Stage II/III breast cancer patients treated with breast-conserving-surgery (BCS) plus radiotherapy in a publicly-funded healthcare system had a decreased hazard of all-cause and breast cancer-specific mortality compared to those who received mastectomy. Greater efforts to educate patients in the benefits of BCS plus radiotherapy over mastectomy when medically feasible and appropriate are needed.
Keywords: breast cancer, population-based, surgery, survival
Abstract
Background
Recent investigations of breast cancer survival in the United States suggest that patients who receive mastectomy have poorer survival than those who receive breast-conserving surgery (BCS) plus radiotherapy, despite clinically established equivalence. This study investigates breast cancer survival in the publicly funded health care system present in Alberta, Canada.
Patients and methods
Surgically treated stage I–III breast cancer cases diagnosed in Alberta from 2002 to 2010 were included. Demographic, treatment and mortality information were collected from the Alberta Cancer Registry. Unadjusted overall and breast cancer-specific mortality was assessed using Kaplan–Meier and cumulative incidence curves, respectively. Cox proportional hazards models were used to calculate stage-specific mortality hazard estimates associated with surgical treatment received.
Results
A total of 14 939 cases of breast cancer (14 633 patients) were included in this study. The unadjusted 5-year all-cause survival probabilities for patients treated with BCS plus radiotherapy, mastectomy, and BCS alone were 94% (95% CI 93% to 95%), 83% (95% CI 82% to 84%) and 74% (95% CI 70% to 78%), respectively. Stage II and III patients who received mastectomy had a higher all-cause (stage II HR = 1.36, 95% CI 1.13–1.48; stage III HR = 1.74, 95% CI 1.24–2.45) and breast cancer-specific (stage II HR = 1.39, 95% CI 1.09–1.76; stage III HR = 1.79, 95% CI 1.21–2.65) mortality hazard compared with those who received BCS plus radiotherapy, adjusting for patient and clinical characteristics. BCS alone was consistently associated with poor survival.
Conclusions
Stage II and III breast cancer patients diagnosed in Alberta, Canada, who received mastectomy had a significantly higher all-cause and breast cancer-specific mortality hazard compared with those who received BCS plus radiotherapy. We suggest greater efforts toward educating and encouraging patients to receive BCS plus radiotherapy rather than mastectomy when it is medically feasible and appropriate.
introduction
Randomized clinical trials have suggested survival is equivalent for women diagnosed with early-stage breast cancer who receive mastectomy or breast-conserving surgery (BCS) followed by radiotherapy [1–3]. Results from clinical trials, however, do not always translate to the population since few cancer patients enroll in trials and those that do tend to be younger, healthier and less racially and ethnically diverse than the typical patient population [4]. The investigation of current breast cancer patient survival in a population-based context is, therefore, of interest.
Three recent studies from the United States have reported better survival with receipt of BCS plus radiotherapy compared with mastectomy among stage I and II breast cancer patients [5–7]. This is concerning, for significant variation in the receipt of BCS has been found within Canada [8]. In Quebec, 64% of breast cancer patients received BCS from 2007 to 2010 compared with 29% in Newfoundland. In Alberta, where 46% of patients received BCS, significant variation within the province has also been reported [9]. Here, we investigate breast cancer patient survival by surgery received within the publicly funded health care system present in Alberta, Canada.
The purpose of this study was to assess: (i) the all-cause and breast cancer-specific survival rates of nonmetastatic breast cancer patients surgically treated with mastectomy, BCS alone and BCS plus radiotherapy and (ii) identify the relationship between several factors, including treatment, with mortality among surgically treated breast cancer patients diagnosed in Alberta, Canada.
methods
study population
The Alberta Cancer Registry was used to identify all stage I, II and III breast cancer cases (International Classification of Diseases for Oncology (ICD-O-3) site code c50 [10]) diagnosed in adult women in Alberta from 2002 to 2010 who received breast cancer surgery. Breast cancer cases were excluded if: (i) the cancer was not the first primary diagnosis in a given breast; (ii) histology was not consistent with a solid breast tumor, including sarcoma, lymphoma and hematopoietic tumors and (iii) the patient had another cancer diagnosis within 6 months before the breast cancer case, as this could influence treatment and/or survival.
data source and variables
Demographic, clinical and treatment information were obtained from the Alberta Cancer Registry including: date of diagnosis, age at diagnosis, estrogen and progesterone receptor (ER/PR status), cancer stage, tumor size, nodal status, type of surgery, geographic region in which surgery took place, receipt of neoadjuvant and adjuvant chemotherapy, receipt of hormone therapy, receipt of postoperative radiotherapy, date of death, cause of death. The American Joint Committee on Cancer (AJCC) 5th edition was used to determine cancer stage for patients diagnosed in years 2002 and 2003, while the 6th edition was used for years 2004–2010 [11, 12]. Geographic region of surgery was categorized into the five administrative health zones of Alberta. The province of Alberta consists of an area of 666 000 km2 and has a population of 3.7 million. Approximately 85% of the population is White; the 15% visible minority population primarily resides in one of the two major cities [13]. Two zones are urban and suburban in population size and density (Edmonton and Calgary) and three zones are a combination of suburban, rural and remote regions (South, Central and North).
