Abstract
Immunochemotherapeutics, epirubicin-(C3-amide)-SS-[anti-HER2/neu] with an internal disulfide bond, and epirubicin-(C3-amide)-[anti-HER2/neu] were synthesized utilizing succinimidyl 2-[(4,4′-azipentanamido) ethyl]-1,3′-dithioproprionate or succinimidyl 4,4-azipentanoate respectively. Western blot analysis was used to determine the presence of any immunoglobulin fragmentation or IgG-IgG polymerization. Retained HER2/neu binding characteristics of epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] were validated by cell-ELISA using a mammary adenocarcinoma (SKBr-3) population that highly over-expresses trophic HER2/neu receptor complexes. Cytotoxic anti-neoplastic potency of epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] between epirubicin-equivalent concentrations of 10−10 M and 10−6 M was determined by measuring the vitality/proliferation of chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3 cell type). Cytotoxic anti-neoplastic potency of benzimidazoles (albendazole, flubendazole, membendazole) and griseofulvin were assessed between 0-to-2 μg/ml and 0-to-100 μg/ml respectively while mebendazole and griseofulvin were analyzed at fixed concentrations of 0.35 μg/ml and 35 g/ml respectively in dual combination with gradient concentrations of epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu].
Cytotoxic anti-neoplastic potency for epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) was nearly identical at epirubicin-equivalent concentrations of 10−10 M and 10−6 M. The benzimadazoles also possessed cytotoxic anti-neoplastic activity with flubendazole and albendazole being the most and least potent respectively. Similarly, griseofulvin had cytotoxic anti-neoplastic activity and was more potent than methylselenocysteine. Both mebendazole and griseofulvin when applied in dual combination with either epirubicin-(C3-amide)-[anti-HER2/neu] or epirubicin-(C3-amide)-SS-[anti-HER2/neu] produced enhanced levels of cytotoxic anti-neoplatic potency.
Keywords: anthracycline (epirubicin), chemotherapeutic-resistant, covalent immunochemotherapeutic, cytotoxic anti-neoplastic potency, mammary adenocarcinoma, selective “targeted” delivery, benzimidazoles, griseofulvin
1. Introduction
A high percentage of aggressive and resistant forms of breast cancer over-express EGFR and/or HER2/neu (Cameron & Stein, 2008; Medina & Goodin, 2008; Widakowich, Dinh, de Azambuja, Awada, & Piccart-Gebhart, 2008) which is frequently associated with chemotherapeutic-resistance, elevated cancer cell survival characteristics, and increased proliferation rates (Loew, Schmidt, Unterberg, & Halatsch, 2009; Slamon, Clark, & Wong, 1987). Resistant forms of breast cancer that over-express EGFR and HER2/neu are often less vulnerable to the cytotoxic potency of chemotherapeutics due to simultaneous over-expression of P-glycoprotein functioning as a non-selective trans-membrane “pump” complex for many pharmaceutical substrates (Chekhun, Zhylchuk, Lukyanova, Vorontsova, & Kudryavets, 2009; Gonzalez-Angulo, Morales-Vasquez, & Hortobagyi, 2007; Liu et al., 2010; Pasquier, Magal, Boulangé-Lecomte, Webb, & Foll, 2011; Patwardhan, Gupta, Huang, Gu, & Liu, 2010; Shen, Lee, & Gan, 2011). Monoclonal immunoglobulin or pharmaceuticals with binding-avidity for HER2/neu (e.g. anti-HER2/neu: trastuzumab, pertuzumab) (Gong et al., 2011; Phillips et al., 2008; Pandya et al., 2011; Scaltriti et al., 2011; Sliwkowski et al., 1999), EGFR (e.g. anti-EGFR: cetuximab, gefitinib) (Morgillo, Woo, Kim, Hong, & Lee, 2006; Morgillo et al., 2007; Sartore-Bianchi et al., 2009; Weickhardt, Tebbutt, & Mariadason, 2010), monoclonal