Abstract
Neuropeptide Y (NPY) is expressed in certain primary afferent fibers, is up-regulated in response to tissue injury and is capable of inhibiting nociceptive behavior at the spinal level. However, the spinal mechanism(s) for NPY-evoked antinociception is unknown. In this study, we evaluated the hypothesis that agonists at the NPY Y1 receptor subtype (Y1-R) inhibit exocytosis from the capsaicin-sensitive class of nociceptors. Using in vitro superfusion of rat dorsal spinal cord slices, pre-treatment with the Y1-R agonist [Leu31Pro34]NPY significantly inhibited capsaicin-evoked release of immunoreactive calcitonin gene-related peptide with an EC50 value of 10.6 nM. This inhibitory effect was concentration dependent, significantly attenuated by pre-treatment with the Y1 receptor antagonist BIBP3226 and reproduced by synthetic NPY. Examination of adult rat dorsal root ganglia using double immunofluorescent labeling revealed frequent co-localization of Y1 receptor immunoreactivity in vanilloid receptor type 1-immunoreactive neurons, indicating that Y1 agonists may directly modulate the capsaicin-sensitive class of nociceptors. Collectively, these results indicate that NPY is capable of inhibiting capsaicin-sensitive neurons via a Y1 receptor mechanism, suggesting the mechanisms for spinal NPY-induced antinociception is due, at least in part, to inhibition of central terminals of capsaicin-sensitive nociceptors.
Keywords: superfusion, pain, spinal cord, dorsal root ganglion, exocytosis
Neuropeptide Y (NPY) is a 36 amino-acid neuropeptide that is highly expressed throughout the central and peripheral nervous systems (Balasubramaniam, 1997; Larhammar, 1996; Malstrom, 1997). NPY has been proposed to mediate several physiologic systems including the processing of nociceptive signaling at the level of the spinal cord (Duggan et al., 1991; Mark et al., 1997; Balasubramaniam, 1997; Hokfelt et al., 1998). The NPY Y1 and Y2 receptors are expressed in the dorsal horn of the spinal cord as well as in dorsal root ganglia (DRG) (Kar and Quirion, 1992; Mantyh et al., 1994). The NPY Y1 receptor (Y1-R) is expressed primarily in small- and medium-sized neurons that express calcitonin gene related peptide (CGRP) in the DRG as well as in the dorsal spinal cord in inner lamina II (Zhang et al., 1994). The NPY Y2 receptor is also expressed in DRG neurons, but primarily in large-sized neurons not thought to be involved with nociception under normal conditions (Zhang et al., 1997).
The intrathecal administration of NPY or its analogs produces both antinociception and antihyperalgesia (Hua et al., 1991; Taiwo and Taylor, 2002). Recent studies utilizing Y1-R knockout mice or specific NPY receptor antagonists indicate that the intrathecal effects of NPY are due primarily to activation of the Y1-R (Naveilhan et al., 2001; Taiwo and Taylor, 2002). However, the target neuronal populations mediating this effect have yet to be determined.
Numerous studies have demonstrated that CGRP is involved in spinal nociception (see Vasko, 1995; van Rossum et al., 1997 for reviews). CGRP is primarily expressed by primary afferents involved in the transmission of pain, namely the unmyelinated c fibers and lightly myelinated Aδ fibers (Pohl et al., 1990; Willis and Coggeshall, 1991). Noxious mechanical and thermal stimuli evoke the release of CGRP from the superficial dorsal horn (Morton and Hutchison, 1989), and carrageenan inflammation enhances CGRP release (Garry and Hargreaves, 1992). Intrathecal administration of a CGRP-1 receptor antagonist significantly inhibits the development of capsaicin-evoked mechanical allodynia (Sun et al., 2003) Finally αCGRP knockout mice demonstrate a diminished pain behavior in response to capsaicin injection into the hind paw (Salmon et al., 2001). These studies and numerous others demonstrate that CGRP release in the dorsal horn of the spinal cord is a critical component of nociceptive transmission.
In the present study, we evaluated the hypothesis that NPY, via activation of the Y1-R, inhibits exocytosis of CGRP from central terminals of capsaicin-sensitive primary afferent fibers. We also evaluated the hypothesis that this effect may be due to the activation of Y1-Rs located on vanilloid receptor type 1 (VR-1)-expressing, capsaicin-sensitive sensory neurons.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague-Dawley rats (200–250 g; Harlan, Indianapolis, IN, USA) were used in all experiments. Animals were housed on a 12-h light/dark cycle with free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee of The University of Texas Health Science Center at San Antonio. All experiments conformed to IACUC and federal guidelines on the ethical use of animals. Experiments were designed to minimize the number of animals used and their suffering.
In vitro superfusion
The superfusion of spinal dorsal horn slices was performed as previously described (Garry et al., 1994). In brief, animals were decapitated and the spinal cord was removed by hydraulic extrusion. The dorsal half of the lumbar enlargement or the whole cord was dissected and then sliced into 200 µm2 sections using a McIlwain tissue chopper (Mickel Laboratory Engineering, Gomshall, UK), weighed (approximately 30 mg tissue/chamber) and placed in a chamber in which it was continuously superfused with oxygenated Krebs buffer (pH = 7.6, 37 °C) at a rate of 0.5 ml/ min, with collection of 3 min fractions. The Krebs buffer consisted of NaCl (135 mM), KCl (5 mM), CaCl2 (2.5 mM), MgCl (1.2 mM), NaHCO3 (25 mM), NaH2PO4 (1 mM), dextrose (10 mM), HEPES (15 mM), thiorphan (16 µM; Bachem, Torrence, CA, USA), ascorbate (100 µM), and bovine serum albumin (0.1%; Sigma, St. Louis, MO, USA). Following equilibration of the tissue (1 h), NPY (Peninsula Laboratories, San Carlos, CA, USA), the Y1 selective agonist [Leu31Pro34]NPY (Peninsula Laboratories) or vehicle was administered for 3 min. Capsaicin (300 nM) or vehicle (0.05% ethanol in Krebs) was then co-administered in the presence of the pretreated drug or vehicle for an additional 3 min. In a separate experiment, the specific Y1 antagonist, BIBP3226 (Peninsula Laboratories) or vehicle was administered for 3 min prior to and concurrent with administration of either [Leu31Pro34]NPY or vehicle.