Patients diagnosed in 2002 and 2003 who received hormone therapy were classified as ER/PR positive, while those who did not receive hormone therapy were classified as ER/PR negative, since ER/PR status was not collected in these years. If ER/PR status was missing in patients diagnosed from 2004 to 2010, the case was assumed to be ER/PR positive (N = 107), since roughly 75% of breast cancers in North America are ER/PR positive [14]. Sensitivity analyses found that these assumptions did not affect the study results. Patients with missing tumor size (N = 234) or nodal status (N = 226) were randomly assigned a value proportionately based on the non-missing information. The North American Association of Central Cancer Registries has awarded the Alberta Cancer Registry the highest level of certification in all years of the study for its high level of completeness and for the timeliness of data collection and reporting.
statistical analysis
Three treatment categories were defined based on the surgery type and radiotherapy treatments received: BCS alone, BCS plus adjuvant radiotherapy and mastectomy. Descriptive statistics were calculated for the demographic, clinical and treatment characteristics of the breast cancer cases by treatment category, stratified by cancer stage. Statistical differences were determined by χ2 and Fisher's exact tests.
Kaplan–Meier curves were generated to compare overall survival of patients by treatment category, stratified by cancer stage using the first cancer case per patient only. Cumulative incidence curves were used to describe the cumulative mortality from breast cancer-specific deaths, treating other causes of death as competing risk. Since patient deaths occurring within 30 days of surgical treatment are likely due to complications, the start time for the curves was 30 days post-surgery. Deaths before this start time were excluded. The log-rank and Gray's test statistics were used to assess differences in overall survival and breast cancer-specific survival, respectively.
Cox proportional hazards regression models were fitted to calculate the adjusted hazard ratios of overall and breast cancer-specific mortality by treatment category, stratified by stage and adjusting for age at diagnosis, geographic region of surgery, year of diagnosis, ER/PR status and hormone therapy, neoadjuvant chemotherapy, adjuvant chemotherapy, tumor size and nodal status. These models were also fitted using 30 days post-surgery as the start time. Correlation of outcomes for patients with two breast cancer cases was taken into account through use of the counting process [15]. Wald test statistics were used to assess hazard ratio differences. Regression models were also run with the additional stratification of mastectomy patients by radiotherapy status. The proportional hazards assumption was checked for all models.
All statistical analyses were carried out using SAS statistical software version 9.3 (SAS Institute Cary, NC) and R version 3.0.3 (R foundation for Statistical Computing, Vienna, Austria).
results
There were 15 252 cases (14 933 patients) of stage I, II or III breast cancer diagnosed in female residents of Alberta, excluding non-solid tumors and patients who had another cancer diagnosis within 6 months before the breast cancer case of interest. The following number of cases/patients were excluded for the following reasons: 294 cases and 281 patients did not receive surgery; 6 cases/patients had an unknown or other type of surgery; 13 cases/patients died within 30 days of surgery. The final cohort, therefore, included 14 939 cases of breast cancer (14 633 patients). The median follow-up time was 4.2 years. A total of 1853 (12%) patients died during the study period: 980 (7%) from breast cancer and 873 (6%) from other causes.
Table 1 shows the distribution of demographic, clinical and treatment characteristics for stage I, II and III breast cancer cases by treatment category. BCS plus adjuvant radiotherapy was received by 50% (3652), 32% (1792) and 14% (278) of stage I, II and III cases, respectively. Younger patients were most likely to receive BCS plus radiotherapy, while older patients were more likely to receive mastectomy, regardless of cancer stage. The proportion of stage I and II cases that received BCS alone was low in all age categories (1%–7%) except in the most elderly patients, ≥80 years of age, in which 27% and 15% received BCS alone, respectively. Cases that received surgery in one of the two largest urban areas in the province (Edmonton or Calgary) were more likely to receive BCS plus adjuvant radiotherapy than those living in a more rural area, regardless of cancer stage. The region with the highest proportion of mastectomy in all cancer stages was Central Alberta (62%, 77% and 89% for stage I, II and III patients, respectively).
Table 1.