immunoglobulin with dual HER2/neu and EGFR binding-avidity (e.g. anti-HER2/neu and anti-EGFR properties: panitumumab) (Dempke & Heinemann, 2010; Modjtahedi & Essapen, 2009; Sartore-Bianchi, 2009; Weickhardt, Tebbutt, & Mariadason, 2010), or monoclonal immunoglobulin inhibitors of other trophic receptors are all effective treatment options for forms of cancer affecting the breast, intestinal tract, lung and prostate. The obvious advantage of these therapeutic monoclonal immunoglobulins is their unique mechanism-of-action and their administration avoids many of the sequelae commonly associated with conventional chemotherapeutics. Unfortunately, most monoclonal immunoglobulin-based therapies that inhibit anti-trophic receptor function are usually only capable of promoting cytostatic properties and are almost invariably plagued by an inability to evoke cytotoxic activity sufficient to resolve most aggressive or advanced forms of neoplastic disease (Chen, Xia, & Spector, 2008; Cobleigh et al., 1999; Kute et al., 2009; Lewis Phillips et al., 2008; Lin et al., 2008; Marches & Uhr, 2004; Mitra et al., 2009; Nanda, 2007; Narayan et al., 2009; Pietras, Pegram, Finn, Maneval, & Slamon, 1998; Ritter et al., 2007; Sliwkowski et al., 1999; Vogel et al., 2002). Exceptions include scenarios where they are administered in combination with conventional chemotherapeutics or other cancer treatment modalities (García-Sáenz et al., 2008; Harris, Ward, Dobbins, Drew, & Pearson, 2011; Slamon et al., 2001). Lack of cytotoxic efficacy of the anti-trophic receptor immunoglobulins has been attributed to increases in cell-cycle G1-arrest, increased cell transformation into states of apoptosis-resistance (Marches & Uhr, 2004) and selection for resistant sub-populations (Lewis Phillips et al., 2008; Sliwkowski et al., 1999) that is frequently complicated by reversal of tumor growth inhibition (Sliwkowski et al., 1999) and relapse trophic receptor over-expression (Pietras et al., 1998) following discontinuation of therapy.
The anthracycline class of chemotherapeutics is commonly administered for the treatment of breast cancer and many other neoplastic conditions due to their superior level of potency. One of the most common dose-limiting side effects of anthracycline administration is cardiotoxicity (doxorubicin ≫ epirubicin). Even with the anthracyclines a complete clinical resolution of breast cancer, (particularly resistant forms), is rarely attainable especially when utilized as a monotherapy. Combination chemotherapy regimens are almost invariably more potent in suppressing the growth and metastasis of neoplastic cell types, significantly prolonging quality-of-life, delaying the onset of disease relapse, combating chemotherapeutic resistance, extending the duration of disease remission, and facilitating complete neoplastic disease elimination. Chemotherapeutic resistance is a particularly important development that hinders successful treatment of breast cancer because approximately 20–30% of all affected cases develop metastatic brain lesions which characteristically display moderate-to-high levels refractoriness to chemotherapeutic intervention (Honig et al., 2005). Despite the advantages of combination chemotherapy regimens, they still suffer from a high frequency of toxic sequelae that can limit the extent and duration of administration (Azad, Posadas et al., 2008; Balayssac et al., 2011; Ceresa & Cavaletti, 2011; Chang et al., 2001; Iarussi, Indolfi, Galderisi, & Bossone, 2000; Raschi et al., 2010; Scully & Lipshultz, 2007; Stavridi & Palmieri, 2008; Vantelon et al., 2001; Wachters, Van Der Graaf, & Groen, 2004).
