Abstract
Objective
To estimate the lifetime risk of symptomatic knee osteoarthritis (OA), overall and stratified by sex, race, education, history of knee injury, and body mass index (BMI).
Methods
The lifetime risk of symptomatic OA in at least 1 knee was estimated from logistic regression models with generalized estimating equations among 3,068 participants of the Johnston County Osteoarthritis Project, a longitudinal study of black and white women and men age ≥45 years living in rural North Carolina. Radiographic, sociodemographic, and symptomatic knee data measured at baseline (1990–1997) and first followup (1999–2003) were analyzed.
Results
The lifetime risk of symptomatic knee OA was 44.7% (95% confidence interval [95% CI] 40.0–49.3%). Cohort members with history of a knee injury had a lifetime risk of 56.8% (95% CI 48.4–65.2%). Lifetime risk rose with increasing BMI, with a risk of 2 in 3 among those who were obese.
Conclusion
Nearly half of the adults in Johnston County will develop symptomatic knee OA by age 85 years, with lifetime risk highest among obese persons. These current high risks in Johnston County may suggest similar risks in the general US population, especially given the increase in 2 major risk factors for knee OA, aging, and obesity. This underscores the immediate need for greater use of clinical and public health interventions, especially those that address weight loss and self-management, to reduce the impact of having knee OA.
INTRODUCTION
Arthritis is among the leading causes of disability in the US (1), and osteoarthritis (OA) is the most common type of arthritis (2). Knee OA, the most frequent form of lower extremity OA (3), was the primary diagnosis for 430,000 hospital discharges and $14 billion of hospital charges in the 2004 US National Inpatient Sample (4), and 12.1% (annualized prevalence) of Americans age ≥60 years had symptomatic radiographic knee OA in the 1991–1994 National Health and Nutrition Examination Survey (5). Although these population-based measures demonstrate the profound clinical and public health burden of knee OA, they are poor at conveying the person-level risk to the general public.
One statistic that has been used extensively to describe person-level risk is lifetime risk, which is the probability of developing a condition over the course of a lifetime. Lifetime risk has been reported for various chronic conditions and risk factors, including coronary heart disease (6), hypertension (7), diabetes (8), and breast cancer (9). This measure conveys the risk of these conditions in terms that are understandable to both clinical and lay audiences.
The purpose of this study was to estimate the lifetime risk of developing a disabling and relatively common type of arthritis, symptomatic knee OA, therefore increasing the general public’s understanding of the risk of arthritis.
PARTICIPANTS AND METHODS
Study population
We analyzed data from the Johnston County Osteoarthritis (OA) Project, a population-based, longitudinal, prospective study of the occurrence and natural history of knee and hip OA in Johnston County, North Carolina, an area currently transitioning from rural to suburban. The study was designed to be representative of the civilian, noninstitutionalized, English-speaking black and white population age ≥45 years who were residents of 1 of 6 selected townships of Johnston County for at least 1 year. Participants had to be physically and mentally capable of completing the study protocol. The study protocol was approved by the Institutional Review Boards of the Centers for Disease Control and Prevention and the University of North Carolina School of Medicine. The study has been described elsewhere in detail (10,11).
The study protocol at both baseline (1990–1997) and first followup (1999–2003) included an initial home interview, clinical examination (with radiographs and height and weight measurements), and a second home interview ~2 weeks following clinical examination. Radiograph results included anteroposterior views of the knees with weight-bearing and foot map positioning, which were read by a single bone and joint radiologist (JBR) for radiographic knee OA using Kellgren/Lawrence (K/L) scale grades. A K/L scale grade of ≥2 indicates the presence of at least mild radiographic OA (12). K/L scale grades, the most commonly used measures in epidemiologic studies of radiographic knee OA (13), have been demonstrated to have high intrarater and interrater reliability (13), are highly correlated with knee pain (13,14), and are the recommended standard for population-based studies of radiographic knee OA (14). The intrarater and interrater reliabilities of the Johnston County OA Project radiologist were previously determined to be high, with weighted kappas of 0.89 and 0.86, respectively (10).
