Abstract
Chronic inflammation and infection are major risk factors for gastric carcinogenesis in adults. As chronic gastritis is common in Mexican children, diagnosis of Helicobacter pylori and other causes of gastritis are critical for the identification of children who would benefit from closer surveillance. Antral biopsies from 82 Mexican children (mean age 8.3±4.8y) with chronic gastritis (36 H. pylori +, 46 H. pylori -) were examined for gastritis activity, atrophy, intestinal metaplasia, and immunohistochemical expression of gastric carcinogenesis biomarkers CDX2, ephrin type-B receptor 4, matrix metalloproteinase 3 (MMP3), macrophage migration inhibitory factor (MIF), p53, β-catenin, and E-cadherin. Atrophy was diagnosed in 7/82 (9%) and intestinal metaplasia in 5/82 (6%) by routine histology, while 6 (7%) additional children (3 H. pylori +) exhibited aberrant CDX2 expression without intestinal metaplasia. Significant positive correlations were seen between EphB4, MMP3, and MIF (p<0.0001). Atrophy and follicular pathology were more frequent in H. pylori + biopsies (p<0.0001), while intestinal metaplasia and CDX2 expression showed no significant correlation with H. pylori status. Antral biopsies demonstrating atrophy, intestinal metaplasia, and/or aberrant CDX2 expression were seen in 21.95 % (18/82) of the children, potentially identifying those who would benefit from closer surveillance and preventive dietary strategies. Biomarkers CDX2, EphB4, MMP3, and MIF may be useful in the work-up of pediatric gastritis.
Keywords: antral gastritis, biomarkers, child, Helicobacter pylori, Mexican, surveillance
Introduction
Chronic inflammation is a risk factor for carcinogenesis in several tissues, including the stomach1, 2. Inflammation is a well-coordinated response of the innate and adaptive immune systems following infection or injury1. Deregulation of the inflammatory response leads to unresolved inflammation and a pro-neoplastic microenvironment1. The tissue damage produced by high levels of phagocyte-generated reactive oxygen, nitrogen, and halogen species can cause mutations and cell death and play a key role in the carcinogenic process2.
Chronic gastritis in children has multiple etiologies, including gastroesophageal reflux, food allergies, high intake of spicy food, acid peptic disease, non-steroidal anti-inflammatory drugs, and Helicobacter pylori infection. H. pylori infection increases the production of reactive oxygen and nitrogen species, and the gram-negative microaerophile confers nearly an eleven-fold increased risk of gastric cancer (GC)3. Infection with H. pylori is highly prevalent among socially and economically disadvantaged children. Age, overcrowding, number of siblings, and a low maternal education level increase infection risk4-7.
Globally, GC is the fourth most common cancer and second highest cause of cancer mortality with nearly two-thirds of these deaths occurring in developing nations3. Although we seldom see GC in children, these issues are of keen interest in underdeveloped countries where H. pylori is highly prevalent. Gastric carcinogenesis is hypothesized to be a process involving a number of premalignant genetic and morphologic alterations of gastric mucosa. Busuttil and Boussioutas3 outline the progression from normal stomach to gastritis, and intestinal metaplasia (IM). IM is considered a preneoplastic lesion, although it should be noted not all IM advances to dysplasia, the step previous to GC8,9.
Given the important role of chronic inflammation in carcinogenesis, we sought to determine whether Mexican children with a pathologic diagnosis of chronic antral gastritis exhibited histologic markers associated with adult preneoplastic lesions. Secondly, since Caudal Type Homeobox 2 (CDX2) expression precedes the development of gastric preneoplastic lesions in the setting of IM, we sought to define the H. pylori status and expression of CDX2 in our cohort of children and compare them with Mexican and American adult cohorts. Finally, we seek a panel of candidate biomarkers to use routinely in gastric biopsies in pediatric populations with a high prevalence of H. pylori infection7. Therefore, we selected an immunohistochemical (IHC) protein profile involved in gastric carcinogenesis and progression: Caudal Type Homeobox 2 10, Ephrin Type-B Receptor 4 (EphB4)11-12, Matrix Metalloproteinase 3 (MMP3)13-14, Macrophage Migration Inhibitory Factor (MIF)15, p53 (TP53 tumor ressor gene)16, β-catenin, and E-cadherin17-18. Our ultimate goal is the identification of children with antral lesions who would benefit from closer follow up surveillance, preventive nutritional strategies, and health promotion activities.
Materials and Methods
Patients and Samples
This study was conducted with the approval of the Central Military Hospital and the Medical College of Wisconsin Institutional Review Board (IRB). Consecutive gastric antral biopsy samples were obtained from 82 Mexican children (Table 1) of middle socioeconomic status attending the Central Military Hospital in Mexico City the first 3 months of 1996 and 2009.
Table 1.