Characteristics of stage I, II and III breast cancer cases that received surgery in Alberta from 2002 to 2010
| Stage I |
Stage II |
Stage III |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCS alone, N (%)a | BCS + adjuvant radiotherapy, N (%)a | Mastectomy, N (%)a | Total, N | BCS alone, N (%)a | BCS + adjuvant radiotherapy, N (%)a | Mastectomy, N (%)a | Total, N | BCS alone, N (%)a | BCS + adjuvant radiotherapy, N (%)a | Mastectomy, N (%)a | Total, N | |
| Overall | 470 (6) | 3652 (50) | 3170 (43) | 7292 | 281 (5) | 1792 (32) | 3613 (64) | 5686 | 54 (3) | 278 (14) | 1629 (83) | 1961 |
| Age at diagnosisb | ||||||||||||
| <50 | 60 (4) | 827 (54) | 648 (42) | 1535 | 58 (4) | 601 (36) | 992 (60) | 1651 | 19 (3) | 93 (15) | 520 (82) | 632 |
| 50–59 | 78 (4) | 1109 (58) | 712 (37) | 1899 | 64 (4) | 525 (37) | 847 (59) | 1436 | 7 (1) | 95 (19) | 411 (80) | 513 |
| 60–69 | 79 (4) | 988 (53) | 788 (42) | 1855 | 38 (3) | 395 (34) | 722 (63) | 1155 | 9 (2) | 58 (15) | 308 (82) | 375 |
| 70–79 | 98 (7) | 608 (42) | 729 (51) | 1435 | 37 (4) | 212 (24) | 628 (72) | 877 | 4 (2) | 23 (9) | 230 (89) | 257 |
| ≥80 | 155 (27) | 120 (21) | 293 (52) | 568 | 84 (15) | 59 (10) | 424 (75) | 567 | 15 (8) | 9 (5) | 160 (87) | 184 |
| Geographic region of surgeryb | ||||||||||||
| South | 54 (9) | 239 (41) | 297 (50) | 590 | 33 (7) | 114 (23) | 351 (70) | 498 | 5 (3) | 20 (14) | 122 (83) | 147 |
| Calgary | 156 (6) | 1500 (55) | 1063 (39) | 2719 | 118 (5) | 793 (36) | 1276 (58) | 2187 | 26 (3) | 113 (15) | 612 (81) | 751 |
| Central | 30 (6) | 150 (32) | 295 (62) | 475 | 22 (5) | 90 (19) | 365 (77) | 477 | 3 (2) | 14 (9) | 142 (89) | 159 |
| Edmonton | 213 (6) | 1665 (51) | 1408 (43) | 3286 | 94 (4) | 739 (32) | 1449 (63) | 2282 | 19 (2) | 115 (14) | 677 (83) | 811 |
| North | 17 (8) | 98 (44) | 107 (48) | 222 | 14 (6) | 56 (23) | 172 (71) | 242 | 1 (1) | 16 (17) | 76 (82) | 93 |
| Year of diagnosis | ||||||||||||
| 2002–2004 | 129 (6) | 1158 (50) | 1047 (45) | 2334 | 67 (4) | 566 (32) | 1143 (64) | 1776 | 8 (2) | 78 (15) | 442 (84) | 528 |
| 2005–2007 | 176 (8) | 1159 (49) | 1010 (43) | 2345 | 108 (6) | 564 (30) | 1189 (64) | 1861 | 23 (3) | 102 (14) | 585 (82) | 710 |
| 2008–2010 | 165 (6) | 1335 (51) | 1113 (43) | 2613 | 106 (5) | 662 (32) | 1281 (63) | 2049 | 23 (3) | 98 (14) | 602 (83) | 723 |
| ER/PR status | ||||||||||||
| Positive | 395 (7) | 3061 (50) | 2612 (43) | 6068 | 208 (5) | 1489 (32) | 2916 (63) | 4613 | 46 (3) | 222 (15) | 1250 (82) | 1518 |
| Negative | 75 (6) | 591 (48) | 558 (46) | 1224 | 73 (7) | 303 (28) | 697 (65) | 1073 | 8 (2) | 56 (13) | 379 (86) | 443 |
| Tumor size | ||||||||||||
| T0 | 0 (0) | 0 (0) | 0 (0) | 0 | 1 (10) | 0 (0) | 9 (90) | 10 | 1 (14) | 1 (14) | 5 (71) | 7 |
| T1 | 467 (6) | 3644 (50) | 3157 (43) | 7268 | 76 (5) | 635 (39) | 920 (56) | 1631 | 15 (4) | 117 (34) | 213 (62) | 345 |
| T2 | 0 (0) | 0 (0) | 0 (0) | 0 | 197 (5) | 1131 (29) | 2542 (66) | 3870 | 21 (3) | 125 (17) | 596 (80) | 742 |
| T3 | 0 (0) | 0 (0) | 0 (0) | 0 | 7 (4) | 26 (15) | 142 (81) | 175 | 5 (1) | 18 (4) | 467 (95) | 490 |
| T4 | 0 (0) | 0 (0) | 0 (0) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 | 12 (3) | 17 (5) | 348 (92) | 377 |