Sociodemographic and clinical characteristics of participants, including sex, race, education, income, history of knee injury, weight at age 18 years, and presence of knee symptoms, were assessed in an interviewer-administered questionnaire. Symptoms were determined through a question about each knee: “On MOST days do you have pain, aching, or stiffness in your LEFT (or RIGHT) knee?”
Symptomatic knee OA was defined as a K/L scale radiographic grade of ≥2 (at least mild radiographic OA) and symptoms in the same knee. We focused on symptomatic knee OA because it is relevant for both clinical diagnosis and measuring the public health burden of knee OA. The primary indication for most knee joint replacements is symptomatic OA (15); therefore, knees that had undergone total joint replacement were coded as affected. Knees with radiographic evidence of inflammatory arthritis (i.e., rheumatoid arthritis) were excluded in the analysis.
Statistical analysis
Participants were characterized at baseline by age (categorized in 5-year groupings), sex, race (black or white), education (less than high school, high school completed, or greater than high school), household income (<$10,000, $10,000 to <$20,000, $20,000 to <$35,000, or ≥$35,000), history of knee injury (no/yes), and presence of symptomatic knee OA. Body mass index (BMI) of participants was examined at 2 time points: age 18 years (<25 kg/m2 [underweight/normal weight] or ≥25 kg/m2 [overweight/obese, aggregated due to limited sample size]) and baseline (<25 kg/m2 [underweight/normal weight], 25 to <30 kg/m2 [overweight], or ≥30 kg/m2 [obese]).
Lifetime risk estimates were derived from logistic regression models with generalized estimating equations (GEEs). Age was the predictor variable, symptomatic knee OA was the outcome variable, and lifetime risk was the predicted probability of developing OA in at least 1 knee by age 85 years. Stratified lifetime risk estimates were also generated for the following demographic variables: sex, race, education, BMI, and history of knee injury. BMI was analyzed in 2 forms: as a summary of BMI over the life course (categorized as <25 kg/m2 or ≥25 kg/m2 at age 18 years, baseline, and followup), and BMI at baseline and followup (each categorized as <25 kg/m2, ≥25 to <30 kg/m2, or ≥30 kg/m2). Lifetime risks were derived from models with only age and the stratified variable of interest as independent variables. The 95% confidence intervals (95% CIs) for all estimates were derived using the SEs of the model-predicted probabilities.
OA symptoms may be intermittent (16). To determine the degree to which changes in symptom status would lead to underestimation of lifetime risk, we examined the distribution of symptoms at baseline and followup among those with a radiographic K/L scale grade of ≥2 for OA at baseline and/or followup.
Sampling weights were applied during modeling to adjust the sample data to the 1990 Johnston County population distribution. The methods for calculating the sampling weights have been described elsewhere (11). During the statistical modeling, adjustment was made for the 3 sources of correlated error resulting from the survey design: repeated measures across study participants, multiple participants per household, and the 2-stage clustered sample design. Lifetime risks were estimated using SUDAAN software (17). Additional details on the statistical methods are shown in Appendix A (available at the Arthritis Care & Research Web site at http://www.interscience.wiley.com/jpages/0004-3591:1/suppmat/index.html).
Longitudinal studies (i.e., baseline and followup data) pose special design and analytic challenges. First, participants with baseline and followup data have repeated measurements that may be correlated; therefore, GEE methods were used because this modeling procedure correctly accounts for repeated measures and their correlation with each other (18,19). Followup data were obtained for a subset (52%) of the baseline cohort, and use of the GEE approach enabled estimation of risk from individuals with single (either baseline or followup) or multiple (both baseline and followup) outcome measures. Second, attrition is a universal issue in longitudinal cohort studies. The Johnston County OA Project used various strategies to minimize cohort attrition (e.g., annual newsletters, notices in local media and in medical and community settings, contacts, medical providers, and community inquiries) and to identify people who had died between baseline and followup (e.g., review of local obituaries, word of mouth, knowledge of local project staff, and local, state, and national [National Death Index] death records). Nevertheless, followup data were unavailable for some of the inception cohort, and therefore we conducted sensitivity analyses (see Appendix B, available at the Arthritis Care & Research Web site at http://www.interscience.wiley.com/jpages/0004-3591:1/suppmat/index.html) to examine the potential bias resulting from cohort attrition.