Immunoreactive CGRP (iCGRP) radioimmunoasssay
Aliquots of the superfusate were preincubated for 24 h at 4 °C with 100 µl of CGRP antisera (kindly donated by Dr. M. Iadarola, NIDCR, NIH, Bethesda, MD, USA). Then 100 µl of [125I]-CGRP28–37 (approximately 20,000–25,000 CPM) and 50 µl of goat anti-rabbit antisera coupled to magnetic beads (PerSeptive Diagnostics, Cambridge, MA, USA) were added to the tubes, vortexed and allowed to incubate another 24 h. Bound and free [125I]-CGRP28–37 were then separated by immunomagnetic separation. All drugs were tested for interference with the RIA, and no cross-reactivity was observed in this study.
Immunohistochemistry
Rats were deeply anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine i.p. and then perfused transcardially, first by a normal saline solution followed by a 4% paraformaldehyde solution. The L4–L6 DRG were carefully dissected and postfixed for 1 h. Tissues were placed in a 20% sucrose solution until completely submerged. Tissues were then embedded in Tissue-Tek O.C.T. (Sakura Finetek, Torrence, CA, USA), frozen, cut into 20 µm sections and thaw mounted onto Superfrost plus glass slides. Sections were permeabilized for 1 h using 0.2% Triton X-100 and blocked for 30 min using 10% normal goat serum (NGS) in phosphate-buffered saline. Tissues were then incubated for 18 h at 4 °C with polyclonal guinea-pig anti-VR1 serum (Neuromics, Minneapolis, MN, USA) diluted 1:5000 in 10% NGS containing 0.1% NaN3 followed by incubation with a FITC-labeled goat anti-guinea-pig secondary antibody at 25 °C for 1 h (Vector Laboratories, Burlingame, CA, USA). The tissue was then incubated for 18 h at 4 °C with polyclonal rabbit anti-Y1 serum (Dia Soren, Stillwater, MN, USA), diluted 1:200 as above, followed by biotinylated goat anti-rabbit antibody for 1 h at 25° (Vector Laboratories) and Avidin–Texas Red for 1 h at 25 °C (Vector Laboratories). Appropriate control experiments were performed, including incubation of tissue with primary antibody that was preadsorbed with blocking peptide or by substituting primary antibody with normal rabbit serum.
Image acquisition and quantification
Images were acquired using a Nikon E600 microscope (Melville, NY, USA) equipped with appropriate filters and a Photometrics SenSys CCD-cooled digital camera (Roper Scientific, Tuscan, AZ, USA) connected to a computer equipped with Metamorph V4.1 image analysis software (Universal Image Corporation, Downingtown, PA, USA). Several sections of lumbar DRG (separated by at least 100 µm) from three different animals were counted. A total of 2357 neuronal profiles were counted. For double labeling experiments, only strongly labeled cells were counted. Cells without a clearly defined nucleus were excluded. Images were taken across an entire ganglion and data are expressed as mean±S.D.
Statistics
Superfusion data were analyzed with repeated measures analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test. Concentration-response curve data were analyzed by non-linear regression using GraphPad Prism software (San Diego, CA, USA) to calculate EC50 values. Results were considered significant when the probability that they occurred due to chance alone was less than 5% (i.e. P<0.05). Data are reported as mean±S.E.M.
RESULTS
Effect of [Leu31Pro34]NPY on capsaicin-evoked iCGRP release
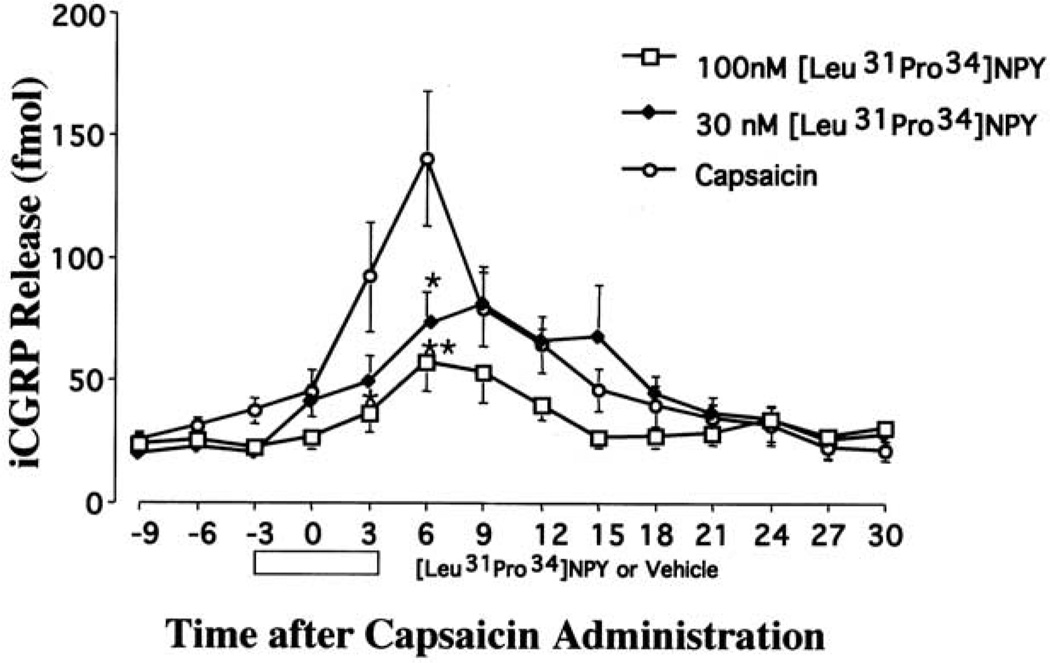
To test the hypothesis that a Y1 agonist inhibits capsaicin-evoked exocytosis from central terminals of certain nociceptive primary afferent fibers, experiments were conducted measuring capsaicin-evoked iCGRP release from isolated rat dorsal spinal cord. Spinal cord tissue was pretreated with either vehicle or the Y1 agonist [Leu31Pro34]NPY (at 30 or 100 nM) and then stimulated with capsaicin (Fig. 1). Pretreatment with [Leu31Pro34]NPY had no effect on basal CGRP release. During peak release, capsaicin evoked a 4–5-fold increase in iCGRP release over baseline. Baseline represents the average CGRP release in the first three fractions (9 min) of the experiment, before any drug was administered. A two-way ANOVA (drug×fraction) revealed a significant effect for [Leu31Pro34]NPY pretreatment (F2,148=10.3, P<0.0001). Post hoc analyses indicate that either 30 nM or 100 nM [Leu31Pro34]NPY significantly inhibited capsaicin-evoked iCGRP release compared with vehicle pretreatment.