| Nodal status | ||||||||||||
| N0 | 470 (7) | 3651 (51) | 3167 (44) | 7215 | 153 (6) | 774 (32) | 1492 (62) | 2419 | 6 (10) | 6 (10) | 46 (79) | 58 |
| N1 | 0 (0) | 1 (33) | 3 (66) | 3 | 128 (4) | 1018 (31) | 2121 (65) | 3267 | 7 (2) | 15 (4) | 386 (95) | 408 |
| N2 | 0 (0) | 0 (0) | 0 (0) | 1 | 0 (0) | 0 (0) | 0 (0) | 0 | 33 (3) | 185 (19) | 767 (78) | 985 |
| N3 | 0 (0) | 0 (0) | 0 (0) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 | 88 (17) | 72 (14) | 430 (84) | 510 |
| Neoadjuvant chemotherapyb | ||||||||||||
| Received | 1 (3) | 6 (16) | 31 (82) | 38 | 7 (2) | 60 (21) | 222 (77) | 289 | 8 (2) | 20 (5) | 389 (93) | 417 |
| Not received | 469 (6) | 3646 (50) | 3139 (43) | 7254 | 274 (5) | 1732 (32) | 3391 (63) | 5397 | 46 (3) | 258 (17) | 1240 (80) | 1544 |
| Adjuvant chemotherapyb | ||||||||||||
| Received | 30 (2) | 668 (52) | 587 (46) | 1285 | 74 (2) | 1168 (37) | 1902 (60) | 3144 | 12 (1) | 224 (20) | 860 (78) | 1096 |
| Not received | 440 (7) | 2984 (50) | 2583 (43) | 6007 | 207 (8) | 624 (25) | 1711 (67) | 2542 | 42 (5) | 54 (6) | 769 (89) | 865 |
| Hormone therapyb | ||||||||||||
| Received | 186 (4) | 2379 (53) | 1909 (43) | 4474 | 120 (3) | 1417 (34) | 2594 (63) | 4131 | 25 (2) | 215 (16) | 1133 (83) | 1373 |
| Not received | 284 (10) | 1273 (45) | 1261 (45) | 2818 | 161 (10) | 375 (24) | 1019 (66) | 1555 | 29 (5) | 63 (11) | 496 (84) | 588 |
aPercentages are row percentages.
bP value from χ2 test was <0.001.
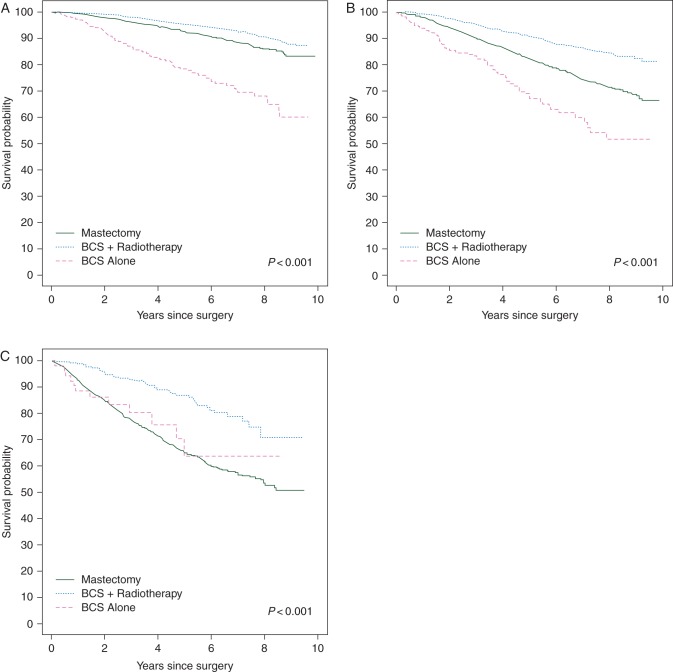
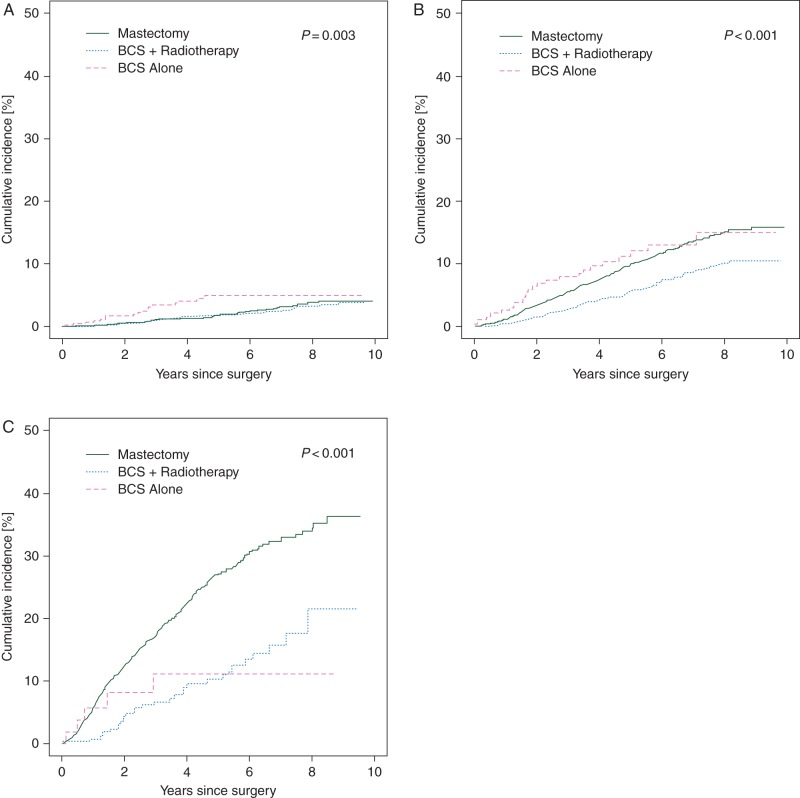
Figures 1 and 2 show Kaplan–Meier and cumulative incidence curves for all-cause survival and breast cancer-specific mortality, respectively, by treatment category stratified by tumor stage. Stage II and III patients who received mastectomy had a greater risk of all-cause and breast cancer-specific mortality than those who received BCS plus radiotherapy. BCS alone was consistently associated with the worst survival. The overall 5-year all-cause survival probabilities for patients treated with BCS plus radiotherapy, mastectomy, and BCS alone were 94% (95% CI 93% to 95%), 83% (95% CI 82% to 84%) and 74% (95% CI 70% to 78%), respectively. Among stage I, II and III patients, 5-year cumulative incidence of breast cancer-specific death was 1.8% (95% CI 1.2% to 2.4%), 9.9% (95% CI 8.7% to 11.1%) and 27.1% (95% CI 24.4% to 29.8%) among those who received mastectomy and 1.9% (95% CI 1.4% to 2.4%), 5.6% (95% CI 4.3% to 6.9%) and 10.2% (95% CI 6.1% to 14.3%) among those who received BCS plus radiation, respectively.
Figure 1.
Kaplan–Meier survival probability by treatment category for stage I (A), II (B) and III (C) breast cancer patients.
Figure 2.
Cumulative breast cancer mortality by treatment category for stage I (A), II (B) and III (C) breast cancer patients.