Lifetime risk is the cumulative probability, or cumulative incidence, of a condition in a cohort. In this study, cumulative incidence equals lifetime prevalence because symptomatic knee OA is a persistent, low-mortality condition. Lifetime risk differs from point prevalence in that point prevalence captures cases in a cohort at a single point in time, whereas lifetime risk is a cumulative measure (i.e., collected at multiple time points) of information among cohort members and includes persons who may subsequently die or leave the cohort for other reasons (e.g., move outside the catchment area). All cohort members (n = 3,068), regardless of OA status at baseline, were included in this analysis. The inclusion of prevalent and incident cases is also necessary in estimating lifetime risk of symptomatic OA, because symptoms may be intermittent or abate (e.g., response to treatment of symptoms); this approach ensures a greater likelihood that persons who have ever had symptomatic OA are included in the estimate.
RESULTS
The mean age of the baseline sample was 61 years (range 45–93), and the mean time between baseline and followup was 6.0 years (range 3.5–13.2). The baseline cohort comprised a higher weighted proportion of women than men (57.4% versus 42.6%), and more whites than blacks (81.6% versus 18.4%) (Table 1). Two-thirds of the sample were married, 17.6% had an annual household income ≥$35,000, and the majority of patients had at least a high school education (60.1%). Whereas almost two-thirds (63.5%) of the sample were overweight or obese as measured at baseline, only one-tenth (10.4%) reported to be so at age 18 years. A history of knee injury was reported by 15.7% of respondents. The prevalence of symptomatic knee OA was 15.2% in the baseline cohort and 5.4% of the respondents with symptomatic knee OA had radiographic evidence of knee replacements. Of the 2,228 participants eligible for inclusion in the first followup, 1,590 (71.3%) participated in both a clinical examination and a household interview (Figure 1). Of the participants with a radiographic K/L scale grade of ≥2 for knee OA at baseline and/or followup, 80% were symptomatic at baseline and/or followup, and 63% of the sample with radiographic knee OA were symptomatic at both time points. The un-weighted frequency of symptomatic knee OA status at baseline and followup by 5-year age groups is shown in Appendix C (available at the Arthritis Care & Research Web site at http://www.interscience.wiley.com/jpages/0004-3591:1/suppmat/index.html).
Table 1.
Distribution of sociodemographic and clinical characteristics among the Johnston County cohort at baseline (n = 3,068), weighted*
| Variable | Percentage† |
|---|---|
| Age, years | |
| 45–49 | 15.8 |
| 50–54 | 17.7 |
| 55–59 | 13.3 |
| 60–64 | 13.7 |
| 65–69 | 14.0 |
| 70–74 | 12.8 |
| ≥75 | 12.8 |
| Men | 42.6 |
| Women | 57.4 |
| Race | |
| Black | 18.4 |
| White | 81.6 |
| Marital status | |
| Never married | 3.3 |
| Married/common-law | 65.5 |
| Separated/divorced | 10.1 |
| Widowed | 21.1 |
| Income | |
| <$10,000 | 23.7 |
| $10,000–<$20,000 | 18.4 |
| $20,000–<$35,000 | 19.5 |
| ≥$35,000 | 17.6 |
| Missing | 20.7 |
| Education | |
| Less than high school | 39.9 |
| High school completed | 32.6 |
| Greater than high school | 27.5 |
| BMI at age 18 years, kg/m2‡ | |
| <25 (underweight and normal weight) | 86.9 |
| ≥25 (overweight and obese) | 10.4 |
| Missing | 2.7 |
| BMI, kg/m2 | |
| <25 (underweight and normal weight) | 32.9 |
| 25–<30 (overweight) | 39.5 |
| ≥30 (obese) | 24.0 |
| Missing | 3.4 |
| History of knee injury | |
| No | 80.9 |
| Yes | 15.7 |
| Missing | 3.4 |
| Pain, aching, or stiffness in at least 1 knee§ |
42.5¶ |
| Symptomatic OA in at least 1 knee# | 15.6 |
| Total joint replacement in at least 1 knee |
0.9 |
Weighted to Johnston County population distribution in 1990 US Census. BMI = body mass index; OA = osteoarthritis.
