Abstract
Background
This study examines real-world drug utilization patterns, health care resource use, and costs among patients receiving adjuvant treatment with IFN versus patients receiving no treatment (“observation”) for malignant melanoma following surgery.
Methods
A retrospective cohort study was conducted using administrative claims from Truven Health Analytics (MarketScan®) to identify all adjuvant melanoma patients (aged ≥18 years) diagnosed between June 2007 and June 2011 who had a lymph node dissection (ie, index surgery) and were treated with IFN or subsequently observed. Health care resource use and costs of services were converted to 2012 US dollars and were evaluated and compared using multivariable regression.
Results
Of 1,999 eligible subjects with melanoma surgery claims, 179 (9.0%) were treated with IFN and 1,820 (91.0%) were observed. The median duration (days) and number of doses of IFN therapy were 73 and 36, respectively. Among IFN-treated patients, only 10.6% completed ≥80% of maintenance therapy. The total average cost for patients treated with IFN was US$60,755±$3,972 (n=179); significantly higher than for patients undergoing observation ($31,641±$2,471; P<0.0001). Similar trends were observed when evaluating total cost components, including melanoma-related and non-melanoma–related medical costs. Among the melanoma-related medical costs, outpatient services, including office visits and laboratory testing, represented between 33% and 53% of total costs and demonstrated the largest difference between IFN-treated and observation patients. Outpatient service costs for IFN-treated patients were $32,414±$2,498, over three times greater than those for observation patients ($10,556±$1,128; P<0.0001).
Conclusion
The majority of adjuvant melanoma patients in this study was treated with observation versus IFN treatment. Among those who attempted IFN treatment, most could not complete the recommended course of therapy. Health care costs were significantly greater for patients treated with IFN, with the greatest differences being for melanoma-related medical cost components. These findings illustrate the significant economic burden borne by adjuvant melanoma patients and their health insurers.
Keywords: adjuvant melanoma, interferon, claims data, cost analysis
Introduction
Over the last decade, the incidence of melanoma has increased faster than that of any other solid tumor.1 Currently, the lifetime risk of developing melanoma is one in 50, and in 2013 nearly 80,000 new cases of melanoma were diagnosed in the USA alone.2 Although early recognition and surgical excision clearly represent the best opportunity for cure, there are also patients who present at more advanced stages.
Patients with thick or ulcerated primary lesions (American Joint Committee on Cancer stage IIB or IIC) and patients with pathological or clinical evidence of regional nodal metastasis (American Joint Committee on Cancer stage III) represent a significant treatment challenge, with a reported 5-year survival rate ranging from 30% to 70%.3 According to US national guidelines, adjuvant therapy is offered to patients with stage IIB, IIC, or III disease who have undergone surgery, consisting exclusively of treatment with IFN-α under different formulations, such as high-dose IFN (HDI) and pegylated IFN. However, according to the National Comprehensive Cancer Network, the impact of adjuvant IFN on overall survival (OS) remains unclear.4 Enrollment in clinical trials and observation are also valid treatment options for patients with stage IIB, IIC, or III melanoma.4 For both physicians and patients, the factors that drive decision making in these cases are complex.
The basis for adjuvant treatment of stage IIB, IIC, or III disease with IFN-α rests on a randomized, multicenter, national trial, E1684, that was conducted by the Eastern Cooperative Oncology Group in 1995 (n=287).5 Data from this trial demonstrated significantly improved relapse-free survival (RFS) and OS with the use of adjuvant HDI in patients with non-metastatic but locally advanced melanoma. Based on these data, the US Food and Drug Administration approved HDI for post-surgical adjuvant treatment of high-risk melanoma patients; however, data in subsequent trials concerning the adjuvant use of IFN-α have been less conclusive. For example, in trial E1690 (n=642), conducted by the same investigators when commercial IFN became available as a post-study drug, HDI did not exhibit a significant OS benefit when compared with observation alone (hazard ratio [HR] =1.0; P=0.995).6 A similar finding was reported when comparing low-dose IFN-α versus observation. Another trial designed to evaluate HDI versus observation for 1 month (E1697; n=1,150) was discontinued at interim analysis due to lack of IFN treatment benefit.7 Conversely, a formal meta-analysis of 18 randomized controlled trials enrolling over 10,000 participants (published between 1995 and 2011) demonstrated that adjuvant IFN was associated with significantly improved disease-free survival (HR =0.83; 95% confidence interval [CI] 0.78–0.87; P<0.00001) and OS (HR =0.91; 95% CI 0.85–0.97; P=0.003).8 As a result, an inconsistent standard of care has emerged, with North American guidelines promoting the use of HDI for stage IIB, IIC, or III disease, while European oncologists and other health care decision-makers have concluded that routine use of IFN cannot be recommended.9,10
In addition to the clinical debate around the use of HDI for stage IIB, IIC, or III melanoma, controversies exist from an economic standpoint. Whereas some investigators have found that adjuvant use of HDI for non-metastatic melanoma is cost-effective, with cost-utility ratios comparable to those for other cancer interventions,11 others have suggested that it may be cost-effective in only the most advanced adjuvant sub-stages.12
Despite these contradictory findings and the substantive debate in the melanoma literature, IFN-α remains an important treatment option for many patients with non-metastatic but locally advanced melanoma in the USA. There are other factors to consider in addition to clinical efficacy, including tolerability and cost. The most prominent acute toxicities of IFN comprise an influenza-like syndrome and include fatigue, depression, and myalgia, among others.13,14
Real-world experience with IFN therapy and its associated costs are increasingly of interest, not only to payers but also to physicians and patients.15 Real-world data can supplement results from randomized clinical trials with additional information on comparative effectiveness, safety, and cost, helping to optimize the management of patients with cancer.16 However, real-world research with IFN therapy in melanoma patients is limited.15 Our study, which builds upon a similar, retrospective, real-world study in this setting,15 used a US-based administrative claims dataset to examine the real-world treatment patterns associated with IFN therapy and to examine health care resource use (HCRU) and direct medical costs among patients receiving adjuvant IFN treatment versus patients receiving no treatment (observation) for non-metastatic but locally advanced malignant melanoma.