Due largely to their relatively high potency against many common neoplastic conditions, the anthracyclines have long been one of the most common chemotherapeutic classes utilized in the molecular design and synthesis of therapeutic modalities that possess properties of selective “targeted delivery with the potential of improving treatment effectiveness and reducing deposition within innocent tissues and organ systems (Coyne, Jones, Sygula, Bailey, & Pinchuk, 2011; Coyne, Ross, Bailey, & Jones, 2009; Diener, Diner, Sinha, Xie, & Vergidis, 1986; Dillman, Johnson, Ogden, & Beidler, 1989; King et al., 1999; Kratz et al., 2002; Liu, Zhao, Volk, Klohr, Kerns, & Lee, 1996; Muldoon & Neuwelt, 2003; Page, Thibeault, Noel & Dumas, 1990; Thorpe et al., 1987; Worrell et al., 1986). Covalent bonding of anthracycline chemotherapeutics to monoclonal immunoglobulin therefore collectively facilitates selective “targeted” delivery, maximizes cancer cell chemotherapeutic deposition, promotes progressive intracellular chemotherapeutic accumulation, and reduces the risk and frequency of severe sequellae. In addition, the implementation of molecular platforms that fascilitate mechanisms of selective “targeted” chemotherapeutic delivery provides opportunities for attaining additive and synergistic levels of cytotoxic anti-neoplastic potency (Pegram, Lopez, Konecny, & Slamon, 2000; Slamon et al., 2001).
Covalent immunochemotherapeutics designed to selectively bind to external surface membrane receptors whereby the entire complex is internalized by mechanisms of receptor-mediated-endocytosis ultimately liberates the chemotherapeutic moiety through various processes within the acidic endolysosome environment (pH 5.0–5.5). A previously synthesized covalent anthracycline immunochemotherapeutic, epirubicin-[anti-HER2/neu] designed to contain a synthetically introduced acid-labile hydrazone (C13-imino) bond structure did not have any detectably greater cytotoxic anti-neoplastic potency against mammary adenocarcinoma (SKBr-3) populations (Coyne, Jones, & Pharr, 2011). Similar covalent anthracycline immunochemotherapeutics with acid-labile/acid-sensitive properties reportedly afford increased or accelerated liberation (cytosol bioavailability) of only 40% of their total chemotherapeutic content within the low pH of endolysosomal environments found in many cancer cell types. In parallel with the concept of acid-sensitive anthracycline immunochemotherapeutics both epirubicin-(C3-amide)-SS-[anti-HER2/neu] and epirubicin-(C3-amide)-[anti-HER2/neu] were synthesized in order to determined if the synthetic introduction of a disulfide bond structure created an enzyme or acid-sensitive covalent immunochemotherapeutic. Similar covalent maytansinoid immunochemotherapeutics with an integral disulfide bond structure have been synthesized that variably provide increases in intracellular chemotherapeutic bioavailability (Erickson et al., 2010; Kellogg et al., 2011).
In combination chemotherapy protocols the anthracyclines are frequently applied in concert with conventional tubulin/microtubule inhibitor chemotherapeutics to attain increased cytotoxic anti-neoplastic potency and improve the probability of clinically resolving breast cancer and other common neoplastic conditions (Gonzalez-Angulo, 2007; Hatzis et al., 2011; Honig et al., 2005; Hudis & Schmitz, 2004; Kim et al., 2011; Manzoni et al., 2010; Neskoviæ-Konstantinoviæ, Bosnjak, Raduloviæ, & Mitroviæ, 1996; Tang, 2009; Wong & Chiu, 2010). Conventional tubulin/microtubule inhibitor chemotherapeutics include colchicine (Levy, Spino, & Read, 1991), the vinca alkaloids (Antón et al., 2011; Dorsey et al., 2010; Honig et al., 2005; Hudis et al., 2004; Wong et al., 2010), taxanes (e.g. paclitaxel) (Chan, Miles, & Pivot, 2010; Dorsey et al., 2010; Rak Tkaczuk, 2011), podophyllotoxins (e.g. etoposide: semi-synthetic derivative) (Desbène & Giorgi-Renault, 2002; Neskoviæ-Konstantinoviæ et al., 1996), and monomethyl auristatin E (MMAE) (Naumovski & Junutula, 2010). The narrow margin-of-safety (therapeutic index) and relative lack of efficacy for colchicine restricts its wide-spread application for breast cancer treatment (Finkelstein et al., 2010; Levy et al., 1991; Terkeltaub, 