Sum of percentages do not add up to 100% because of rounding.
Weight at age 18 years was self-reported.
Participants who reported pain, aching, and stiffness in at least 1 knee, regardless of radiographic status of the knee.
Baseline knee symptom status unknown for 62 (2.0%) persons.
Includes knees with total joint replacement. Baseline knee radiographic status unknown for 53 (1.4%) persons. Kellgren/Lawrence scale radiographic grade ≥2
Figure 1.
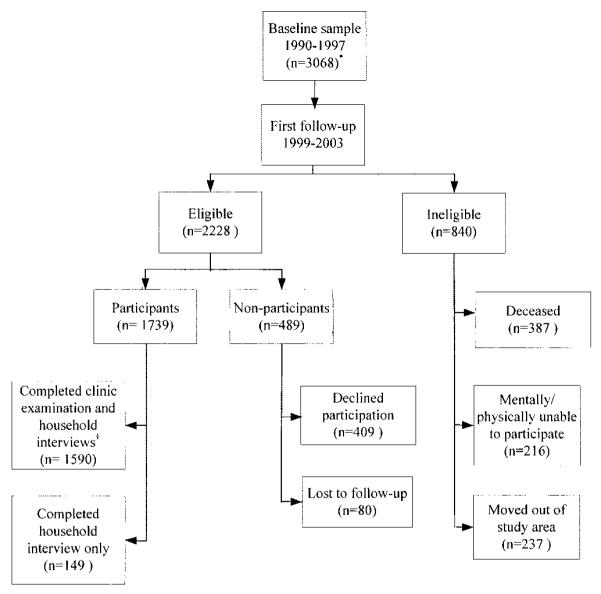
Status of Johnston County Osteoarthritis Project baseline participants at first followup. * = baseline response rate 3,068 (60%) out of 5,138, clinic completion rate 3,068 (83%) out of 3,690; † = first followup response rate 1,590 (71%) out of 2,228, clinic completion rate 1,590 (91%) out of 1,739.
The lifetime risk of symptomatic knee OA (i.e., developing the condition by age 85 years) was 44.7% (95% CI 40.0–49.3%) overall (Table 2). Although there were no significant differences by sex, race, and education, obese participants had a significantly higher lifetime risk (60.5% compared with 30.2% and 46.9% among those who were normal weight and overweight, respectively), as did those with a history of knee injury (56.8% compared with 42.3% without a history of knee injury). In the sensitivity analyses, which estimated the influence of missing data for 6 followup groups, we observed a lifetime risk of 41.3% when all groups were recoded, and 45.3% for 5 groups (excluding those who were deceased at followup) (Appendix B).
Table 2.