Materials and methods
Design and setting
This was a retrospective cohort analysis using the Truven Health MarketScan Claims Data for the period June 1, 2007 to June 30, 2011. The MarketScan database captures person-specific clinical utilization, expenditures, and enrollment across inpatient, outpatient, prescription drug, and carve-out services from a selection of large employers, health plans, and government and public organizations. It links paid claims with encounter data and includes private sector health data from approximately 100 payers. These data represent the medical experience of insured employees and their dependents for active employees, early retirees, Consolidated Omnibus Budget Reconciliation Act continuers, and Medicare-eligible retirees with employer-provided Medicare supplemental plans for approximately 43 million individuals. It also includes inpatient and outpatient diagnoses and procedures as well as retail and mail order prescription records. Available data on prescription records include the National Drug Code and the quantity of the medication dispensed. Charged, allowed, and paid amounts are available for all services rendered, as are dates of service for all claims. Additional data elements include demographic variables (age, sex, and geographic region), product type (eg, health maintenance organization, preferred provider organization), payer type (eg, commercial, self-pay), provider specialty, and eligibility dates related to plan enrollment and participation.
Subjects and treatments
The study identified non-metastatic melanoma patients following local-regional lymph node dissection (ie, non-metastatic, post-surgical lymph node dissection). Since staging information was not available in the database, an operational definition for non-metastatic, post-surgical melanoma was used, with specific inclusion criteria as follows: a) procedure code for melanoma-related surgical intervention (ie, lymph node dissection; Table S1) any time during the identification period between June 1, 2007 and June 30, 2011 (index surgery); b) primary diagnosis based on International Statistical Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes (172.0–172.9 or V10.82) any time during 90 days prior to or after the index surgery; c) age ≥18 years at the time of the index surgery; and d) continuous enrollment for 6 months prior to and 3 months after the index surgery. Patients were excluded if they received systemic chemotherapy during the 180 days prior to or 60 days after the index surgery (ie, lymph node dissection; Table S1), or if they had an ICD-9 diagnosis code for any other primary cancer (140.x-171.x, 174.x-195.x, 199.x, 209.x) on ≥1 inpatient claims ≥2 outpatient visit claims, during the 180 days prior to and including or 60 days after the index date. Patients were also excluded if they had an ICD-9 diagnosis code for any secondary cancer on ≥1 inpatient claims, or ≥2 outpatient visit claims, during the 180-day period prior to and including the index date. This last criterion was not applied if the ICD-9 diagnosis code was for a metastasis involving a site common to melanoma (196.0, 196.3, 196.5) or was unlikely to be associated with another primary cancer (Table S2).
Eligible patients with receipt of melanoma-related systemic chemotherapy, defined as a claim for dacarbazine and/or temozolomide and/or paclitaxel and/or carmustine and/or cisplatin and/or carboplatin and/or vinblastine and/or ipilimumab and/or vemurafenib (Table S1), beyond 60 days from the index surgery were right censored. Patients were followed until the end of continuous enrollment or the end of the patient data.
The non-metastatic, post-surgical melanoma cohort included all patients identified per the inclusion/exclusion criteria above and constituted the overall study population of non-metastatic, post-surgical melanoma patients. This cohort was sub-classified into two cohorts: a) the IFN cohort: patients with evidence of treatment with IFN within 120 days of the index surgery and b) the non-IFN (observation) cohort: patients without evidence of treatment with IFN within 120 days of the index surgery.
Data analyses
The available IFN claims data were used to assess compliance with established recommendations for IFN treatment. Adjuvant IFN therapy for non-metastatic melanoma is 12 months in duration and consists of induction and maintenance phases. The induction phase requires intravenous administration of IFN five times per week for 4 weeks (20 doses), followed by a maintenance phase consisting of self-administered subcutaneous injections three times per week for 48 weeks (144 doses).13 We implemented a 2-week window to allow for real-world variation in dosing during induction. Consequently, the induction phase was regarded as the first 42 days following initiation of IFN therapy, while days 43–365 were taken to comprise the maintenance phase. Patients were censored before day 365 if they received chemotherapy, lacked continuous enrollment, or were lost to observation.
Treatment patterns with IFN therapy
For patients in the IFN cohort, the duration and frequency of dosing were observed. Based on the frequency of dosing, completion of IFN therapy was defined as receipt of 20 doses intravenously during the induction phase and 144 doses of self-administered subcutaneous injections during the maintenance phase.15 The percentages of patients receiving ≥80% (treatment compliance), ≥50%, and <50% (treatment discontinuation) of the expected 164 doses during the induction and maintenance phases were reported.
Resource utilization and cost
Economic measures of interest included costs associated with the index surgery, follow-up surgeries, IFN therapy, melanoma-related outpatient care, ancillary services, and hospital admissions. Imaging costs were not considered. Additionally, non-melanoma–related costs, such as costs of medications other than IFN and outpatient and inpatient costs without a listed melanoma diagnosis, were considered. Costs were assessed using the amount paid to providers for a claim or health service, including copayment(s) and/or coinsurance, and were reported on a per-patient per-year basis. All costs were expressed in 2012 US dollars and were adjusted using the medical care component of the US Consumer Price Index.17
Melanoma-related (index) surgery resource use and costs included all index surgery, anesthesia, pathology, and hospital care-related claims and associated costs. Follow-up (post-index) surgery resource use costs included all post-index surgery, anesthesia, pathology, and hospital care-related claims and associated costs. IFN therapy resource use and costs included drug costs along with the services required to monitor IFN therapy (complete blood count panels, thyroid-releasing hormone stimulation tests, infusion administrations, and associated office visits). Finally, melanoma-related medical service resource use and costs included outpatient care as well as ancillary/laboratory and radiation therapy services associated with a melanoma ICD-9 diagnosis. A claim was considered to be melanoma-related as long as it was associated with a diagnosis of melanoma regardless of its position (ie, primary diagnosis or not).