Patient age, gender, and Helicobacter pylori status
| Variables | Children (n: 82) | Adults (n: 35) |
|---|---|---|
| Age [mean years (range)] | 8.1 (0.3 to 17) | 66.3 (50 to 81) |
| Gender (female, male) | 47, 35 | 18, 17 |
| H. pylori (+, -) | 36, 46 | 21, 14 |
Patients presented with one or more of the following symptoms: chronic epigastric or abdominal pain, pyrosis, or gastrointestinal bleeding. Gastroesophageal junction, antrum, and duodenum biopsies were examined by an attending hospital pathologist. Our cases did not include autoimmune gastritis, chemical gastritis, primary bile reflux gastritis, inadvertent sampling of the gastroduodenal junction, or postoperative gastritis, and none had received H. pylori eradication therapy. The adult biopsies were used as controls to compare CDX2 and the gastritis criteria with the children’s biopsy results. Thirty-five adult antral specimens were obtained from either Froedtert Hospital in Milwaukee, WI (n=14) or the Mexican Institute of Social Security (n=21) (Table 1).
Immunohistochemical Staining
Biopsy specimens were fixed in 10% buffered formalin and embedded in paraffin. Four micrometer thick sections were deparaffinized in xylene, hydrated in descending dilutions of ethanol, and exposed to heat-induced epitope retrieval. See Table 2 for details.
Table 2.
Antibodies and pretreatment used
| Antigen | Clone | Source | HIER* | Dilution |
|---|---|---|---|---|
| β-Catenin | β-Catenin-1 | Dako | TRS pH 9.0 | RTU** |
| CDX2 (children) | AMT28 | Leica Microsystems | Citrate buffer pH 6.0 | 1/100 |
| CDX2 (adults) | DAK-CDX2 | Dako | TRIS pH 9 | RTU |
| E-Cadherin | NCH-38 | Dako | TRIS pH9 | RTU |
| EphB4 | 3D7G8 | InVitrogen | TRIS pH9 | 1/50 |
| H. pylori | Rabbit | Dako | Citrate pH 6 | RTU |
| MIF | D-2 | Santa Cruz Biotech | TRIS pH 9 | 1:200 |
| MMP3 | 10D6 | R&D Systems | TRIS pH 9 | 1:50 |
| P53 | Pab 1801 | Leica Microsystems | Citrate pH 6 | 1:500 |
HIER:heat-induced epitope retrieval;
RTU: Ready to use
Pediatric CDX2 and p53 IHC staining was performed manually. IHC for β-catenin, E-cadherin, adult CDX2, EphB4, MIF, MMP3, and H. pylori was performed using reagents from the Dako Envision FLEX High pH kit and the Dako Autostainer Plus. Following pretreatment with Target Retrieval Solution (TRS pH 9.0), tissue was blocked with peroxidase-blocking reagent for 5 min, treated with PBS/BSA (EphB4, MIF, MMP3) for 30 min, incubated with primary antibody for 10 min (CDX2) or 30 min (β-catenin, E-cadherin, EphB4, MIF, MMP3) at room temperature, followed by 20 min horseradish peroxidase, 10 min DAB, and EnVision FLEX hematoxylin (Dako, Carpinteria, CA, US) counterstain.
Immunohistochemistry Analysis
The percentage of total gastric gland epithelial cells staining positive was determined (EphB4, MIF, MMP3). The samples were analyzed by a pathologist (ACM and/or ALG) blind to gastritis parameter scores or H. pylori infection status. To validate IHC, we stained various adult tissue specimens. These staining patterns were used as reference intensities for the gastric staining and scoring. β -catenin, E-cadherin, and CDX2 were scored as either 1 (low) or 2 (high) staining intensity. β-catenin expression was evaluated in membranous or nuclear location. Staining intensity for EphB4, MIF, and MMP3 was determined as: 0 (none), 1 (mild), 2 (moderate), and 3 (strong). The most intense stain covering at least 10% of the biopsy was used as the intensity score. p53 was evaluated based on presence of nuclear positivity. Positive control tissues included: breast cancer (p53), colon (β-catenin, E-cadherin, EphB4), duodenum (CDX2), and liver (MIF, MMP3).
Four criteria of chronic gastritis—gastritis activity, atrophy, follicular pathology, and IM—were evaluated by a pathologist (ALG). Histological classification of all biopsies’ H&E stains was done according to the Updated Sidney System19.
Warthin-Starry stain was used for the detection of H. pylori in the U.S.A. adult samples. A modified Giemsa stain was used to detect H. pylori in the specimens from the Mexican children and adults. All pediatric samples that were H. pylori negative were confirmed with H. pylori IHC.
Statistical Analysis
Statistical analyses were performed using SAS 9.2 Statistical software. The correlation between the antibodies and gastritis parameters was measured by Spearman Rank-order test. The association between H. pylori status and gastritis criteria was investigated by Pearson Chi-square test. The association of antibody staining intensities with H. pylori infection status as well as the association of CDX2 with H. pylori infection status for children and adults was tested by Fisher’s exact test. Significance was set at p <0.05.
Results
The distribution of selected gastritis histopathology and immunohistochemistry variables in the pediatric cohort is shown in Table 3. Antral atrophy was seen in 7/82 (8.5%) and IM in 5/82 (6.1 %) by H&E staining (Figure 3). The IM was classified as Type I for 4/5 cases and type II for the fifth case20. Of the 7 children with atrophy, 6 had H. pylori + biopsies, while of the 5 children with IM, one was H. pylori +.
Table 3.