2009). Administration of the vinca alkaloids like vinblastine and vincristine is often complicated by neurotoxicity (Broyl et al., 2010; Cavaletti et al., 2000; Meyer, Patte-Mensah, Taleb, & Mensah-Nyagan, 2010; Windebank, 1999), bone marrow suppression (Lowe et al., 2009; van Tellingen, Buckle, Jonker, van der Valk, & Beijnen, 2003), and emergence of therapeutic resistance patterns (Cavaletti et al., 2000; Dorsey et al., 2010; Dumontet & Sikic, 1999; He, Li, Kanwar, & Zhou, 2011; Kars, Iseri, & Gündüz, 2011; Kim et al., 2011; O’Brien et al., 2008; Svirnovski et al., 2009; VanderWeele, Zhou, & Rudin, 2004). The taxane class of anti-tubulin chemotherapeutics (e.g. paclitaxel, docetaxael) suffers from very similar disadvantages including acquired/intrinsic resistance (Antón et al., 2011; Dorsey et al., 2010; Dumontet, 1999; Kars et al., 2011) secondary to multidrug resistance protein (e.g. P-glycoprotein) over-expression (Harrison & Swanton, 2008; Overmoyer, 2008; Shen et al., 2011), hypersensitivity reactions (Feldweg, Lee, Matulonis, & Castells, 2005; Karacan, Eyüboglu, Akçay, & Ozyilkan, 2004; Lee, Gianos & Klaustermeyer, 2009; Rizzo, Spaggiari et al., 2010), hematopoietic toxicity (dose limiting feature) (Escuin et al., 2011; Manzoni, 2010; Pullarkat et al., 2009), and cumulative neurotoxicity (Cavaletti et al., 2000; Hershman et al., 2011; Windebank, 1999) that can all lead to termination of treatment protocols (Tang, 2009). Podophyllotoxins (e.g. etoposide semi-synthetic derivative) induce a moderately high frequency of dose-limiting leucopenia (Chamberlain, Tsao-Wei, & Groshen, 2006; Herzig, 1991; Neskoviæ-Konstantinoviæ et al., 1996) and gastrointestinal disturbances (nausea, vomiting, stomatitis). Monomethyl auristatin E (MMAE) is too toxic for systemic administration so it instead must be covalently bonded to a large molecular weight “platform” like immunoglobulin (e.g. anti-GPNMB/anti-CRO11/glembatumumab, anti-CD30/brentuximab).
Due to the effectiveness of conventional tubulin/microtubule inhibitors in combination with the anthracyclines, and because of their different spectrums of dose-limiting toxic sequelae, there is a distinct need to identify and evaluate alternative tubulin/microtubule inhibitors that have potent cytotoxic anti-neoplastic potency. To date, very little is known about the cytotoxic anti-neoplastic potency of the benzimidazoles (anthelmintic) or griseofulvin (anti-fungal agent) tubulin/microtubule inhibitors against aggressive and resistant breast cancer or their capacity to produce additive or synergistic levels of cytotoxic anti-neoplastic potency when applied in dual combination with covalent anthracycline immunochemotherapeutics.
2. Materials and Methods
2.1 Synthesis of Covalent Epirubicin-(C3-amide)-[Anti-HER2/neu] Immunochemotherapeutics
2.1.1 Phase-I Synthesis Reaction for UV-Photoactivated Epirubicin-(C3-amide) Intermediates
The C3 monoamine group of epirubicin (3.02 × 10−7 mg, 1.75 × 10−4 mmoles) was reacted at a 5:1 molar-ratio with the amine-reactive N-hydroxysuccinimide ester “leaving” complex of either succinimidyl 4,4-azipentanoate (1.55 × 10−7 mg, 3.5 × 10−5 mmoles) or it’s disulfide analog succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithioproprionate (2.67 × 10−7 mg, 3.5 × 10−5 mmoles) in the presence of triethylamine (≥ 50 mM final concentration) utilizing dimethylsulfoxide (DMSO) as an anhydrous organic solvent system (Coyne, Jones, & Bear 2012). Separate stock solutions were used to formulate reaction mixtures of epirubicin with succinimidyl 4,4-azipentanoate, or epirubicin with succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithioproprionate each of which was continually stirred gently over a 4-hour incubation period at 25°C in the dark and protected from light exposure. The relatively prolonged incubation period of 4 hours was utilized to maximize degradation of the ester group within any residual succinimidyl 4,4-azipentanoate or succinimidyl 2-[(4,4′-azipentanamido) ethyl]-1,3′-dithioproprionate that may not of reacted in the first 30 to 60 minutes with the C3 monoamine group of epirubicin. The reaction can alternatively be performed with the addition of PBS (10% v/v, pH 7.4) as a substitute for trieithylamine.