Comparison of post-index costs
Aggregate melanoma-related direct medical costs were examined separately for IFN- and non IFN-treated patients. In addition to unadjusted comparisons, generalized linear modeling was employed to assess differences in resource use and costs while controlling for potential confounding factors. Observed data patterns provided guidance as to the selection of model specifications (eg, negative binomial for resource use and gamma for costs). In addition, univariate results provided guidance as to which resource-use and cost measures were modeled. Explanatory variables in the base model included: a) age (18–34, 35–44, 45–54, 55–64, or 65+ years); b) sex; c) plan type (health maintenance organization, preferred provider organization, point of sale, indemnity, or unknown); d) geographic region; e) payer type (commercial, Medicaid, Medicare, self-insured, or other); f) physician specialty; and g) Charlson comorbidity index score.18 Candidate variables were retained in the model if significant (P<0.05) or if there was a strong clinical rationale for their inclusion. The coefficient of determination and scaled deviance were used to assess model goodness of fit. Since patients had variable follow-up, costs were annualized. The annualized cost was calculated as the total cost divided by duration of follow-up with the resulting quotient being extrapolated to a 12-month time horizon.
Results
Patient population
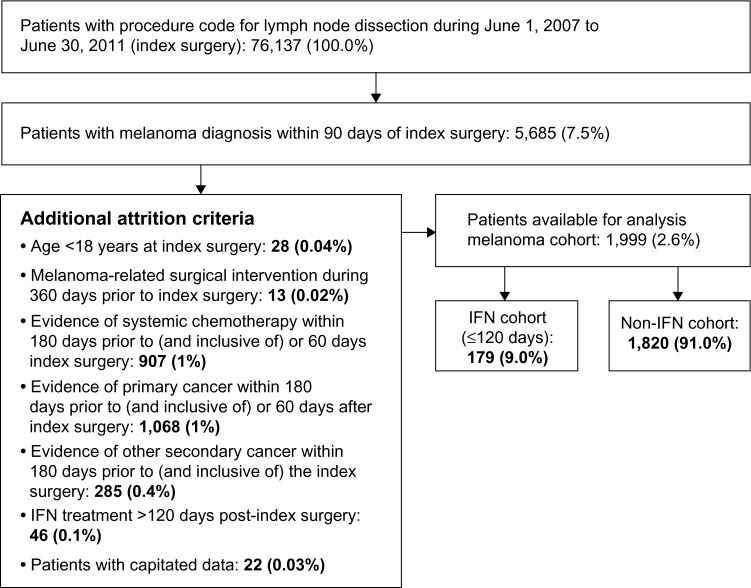
An index surgery event was identified for 76,137 patients (Figure 1), of whom 5,685 had a confirmed claim for a melanoma diagnosis within 90 days of the index surgery. Additional selection criteria reduced the final cohort for analysis to 1,999 non-metastatic, post-surgical patients. Additional attrition criteria included: 1) age <18 years (n=28); 2) melanoma-related surgical interventions (n=13); 3) evidence of systemic chemotherapy in the pre-index or post-index periods (n=907); 4) evidence of other primary (n=1,068) or secondary (n=285) cancer in the pre-index or post-index periods; 5) patients with IFN treatment 120 days beyond the index surgery (n=46); and 6) patients with capitated data (n=22) (Figure 1). Of the 1,999 patients who comprised our final cohort, 179 (9.0%) received IFN treatment and 1,820 (91.0%) did not receive adjuvant IFN therapy and were classified as observed. Clinical and demographic characteristics for the IFN and non-IFN cohorts are presented in Table 1. The two groups differed significantly with regard to age at first melanoma-related surgery, geographic region, and the presence of cerebrovascular disease prior to index surgery, but not sex, physician specialty at index, other pre-index comorbidities, or comorbidity index score. The number (percent) of patients who received chemotherapy agents during their follow-up period was 18 (10.1%) and 127 (7.0%) in the IFN and non-IFN cohorts, respectively. Mean (standard deviation) follow-up was 738 days (404.8) and 704 days (421.6) in the IFN and non-IFN cohorts, respectively, while median (range) follow-up was 680 days (109–1,780) and 619 days (62–1,825), respectively.
Figure 1.
Attrition of sample size, by reason.
Table 1.
Demographic and clinical characteristics
| Measure | Non-metastatic, post-surgery melanoma cohort
|
P-value
|
|||
|---|---|---|---|---|---|
| Patients with IFN therapy following index surgery (N=179)
|
Patients without IFN therapy following index surgery (N=1,820)
|
IFN treatment vs non-IFN
|
|||
| N | Column % | N | Column % | ||
| Total patients | 179 | 100 | 1,820 | 100 | |
| Age at first melanoma-related surgery | <0.0001 | ||||
| 18–54 years | 104 | 58.1 | 714 | 39.2 | |
| 55–64 years | 56 | 31.3 | 546 | 30.0 | |
| 64–74 years | 14 | 7.8 | 265 | 14.6 | |
| 75–84 years | 5 | 2.8 | 233 | 12.8 | |
| ≥85 years | 0 | 0.0 | 62 | 3.4 | |
| Mean | 53 | 58 | <0.0001 | ||
| SD | 10.7 | 14.8 | |||
| Median | 53 | 59 | |||
| Minimum | 21 | 19 | |||
| Maximum | 78 | 96 | |||
| Sex | |||||
| Male | 102 | 57.0 | 1,070 | 58.8 | |
| Female | 77 | 43.0 | 750 | 41.2 | |
| Geographic region | 0.0116 | ||||
| Northeast | 26 | 14.5 | 299 | 16.4 | |
| North Central | 50 | 27.9 | 434 | 23.8 | |
| South | 77 | 43.0 | 667 | 36.6 | |
| West | 22 | 12.3 | 402 | 22.1 | |
| Unknown | 4 | 2.2 | 18 | 1.0 | |
| Physician specialty at index | |||||
| Dermatology | 0 | 0.0 | 5 | 0.3 | |
| Oncology | 2 | 1.1 | 40 | 2.2 | |
| Other | 35 | 19.6 | 331 | 18.2 | |
| Primary care | 15 | 8.4 | 185 | 10.2 | |
| Surgeon | 116 | 64.8 | 1,163 | 63.9 | |
| Unknown | 11 | 6.1 | 96 | 5.3 | |
| Comorbidities of interesta | |||||
| Diabetes mellitus | 25 | 14.0 | 284 | 15.6 | |
| Hyperlipidemia | 59 | 33.0 | 625 | 34.3 | |
| Essential hypertension | 71 | 39.7 | 779 | 42.8 | |
| Congestive heart failure | 4 | 2.2 | 81 | 4.5 | |
| Cerebrovascular disease | 3 | 1.7 | 107 | 5.9 | 0.0150 |
| Chronic pulmonary disease | 17 | 9.5 | 191 | 10.5 | |
| Dementia | 0 | 0.0 | 5 | 0.3 | |
| Osteoporosis | 5 | 2.8 | 54 | 3.0 | |
| Charlson comorbidity indexb | |||||
| 0 | 150 | 83.8 | 1,414 | 77.7 | |
| 1 | 16 | 8.9 | 216 | 11.9 | |
| 2 | 9 | 5.0 | 118 | 6.5 | |
| 3 | 2 | 1.1 | 48 | 2.6 | |
| 4+ | 2 | 1.1 | 24 | 1.3 | |
Notes:
Measured during 365 days prior to index surgery;
for patients who used chemotherapy after index surgery, their follow-up period is censored at the first chemotherapy.