Histopathologic and immunostain findings in pediatric cohort (n=82)
| Variable | Score | n | % |
|---|---|---|---|
| Gastritis | 0 (No Activity) | 39 | 47.6 |
| 1 (Mild) | 30 | 36.6 | |
| 2 (Moderate) | 6 | 7.3 | |
| 3 (Strong) | 7 | 8.5 | |
| Atrophy * | No | 75 | 91.5 |
| Yes | 7 | 8.5 | |
| Follicular pathology * | none | 70 | 85.4 |
| Mild | 11 | 13.4 | |
| Marked | 1 | 1.2 | |
| Intestinal metaplasia * | No | 77 | 93.9 |
| Yes | 5 | 6.1 | |
| H pylori | Negative | 46 | 56.1 |
| Positive | 36 | 43.9 | |
| p53 | Negative | 82 | 100 |
| CDX2 | Low | 76 | 92.7 |
| High | 6 | 7.3 | |
| MIF | 0 | 30 | 34.2 |
| 1+ | 45 | 55.7 | |
| 2+ | 5 | 6.3 | |
| MMP3 | 0 | 3 | 3.8 |
| 1+ | 15 | 19 | |
| 2+ | 14 | 17.7 | |
| 3+ | 32 | 59.4 | |
| EphB4 | 0 | 8 | 10.1 |
| 1+ | 36 | 45.6 | |
| 2+ | 31 | 39.2 | |
| 3+ | 4 | 5.0 | |
| β-catenin, membranous | 0 | 3 | 4.0 |
| 1+ | 46 | 60.5 | |
| 2+ | 27 | 35.5 | |
| E-cadherin | 1+ | 8 | 10.1 |
| 2+ | 71 | 89.9 |
Four criteria of chronic— gastritis gastritis activity, atrophy, follicular pathology, and intestinal metaplasia were evaluated according to the Updated Sidney System.
Figure 3. Representative pediatric gastric biopsies showing gastritis criteria.
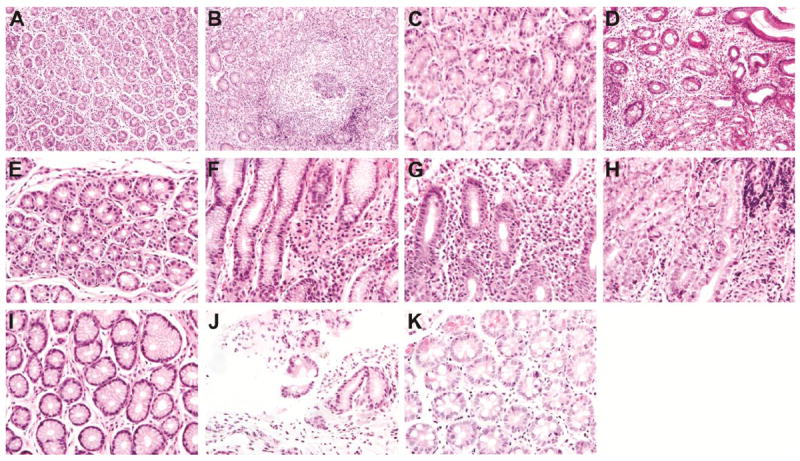
(A) Normal follicles. (B) Hyperplastic follicles. (C) Absent atrophy. (D) Atrophy present. (E) Absent inflammation. (F) Mild inflammation. (G) Moderate inflammation.
(H) Marked inflammation. (I) Absent intestinal metaplasia. (J) Incomplete intestinal metaplasia.
(K) Complete intestinal metaplasia.
Expression of β-catenin and E-cadherin expression occurred in 96% and 100% of cases respectively and was membranous (Figure 1). There was no nuclear β-catenin staining. No biopsies exhibited p53 nuclear positivity. Six additional biopsies exhibited aberrant CDX2 expression without histologic evidence of IM (Figure 1), and CDX2 expression did not correlate with H. pylori infection. Expression of MMP3, EphB4, and MIF was present in 96%, 90% and 62 % of biopsies, respectively (Figure 2). There was a positive correlation between cases with EphB4 positivity and MMP3 and MIF positivity (p<0.0001).
Figure 1. β-catenin, E-cadherin, & CDX2 (400x).