Fig. 1.
Effect of [Leu31Pro34]NPY (30 or 100 nM or vehicle) on capsaicin-evoked immunoreactive CGRP release from the dorsal half of rat lumbar spinal cord. Spinal cord slices were collected and superfused as described in the Experimental Procedures. Samples were collected every 3 min. For example the CGRP levels at t = 0 min after capsaicin represent the total CGRP released from 0 to 2.59 min of capsaicin. After equilibration and three fractions of baseline (9 min), the tissue was pre-treated with vehicle (n = 16), 30 nM [Leu31Pro34]NPY (n = 12) or 100 nM [Leu31Pro34]NPY (n = 12) for 3 min. Tissue was then stimulated with 300 nM capsaicin in the presence of the pretreated drug or vehicle for 3 min. Superfusates were assayed for iCGRP using radioimmunoassay. Data are presented as mean±S.E.M. with both P<0.05 (*) and P<0.01 (**) versus capsaicin alone group as indicated.
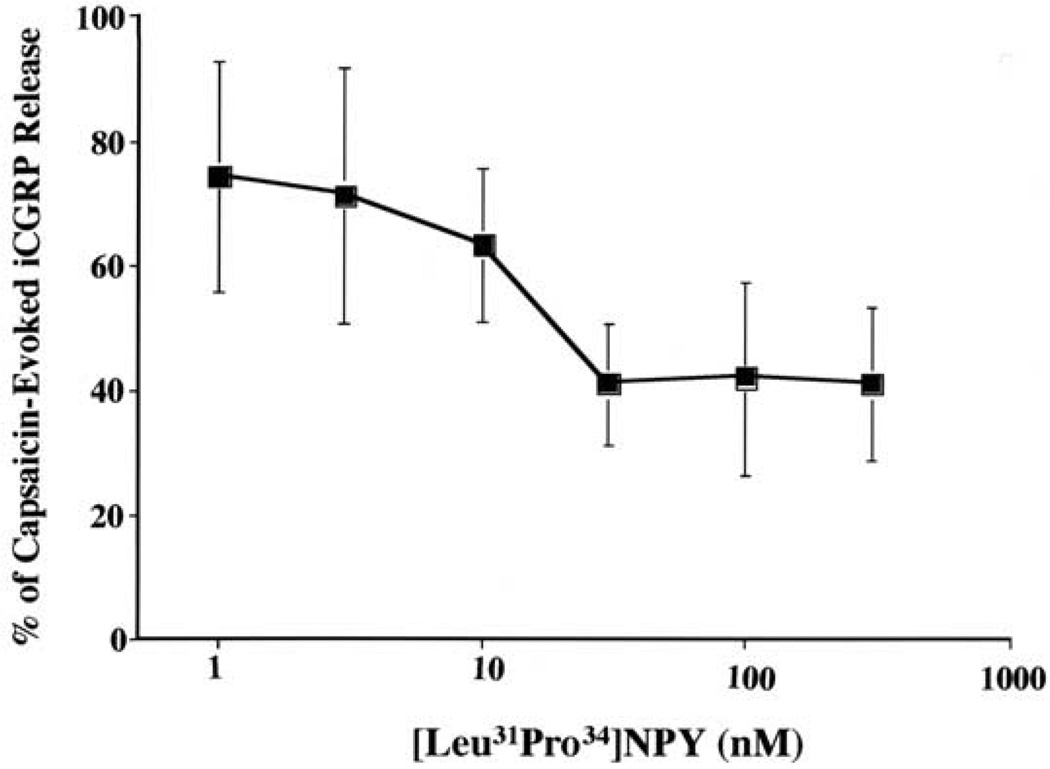
We next characterized the concentration-response relationship for Y1 agonist-mediated inhibition of capsaicin-evoked iCGRP release (Fig. 2). The data are normalized such that the 100% value represents the 4–5-fold peak increase in iCGRP release observed in the vehicle/capsaicin group. Pretreatment with various concentrations of the Y1 agonist [Leu31Pro34]NPY inhibited capsaicin-evoked iCGRP release in a concentration dependent manner. The concentrations of [Leu31Pro34]NPY tested had no effect on basal iCGRP release (data not shown). The EC50 for [Leu31Pro34]NPY inhibition of capsaicin-evoked exocytosis was 10.6 nM, with a maximal effect of 60% reduction in capsaicin-evoked exocytosis.
Fig. 2.
Effect of pretreatment with [Leu31Pro34]NPY on capsaicin-evoked iCGRP from the dorsal half of lumbar spinal cord in rat. Tissue was collected and superfused as described in the Experimental Procedures. After equilibration, the tissue was treated with selected concentrations of [Leu31Pro34]NPY or with vehicle for 3 min. Tissue was then stimulated with 300 nM capsaicin in the presence of pretreated drug or vehicle for 3 min. Data are presented as percentage of capsaicin-evoked iCGRP release. EC50=10.6 nM.