Abbreviations: SD, standard deviation; vs, versus.
Adjuvant IFN therapy treatment patterns
Of the 179 patients who received adjuvant IFN treatment, only 100 (55.9%) successfully completed the 20-dose induction phase (Table 2). Among the 79 patients who did not complete the induction phase, the average (median) duration of follow-up was 591 (537) days. One hundred and ten (61.5%) of the original 179 patients in this IFN cohort went on to receive maintenance dosing. During the maintenance phase, only 19 patients (10.6%) completed at least 80% of the required 144 doses. Among the patients who initiated maintenance treatment, the average (median) duration of follow-up was 718 (627) days. Fifty-one patients (28.5%) discontinued IFN maintenance therapy as indicated by receipt of <50% of the required maintenance dose administrations. Among patients in the IFN cohort, 27.9% were on therapy for 2–29 days, 21.2% for 30–90 days, and 9.5% for 91–180 days. Less than one-third of patients (n=57) continued therapy for between 181 and 365 days. A small minority of IFN-treated patients (5%) had evidence of maintenance therapy lasting beyond 365 days for reasons that could not be explained by the data. The average duration of IFN therapy was 143±136 days, and the average number of completed doses was 57±50 (Table 2).
Table 2.
Treatment patterns of IFN therapy
| Measure | Non-metastatic, post-surgery melanoma cohort
|
|
|---|---|---|
| Patients with IFN therapy following index surgery (N=179)
| ||
| N | Column % | |
| Total patients | 179 | 100 |
| Total patients completing induction phasea | 100 | 55.9 |
| Total patients starting maintenance phaseb | 110 | 61.5 |
| Patients who completed ≥80% of maintenance dose | 19 | 10.6 |
| Patients who completed <50% of maintenance dosec | 60 | 33.5 |
| Patients who discontinued maintenance phase because end of enrollmentc,d | 9 | 5.0 |
| Patients who completed <50% of maintenance dose (discontinuation) | 51 | 28.5 |
| Patients who completed maintenance phase | 0 | 0.0 |
| Total duration of IFN therapy | ||
| 1 day (only dose of IFN) | 8 | 4.5 |
| 2–29 days | 50 | 27.9 |
| 30–90 days | 38 | 21.2 |
| 91–180 days | 17 | 9.5 |
| 181–365 days | 57 | 31.8 |
| >365 days | 9 | 5.0 |
| Duration of IFN therapy (days) | ||
| Mean: 143 | ||
| SD: 135.7 | ||
| Median: 73 | ||
| Minimum: 1 | ||
| Maximum: 568 | ||
| Total IFN doses | ||
| Mean: 57 | ||
| SD: 50.4 | ||
| Median: 36 | ||
| Minimum: 1 | ||
| Maximum: 168 | ||
Notes:
Induction phase is defined as first 42 days from the start of IFN therapy (20 doses in the induction phase is considered complete);
maintenance phase is defined as the period from the 43rd to 365th day from the start of IFN therapy (144 doses in the maintenance phase is considered complete; fewer than 72 doses [50% of 144] is considered a discontinuation);
National Drug Code is counted according to the days of supply: 28 days of supply =12 doses (j codes counted as single dose);
if final prescription (date + days of supply) is within 60 days of final enrollment date, it is not considered a discontinuation.
Abbreviation: SD, standard deviation.
Adjuvant IFN therapy HCRU and costs
HCRU and adjusted average treatment costs per melanoma patient per year are reported in Table 3 for both IFN-treated and observation patients, and include those associated with the index surgery, any follow-up surgeries, and melanoma-related medical care. Total costs are also shown. The total average cost per IFN-treated patient was $60,755±$3,972 (n=179); significantly higher than the total average cost of patients undergoing observation ($31,641±$2,471; P<0.0001). The adjusted ratio of total IFN-related treatment costs to observation-related treatment costs was 1.92. HCRU and medical costs for melanoma-related medical services and non-melanoma medical services were also significantly higher for IFN-treated patients compared to observation patients. Melanoma-related medical costs were $38,730±$4,254 for IFN-treated patients versus $13,995±$2,676 for observation patients (P<0.001), and non-melanoma–related medical costs were $22,415±$12,668 for IFN-treated patients versus $18,442±$13,438 for observation patients (P<0.0001). Costs for outpatient services, including office visits and laboratory testing, comprised 33%–53% of all melanoma-related medical costs and, among all costs, exhibited the greatest difference between IFN-treated and observation patients. Outpatient service costs for IFN-treated patients were $32,414±$2,498 and were over three times greater than those for observation patients ($10,556±$1,128; P<0.0001).