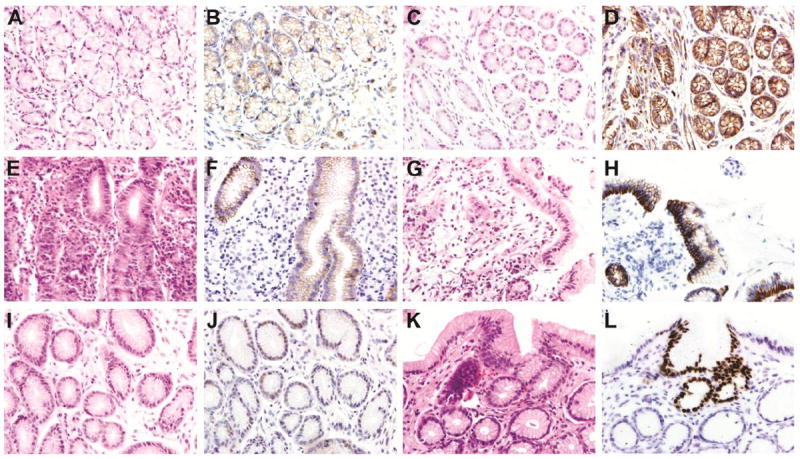
(A) Unremarkable antral biopsy from four-year-old girl, H&E. (B) β-catenin. Membranous positivity is weak with a patchy distribution. (C) 6 month old girl with H. pylori positive biopsy and focal glandular atrophy, H&E. (D) β-catenin. The membranous positivity is strong. There is no nuclear positivity. (E) Fifteen-year-old boy with active severe inflammatory response and H pylori positivity, H&E. Elsewhere in the biopsy there was focal atrophy and follicular pathology. (F) E-cadherin. Weak focal membranous immunoreactivity. (G) Fifteen-year-old-boy with abdominal pain and a finely granular gastric mucosa at endoscopy, H&E. Mild chronic inflammation and elsewhere follicular pathology. (H) E-cadherin. Strongly positive membranous staining. (I) Eight-year-old girl with an unremarkable, H. pylori negative antral biopsy, H&E. (J). CDX2. Positive weak nuclear staining in mostly dilated gastric glands. (K) Fifty-nine-year old with H. pylori positive biopsy and intestinal metaplasia, H&E. (L) CDX2. Nuclear immunoreactivity is strong in the area of intestinal metaplasia.
Figure 2. EphB4, MMP3, & MIF (400x).
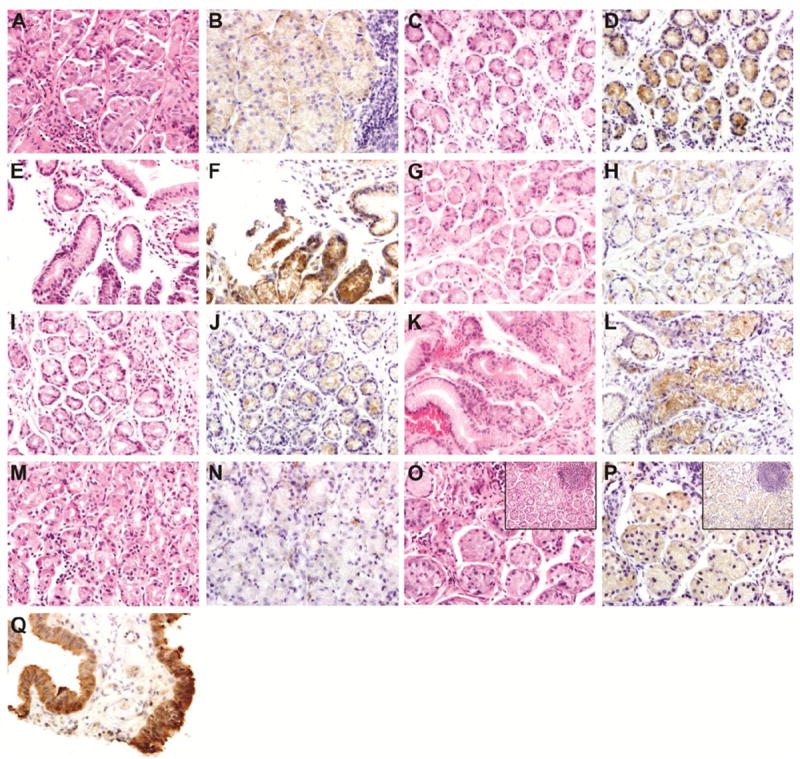
(A) Eight-year-old girl with a history of abdominal pain and H. pylori positive biopsy. Moderately active gastritis, H&E. (B) EphB4. Weak immunoreactivity of EphB4 in gastric glands surrounded by focal severe chronic inflammatory infiltrates. Inset shows a hyperplastic follicle H&E × 200. (C) Nineteen month-old boy with a history of abdominal pain, normal gastric mucosa at endoscopy and negative H. pylori. Mild inflammation, H&E. (D) EphB4. Moderate immunoreactivity is seen in glands surrounded by mild inflammatory activity. (E) Eight year old girl, biopsy negative for H. pylori. Mild inflammatory activity, H&E. (F) EphB4. Strong EphB4 immunoreactivity is seen in glands surrounded by mild inflammation.
(G) Twelve year old girl with a history of chronic abdominal pain, mild gastritis by endoscopy and antral biopsy positive for H pylori. Mild inflammatory activity, H&E. (H) MMP3. Scattered glands with weak cytoplasmic staining. (I) Three year old boy with a clinical history of chronic abdominal pain, mild gastritis by endoscopy. and antral biopsy positive for H. pylori, H&E. There is mild inflammatory activity. (J) MMP3. A moderate number of gastric glands showed cytoplasmic immunoreactivity. (K) Seventeen-year-old girl with a normal endoscopy and positive H. pylori. Mild inflammatory activity, H&E. (L) MMP3. Strong immunoreactivity of gastric epithelial cells in association with moderate focal chronic inflammation. (M) Nine-year-old girl with H. pylori positive biopsy. Mild inflammation, H&E. (N) MIF. Scattered MIF-positive glands. (O) Eight-year-old girl with a history of abdominal pain and a granular antral biopsy at endoscopy. Moderate inflammation, H&E. Inset: hyperplastic follicle. (P) MIF. Moderate cytoplasmic MIF staining. (Q) MIF control with strong positivity.