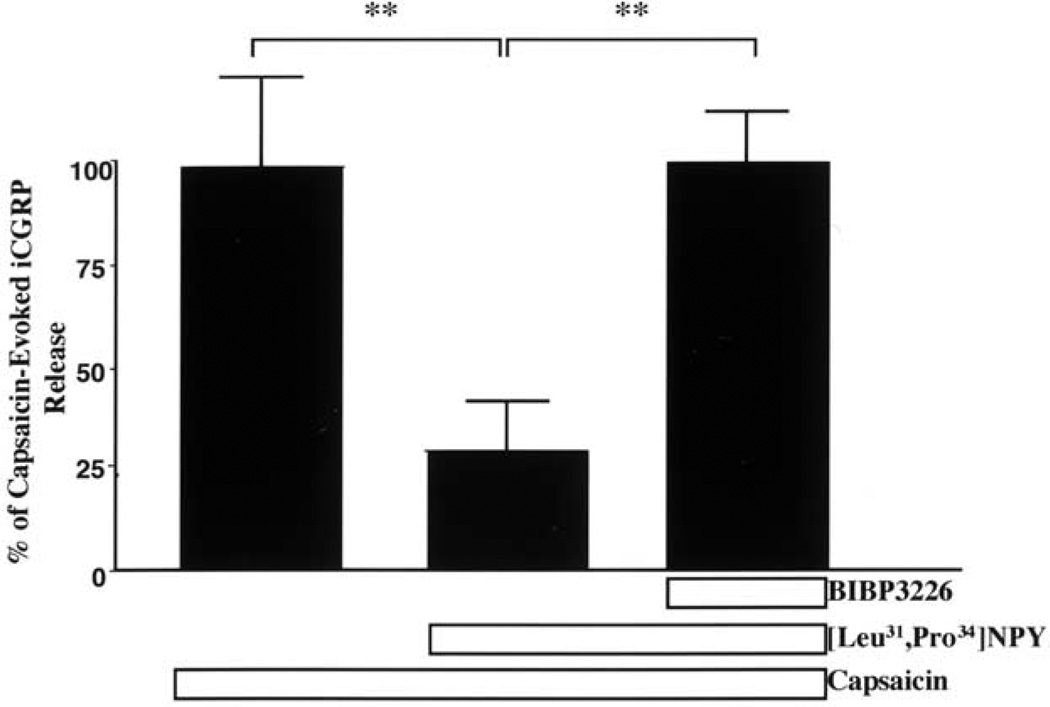
To determine whether this effect of inhibiting capsaicin-evoked exocytosis was mediated via activation of the Y1-R receptor, we determined whether pretreatment with a competitive Y1-R antagonist blocked the effect of the Y1 agonist (Fig. 3). The data are normalized so that the 100% value represents the 4–5-fold increase in iCGRP release observed in the vehicle/capsaicin group. Pretreatment of isolated rat dorsal spinal cord with the Y1 antagonist BIBP3226 completely blocked [Leu31Pro34]NPY-mediated inhibition of capsaicin-evoked iCGRP release (F2,21=6.5, P<0.01). Administration of BIBP3226 alone had no effect on spontaneous iCGRP release.
Fig. 3.
Effect of [Leu31Pro34] NPY±the Y1-R antagonist BIBP3226 on capsaicin-evoked iCGRP release from the dorsal half of the rat lumbar spinal cord. Tissue was collected and superfused as described in the Experimental Procedures. After equilibration, the tissue was treated with vehicle or 100 nM BIBP3226 for 3 min (fraction 4). Tissue was then treated with vehicle, 10 nM [Leu31Pro34] NPY/100 nM BIBP3226, or 10 nM [Leu31Pro34] NPY/vehicle for 3 min (fraction 5). Tissue was then stimulated with 300 nM capsaicin in the presence of the pretreated drug or vehicle for 3 min (fraction 6). Superfusate was assayed using radioimmunoassay for iCGRP. Data are presented as mean±S.E.M. Post hoc test with Newman-Keuls multiple comparison test with P<0.05 (*; [Leu31Pro34] NPY/Cap vs. BIBP3226/NPY/Cap and P<0.01(**; [Leu31Pro34] NPY/Cap vs Cap).
Effect of BIBP3226 on NPY mediation of capsaicin-evoked iCGRP release
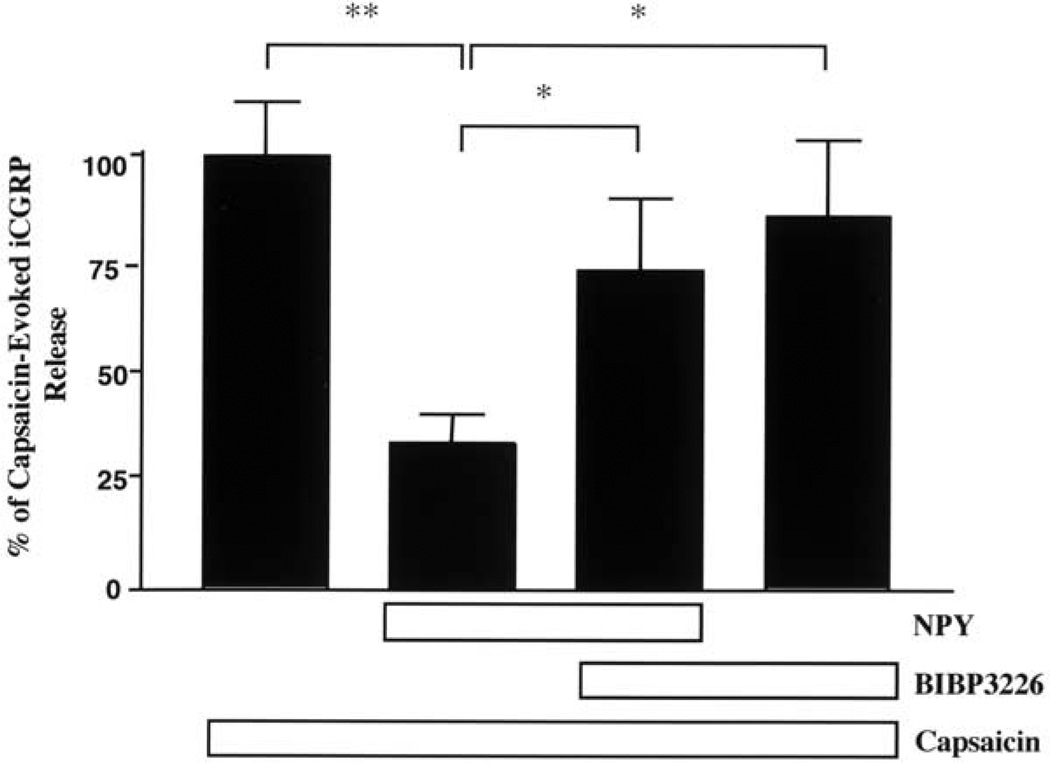
We next evaluated whether synthetic NPY inhibits capsaicin-evoked iCGRP release from rat spinal cord via Y1-R activation (Fig. 4). Again, data are normalized such that 100% represents the 4–5-fold increase in iCGRP release evoked by vehicle/capsaicin treatment. Pretreatment with NPY significantly reduced capsaicin-evoked iCGRP release by 66%, and the Y1 antagonist BIBP3226 blocked approximately 60% of the NPY effect (F3,41=5.9, P<0.005). Treatment with BIBP3226 alone had no significant effect on capsaicin-evoked iCGRP release. Neither BIBP3226 nor NPY affected basal CGRP release (data not shown). The group receiving capsaicin alone did not differ significantly from the group that received BIBP3226, NPY, and capsaicin. Also the group treated with capsaicin and BIBP3226 did not differ significantly from the group treated with capsaicin, BIBP3226, and NPY.
Fig. 4.