Lifetime risk probabilities and 95% CIs for symptomatic knee OA, stratified and overall*
| Lifetime risk percentage (95% CI) |
|
|---|---|
| Stratified | |
| Men | 39.8 (32.2–47.3) |
| Women | 46.8 (41.2–52.5) |
| Race | |
| Black | 50.1 (41.8–58.4) |
| White | 43.8 (38.4–49.1) |
| Education† | |
| Less than high school | 43.5 (37.5–49.5) |
| High school completed | 51.9 (42.2–61.6) |
| Greater than high school | 34.8 (25.8–43.8) |
| BMI, kg/m2† | |
| <25 (underweight and normal weight) |
30.2 (23.0–37.4)‡ |
| 25–<30 (overweight) | 46.9 (39.3–54.5)§ |
| ≥30 (obese) | 60.5 (53.0–68.1) |
| History of knee injury† | |
| No | 42.3 (37.2–47.4) |
| Yes | 56.8 (48.4–65.2)¶ |
| Overall | 44.7 (40.0–49.3) |
Symptomatic knee OA is Kellgren/Lawrence scale radiographic grade ≥2. 95% CI = 95% confidence interval; OA = osteoarthritis; BMI = body mass index.
Education, BMI, and history of knee injury in these models were time dependent (e.g., education of participants at each time point was analyzed).
Comparison of lifetime risk, normal weight and obese, P < 0.0001.
Comparison of lifetime risk, overweight and obese, P = 0.01.
Comparison of lifetime risk, history of knee injury (no and yes), P = 0.002.
The analysis of BMI across the life course indicated that those who had a normal weight at the 3 time points examined (age 18 years, baseline, and followup) had the lowest lifetime risk (29.2%; 95% CI 15.8–42.7%), whereas those who reported a normal weight at age 18 years but were overweight or obese at the 2 later time points had the highest risk (59.9%; 95% CI 51.8–68.1%). These estimates were significantly different (P < 0.001) (Figure 2).
Figure 2.
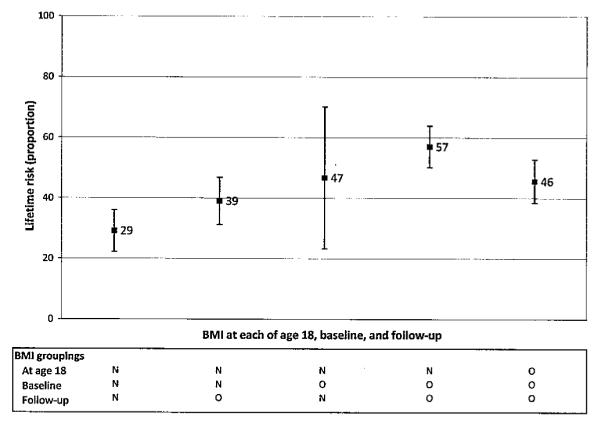
Lifetime risk of symptomatic knee osteoarthritis by body mass index (BMI) at age 18 years, baseline, and followup (n = 1,523). Baseline and followup height and weight measured by study staff during clinic visit. Comparison of NNN with NOO, P < 0.001. N = normal (BMI <25 kg/m2); O = overweight or obese (BMI ≥25 kg/m2).
DISCUSSION
In this study, lifetime risk was the probability of a person developing symptomatic OA in at least 1 knee by age 85 years. We found the lifetime risk of symptomatic knee OA to be nearly 1 in 2 overall, more than 1 in 2 among those with history of a knee injury, and nearly 2 in 3 for obese persons. Comparison of BMI patterns over the life course suggested that higher BMI at later ages accounted for the greatest variability in lifetime risk estimates (Figure 2); for example, the lifetime risk among those who had a normal BMI at age 18 years but who were overweight/obese at baseline, and followup was twice that of those who had a normal BMI at each of the 3 time periods. The latter results are comparable with those of Dawson et al, who examined the association between life-course BMI and the prevalence of symptomatic knee OA, and reported that BMI >25 kg/m2 at older ages (i.e., 36–40 years, 51–56 years) was most strongly associated with symptomatic knee OA (20).