Significant negative correlations were noted between the severity of gastritis activity and MMP3 (p=0.0255) and EphB4 (p=0.0432), as well as between atrophy and MMP3 percent positivity (p=0.0370).
H. pylori Positive and H. pylori Negative Children Groups
In children, H. pylori positive versus negative biopsies differed significantly with respect to atrophy (Chi-square 56.5744, Asymptotic Pr>Chi-Sq p<0.0001) and follicular pathology (Chi-square 11.3981, Asymptotic Pr>Chi-Sq p<0.0007), but not with respect to IM (Chi-square 1.2353, Asymptotic Pr>Chi-Sq p=0.2664). Membranous β-catenin intensity was significantly higher in biopsies from H. pylori positive patients compared with those from H. pylori negative patients (p=0.0026). In H. pylori positive biopsies, negative correlations were noted between MMP3 and atrophy (p=0.0484) and EphB4 and gastritis activity (p=0.0475). In H. pylori negative biopsies, MMP3 staining correlated negatively with atrophy (p=0.0256) and gastritis activity (p=0.0015).
CDX2 Expression: Child and Adult
CDX2 was positive in 6 (7%) of children’s gastric biopsy specimens (3 H. pylori + and 3 H. pylori -), and exhibited no significant associations with any of the gastritis criteria. CDX2 expression was more frequent in adult biopsies than in children’s biopsies regardless of infection status (p<0.0001), as well as within H. pylori + (p=0.0122) and H. pylori - (p<0.0001) adult cohorts. Adult CDX2 percent positivity showed significant positive correlations with both IM (p=0.0019) and atrophy (p=0.0433).
Discussion
We found that 18 of 82 antral biopsies from children had either atrophy, intestinal metaplasia (IM), and/or ectopic CDX2 expression. This is notable considering that IM and atrophy are relatively rare in other reported studies of pediatric gastritis6,21, 22. As CDX2 is expressed early in the IM progression pathway, ectopic antral CDX2 probably reflects pathologic changes leading to IM3. In transgenic mice, gastric expression of CDX2 alone can induce IM25. Children’s biopsies had significantly less CDX2 positivity than adults’ biopsies, regardless of infection status. Adults also showed a significant positive correlation of CDX2 with both IM and atrophy. In contrast, children’s biopsies with CDX2 positivity exhibited no significant associations with these two gastritis parameters or with H. pylori status. Current literature suggests IM regression is rare. While elimination of H. pylori is associated with regression of gastric inflammation and atrophy, IM usually persists23. Since 80% of gastric carcinomas arise in the context of IM and its presence results in a two to six-fold increased risk for cancer development23,26, the finding of CDX2 positivity in 7% of children warrants further exploration for its association with either H. pylori or other chronic gastritis etiologies.
As the regulatory mechanisms involved in triggering and maintaining gastric CDX2 expression are not entirely clear27, we were interested in two recent papers describing novel CDX2 regulatory mechanisms relevant to this work. Barros et al. suggested an auto-regulatory CDX2 loop, which may have a major impact on the stability of human IM, possibly resulting in progression along the gastric carcinogenesis pathway23. Results from Camilo et al. provided a link between H. pylori infection and the Bone Morphogenetic Protein (BMP) pathway in the regulation of intestinal and gastric-specific genes that might be relevant for gastric IM24. It remains to be seen what CDX2 regulatory mechanisms participate in the absence of H. pylori25-28.
Atrophy exhibited a strong correlation with H. pylori positive status in our children. Chronic atrophic gastritis has been considered a progressive disease worsened by H. pylori infection, use of non-steroidal anti-inflammatory drugs and proton pump inhibitors, and with the intake of carbonated drinks and fast food29. This is relevant to our cohort’s diet and high consumption of soft drinks in Mexico—an average of 163 L per capita per year compared to 118L in the U.S.A.).30
The significant positive associations between EphB4, MMP3, and MIF (p<0.0001) suggest interaction between the EphB pathway which regulates the degradation of extracellular matrix proteins, cell adhesion proteins, and an inflammatory cytokine in the progression from chronic inflammation to carcinogenesis. EphB4 is part of the Eph (erythropoietin-producing hepatoma) receptor tyrosine kinase family regulating cell migration during embryonic development and adhesion and migration of cancer cells. It is fundamental for angiogenesis, vessel maturation, and pericyte recruitment11. It has been consistently found in most epithelial cancers, including gastric cancer12. The expression of EphB4 in 90% of antral samples is pertinent to the recently emerging unifying theme outlined in Wang et al., in which an evolving cancer cell may either directly eliminate the anti-migratory effects of the activated Eph receptors, or the Eph receptors aid in migration and invasion31. This novel concept is highly relevant in the setting of gastric carcinogenesis because epithelial cells with high expression of Ephs and ephrins could have a survival advantage and participate in the tumor progression selection31. The persistence of high EphB4 immunoreactivity in gastric glands in the scenario of IM and gastric atrophy raises questions about malignant progression, particularly if the high expression is not ameliorated by treatment31,32.