Adjusted all-cause mortality hazard ratios by cancer stage are shown in Table 2. Mastectomy was associated with an increased all-cause mortality hazard (stage I HR = 1.21, 95% CI 1.00–1.48; stage II HR: 1.36, 95% CI 1.13–1.63; stage III HR: 1.74, 95% CI 1.24–2.45) compared with those who received BCS plus radiotherapy. Mastectomy remained associated with increased all-cause mortality for stage II and III patients after stratifying patients who received mastectomy by radiotherapy status (supplementary Table S1, available at Annals of Oncology online). Patients with stage I and II cancers treated with BCS alone had twice the all-cause mortality hazard as those treated with BCS plus radiotherapy (stage I HR = 2.39, 95% CI 1.83–3.12; stage II HR = 1.74, 95% CI 1.30–2.33). All-cause mortality was inversely associated with later year of diagnosis in all disease stages.
Table 2.
Adjusteda Cox PH models assessing all-cause mortality by treatment category for stage I, II and III breast cancer patients
| Adjusteda hazard ratios (95% CI) |
|||
|---|---|---|---|
| Stage I | Stage II | Stage III | |
| Treatment category | P < 0.001 | P < 0.001 | P = 0.001 |
| BCS + adjuvant radiotherapy | 1.00 | 1.00 | 1.00 |
| Mastectomy | 1.21 (1.00–1.48) | 1.36 (1.13–1.63) | 1.74 (1.24–2.45) |
| BCS | 2.39 (1.83–3.12) | 1.74 (1.30–2.33) | 1.15 (0.60–2.21) |
| Age at diagnosis | P < 0.001 | P < 0.001 | P = 0.003 |
| <50 | 1.00 | 1.00 | 1.00 |
| 50–59 | 1.48 (1.00–2.19) | 1.42 (1.12–1.79) | 1.01 (0.78–1.31) |
| 60–69 | 2.39 (1.64–3.49) | 1.49 (1.16–1.92) | 0.98 (0.74–1.30) |
| 70–79 | 5.28 (3.65–7.63) | 2.57 (1.96–3.38) | 1.33 (0.97–1.84) |
| ≥80 | 11.72 (7.95–17.29) | 4.11 (3.10–5.45) | 1.91 (1.34–2.71) |
| Geographic region of surgery | P = 0.10 | P = 0.28 | P = 0.29 |
| Calgary | 1.00 | 1.00 | 1.00 |
| South | 1.06 (0.77–1.45) | 1.27 (1.00–1.60) | 0.96 (0.69–1.33) |
| Central | 1.37 (1.00–1.89) | 1.05 (0.82–1.35) | 1.14 (0.84–1.54) |
| Edmonton | 1.06 (0.87–1.29) | 1.09 (0.93–1.29) | 0.84 (0.69–1.04) |
| North | 0.53 (0.27–1.05) | 1.25 (0.92–1.71) | 1.03 (0.68–1.58) |
| Year of diagnosis | P < 0.001 | P < 0.001 | P = 0.010 |
| 2002–2004 | 1.00 | 1.00 | 1.00 |
| 2005–2007 | 0.64 (0.52–0.79) | 0.60 (0.50–0.71) | 0.90 (0.73–1.12) |
| 2008–2010 | 0.36 (0.26–0.49) | 0.35 (0.26–0.45) | 0.61 (0.44–0.84) |
| ER/PR status and hormone therapy | P < 0.001 | P < 0.001 | P < 0.001 |
| ER/PR positive and received hormone | 1.00 | 1.00 | 1.00 |
| ER/PR positive and no hormone | 1.24 (0.99–1.55) | 2.00 (1.58–2.53) | 1.91 (1.38–2.65) |
| ER/PR negative | 1.50 (1.22–1.85) | 2.32 (1.97–2.72) | 2.24 (1.85–2.72) |
| Neoadjuvant chemotherapy | P = 0.95 | P = 0.66 | P = 0.07 |
| Not received | 1.00 | 1.00 | 1.00 |
| Received | – | 0.92 (0.64–1.32) | 0.75 (0.55–1.02) |
| Adjuvant chemotherapy | P = 0.02 | P < 0.001 | P < 0.001 |
| Not received | 1.00 | 1.00 | 1.00 |
| Received | 1.44 (1.05–1.96) | 0.64 (0.51–0.79) | 0.39 (0.30–0.52) |
| Tumor size | P < 0.001 | P = 0.006 | |
| T0/T1 | – | 1.00 | 1.00 |
| T2 | – | 2.02 (1.67–2.44) | 1.23 (0.93–1.64) |
| T3 | – | 1.83 (1.15–2.90) | 1.33 (0.96–1.83) |
| T4 | – | – | 1.79 (1.28–2.52) |
| Nodal status | P < 0.001 | P < 0.001 | |
| N0 | – | 1.00 | 1.00 |
| N1 | – | 1.78 (1.52–2.09) | 1.12 (0.67–1.86) |
| N2 | – | – | 1.25 (0.75–2.10) |
| N3 | – | – | 2.20 (1.31–3.70) |
aAdjusted for all variables shown in the table.