Our estimates of lifetime risk are potentially underestimated. First, the youngest Johnston County OA Project cohort members were age 45 years at baseline, the lower age limit for the OA Project, because OA is very uncommon among people age <45 years (3,21). Nevertheless, the omission of younger age groups may contribute to a slight underestimation of lifetime risk. Second, previous studies indicate that OA symptoms may be intermittent (16). Of those respondents with a radiographic K/L scale grade of ≥2 for OA at baseline and/or followup, 20% did not report symptoms at baseline or followup. Although analysis of symptomatic disease status at 2 points increased the likelihood of detecting symptomatic and radiographic knee OA, any of these respondents who experienced knee symptoms other than at baseline and followup were coded as unaffected in this analysis. Third, this analysis is based on multiple birth cohorts, which assume no period or cohort effects in OA onset, and the fact that the age-specific disease rates experienced by older members of the sample will be the same for the younger members across time. However, an increase in the prevalence of a major risk factor for OA—overweight/obesity—among the younger cohort members would result in an even greater estimate of lifetime risk. Finally, radiographic patellofemoral joint OA was not measured in this study, although it also contributes to symptomatic knee OA.
This study has several limitations. To our knowledge, the Johnston County OA Project is one of the largest longitudinal population-based studies of knee and hip OA in the US. Nevertheless, the limited sample size decreased the precision of the point estimates, and likely prevented detection of statistically significant differences between subgroups (e.g., comparison of BMI across the life course). Also, although baseline and followup weights were measured by clinic staff to ensure accuracy of information, weight at age 18 years was self-reported and was likely underestimated by many respondents. Our analysis examined multiple factors for which there is at least moderate evidence of an etiologic association with symptomatic knee OA (20,22,23). We did not analyze lifetime risk in association with work history, because such an analysis is complex and outside the scope of this study.
This study also has several strengths. First, the use of GEE repeated-measures modeling reduced selection bias and increased statistical power, because data for all cohort members were analyzed regardless of followup status. Second, the mean followup time of 6.0 years is sufficient to detect new cases, because several recent longitudinal studies of OA progression have had similar or even less followup time (range of mean followup time 18 months to 6.6 years) (24–28). Third, the Johnston County OA Project is not only one of the largest longitudinal studies of patients with OA, but it is also one of only a few studies to include both blacks and whites; the data provide a limited opportunity to estimate lifetime risk in a racially-mixed sample. Fourth, various strategies were used to maximize survey participation, which resulted in a response rate that was the same as, or higher than, other community-based, racially-mixed longitudinal studies of middle-aged and older adults (29–31). The sensitivity analyses, designed to assess the effect of missing data encountered in all such longitudinal studies, found a mortality-adjusted lifetime risk of 45.3%, a result so similar to the main analysis (45.5%) that study results were unlikely to be biased by the missing followup data.
Longitudinal studies of knee OA have been confined to small geographic areas because knee OA is difficult to diagnose without costly knee radiographs. Self-reported knee pain is not an accurate method of identifying knee OA, given the competing causes of knee pain (e.g., soft-tissue and widespread pain disorders and other arthropathies). Sampling weights were applied to derive estimates generalizable to the 1990 Johnston County population, but generalizing the Johnston County findings to the US population should be approached with caution. Although the distribution of age, sex, and BMI of the Johnston County and US populations were similar in 1990 (one-third of residents in both jurisdictions were age ≥45 years; 12.6% and 12.1% were age ≥65 years, respectively), approximately 51% were women, and two-thirds were overweight (66% in the study sample and 65% among US black and white adults age ≥45 years [Third National Health and Nutrition Examination Survey, 1988–1994]) (32–34), the Johnston County population was more likely than the US population to be black (18% versus 12%), live in a rural area (76% versus 25%), be less educated (35% versus 25% had not completed high school), and have a lower income (median income $25,169 versus $30,056).