MMP3 upregulation has also been associated with gastric carcinogenesis13,14. In the Economescu et al. study13, MMP3 upregulation was associated with gastric tumor progression, while Rajkumar et al. 14 demonstrated MMP3 upregulation in gastric carcinomas but not in the adjacent non-neoplastic gastric mucosa. Moreover, MMP3 promoter polymorphisms (MMP3707 G/G and MMP3-1612 5A/6A) are potential independent predictors of gastric cancer risk development33.
Macrophage migration inhibitory factor (MIF) is a multifunctional cytokine which plays important roles in inflammation and tumorigenesis. Polymorphisms such as MIF-173 and MIF-794-CATT have been associated with risk for severe chronic atrophic gastritis34. Mice studies have found both serum and gastric MIF immunoexpression progressively increase in H. pylori-induced gastritis, IM, and gastric cancer, and even in gastric injury due to non-steroidal anti-inflammatory drugs35,36. Fehlings et al. demonstrated that MIF suppression by H. pylori infected monocyte-derived dendritic cells enables immune evasion mechanisms, promoting the bacterium’s persistence37. MIF knockout mice, on the other hand, did not develop gastritis after H. pylori infection—the inhibition of H. pylori-induced innate immune responses and Th1 -- mediated immune injury playing a probable role38. Given the uncertainty of the role of MIF in pediatric antral biopsies, this marker should be further explored in the evaluation of these specimens.
High membranous β-catenin expression was significantly associated with H. pylori positive biopsies, while E-cadherin expression was not associated with H. pylori status. Infection with H. pylori is associated with deregulated accumulation of nuclear β-catenin and promotes malignant transformation, while mutations of CTNNB1 genes occur early in GC development and contribute to gastric carcinogenesis18,39. The Wnt/ β-catenin pathway, among its many vital developmental roles, is also involved in the development of cancer and in supporting cadherin-mediated cell adhesion40. It will be of interest to expand this observation to larger cohorts, and especially with longitudinal follow-up. H. pylori-induced calpain activation results in cleavage of E-cadherin to produce a truncated form and induce relocalization of E-cadherin and β-catenin and inhibition of TLR2 prevented H. pylori-induced calpain activation and adherens junctions (AJ) disassembly41. O′Connor suggested H. pylori activates calpain via TLR2 to disrupt gastric epithelial AJ structure—in turn, the disruption of AJ structure favors severe disease41.
The presence of atrophy, IM and CDX2 positive cells independent of H. pylori status suggest a need for more intense clinical surveillance in this pediatric cohort. Close follow-up should be indicated for children with CDX2 nuclear positivity even in the absence of intestinal metaplasia by H&E, high expression of EphB4 31, and those with atrophy. Biopsies with atrophy should be checked for H. pylori by modified Giemsa, Warthin-Starry, or H. pylori antibodies. Moreover, since intestinal metaplasia rarely regresses and there are no published children’s studies indicating the natural history of IM, follow-up is warranted.
Alongside the follow-up pathology and clinical surveillance, use of probiotics42,43, avoiding the use of unnecessary antibiotics that wipe out beneficial bacteria44, preventive dietary strategies i.e., diets high in vegetables and reduced intake of carbonated drinks and spicy food29, along with health promotion activities (i.e., introduction of free, potable drinking-water fountains in schools and public spaces, educating parents on improving sanitation conditions, and nutritional guidance to mothers) should be encouraged.
The importance of our findings is limited by the absence of long-term clinical and pathologic follow-up. Expanding the number of cases and having access to sequential biopsies will allow us to define the progression of the preneoplastic lesions and the use of selected markers in future studies. Prospective studies of pediatric antral biopsies would benefit from differential gene expression profiling of key markers using laser microdissection a45, and the use of DNA microarrays of preneoplastic lesions. Longitudinal studies plus molecular techniques will disclose the complex interplay of environmental and genetic factors in the development of gastric preneoplastic lesions in children.