Table 3 provides adjusted breast cancer-specific mortality hazard ratios by cancer stage. The breast cancer-specific mortality hazard for patients with stage II and III cancers treated with mastectomy was significantly increased compared with those treated with BCS plus radiotherapy (stage II HR = 1.39, 95% CI 1.09–1.76; stage III HR = 1.79, 95% CI 1.21–2.65). Mastectomy remained associated with increased breast cancer-specific mortality among stage II and III patients after stratifying patients who received mastectomy by radiotherapy status (supplementary Table S2, available at Annals of Oncology online). Stage I and II patients treated with BCS alone also had an increased hazard of breast cancer-specific death compared with patients who received BCS plus radiotherapy (stage I HR = 1.82, 95% CI 1.06–3.15; stage II HR = 1.46, 95% CI 0.94–1.94). Breast cancer-specific mortality was inversely associated with later year of diagnosis in all stages; however, this association was only significant for those with stage II breast cancer.
Table 3.
Adjusteda Cox PH model assessing breast cancer mortality by treatment category for stage I–III breast cancer patients
| Adjusteda hazard ratios (95% CI) |
|||
|---|---|---|---|
| Stage I | Stage II | Stage III | |
| Treatment category | P = 0.03 | P = 0.04 | P < 0.001 |
| BCS + adjuvant radiotherapy | 1.00 | 1.00 | 1.00 |
| Mastectomy | 1.04 (0.74–1.47) | 1.39 (1.09–1.76) | 1.79 (1.21–2.65) |
| BCS alone | 1.82 (1.06–3.15) | 1.46 (0.94–2.28) | 0.79 (0.32–1.94) |
| Age at diagnosis | P < 0.001 | P < 0.001 | P = 0.73 |
| <50 | 1.00 | 1.00 | 1.00 |
| 50–59 | 0.65 (0.38–1.11) | 1.33 (1.02–1.75) | 0.87 (0.66–1.16) |
| 60–69 | 1.05 (0.63–1.76) | 1.30 (0.96–1.77) | 0.92 (0.68–1.24) |
| 70–79 | 2.11 (1.27–3.49) | 1.89 (1.32–2.69) | 1.08 (0.75–1.54) |
| ≥80 | 2.80 (1.49–5.24) | 2.34 (1.57–3.47) | 1.13 (0.74–1.73) |
| Geographic region of surgery | P = 0.11 | P = 0.72 | P = 0.29 |
| Calgary | 1.00 | 1.00 | 1.00 |
| South | 1.31 (0.73–2.36) | 1.23 (0.89–1.71) | 0.88 (0.60–1.29) |
| Central | 1.96 (1.14–3.35) | 0.95 (0.67–1.36) | 0.92 (0.64–1.32) |
| Edmonton | 1.12 (0.78–1.63) | 1.03 (0.83–1.29) | 0.77 (0.61–0.97) |
| North | 0.61 (0.19–1.96) | 1.14 (0.75–1.73) | 0.92 (0.57–1.50) |
| Year of diagnosis | P = 0.16 | P < 0.001 | P = 0.12 |
| 2002–2004 | 1.00 | 1.00 | 1.00 |
| 2005–2007 | 0.82 (0.56–1.19) | 0.66 (0.53–0.84) | 0.92 (0.72–1.17) |
| 2008–2010 | 0.58 (0.33–1.03) | 0.40 (0.27–0.59) | 0.68 (0.47–0.99) |
| ER/PR status and hormone therapy | P < 0.001 | P < 0.001 | P < 0.001 |
| ER/PR positive and received hormone | 1.00 | 1.00 | 1.00 |
| ER/PR positive and no hormone | 1.32 (0.84–2.08) | 1.99 (1.38–2.88) | 1.93 (1.31–2.84) |
| ER/PR negative | 2.38 (1.65–3.42) | 2.93 (2.38–3.61) | 2.49 (2.01–3.10) |
| Neoadjuvant chemotherapy | P = 0.97 | P = 0.049 | P = 0.33 |
| Not received | 1.00 | 1.00 | 1.00 |
| Received | – | 1.52 (1.00–2.30) | 0.84 (0.59–1.19) |
| Adjuvant chemotherapy | P = 0.001 | P = 0.31 | P < 0.001 |
| Not received | 1.00 | 1.00 | 1.00 |
| Received | 2.06 (1.32–3.22) | 0.87 (0.65–1.15) | 0.42 (0.30–0.58) |
| Tumor size | P < 0.001 | P = 0.001 | |
| T0/T1 | – | 1.00 | 1.00 |
| T2 | – | 2.37 (1.85–3.05) | 1.24 (0.89–1.73) |
| T3 | – | 1.74 (0.85–3.56) | 1.42 (0.98–2.06) |