Table 3.
Per-patient per-year health care utilization and costsa
| Measure
|
Non-metastatic, post-surgery melanoma cohort
|
Ratio IFN treatment/non-IFN treatment b | P-value for mean cost | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with IFN therapy following index surgery (N=179)
|
Patients without IFN following index surgery (N=1,820)
|
|||||||||||
| Resource | N | Mean | SD | IQR | Median | N | Mean | SD | IQR | Median | ||
| Index surgery | ||||||||||||
| Anesthesia | 172 | $1,014 | $640 | $458 | $869 | 1,733 | $679 | $444 | $379 | $613 | 1.49 | <0.0001 |
| Surgery | 179 | $2,030 | $1,079 | $777 | $1,740 | 1,818 | $1,419 | $742 | $677 | $1,249 | 1.43 | <0.0001 |
| Pathology | 174 | $370 | $198 | $155 | $330 | 1,740 | $400 | $239 | $180 | $362 | 0.93 | |
| Hospital care | 36 | $454 | $274 | $372 | $372 | $229 | $433 | $311 | $309 | $329 | 1.05 | |
| Follow-up surgery/surgeries | ||||||||||||
| Anesthesia | 80 | $762 | $409 | $360 | $663 | 672 | $636 | $376 | $362 | $592 | 1.20 | 0.0054 |
| Surgery | 7 | $1,688 | $1,051 | $1,552 | $1,380 | 77 | $2,032 | $2,751 | $1,457 | $1,195 | 0.83 | |
| Pathology | 130 | $376 | $107 | $88 | $359 | 1,219 | $321 | $98 | $99 | $310 | 1.17 | <0.0001 |
| Hospital care | 56 | $490 | $252 | $243 | $430 | 418 | $549 | $268 | $300 | $497 | 0.89 | |
| Melanoma-related medical services | ||||||||||||
| Outpatient services | 178 | $32,414 | $2,498 | $3,490 | $32,200 | 1,791 | $10,556 | $1,128 | $1,504 | $10,444 | 3.07 | <0.0001 |
| Office visits | 177 | $1,995 | $709 | $697 | $1,824 | 1,771 | $1,048 | $409 | $358 | $972 | 1.90 | <0.0001 |
| Outpatient laboratory | 165 | $277 | $136 | $167 | $248 | 1,163 | $79 | $42 | $47 | $72 | 3.50 | <0.0001 |
| Impatient services | 80 | $10,868 | $4,716 | $5,369 | $10,798 | 448 | $11,358 | $6,241 | $8,554 | $10,626 | 0.96 | |
| Radiation therapy | 33 | $7,627 | $5,006 | $4,406 | $5,948 | 148 | $8,360 | $5,836 | $6,836 | $6,681 | 0.91 | |
| Total cost | ||||||||||||
| Melanoma-related medical | 178 | $38,730 | $4,254 | $2,679 | $39,140 | 1,797 | $13,995 | $2,676 | $5,411 | $15,276 | 2.77 | <0.0001 |
| Non-melanoma-related medical | 179 | $22,415 | $12,668 | $10,204 | $19,026 | 1,811 | $18,442 | $13,438 | $7,753 | $15,416 | 1.22 | <0.0001 |
| Overall total cost | 179 | $60,755 | $3,972 | $7,283 | $58,968 | 1,818 | $31,641 | $2,471 | $6,182 | $31,214 | 1.92 | <0.0001 |
Notes:
Adjusted for age index date (group), insurance type at index date, region, sex, specialty at index date, year of diagnosis, year of index, listed comorbidities, and listed chemotherapy;
ratio IFN treatment/non-IFN treatment was based on mean values. Costs reported in 2012 USD.
Abbreviations: SD, standard deviation; IQR, interquartile range.
Discussion
The objective of our study was to use a US-based administrative claims dataset (Truven Health MarketScan Claims Data; June 1, 2007 to June 30, 2011) to examine real-world treatment patterns associated with IFN therapy as well as to examine HCRU and medical costs among patients receiving adjuvant treatment with IFN versus patients receiving no treatment (observation). Our analysis found that the majority of patients (91.0%) did not receive adjuvant IFN therapy and were instead observed. The rate of IFN use was lower than would be expected for patients with stage II or III disease, and may limit the generality of our findings. Additionally, among patients who did receive IFN treatment, the vast majority were unable to successfully complete either the induction phase (44.1%) or maintenance phase (89.4%) of therapy. Treatment costs were considerably higher for IFN-treated patients, with a total average cost per IFN-treated patient nearly twice the total average cost per observation patient. Melanoma-related medical costs, including office visits and laboratory testing, comprised a significant proportion of total treatment costs for both IFN-treated patients and observation patients. However, these costs were more than three times higher for IFN-treated patients than patients who were not treated with IFN.
Despite conflicting evidence in the clinical5–8 and economic literature,11,12 there have been relatively few published studies of the real-world patterns of use and costs associated with adjuvant IFN in non-metastatic melanoma patients.15,19,20 A real-world, retrospective, US study by Hackshaw et al using administrative claims described treatment patterns, health care resource utilization, and costs for patients with malignant melanoma diagnosed between 2004 and 2008 who received IFN therapy following surgery.15 In that study, approximately half of patients with malignant melanoma on IFN therapy following surgery did not complete the recommended 1-year treatment course, potentially compromising the full therapeutic benefits of IFN and underscoring an unmet treatment need. For patients to achieve the full clinical and economic benefits of IFN treatment, it is important that they adhere to the recommended therapy regimen. This is especially true for the induction phase; induction is considered an essential element in the treatment schedule because the separation of Kaplan–Meier curves for RFS occurs very early in treatment.21 In our study, fewer than 60% of patients completed the induction phase of treatment according to the predefined criteria. The authors of another study evaluating a 4-week course of IFN therapy, equivalent to the induction phase alone, reported no improvement in RFS (6.8 years vs 7.3 years for observation).7,22 These findings suggest that a longer duration of treatment may be important. In our analysis, approximately 10% of patients completed the maintenance phase of IFN therapy, with an average duration of treatment of 143 days and an average of <60 doses completed. The main reasons for IFN discontinuation, although unknowable in this claim-based analysis, are likely to be related to disease progression and toxicity.5 Possible additional factors contributing to discontinuation may include the inconvenience of infusion administration and frequency of therapy during the induction phase.23 Reasons for discontinuation could not be determined in our study due to the data source used.