Effect of NPY±the Y1-R antagonist BIBP3226 on capsaicin-evoked iCGRP release from the dorsal half of rat lumbar spinal cord. Tissue was collected, pooled, and then superfused. After equilibration, the tissue was treated with vehicle or 30 nM BIBP3226 for 3 min (fraction 4). Tissue was then treated with vehicle, 3 nM NPY/30 nM BIBP3226 or 3 nM NPY/vehicle for 3 min (fraction 5). Tissue was then stimulated with 300 nM capsaicin in the presence of the pretreated drug or vehicle for 3 min (fraction 6). Superfusate was assayed using radioimmunoassay for immunoreactive CGRP. Data are presented as mean±S.E.M. Post hoc test with Newman-Keuls multiple comparison test with P<0.05 (*; NPY/Cap vs. BIBP3226/NPY/Cap) and P<0.01 (**; NPY/Cap vs Cap). None of the other effects were statistically significant.
NPY Y1-R and VR1 in lumbar DRG and spinal cord
We next determined whether there is evidence at the cellular level for a direct mechanism for Y1-R-mediated inhibition of capsaicin-sensitive neurons. We tested this hypothesis using double-label immunohistochemistry for evaluating the co-localization of the Y1 and VR1 receptors in the same afferent neuronal somata in rat DRG and spinal dorsal horn neurons.
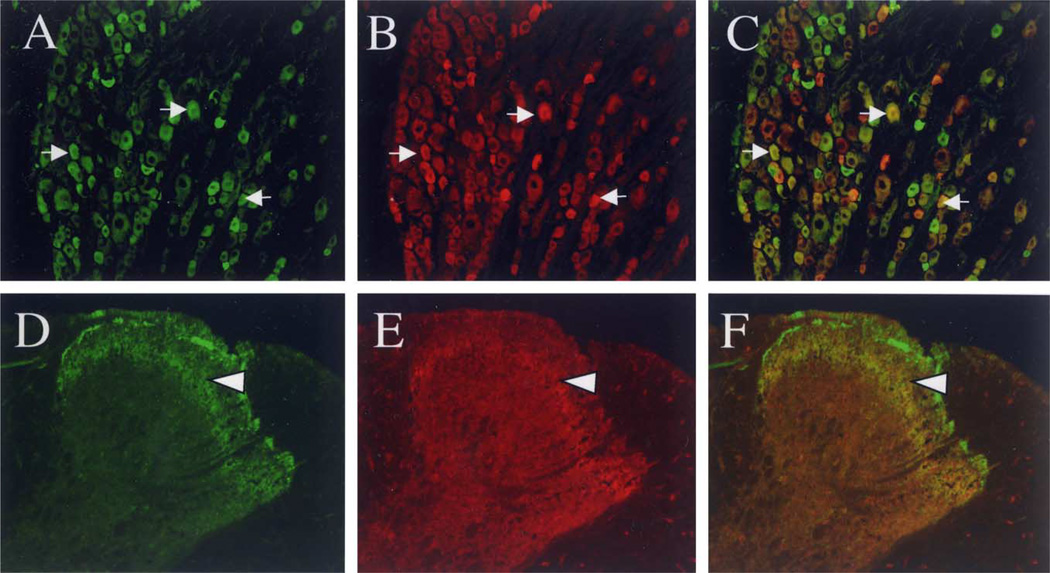
In rat lumbar DRG, 30% of all counted neuronal profiles (n = 2043) were strongly immunoreactive for VR1 (Fig. 5A) and these neurons were primarily small- and medium-diameter neuronal profiles. Pre-adsorption control experiments with the cognate peptide blocked VR1 staining (data not shown). Approximately 20% of all DRG neurons were strongly immunoreactive for the Y1-R (Fig. 5B), and the majority of these neurons also had medium- and small-sized profiles (Table 1). Immunoreactivity for the two receptors was frequently expressed in the same cell (Fig. 5C). Of all cells that expressed the Y1-R, approximately 35% also expressed VR1. Of all cells that expressed VR1, at least 20% also expressed the Y1-R.
Fig. 5.
Co-localization of VR1 immunoreactivity with Y1 immunoreactivity in DRG neurons and in the superficial dorsal horn. (A–C) Double labeling of VR1 (A; green) with Y1 (B; red) and overlay (C) in DRG (magnification = 20×). Co-localization is shown in yellow and by arrows. (D–F) Double labeling of VR1 (D; green) with Y1 (E; red) and overlay (F; magnification = 20×). Immunoreactivity for Y1 and VR1 are both localized in inner lamina II, as indicated by yellow and arrowhead. These images were digitally sharpened using Adobe Photoshop 5.0.
Table 1.
Number of trigeminal neurons expressing Y1-R and VR1-R immunoreactivity
| Immuno- reactive neurons |
Total # of IR-neurons |
% IR neuronsa |
# of co-VR1 IR neurons/total # of neurons |
% of co-VR1-IR neuronsb |
# of co-Y1 IR neurons/total # of neurons |
% of co-Y1-IR neuronsc |
|---|---|---|---|---|---|---|
| Y1 receptor | 435/2357 | 18.4 ± 1.8d | 110/335 | 33.7 ± 7.1 | – | – |
| VR1 receptor | 640/2357 | 27.3 ± 3.6 | – | – | 110/528 | 21.0 ± 2.8 |
Percentage of total neurons counted expressing either Y1 or VR1 immunoreactivity; represents counts of 5 entire sections from 3 different animals.
Percentage of Y1-IR neurons co-expressing VR1 immunoreactivity; represents counts of 4 entire tissue sections from 3 different animals.
Percentage of VR1-IR neurons co-expressing Y1 immunoreactivity; represents counts of 4 entire tissue sections from 3 different animals.
Data are expressed as mean±SD, of percentages from each section.
We also determined whether Y1-R and VR1 were located in the same region of the dorsal horn of the spinal cord. Immunoreactivity for VR1 was localized in lamina I and inner lamina II (Fig. 5D). Y1-R immunoreactivity was most prominent in inner lamina II (Fig. 5E). Immunoreactivity for Y1 and VR1 were both found in inner lamina II (Fig, 5F).