| T4 | – | – | 2.06 (1.40–3.04) |
| Nodal status | P < 0.001 | P < 0.001 | |
| N0 | – | 1.00 | 1.00 |
| N1 | – | 2.41 (1.93–3.01) | 1.77 (0.91–3.46) |
| N2 | – | – | 1.94 (0.98–3.83) |
| N3 | – | – | 3.45 (1.74–6.82) |
aAdjusted for all variables shown in the table.
discussion
In this study, stage II and III breast cancer patients treated with BCS plus radiotherapy had a decreased hazard of all-cause and breast cancer-specific mortality compared with those who received a mastectomy. Three recent studies from the United States have also reported better survival with receipt of BCS plus radiotherapy compared with mastectomy among stage I and II breast cancer patients [5–7]. We believe our study is the first to investigate and find this survival advantage specifically in stage III patients. To our knowledge, there have not been any cohort studies demonstrating a survival advantage of mastectomy over BCS plus radiotherapy.
Radiotherapy has been deemed an important component of treatment of many breast cancer patients who receive mastectomy [16, 17]. One-third of the mastectomy patients in the current study received adjuvant radiotherapy treatment and, therefore, a potential explanation for the observed survival disparity is the inappropriate lack of post-mastectomy radiotherapy treatment in some patients. To assess this hypothesis, we ran additional analyses stratifying the mastectomy patients by radiotherapy receipt (see supplementary Tables, available at Annals of Oncology online). Among stage II and III patients who received mastectomy, the adjusted all-cause and breast cancer-specific survival was similar in both those that received and did not receive radiotherapy treatment and significantly worse compared with those who received BCS plus radiotherapy. This result is consistent with that found by Agarwal et al. [7]. Inappropriate lack of post-mastectomy radiotherapy, therefore, does not explain the observed survival difference.
A small proportion of patients of each stage received BCS without radiotherapy. This may be attributed in part to unclear advantages of radiotherapy in women greater than 70 years of age when life expectancy, potential toxicity and comorbidities are taken in to account [18]. Also, some women may choose to forgo radiotherapy due to distance to these facilities, thus negatively affecting their survival [19].
Confounding by indication may explain the observed survival discrepancy, as receipt of mastectomy may be associated with higher risk patients in practice. We were unable to account for factors such as tumor-to-breast ratio, tumor aggressiveness, contraindications to BCS or radiation therapy or patient socioeconomic status, all of which may be associated with both receipt of mastectomy and poor survival [5, 20, 21]. Surgeon and hospital factors may also play a role. Surgeon preferences, surgeon and hospital volume and hospital teaching status have been found to be associated with type of surgery and may also be associated with survival [22–24]. Further investigation into these factors is merited, as results may have potential for policy implications.