Acknowledgments
The Federation of American Societies for Experimental Biology (FASEB), the American Society for Investigative Pathology (ASIP) and their Minority Access to Research Careers (MARC) Summer Research Opportunities Program in Pathology (SROPP) generously supported Rodolfo Villarreal-Calderon to conduct research for this study at the Medical College of Wisconsin in Milwaukee, WI under the mentorship of Dr. Alexander Craig Mackinnon, Jr. This project was supported, in part, by grant 1UL1RR031973 from the Clinical and Translational Science Award (CTSI) program of the National Center for Research Resources, NIH, which allowed for the biostatistics work at the Medical College of Wisconsin Institute for Health and Society, with the help of Assistant Professor Dr. Sergey Tarima.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 2.Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int J Cancer. 2011;128:1999–2009. doi: 10.1002/ijc.25815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busuttil RA, Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol. 2009;24:193–201. doi: 10.1111/j.1440-1746.2008.05774.x. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz L, Camorlinga M, Hernandez R, et al. Immune and proliferative cellular responses to Helicobacter pylori infection in the gastric mucosa of Mexican children. Helicobacter. 2007;12:224–230. doi: 10.1111/j.1523-5378.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 5.Vilchis J, Duque X, Mera R, et al. Association of Helicobacter pylori infection and height of Mexican children of low socioeconomic level attending boarding schools. Am J Trop Med Hyg. 2009;81:1091–1096. doi: 10.4269/ajtmh.2009.09-0107. [DOI] [PubMed] [Google Scholar]
- 6.Jaramillo-Rodríguez Y, Nares-Cisneros J, Martínez-Ordaz VA, et al. Chronic gastritis associated with Helicobacter pylori in Mexican children: histopathological patterns. Pediatri Dev Pathol. 2011;14:93–98. doi: 10.2350/09-12-0754-OA.1. [DOI] [PubMed] [Google Scholar]
- 7.Duque X, Vilchis J, Mera R, et al. Natural history of Helicobacter pylori infection in Mexican school children: Incidence and spontaneous clearance. J Pediatr Gastroenterol Nutr. 2012;55:209–216. doi: 10.1097/MPG.0b013e318248877f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rugge M, Capelle LG, Cappellesso R, et al. Precancerous lesions in the stomach: from biology to clinical patient management. Best Pract Res Clin Gastroenterol. 2013;27:205–223. doi: 10.1016/j.bpg.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Wang L, Zhang JP, et al. Expression of p53, c-erbB-2 and Ki67 in intestinal metaplasia and gastric carcinoma. World J Gastroenterol. 2010;16:339–344. doi: 10.3748/wjg.v16.i3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto A, Hirahashi M, Osada M, et al. Aberrant activation-induced cytidine deaminase expression is associated with mucosal intestinalization in the early stage of gastric cancer. Virchows Arch. 2011;458:717–724. doi: 10.1007/s00428-011-1086-x. [DOI] [PubMed] [Google Scholar]
- 11.Salvuci O, Tosato G. Essential role of EphB receptors and EphrinB ligands in endothelial cell function and angiogenesis. Adv Cancer Res. 2012;114:21–57. doi: 10.1016/B978-0-12-386503-8.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Zhao ZW, Zhang Y, et al. Over-expression of Ephb4 is associated with carcinogenesis of gastric cancer. Dig Dis Sci. 2011;56:698–706. doi: 10.1007/s10620-010-1346-7. [DOI] [PubMed] [Google Scholar]
- 13.Economescu MC, Necula LG, Dragu D, et al. Identification of potential biomarkers for early and advanced gastric adenocarcinoma detection. Hepatogastroenterology. 2010;57:1453–1464. [PubMed] [Google Scholar]
- 14.Rajkumar T, Vijayalakshmi N, Gopal G, et al. Identification and validation of genes involved in gastric tumorigenesis. Cancer Cell Int. 2010;10:45. doi: 10.1186/1475-2867-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salminen A, Kaarniranta K. Control of p53 and NF-kappaB signaling by WIP1 and MIF: role in cellular senescence and organismal aging. Cell Signal. 2011;23:747–752. doi: 10.1016/j.cellsig.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Gonçalves AR, Carneiro AJ, Martins I, et al. Prognostic significance of p53 protein expression in early gastric cancer. Pathol Oncol Res. 2011;17:349–355. doi: 10.1007/s12253-010-9333-z. [DOI] [PubMed] [Google Scholar]
- 17.Ozawa M, Ringwald M, Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci USA. 1990;87:4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udhayakumar G, Jayanthi V, Devaraj N, et al. Nuclear translocation of beta-catenin correlates with CD44 upregulation in Helicobacter pylori-infected gastric carcinoma. Mol Cell Biochem. 2011;357:283–293. doi: 10.1007/s11010-011-0899-x. [DOI] [PubMed] [Google Scholar]
- 19.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol; International Workshop on the Histopathology of Gastritis, Houston 1994; 1996. pp. 1161–1181. [DOI] [PubMed] [Google Scholar]
- 20.Jass JR, Filipe MI. A variant of intestinal metaplasia associated with gastric carcinoma: a histochemical study. Histopathology. 1979;3:191–199. doi: 10.1111/j.1365-2559.1979.tb02996.x. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho MA, Machado NC, Ortolan EV, et al. Upper gastrointestinal histopathological findings in non-ulcer dyspeptic children and adolescents with Helicobacter pylori infection. J Pediatric Gastroenterol Nutr. 2012;55:523–529. doi: 10.1097/MPG.0b013e3182618136. [DOI] [PubMed] [Google Scholar]
- 22.Hoepler W, Hammer K, Hammer J. Gastric phenotype in children with Helicobacter pylori infection undergoing upper endoscopy. Scand J Gastroenterol. 2011;46:293–298. doi: 10.3109/00365521.2010.533383. [DOI] [PubMed] [Google Scholar]
- 23.Barros R, da Costa LT, Pinto-de-Sousa J, et al. CDX2 autoregulation in human intestinal metaplasia of the stomach: impact on the stability of the phenotype. Gut. 2011;60:290–298. doi: 10.1136/gut.2010.222323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilo V, Barros R, Sousa S, et al. Helicobacter pylori and the BMP pathway regulate CDX2 and SOX2 expression in gastric cells. Carcinogenesis. 2012;33:1985–1992. doi: 10.1093/carcin/bgs233. [DOI] [PubMed] [Google Scholar]
- 25.Silberg DG, Sullivan J, Kang E, et al. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 26.Asfeldt AM, Steigen SE, Lochen ML, et al. The natural course of Helicobacter pylori infection on endoscopic findings in a population during 17 years of follow-up: the Sorreisa gastrointestinal disorder study. Eur J Epidemiol. 2009;24:649–658. doi: 10.1007/s10654-009-9371-6. [DOI] [PubMed] [Google Scholar]
- 27.de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 28.Barros R, Pereira B, Duluc I, et al. Key elements of the BMP/SMAD pathway co-localize with CDX2 in intestinal metaplasia and regulate CDX2 expression in human gastric cell lines. J Pathol. 2008;215:411–420. doi: 10.1002/path.2369. [DOI] [PubMed] [Google Scholar]
- 29.Chooi EY, Chen HM, Miao Q, et al. Chronic atrophic gastritis is a progressive disease: analysis of medical reports from Shanghai 1985-2009. Singapore Med J. 2012;53:318–324. [PubMed] [Google Scholar]
- 30.Mexico Leads World in Consumption of Sugary Drinks, Study Says. Health. 2011 Retrieved from Fox News Latino http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&ved=0CCkQFjAA&url=http%3A%2F%2Flatino.foxnews.com%2Flatino%2Fhealth%2F2011%2F09%2F06 %2Fmexico-leads-world-in-consumption-sugary-drinks-study-says%2F&ei=t5-0UvWQDuWe2wWa44HACA&usg=AFQjCNEBAhHpwYjBzusKpoho18treEjr4g&bvm=bv.58 187178,d.b2I.