DISCUSSION
This study demonstrates that that the Y1 agonist [Leu31Pro34]NPY inhibits neuropeptide exocytosis from central terminals of capsaicin sensitive afferent fibers innervating the dorsal horn of the lumbar spinal cord in rat. This effect was concentration-dependent and completely blocked by pretreatment with the competitive Y1 antagonist BIBP3226, indicating that this effect is mediated by a Y1-R mechanism. We also show that the Y1-R is the primary, but not exclusive receptor involved in NPY mediated inhibition of capsaicin-evoked iCGRP release in the spinal cord, by demonstrating that BIBP3226 partially blocks NPY-mediated inhibition of capsaicin-evoked iCGRP release in rat spinal cord.
Several studies have demonstrated that intrathecally administered NPY is anti-nociceptive under basal conditions or anti-hyperalgesic after rat hindpaw inflammation (Hua et al., 1991; Xu et al., 1999; Naveilhan et al., 2001; Taiwo and Taylor, 2002). Studies using either Y1-R antagonists or the Y1-R knockout mouse indicate that this effect is mediated by a Y1-R receptor mechanism. Collectively, these studies indicate that activation of Y1-Rs in the spinal dorsal horn plays a substantial role in modulating nociception.
Although the neuronal populations expressing the Y1-R are not fully characterized, the prevailing hypothesis has been that these receptors are expressed by intrinsic dorsal horn neurons involved in nociceptive processing (Ji et al., 1994; Zhang et al., 1994). A presynaptic site of action on central terminals of sensory nociceptors has not been considered due to the interpretation of data suggesting that the Y1-R was restricted to the somata of afferent neurons (Hokfelt et al., 1997). However, more recently, the Y1-R has been reported to be localized at pre-synaptic sites as well (Pickel et al., 1998; Marchand et al., 1999; St. Pierre et al., 2000; Brumovsky et al., 2002). Results from the present study on CGRP release are consistent with the hypothesis that Y1-Rs act presynaptically to inhibit capsaicin-evoked exocytosis from capsaicin-sensitive nociceptors. Our data provide strong experimental support for this hypothesis because activation of Y1-Rs inhibited neurosecretion from capsaicin-sensitive nociceptors. Previous studies have demonstrated that the presence of VR1 receptor in the dorsal horn is of primary afferent origin (Guo et al., 1999). The expression of the Y1-R on a proportion of the VR1 positive nociceptor population supports the hypothesis that the inhibitory effect of Y1-R inhibition of capsaicin-sensitive neurons at least partially occurs at a presynaptic site of action.
To our knowledge this is the first report demonstrating that a sub-population of capsaicin-sensitive nociceptors expresses the Y1-R. The co-localization of the Y1-R with the VR1-expressing class of nociceptors indicates that activation of the Y1-R may directly inhibit capsaicin-evoked exocytosis. Interestingly the degree of co-localization of Y1 and VR1 in DRG (about 20%) did not correlate to the magnitude of Y1-R mediated inhibition of capsaicin stimulated iCGRP release from dorsal spinal cord (about 60%). Several hypotheses may explain this difference. First, there may be a difference in assay sensitivity between the RIA and immunohistochemistry protocols. It is possible that the extent of co-localization is underestimated due to the stringent criteria used to identify a positively labeled cell. In this study, only strongly labeled cells were counted, so it is possible that cells with a functionally relevant density of co-localized receptors may not have been counted. Second, it is possible that Y1-R mediated inhibition of capsaicin-stimulated exocytosis in the dorsal horn is partially mediated by an indirect mechanism. A significant amount of the Y1-Rs found in the dorsal horn is not expressed on primary afferent fibers, as evidenced by maintenance of some Y1 immunoreactivity after rhizotomy (Brumovsky et al., 2002). These hypotheses are not mutually exclusive and it is possible that both contribute to our observations.
Our results are consistent with studies using the Y1 knockout mouse, demonstrating that the Y1-R is necessary for the anti-nociceptive effects of spinally administered NPY (Naveilhan et al., 2001). However, studies using the Y1 knockout mouse have reported that the antinociceptive effects of intrathecal NPY are exclusively due to the Y1-R. In contrast, our results demonstrate that approximately 40% of NPY-mediated inhibition of capsaicin-evoked CGRP was not blocked by pretreatment with a Y1-R antagonist. There are at least two hypotheses that may account for this difference. First, it is possible that the antagonist BIBP3226 may not have completely blocked the Y1-R pool (Doods et al., 1996). In order to minimize non-specific effects, we used a 100 nM concentration of BIBP3226 (Vanderheyden et al., 1998; Van Liefde et al., 2001; Mollereau et al., 2001). The antagonist BIBP322 has a reported pA2 of 7.9 (Tough and Cox, 1996). Thus, concentrations of the antagonist were only one to two orders of magnitude greater than the pA2 values and, depending upon the spare receptor pool of Y1, it is possible that at least some small proportion of the receptor pool was available for activation. However, it should be recognized that similar concentrations of BIBP3226 were sufficient to completely block the effects of the Y1 selective agonist [Leu31Pro34]NPY. A second possibility is that NPY inhibits CGRP release through both Y1 and non-Y1-Rs. The NPY Y2 receptor is a possible candidate since NPY binds the Y1 and Y2 receptor with similar affinity (Larhammar, 1996; Blomqvist and Herzog, 1997). The mRNA of the Y2 receptor is present in sensory dorsal horn neurons, although they have not been previously identified on the capsaicin-sensitive population of neurons (Mantyh et al., 1994; Zhang et al., 1997).
In our study the Y1 antagonist did not have a significant effect on capsaicin-evoked iCGRP release (Fig. 4). Studies using a Y1 knockout mouse indicated that the Y1-R is necessary for capsaicin-evoked substance P (SP) release (Naveilhan et al., 2001). There are several possible reasons for the dissimilar findings concerning the role of the Y1-R on capsaicin-evoked exocytosis. First, our study evaluated the effect of the Y1-R on capsaicin-evoked release at central terminals, while the knock-out study evaluated release from peripheral terminals. Second, we were evaluating CGRP release while the knockout study evaluated SP release. Finally, it is possible the finding that the Y1-R is necessary for capsaicin-evoked SP release is the result of developmental compensation in the knockout mouse. In fact studies of the NPY knock-out mouse have demonstrated significant differences in receptor expression in specific brain regions when compared with wild type (Trivedi et al., 2001).
Although CGRP is only one of several substances released from capsaicin-sensitive nociceptors, numerous studies using electrophysiologic, receptor antagonist, immunoneutralization and knockout approaches have all indicated that CGRP facilitates nociceptive processing in the spinal dorsal horn (Cridland and Henry, 1988; Gschossmann et al., 2001; Yu et al., 2002; Zhang et al., 2001). Thus inhibition of neurotransmission from capsaicin-sensitive nociceptors may mediate NPY-induced antinociception and antihyperalgesia. Collectively, the results of these studies indicate that Y1-R mediated inhibition of exocytosis from central terminals of capsaicin-sensitive neurons is a major mechanism mediating the antinociceptive and antihyperalgesic effects of NPY agonists.