Limitations
The results of this research should be interpreted in light of the study design. There are important limitations to be considered when evaluating the results of a retrospective administrative claims database study. Notably, disease stage could not be confirmed due to the absence of staging information in the database. We assumed that receipt of IFN therapy (or observation) and the absence of systemic chemotherapy following lymph node surgery among melanoma patients was representative of regional disease. We were also unable to fully discriminate between sentinel lymph node biopsy and lymph node dissection in this analysis. This last limitation may have led to an oversampling of patients with less advanced disease, limiting the generalizability of the results. That said, a similar approach for identifying adjuvant IFN eligible patients was adopted and published by Hackshaw et al and was shown to be an acceptable method for identifying adjuvant IFN eligible patients given the limitations of claims-based data.15 Furthermore, the rate of IFN use in our study was lower than would be expected, though it was consistent with the findings of Hackshaw et al who reported a prevalence of IFN treatment of 8.4% among 18,075 patients with a confirmed claim of surgery related to melanoma.15 The low apparent rate of IFN treatment can be attributed to the inability to stage patients accurately using claims data. Most surgically resected patients are not candidates for IFN treatment due to being classified with an earlier stage of disease. It is conceivable that the IFN cohort in our study included such patients. Additionally, significant differences in the pre-index characteristics of the IFN and observation cohorts were observed, and these could have contributed in part to the research findings. There was also a lack of congruence between the recommended length of IFN treatment and required duration of follow-up. However, we believe the eligibility criteria that were applied were conservative and that a sensitivity analysis of the inclusion of patients with a minimum of 12 months of follow-up would not be useful due to the expected bias and observed duration of follow-up among most patients.
Choices made in the design of this study affected our ability to assess completion of the induction and maintenance phases of IFN treatment, in a similar manner to the retrospective study by Hackshaw et al.15 Completion of the induction phase was measured as the receipt of ≥20 doses within 42 days after the start of IFN treatment, whereas completion of the maintenance phase was measured as the receipt of ≥144 doses between days 43 and 365 after the start of IFN treatment. Phase completion was assessed among those patients with continuous enrollment. Given that patients were included if IFN treatment was initiated within 120 days of the index surgery, an assessment of the completion of the induction phase required a minimum of 42 and maximum of 162 days of follow-up, while an assessment of the completion of the maintenance phase required a minimum of 365 and maximum of 485 days of follow-up. In spite of the variability in required follow-up, most patients included in the IFN cohort were followed for a sufficient period of time to assess completion of the induction and maintenance phases. Among the 179 patients included in the IFN cohort, 79 did not complete the induction phase, and among these the average (median) duration of follow-up was 591 (537) days. Similarly, 110 of the 179 patients included in the IFN cohort started the maintenance phase, and among these the average (median) duration of follow-up was 718 (627) days.
Assumptions were also made in our study about medication compliance and completion rates, particularly during the self-administered maintenance phase of IFN therapy. Because the induction phase required an intravenous infusion, it was safe to assume that medication claims represented actual administration during induction; however for the self-administered maintenance phase, it was necessary to assume that a medication claim for IFN represented a self-administration of the drug. Although this assumption could not be verified, medication claims are commonly used to assess medication compliance in the published literature.24 While the number of IFN doses received was quantified and related to treatment discontinuation, analyses of IFN dose (ie, units received) were not performed. Finally, costs were measured in our study using amounts paid to providers. Actual costs may have been lower than the reimbursement rates used in our research. It is not known whether any of the patients who contributed data to our study were participating in a clinical trial. If so, then it is possible that participation in a sponsored clinical trial impacted the patient’s claims history, resulting in fewer claims and/or lower costs.
Conclusion
This retrospective cohort study shows that the majority of adjuvant melanoma patients were treated with observation (vs IFN treatment). Among those patients who attempted IFN treatment, most were unable to complete the induction or maintenance phases, which may have limited the clinical effectiveness of their therapy.15 Additionally, total health care costs were significantly greater for patients treated with IFN than for those who were not treated with IFN. These results, which may have implications for payers/decision-makers, suggest a need to consider the implications of adjuvant treatment selection following index surgery and provide information about the costs associated with IFN therapy. Future studies are needed that combine claims and electronic databases, thereby allowing more accurate staging and elimination of contamination of the IFN cohort with patients with less severe disease.
Supplementary materials
Table S1.
