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velthuis JH, Unger WW, Abreu JR, Duinkerken G, Franken K, Peakman M, Bakker AH, Reker-Hadrup S, Keymeulen B, Drijfhout JW, Schumacher TN, Roep BO. Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell–associated epitopes using combinatorial MHC multimers. Diabetes. 2010;59:1721–1730. doi: 10.2337/db09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schloot NC, Roep BO, Wegmann D, Yu L, Chase HP, Wang T, Eisenbarth GS. Altered immune response to insulin in newly diagnosed compared to insulin-treated diabetic patients and healthy control subjects. Diabetologia. 1997;40:564–572. doi: 10.1007/s001250050716. [DOI] [PubMed] [Google Scholar]
- 23.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 24.Velthuis JH, Unger WW, van der Slik AR, Duinkerken G, Engelse M, Schaapherder AF, Ringers J, van Kooten C, de Koning EJ, Roep BO. Accumulation of autoreactive effector T cells and allo-specific regulatory T cells in the pancreas allograft of a type 1 diabetic recipient. Diabetologia. 2009;52:494–503. doi: 10.1007/s00125-008-1237-z. [DOI] [PubMed] [Google Scholar]
- 25.Unger WW, Pearson T, Abreu JR, Laban S, van der Slik AR, der Kracht SM, Kester MG, Serreze DV, Shultz LD, Griffioen M, Drijfhout JW, Greiner DL, Roep BO. Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/scid/IL2RG null mice. PLoS One. 2012;7:e49213. doi: 10.1371/journal.pone.0049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: Evidence for early determination of subsequent diabetes. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.