- 31.Wang B. Cancer cells exploit the Eph-ephrin system to promote invasion and metastasis: tales of unwitting partners. Sci Signal. 2011;4(175):pe28. doi: 10.1126/scisignal.2002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noren NK, Pasquale EB. Paradoxes of the EphB4 receptor in cancer. Cancer Res. 2007;67:3994–3997. doi: 10.1158/0008-5472.CAN-07-0525. [DOI] [PubMed] [Google Scholar]
- 33.Dey S, Stalin S, Gupta A, et al. Matrix metalloproteinase 3 gene promoter polymorphisms and their haplotypes are associated with cancer risk in eastern Indian population. Mol Carcinog. 2012;51(Suppl 1):E42–53. doi: 10.1002/mc.21837. [DOI] [PubMed] [Google Scholar]
- 34.Li ZW, Wu Y, Sun Y, et al. Inflammatory cytokine gene polymorphisms increase the risk of atrophic gastritis and intestinal metaplasia. World J Gastroenterol. 2010;16:1788–1794. doi: 10.3748/wjg.v16.i14.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He XX, Yang J, Ding YW, et al. Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut. 2006;55:797–802. doi: 10.1136/gut.2005.078113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohkawara T, Takeda H, Ohnishi S, et al. Macrophage migration inhibitory factor contributes to development of nonsteroidal anti-inflammatory drugs-induced gastric injury in mice. Int Immunopharmacol. 2011;11:418–423. doi: 10.1016/j.intimp.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Fehlings M, Drobbe L, Moos V, et al. Comparative analysis of the interaction of Helicobacter pylori with human dendritic cells, macrophages and monocytes. Infect Immun. 2012;80:2724–2734. doi: 10.1128/IAI.00381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong BL, Zhu SL, Huang XR, et al. Essential role for macrophage migration inhibitory factor in gastritis induced by Helicobacter pylori. Am J Pathol. 2009;174:1319–1328. doi: 10.2353/ajpath.2009.080708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang BG, Kim WH. Molecular pathology of gastric carcinoma. Pathobiology. 2011;78:302–310. doi: 10.1159/000321703. [DOI] [PubMed] [Google Scholar]
- 40.Archbold HC, Yang YX, Chen L, et al. How do they do Wnt they do?: regulation of transcription by the Wnt/beta-catenin pathway. Acta Physiol. 2011;204:74–109. doi: 10.1111/j.1748-1716.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- 41.O’Connor PM, Lapointe TK, Jackson S, et al. Helicobacter pylori activates calpain via toll-like receptor 2 to disrupt adherens junctions in human gastric epithelial cells. Infect Immun. 2011;79:3887–3894. doi: 10.1128/IAI.05109-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitor JM, Vale FF. Alternative therapies for helicobacter pylori: probiotics and phytomedicine. FEMS Immunol Med Microbiol. 2011;63:153–164. doi: 10.1111/j.1574-695X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- 43.Yang YJ, Sheu BS. Probiotics-containing yogurts suppress Helicobacter pylori load and modify immune response and intestinal microbiota in the Helicobacter pylori-infected children. Helicobacter. 2012;17:297–304. doi: 10.1111/j.1523-5378.2012.00941.x. [DOI] [PubMed] [Google Scholar]
- 44.Blaser M. Antibiotic overuse: Stop the killing of beneficial bacteria. Nature. 2011;476:393–394. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 45.Kouznetsova I, Kalinski T, Meyer F, et al. Self-renewal of the human gastric epithelium: new insights from expression profiling using laser microdissection. Mol Biosyst. 2011;7:1105–1112. doi: 10.1039/c0mb00233j. [DOI] [PubMed] [Google Scholar]