The substantial upregulation of NPY expression and release after peripheral nerve injury or inflammation has been well established (Wakisaka et al., 1991, 1992; Ji et al., 1994; Marchand et al., 1999; Mark et al., 1997; Sten Shi et al., 1999). Injury-induced activation of the NPY system may have important implications in the development of these persistent pain states (Ossipov et al., 2002).
Acknowledgements
This research was funded by NIH grants F30DE14326, and R37DE12888 We would also like to thank the University of Texas Health Science Center Department of Pharmacology for their support.
Abbreviations
- ANOVA
analysis of variance
- CGRP
calcitonin generelated peptide
- DRG
dorsal root ganglion
- iCGRP
immunoreactive calcitonin gene-related peptide
- NGS
normal goat serum
- NPY
neuropeptide Y
- SP
substance P
- VR1
vanilloid receptor type I
- Y1-R
Y1 receptor.
REFERENCES
- Balasubramaniam AA. Neuropeptide Y family of hormones: receptor subtypes and antagonists. Peptides. 1997;18:445–457. doi: 10.1016/s0196-9781(96)00347-6. [DOI] [PubMed] [Google Scholar]
- Blomqvist AG, Herzog H. Y-receptor subtypes: how many more? Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- Brumovsky B, Shi TJ, Matsuda H, Kopp J, Villar MJ, Hokfelt T. NPY Y1 receptors are present in axonal processes of DRG neurons. Exp Neurol. 2002;174:1–10. doi: 10.1006/exnr.2001.7845. [DOI] [PubMed] [Google Scholar]
- Cridland R, Henry J. Effects of intrathecal administration of neuropeptides on a spinal nociceptive reflex in the rat: VIP, galanin, CGRP, TRH, somatostatin and angiontensin II. Neuropeptides. 1988;11:23–32. doi: 10.1016/0143-4179(88)90024-8. [DOI] [PubMed] [Google Scholar]
- Doods HN, Wieland HA, Engel W, Eberlein W, Willem KD, Entzeroth M, Wienen W, Rudolf K. BIBP 3226, the first selective neuropeptide Y1 receptor antagonist: a review of its pharmacological properties. Regul Peptides. 1996;65:71–77. doi: 10.1016/0167-0115(96)00074-2. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Hope PJ, Lang CW. Microinjection of neuropeptide Y into the superficial dorsal horn reduces stimulus-evoked release of immunoreactive substance P in the anaesthetized cat. Neuroscience. 1991;44:733–740. doi: 10.1016/0306-4522(91)90092-3. [DOI] [PubMed] [Google Scholar]
- Garry MG, Hargreaves KM. Enhances release of immunoreactive CGRP and substance P from spinal dorsal horn slices occurs during carrageenan inflammation. Brain Res. 1992;582:139–142. doi: 10.1016/0006-8993(92)90328-7. [DOI] [PubMed] [Google Scholar]
- Garry MG, Richardson JD, Hargreaves KM. Sodium nitroprusside evokes the release of immunoreactive calcitonin gene-related peptide and substance P from dorsal horn slices via nitric oxide-dependent and nitric oxide-independent mechanisms. J Neurosci. 1994;14:4329–4337. doi: 10.1523/JNEUROSCI.14-07-04329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschossmann J, Coutinho S, Miller J, Huebel K, Naliboff B, Wong H, Walsh J, Mayer E. Involvement of spinal calcitonin gene-related peptide in the development of acute visceral hyperalgesia in the rat. Neurogastroenterology. 2001;13:229–236. doi: 10.1046/j.1365-2982.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hua X-Y, Boublik JH, Spicer MA, Rivier JE, Brown MR, Yaksh TL. The antinociceptive effects of spinally administered neuropeptide Y in the rat: systemic studies on structure-activity relationship. J Pharacol Exp Ther. 1991;258:243–248. [PubMed] [Google Scholar]
- Hokfelt T, Zhang X, Xu Z-Q, Ji R-R, Shi T, Corness J, Kerekes N, Landry M, Holmberg K, Broberger K. Phenotype regulation in dorsal root ganglion neurons after nerve injury: focus on peptides and their receptors. In: Borsook D, editor. Molecular neurobiology of pain: progress in pain research and management. Vol. 9. Seattle: IASP Press; 1997. pp. 115–143. [Google Scholar]
- Hokfelt T, Broberger C, Zhang X, Diez M, Kopp J, Xu Z, Lamndr M, Bao L, Schalling M, Koistinaho J, De Armond SJ, Prusiner S, Gong J, Walsh JH. Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev. 1998;26:154–166. doi: 10.1016/s0165-0173(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Ji R, Zhang X, Wiesenfeld-Hallin Z, Hokfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglion following peripheral tissue inflammation. J Neurosci. 1994;14:6423–6434. doi: 10.1523/JNEUROSCI.14-11-06423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S, Quirion R. Quantitative autoradiographic localization of [I125] neuropeptide Y receptor binding sites in rat spinal cord and the effects of neonatal capsaicin, dorsal rhizotomy and peripheral axotomy. Brain Res. 1992;574:333–337. doi: 10.1016/0006-8993(92)90836-x. [DOI] [PubMed] [Google Scholar]
- Larhammar D. Structural diversity of receptors for NPY, peptide YY, pancreatic polypeptide. Regul Peptides. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- Malstrom R. NPY Y1 receptor mechanisms in sympathetic vascular control. Acta Physiol Scan. 1997;636(suppl):1–55. [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Rogers S, DeMaster E, Ghilardi JR, Mosconi T, Kruger L, Mannon PJ, Taylor IL, Vigna SR. Some sensory neurons express neuropeptide Y receptors: potential paracrine inhibition of primary afferent nociceptors following peripheral nerve injury. J Neurosci. 1994;14:3958–3968. doi: 10.1523/JNEUROSCI.14-06-03958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J, Cepeda M, Carr D, Wurm W, Kream R. Alterations in neuropeptide Y, tyrosine hydroxylase, and Y-receptor subtype distribution following spinal nerve injury to rats. Pain. 1999;79:187–200. doi: 10.1016/s0304-3959(98)00165-1. [DOI] [PubMed] [Google Scholar]