To our knowledge, the Johnston County OA Project is the only longitudinal, population-based study of patients with knee and hip OA in the US that includes blacks and whites of both sexes who are middle-aged and older from the same geographic area, and the lifetime risk estimates we have reported were derived from a sociodemographically diverse sample. Estimating prevalence and incidence density among people age ≥85 years is typically challenging in epidemiologic studies because of decreased survival to this age. However, the lifetime risk statistic is a cumulative measure and uses pooled information from across age groups, enabling estimation of disease risk at age 85 years with increased precision. Furthermore, the lifetime risk statistic is an epidemiologic measure that is familiar to the general public, because it has been used to convey the person-level risk of other chronic conditions, including breast cancer; lifetime risk is thought to be a more accessible statistic for describing risk to lay audiences (35). Other lifetime risk studies have reported higher probabilities with increasing observation time (6,7,36), suggesting that the lifetime risks demonstrated here will increase with subsequent followup.
Although Johnston County is a lower-income rural area in the Southern US, the high lifetime risks observed in this population-based sample suggest that the lifetime risk of symptomatic knee OA is likely high in the US and other populations, especially given the aging of the population and the increasing prevalence of obesity (37,38). Results from this study indicate that nearly 1 in 2 people are at risk of developing symptomatic OA in at least 1 knee, and that 2 in 3 obese people are at risk. Weight loss can lead to a decreased risk of symptomatic knee OA (39), and the association between the modifiable risk factor, BMI, and lifetime risk of OA in this study further underscores the need for public health weight loss and management interventions that would contribute to a decreased lifetime risk of OA. Those in the rheumatologic field have lamented the lack of attention given to arthritis in public health planning and policy making, given the high prevalence, tremendous costs, and disability associated with knee OA (40). We have presented the lifetime risk estimate to further illustrate the significant public health burden of this condition.
Supplementary Material
ACKNOWLEDGMENTS
We thank the diligent staff of the Johnston County OA Project, including Janice Woodard, Linda Miles, Edwin Hartman, MD, Erik Myers, and Fang Fang, and we thank the Project participants, without whom this study would not be possible.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Murphy had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Murphy, Helmick, Renner, Dragomir, Luta, Jordan.
Acquisition of data. Helmick, Renner, Dragomir, Luta, Jordan.
Analysis and interpretation of data. Murphy, Schwartz, Helmick, Renner, Tudor, Koch, Dragomir, Kalsbeek, Luta, Jordan.
Manuscript preparation. Murphy, Schwartz, Helmick, Renner, Tudor, Koch, Dragomir, Kalsbeek, Luta, Jordan.
Statistical analysis. Murphy, Schwartz, Koch, Kalsbeek.
The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Centers for Disease Control and Prevention Prevalence of disabilities and associated health conditions among adults: United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:120–5. [PubMed] [Google Scholar]
- 2.Kremers H, Gabriel S. Epidemiology of the rheumatic diseases: Kelley’s textbook of rheumatology. 7th ed Elsevier Saunders; Philadelphia: 2005. pp. 407–25. [Google Scholar]
- 3.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 4.Agency for Healthcare Research and Quality National and regional statistics in the national inpatient sample, 2004. 2006 URL: http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 5.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33:2271–9. [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D’Agostino RB, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94:20–4. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003–10. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 8.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 9.Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel MJ, Tong T. The lifetime risk of developing breast cancer. J Natl Cancer Inst. 1993;85:892–7. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- 10.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis Care Res. 1995;8:242–50. doi: 10.1002/art.1790080407. [DOI] [PubMed] [Google Scholar]
- 11.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–80. [PubMed] [Google Scholar]