The use of a population-based data source is a great strength of this study; investigation of treatments within a nonselected patient population is valuable in assessing the effect of the treatments within a typical clinical practice environment. Furthermore, the nature of the publicly funded health care insurance minimizes bias that may occur due to variation in access to care and treatment decisions. We also were able to adjust for all breast cancer treatments received and several important clinical factors. Limitations not already mentioned include the risk of selection bias introduced by the retrospective design, as well as the lack of information about other prognostic factors such as comorbidities, lifestyle factors, socioeconomic status and race/ethnicity. Other limitations of this study include the relatively short median follow-up time of 4.2 years and possible differential misclassification of cause of death.
conclusion
To our knowledge, this is the first study to assess the relationship between type of surgery and survival for early-stage breast cancer patients in a publicly funded health care system and the first to report a survival advantage specifically for stage III patients who receive BCS plus radiotherapy rather than mastectomy. Given the consistent results from several large unselected population-based studies in both Canada and the United States favoring BCS plus radiotherapy over mastectomy, and given that mastectomy is a more invasive procedure with more sequelae than BCS plus radiotherapy, we suggest greater efforts toward educating and encouraging women to receive BCS plus radiotherapy rather than mastectomy when medically feasible and appropriate.
funding
This work was supported by the Canadian Institutes of Health Research (MOP 111236).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Litere S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomized trial. Lancet Oncol 2012; 13(4): 412–419. [DOI] [PubMed] [Google Scholar]
- 2.Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol 2005; 28(3): 289–294. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trail comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233–1241. [DOI] [PubMed] [Google Scholar]
- 4.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004; 291(22): 2720–2726. [DOI] [PubMed] [Google Scholar]
- 5.Hwang ES, Lichtensztajn DY, Gomez SL, et al. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 2013; 119(7): 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurian AW, Lichtensztain DY, Keegan TH, et al. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. JAMA 2014; 312(9): 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 2014; 149(3): 267–274. [DOI] [PubMed] [Google Scholar]
- 8.Canadian Institute for Health Information. Breast Cancer Surgery in Canada, 2007–2008 to 2009–2010. Ottawa, ON: CIHI; 2012. [Google Scholar]
- 9.Fisher S, Gao H, Yasui Y, et al. Treatment variation in patients diagnosed with early stage breast cancer in Alberta from 2002 to 2010: a population-based study. BMC Health Serv Res 2015; 15(1): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz A, Percy C, Jack A, et al. (eds). International Classification of Diseases for Oncology, 3rd ed Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 11.Irvin DF, Jay SC, Donald EH, et al. (eds). Cancer Staging Manual, 5th ed New York: Spring-Verlag; 1997. [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, et al. (eds). Cancer Staging Manual, 6th ed New York: Springer-Verlag; 2002. [Google Scholar]
- 13.Statistics Canada: Visible minority groups, 2006 counts, for Canada, provinces and territories, and census metropolitan areas and census agglomerations—20% sample data, last modified 6 December 2010.
- 14.Li C, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol 2003; 21(1): 28–34. [DOI] [PubMed] [Google Scholar]
- 15.Gill RD. Understanding Cox's regression model: a martingale approach. J Am Stat Assoc 1984; 70(286): 441–447. [Google Scholar]
- 16.Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001; 19: 1539–1569. [DOI] [PubMed] [Google Scholar]
- 17.Walsh SM, Lowery AJ, Prichard RS, et al. Postmastectomy radiotherapy: indications and implications. Surgeon 2014; 12(6): 310–315. [DOI] [PubMed] [Google Scholar]
- 18.Junkler I. Radiotherapy issues in elderly breast cancer patients. Breast Care (Basel) 2012; 7(6): 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celaya MO, Rees JR, Gibson JJ, et al. Travel distance and season of diagnosis affect treatment choices for women with early-stage breast cancer in a predominately rural population (United States). Cancer Causes Control 2006; 17(6): 851–856. [DOI] [PubMed] [Google Scholar]
- 20.Hiotis K, Ye W, Sposto R, et al. Predictors of breast conservation therapy: size is not all that matters. Cancer 2005; 103(5): 892–899. [DOI] [PubMed] [Google Scholar]
- 21.Lee MC, Rogers K, Griffith K, et al. Determinants of breast conservation rates: reasons for mastectomy at a comprehensive cancer center. Breast J 2009; 15(1): 34–40. [DOI] [PubMed] [Google Scholar]
- 22.Gooiker GA, van Gijn W, Post PN, et al. A systematic review and meta-analysis of the volume-outcome relationship in the surgical treatment of breast cancer. Are breast cancer patients better off with a high volume provider? Eur J Surg Oncol 2010; 36(Suppl 1): S27–S35. [DOI] [PubMed] [Google Scholar]
- 23.Hebert-Croteau N, Brisson J, Lemaire J, et al. Investigating the correlation between hospital of primary treatment and the survival of women with breast cancer. Cancer 2005; 104(7): 1343–1348. [DOI] [PubMed] [Google Scholar]
- 24.Hershman DL, Buono D, Jacobson JS, et al. Surgeon characteristics and use of breast conservation surgery in women with early stage breast cancer. Ann Surg 2009; 249(5): 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.