- Mark MA, Jarrot B, Colvin LA, Duggan AW. The release of immunoreactive neuropeptide Y in the spinal cord of the anaesthetized rat and cat. Brain Res. 1997;754:195–203. doi: 10.1016/s0006-8993(97)00061-9. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Gouarderes C, Dumont Y, Kotani M, Detheux M, Doods H, Parmentier M, Quirion R, Zajac JM. Agonist and antagonist activities on human NPFF(2) receptors of the NPY ligands GR23118 and BIBP3226. Br J Pharmacol. 2001;133:1–4. doi: 10.1038/sj.bjp.0704049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton CR, Hutchison WD. Release of sensory neuropeptides in the spinal cord: studies with calcitonin gene-related peptide and galanin. Neuroscience. 1989;31:807–815. doi: 10.1016/0306-4522(89)90443-0. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Lucas G, Hygge Blakeman K, Hao J-X, Xu X-J, Wiesenfeld-Hallin Z, Thoren P, Ernfers P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409:513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Zhang E-T, Carvajal C, Gardell L, Quirion R, Dumont Y, Lai J, Porreca F. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22:9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Beck-Sickinger AG, Chan J, Wieland A. Y1 receptors in the nucleus accumbens: ultrastructural localization and association with neuropeptide Y. J Neurosci Res. 1998;52:54–68. doi: 10.1002/(SICI)1097-4547(19980401)52:1<54::AID-JNR6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Pohl M, Benoliel JJ, Bourgoin S, Lombard MC, Mauborgne A, Taquet H, Carayon A, Besson JM, Cesselin F, Hamon M. Regional distribution of calcitonin gene-related peptide-, substance P-, cholecystokinin-, met-enkephalin-, and dynorphin A (1–8)-like material in the spinal cord and dorsal root ganglia of adult rats: effects of dorsal rhizotomy and neonatal capsaicin. J Neurochem. 1990;55:1122–1130. doi: 10.1111/j.1471-4159.1990.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in αCGRP deficient mice. Nat Neurosci. 2001;4:357–358. doi: 10.1038/86001. [DOI] [PubMed] [Google Scholar]
- Sten Shi T-J, Cui J-G, Meyerson BA, Linderoth B, Hokfelt T. Regulation of galanin and neuropeptide Y in dorsal root ganglia and dorsal horn in rat mononeuropathic models: possible relation to tactile hypersensitivity. Neuroscience. 1999;93:1999. doi: 10.1016/s0306-4522(99)00105-0. [DOI] [PubMed] [Google Scholar]
- St. Pierre J-A, Nouel D, Dumont Y, Beaudet A, Quirion R. Association of neuropeptide Y Y1 receptors with glutamate-positive and NPY-positive neurons in rat hippocampal cultures. Eur J Neurosci. 2000;12:1319–1330. doi: 10.1046/j.1460-9568.2000.00024.x. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Lawland NB, Willi WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/s0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Taiwo OB, Taylor BK. Antihyperalgesic effects of intrathecal neuropeptide Y during inflammation are mediated by Y1 receptors. Pain. 2002;96:353–363. doi: 10.1016/S0304-3959(01)00481-X. [DOI] [PubMed] [Google Scholar]
- Tough IR, Cox HM. Selective inhibition of neuropeptide Y Y1 receptors by BIBP3226 in rat and human epithelial preparations. Eur J Pharmacol. 1996;310:55–60. doi: 10.1016/0014-2999(96)00372-x. [DOI] [PubMed] [Google Scholar]
- Trivedi PG, Yu H, Trumbauer M, Chen H, Van der Ploeg LH, Guan X. Peptides. 2001;22:395–403. doi: 10.1016/s0196-9781(01)00349-7. [DOI] [PubMed] [Google Scholar]
- Van Liefde I, Vanderheyden PM, De Backer JP, Ebinger G, Vauquelin G. Effects of BIBP3226 and BIBP3435 on cytosolic calcium in neuropeptide Y Y1 receptor-transfected Chinese hamster ovary cells and wild type CHO-K1 cell. J Receptor Sign Transduc Res. 2001;21:11–23. doi: 10.1081/rrs-100107139. [DOI] [PubMed] [Google Scholar]
- Van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization, and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Vanderheyden PM, Van Liefde I, de Backer JP, Vauquelin G. J Receptor Sign Transduc Res. 1998;18:363–385. doi: 10.3109/10799899809047752. [DOI] [PubMed] [Google Scholar]
- Vasko MR. Prostaglandin-induced neuropeptide release from spinal cord. Prog Brain Res. 1995;104:367–380. doi: 10.1016/s0079-6123(08)61801-4. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett. 1991;124:200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennet GJ. Effects of peripheral nerve injuries and tissue inflammation on the levels of neuropeptide Y-like immunoreactivity in rat primary afferent neurons. Brain Res. 1992;598:349–352. doi: 10.1016/0006-8993(92)90206-o. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory mechanism of the spinal cord. New York, NY: Plenum Press; 1991. [Google Scholar]
- Xu IS, Hao J-X, Xu X-J, Hokfelt T, Wiesenfeld-Hallin Z. Brain Res. 1999;833:251–257. doi: 10.1016/s0006-8993(99)01551-6. [DOI] [PubMed] [Google Scholar]
- Yu Y, Lundberg T, Yu L. Role of calcitonin gene-related peptide and its antagonist on the evoked discharge frequency of wide dynamic range neurons in the dorsal horn of the spinal cord of rats. Regul Pept. 2002;103:23–27. doi: 10.1016/s0167-0115(01)00326-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Xu Z-Q, Kopp I, Arvidsson U, Elde R, Hokfelt T. Localization of neuropeptide Y1 receptors in rat nervous system with special reference to somatic receptors on small dorsal root ganglion neurons. Proc Natl Acad Sci USA. 1994;91:11738–11742. doi: 10.1073/pnas.91.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shi T-J, Holmberg K, Landry M, Huang W, Xiao H, Ju G, Hokfelt T. Expression and regulation of the neuropeptide Y [Y2] receptors in sensory ganglia and autonomic regions. Proc Natl Acad Sci USA. 1997;94:729–734. doi: 10.1073/pnas.94.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hoff A, Wimalawansa S, Cote G, Gagel R, Westlund K. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89:265–273. doi: 10.1016/s0304-3959(00)00378-x. [DOI] [PubMed] [Google Scholar]