- 12.Kellgren JH, Lawrence J. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spector TD, Hart DJ, Byrne J, Harris PA, Dacre JE, Doyle DV. Definition of osteoarthritis of the knee for epidemiological studies. Ann Rheum Dis. 1993;52:790–4. doi: 10.1136/ard.52.11.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lethbridge-Cejku M, Scott WW, Jr, Reichle R, Ettinger WH, Zonderman A, Costa P, et al. Association of radiographic features of osteoarthritis of the knee with knee pain: data from the Baltimore Longitudinal Study of Aging. Arthritis Care Res. 1995;8:182–8. doi: 10.1002/art.1790080311. [DOI] [PubMed] [Google Scholar]
- 15.Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004;86-A:1909–16. doi: 10.2106/00004623-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513–7. [PubMed] [Google Scholar]
- 17.Research Triangle Institute . The logistic procedure: SUDAAN user manual release 8.0. ume 2. Research Triangle Park (NC); RTI: 2002. pp. 543–624. [Google Scholar]
- 18.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–75. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 20.Dawson J, Juszczak E, Thorogood M, Marks SA, Dodd C, Fitzpatrick R. An investigation of risk factors for symptomatic osteoarthritis of the knee in women using a life course approach. J Epidemiol Community Health. 2003;57:823–30. doi: 10.1136/jech.57.10.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 22.Bijlsma JW, Knahr K. Strategies for the prevention and management of osteoarthritis of the hip and knee. Best Pract Res Clin Rheumatol. 2007;21:59–76. doi: 10.1016/j.berh.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Bierma-Zeinstra SM, Koes BW. Risk factors and prognostic factors of hip and knee osteoarthritis. Nat Clin Pract Rheumatol. 2007;3:78–85. doi: 10.1038/ncprheum0423. [DOI] [PubMed] [Google Scholar]
- 24.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–95. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 25.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Ann Rheum Dis. 1994;53:565–8. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50:3904–9. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 27.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer GM, van Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56:1204–11. doi: 10.1002/art.22515. [DOI] [PubMed] [Google Scholar]
- 29.Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. Am J Epidemiol. 2003;158:1083–9. doi: 10.1093/aje/kwg263. [DOI] [PubMed] [Google Scholar]
- 30.Nordstrom CK, Diez Roux AV, Schulz R, Haan MN, Jackson SA, Balfour JL. Socioeconomic position and incident mobility impairment in the Cardiovascular Health Study. BMC Geriatr. 2007;7:11. doi: 10.1186/1471-2318-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Psaty BM, Cheadle A, Koepsell TD, Diehr P, Wickizer T, Curry S, et al. Race- and ethnicity-specific characteristics of participants lost to follow-up in a telephone cohort. Am J Epidemiol. 1994;140:161–71. doi: 10.1093/oxfordjournals.aje.a117226. [DOI] [PubMed] [Google Scholar]
- 32.US Census Bureau . DP-1: general population and housing characteristics. Johnston County; North Carolina: 2005. 1990. URL: http://factfinder.census.gov/servlet/QTTable?_bm=y&-context=qt&-qr_name=DEC_1990_STF1_DP1&-ds_name=DEC_1990_STF1_&-CONTEXT=qt&-tree_id=100&-redoLog=true&-all_geo_types=N&-_caller=geoselect&-geo_id=05000US37101. [Google Scholar]
- 33.US Census Bureau . DP-1: general population and housing characteristics. United States: 2005. 1990. URL: http://factfinder.census.gov/servlet/QTTable?_bm=y&qr_name=DEC_1990_STF1_DP1&ds_name=DEC_1990_STF1_&geo_id=05000US37101. [Google Scholar]
- 34.National Center for Health Statistics NHANES III data files, documentation, and SAS code. 2007 URL: http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm.
- 35.Fackelmann K. Refiguring the odds: what’s a woman’s real chance of suffering breast cancer? Science News. 1993;144:76–7. [Google Scholar]
- 36.Beiser A, D’Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Cheng TO. Obesity is a global challenge. Am J Med. 2006;119:e11. doi: 10.1016/j.amjmed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization Global strategy on diet, physical activity and health: obesity and overweight. 2007 URL: http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/
- 39.Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women: the Framingham Study. Ann Intern Med. 1992;116:535–9. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- 40.Badley EM. The economic burden of musculoskeletal disorders in Canada is similar to that for cancer, and may be higher. J Rheumatol. 1995;22:204–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