Identification codes for data analysis
| CPT4 codes for surgical intervention* | |
| 38562 | Removal, pelvic lymph nodes |
| 38564 | Removal, abdomen lymph nodes |
| 38571/38572 | Laparoscopy, lymphadenectomy |
| 38700/38720/38724 | Removal of lymph nodes, neck |
| 38740/38745 | Remove armpit lymph nodes |
| 38746 | Remove thoracic lymph nodes |
| 38747 | Remove abdominal lymph nodes |
| 38760/38765 | Remove groin lymph nodes |
| 38770 | Remove pelvis lymph nodes |
| 38780 | Remove abdomen lymph nodes |
| CPT4 codes for HCRU associated with surgery* | |
| 00100–01999 | All anesthetic codes |
| 88302/88304/88305/88307/88309 | Tissue examination by pathologist |
| 80500/80502 | Laboratory pathology consultation |
| 88399 | Surgical pathology procedure |
| 89240 | Pathology laboratory procedure |
| 99218/99219/99220 | Observation care |
| 99221/99222/99223 | Initial hospital care |
| 99234/99235/99236 | Observation/hospitalization same date |
| 99238/99239 | Hospital discharge day |
| 99251/99252/99253/99254/99255 | Inpatient consultation |
| CPT4 codes for HCRU associated with IFN therapy* | |
| 96413 | Chemotherapy administration, IV infusion |
| 99201/99202/99203/99204/99205 | Office/outpatient visit, new |
| 99211/99212/99213/99214/99215 | Office/outpatient visit |
| CPT4 codes for blood laboratory work associated with IFN therapy* | |
| 85025 | Complete CBC with auto differential WBC |
| 80438/80439/80440 | TRH stimulation panel |
| J codes (chemotherapy) associated with a melanoma diagnosis# | |
| J9214 | Injection, IFN-α-2b |
| J9130 | Dacarbazine, 100 mg |
| J9140 | Dacarbazine, 200 mg |
| J8700 | Temozolomide, oral, 5 mg |
| J9060 | Cisplatin, powder or solution, per 10 mg |
| J9062 | Cisplatin, 50 mg |
| J9045 | Injection, carboplatin, 50 mg |
| J9265 | Injection, paclitaxel, 30 mg |
| J9050 | Injection, carmustine, 100 mg |
| J9360 | Injection, vinblastine sulfate, 1 mg |
| J9228 | Injection, ipilimumab, 1 mg |
Notes:
American Medical Association, 2009;
Centers for Medicare and Medicaid Services, 2009.
Abbreviations: CBC, complete blood count; IV, intravenous; TRH, thyrotropin-releasing hormone; WBC, white blood cell; HCRU, health care resource use.
Table S2.
Improbable sites of lymph node metastasis from selected primary cancers
| Primary cancer | Improbable sites of lymph node metastasis |
|---|---|
| 140–149 | 196.3, 196.5 |
| 150–159 | 196.0, 196.3, 196.5 |
| 160–165 | 196.3, 196.5 |
| 170–171 | None |
| 174–175 | 196.0, 196.5 |
| 176 | None |
| 179–183 | 196.0, 196.3, 196.5 |
| 184 | 196.0, 196.3 |
| 185–186 | 196.0, 196.3, 196.5 |
| 187 | 196.0, 196.3 |
| 188–189 | 196.0, 196.3, 196.5 |
| 190 | 196.3, 196.5 |
| 191–192 | 196.0, 196.3, 196.5 |
| 193 | 196.3, 196.5 |
| 194 | 196.0, 196.3, 196.5 |
| 209.0–209.3 | 196.0, 196.3, 196.5 |
Note: Codes for multiple lymph node sites and unspecified sites (ICD-9-CM 196.8, 196.9) will be considered equally probable for melanoma and other primary cancers and thus are not included in the table.
Abbreviation: ICD-9, International Statistical Classification of Diseases, Ninth Revision.
Acknowledgments
The authors wish to acknowledge Dr Cyril Konto, Global Clinical Research, Bristol-Myers Squibb, and Dr David Battleman, TrueNorth Lifesciences, for their significant contributions to the development and execution of the research and review of the corresponding manuscript. Professional editorial support was provided by Lisa Sullivan and Matthew Dougherty at StemScientific.
Footnotes
Disclosure
The study was funded by Bristol-Myers Squibb. YZ, TKL, JWS, and SK are employees and stockholders of Bristol-Myers Squibb. The authors have no other conflicts of interest in this work.
References
- 1.Rigel DS. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29(4):204–209. doi: 10.1016/j.sder.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Frank SJ, Meyer M. Interferon as adjuvant therapy for high-risk melanoma. Melanoma Lett. 1995;13:1–4. [Google Scholar]
- 4.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Melanoma. National Comprehensive Cancer Network, Version 4; 2011. [Accessed October 30, 2014]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. [Google Scholar]
- 5.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18(12):2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 7.Agarwala SS, Lee SJ, Flaherty LE, et al. Randomized phase III trial of high-dose interferon alfa-2b (HDI) for 4 weeks induction only in patients with intermediate- and high-risk melanoma (Intergroup trial E 1697) J Clin Oncol. 2011;29:527s. [Google Scholar]
- 8.Mocellin S, Lens MB, Pasquali S, Pilati P, Chiarion Sileni V. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev. 2013;6:CD008955. doi: 10.1002/14651858.CD008955.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Punt CJ, Eggermont AM. Adjuvant interferon-α for melanoma revisited: news from old and new studies. Ann Oncol. 2001;12(12):1663–1666. doi: 10.1023/a:1013592219007. [DOI] [PubMed] [Google Scholar]
- 10.Dummer R, Hauschild A, Guggenheim M, Keilholz U, Pentheroudakis G, ESMO Guidelines Working Group Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii86–vii91. doi: 10.1093/annonc/mds229. [DOI] [PubMed] [Google Scholar]
- 11.Hillner BE, Kirkwood JM, Atkins MB, Johnson ER, Smith TJ. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern Cooperative Oncology Group 1684. J Clin Oncol. 1997;15(6):2351–2358. doi: 10.1200/JCO.1997.15.6.2351. [DOI] [PubMed] [Google Scholar]
- 12.Cormier JN, Xing Y, Ding M, et al. Cost effectiveness of adjuvant interferon in node-positive melanoma. J Clin Oncol. 2007;25(17):2442–2448. doi: 10.1200/JCO.2007.10.7284. [DOI] [PubMed] [Google Scholar]
- 13.Intron® [product information] Kenilworth, NJ: Schering-Plough Corporation; 2009. [Google Scholar]
- 14.Kilbridge KL, Cole BF, Kirkwood JM, et al. Quality-of-life-adjusted survival analysis of high-dose adjuvant interferon alpha-2b for high-risk melanoma patients using intergroup clinical trial data. J Clin Oncol. 2002;20(5):1311–1318. doi: 10.1200/JCO.2002.20.5.1311. [DOI] [PubMed] [Google Scholar]
- 15.Hackshaw MD, Krishna A, Mauro DJ. Retrospective US database analysis of drug utilization patterns, health care resource use, and costs associated with adjuvant interferon alfa-2b therapy for treatment of malignant melanoma following surgery. Clinicoecon Outcomes Res. 2012;4:169–176. doi: 10.2147/CEOR.S32349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyman GH. The evolution of clinical trials in oncology: randomised controlled trials to real world studies. Ann Oncol. 2014;25(Supplement 4):iv41–iv42. [Google Scholar]
- 17.US Bureau of Labor Statistics [homepage on the Internet] Consumer price index. 2001. [Accessed February, 2012]. Available from: http://www.bls.gov/cpi/
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Davis KL, Mitra D, Kotapati S, Ibrahim R, Wolchok JD. Direct economic burden of high-risk and metastatic melanoma in the elderly: evidence from the SEER-Medicare linked database. Appl Health Econ Health Policy. 2009;7(1):31–41. doi: 10.1007/BF03256140. [DOI] [PubMed] [Google Scholar]
- 20.Kotapati S, Davis KL, Mitra D, Iloeje U. Treatment patterns in high risk and metastatic melanoma: Evidence from linked electronic medical records and administrative claims data. J Clin Oncol. 2008;26(Suppl) Abstr 17540. [Google Scholar]
- 21.Kefford RF. Adjuvant therapy of cutaneous melanoma: the interferon debate. Ann Oncol. 2003;14(3):358–365. doi: 10.1093/annonc/mdg120. [DOI] [PubMed] [Google Scholar]
- 22.Payne MJ, Argyropoulou K, Lorigan P, et al. Phase II pilot study of intravenous high-dose interferon with or without maintenance treatment in melanoma at high risk of recurrence. J Clin Oncol. 2014;32(3):185–190. doi: 10.1200/JCO.2013.49.8717. [DOI] [PubMed] [Google Scholar]
- 23.Kirkwood JM, Bender C, Agarwala S, et al. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J Clin Oncol. 2002;20(17):3703–3718. doi: 10.1200/JCO.2002.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann A, Aslani P, Ahmed R, et al. Assessing medication adherence: options to consider. Int J Clin Pharm. 2014;36(1):55–69. doi: 10.1007/s11096-013-9865-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Identification codes for data analysis
| CPT4 codes for surgical intervention* | |
| 38562 | Removal, pelvic lymph nodes |
| 38564 | Removal, abdomen lymph nodes |
| 38571/38572 | Laparoscopy, lymphadenectomy |
| 38700/38720/38724 | Removal of lymph nodes, neck |
| 38740/38745 | Remove armpit lymph nodes |
| 38746 | Remove thoracic lymph nodes |
| 38747 | Remove abdominal lymph nodes |
| 38760/38765 | Remove groin lymph nodes |
| 38770 | Remove pelvis lymph nodes |
| 38780 | Remove abdomen lymph nodes |
| CPT4 codes for HCRU associated with surgery* | |
| 00100–01999 | All anesthetic codes |
| 88302/88304/88305/88307/88309 | Tissue examination by pathologist |
| 80500/80502 | Laboratory pathology consultation |
| 88399 | Surgical pathology procedure |
| 89240 | Pathology laboratory procedure |
| 99218/99219/99220 | Observation care |
| 99221/99222/99223 | Initial hospital care |
| 99234/99235/99236 | Observation/hospitalization same date |
| 99238/99239 | Hospital discharge day |
| 99251/99252/99253/99254/99255 | Inpatient consultation |
| CPT4 codes for HCRU associated with IFN therapy* | |
| 96413 | Chemotherapy administration, IV infusion |
| 99201/99202/99203/99204/99205 | Office/outpatient visit, new |
| 99211/99212/99213/99214/99215 | Office/outpatient visit |
| CPT4 codes for blood laboratory work associated with IFN therapy* | |
| 85025 | Complete CBC with auto differential WBC |
| 80438/80439/80440 | TRH stimulation panel |
| J codes (chemotherapy) associated with a melanoma diagnosis# | |
| J9214 | Injection, IFN-α-2b |
| J9130 | Dacarbazine, 100 mg |
| J9140 | Dacarbazine, 200 mg |
| J8700 | Temozolomide, oral, 5 mg |
| J9060 | Cisplatin, powder or solution, per 10 mg |
| J9062 | Cisplatin, 50 mg |
| J9045 | Injection, carboplatin, 50 mg |
| J9265 | Injection, paclitaxel, 30 mg |
| J9050 | Injection, carmustine, 100 mg |
| J9360 | Injection, vinblastine sulfate, 1 mg |
| J9228 | Injection, ipilimumab, 1 mg |
Notes:
American Medical Association, 2009;
Centers for Medicare and Medicaid Services, 2009.
Abbreviations: CBC, complete blood count; IV, intravenous; TRH, thyrotropin-releasing hormone; WBC, white blood cell; HCRU, health care resource use.
Table S2.
Improbable sites of lymph node metastasis from selected primary cancers
| Primary cancer | Improbable sites of lymph node metastasis |
|---|---|
| 140–149 | 196.3, 196.5 |
| 150–159 | 196.0, 196.3, 196.5 |
| 160–165 | 196.3, 196.5 |
| 170–171 | None |
| 174–175 | 196.0, 196.5 |
| 176 | None |
| 179–183 | 196.0, 196.3, 196.5 |
| 184 | 196.0, 196.3 |
| 185–186 | 196.0, 196.3, 196.5 |
| 187 | 196.0, 196.3 |
| 188–189 | 196.0, 196.3, 196.5 |
| 190 | 196.3, 196.5 |
| 191–192 | 196.0, 196.3, 196.5 |
| 193 | 196.3, 196.5 |
| 194 | 196.0, 196.3, 196.5 |
| 209.0–209.3 | 196.0, 196.3, 196.5 |
Note: Codes for multiple lymph node sites and unspecified sites (ICD-9-CM 196.8, 196.9) will be considered equally probable for melanoma and other primary cancers and thus are not included in the table.
Abbreviation: ICD-9, International Statistical Classification of Diseases, Ninth Revision.