2.1.2 Phase-II Synthesis Reaction for Covalent Epirubicin-(C3-amide)-[Anti-HER2/neu] Immunochemotherapeutic Utilizing UV-Photoactivated Anthracycline Intermediates
Monoclonal immunoglobulin (anti-HER2/neu: 1.5 mg, 1.0 × 10−5 mmoles) in buffer (PBS: phosphate 0.1, NaCl 0.15 M, EDTA 10 mM, pH 7.3) was combined at a 1:3.5 molar-ratio with either the UV-photoactivated epirubicin-(C3-amide) or epirubicin-(C3-amide)-SS-intermediates (Phase-1 end products) and allowed to gently mix with constant stirring for 5 minutes at 25°C in the dark. The photoactivated group of the epirubicin-(C3-amide) and epirubicin-(C3-amide)-SS-intermediates were then reacted with amino acid residues within the sequence of anti-HER2/neu monoclonal immunoglobulin during a 15 minute exposure period to UV light at 354 nm (reagent activation range 320–370 nm) in combination with constant gentle stirring (Coyne, Jones, & Bear 2012). Residual epirubicin was removed from epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] applying micro-scale column chromatography pre-equilibrated in PBS (phosphate 0.1, NaCl 0.15 M, pH 7.3).
2.2 Analysis, Characteristics and Properties
2.2.1 General Analysis
Immunoglobulin concentration within preparations of epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] was determined by measuring absorbance at 280 nm (epirubicin-corrected absorbance at 280 nm). Epirubicin concentrations were determined by excitation at 485 nm and measurement of emission at 538 nm which was utilized in concert with a standard reference control curve generated utilizing known epirubicin concentrations (10−9 M to 10−5 M). Detection of non-conjugated “free” epirubicin concentrations contained in covalent epirubicin immunochemotherapeutic preparations was determined by chloroform extraction (Etrych, Mrkvan, Ríhová, & Ulbrich, 2007; Hempel, Schulze-Westhoff, Flege, & Laubrock, 1998; Ulbrich, Etrych, Chytil, Jelinkova, & Rihova, 2003) with the organic phase collected by pipette, evaporated to dryness under a stream of nitrogen gas, and the resulting residue dissolved in Tris buffered saline (50 mM, pH 7.4) prior to spectrophotometric analysis.
2.2.2 Non-Reducing SDS-PAGE Size Separation, Western-Blot Immunodetection, and Chemiluminescent Autoradiography
Standardized amounts and concentrations (60 μg/ml) of covalent epirubicin immunochemotherapeutics and reference control immunoglobulin fractions were combined 50/50 with an equal volume of conventional PAGE sample preparation buffer (Tris/glycerol/bromphenyl blue/sodium dodecyl sulfate) formulated without 2-mercaptoethanol. Each immunoglobulin sample (0.9 μg/well) was processed without boiling and then developed in parallel with a mixture of pre-stained reference control molecular weight markers by non-reducing SDS-PAGE (11% acrylamide, 100 V constant voltage at 3°C for 2.5 hours). Developed non-reducing SDS-PAGE acrylamide gels were then equilibrated in electrophoresis “tank” buffer devoid of methanol. Lateral transfer of SDS-PAGE separated proteins onto sheets of nitrocellulose membrane for Western (immunodetection) blots was performed at 20 volts constant voltage for 16 hours at 2°C to 3°C with the transfer manifold packed in crushed ice.
Nitrocellulose membranes with laterally transferred immunoglobulin fractions for immunodetection and chemiluminescent autoradiographic analyses were equilibrated in Tris buffered saline (TBS: Tris HCl 0.1 M, NaCl 150 mM, pH 7.5, 40 ml) at 4°C for 15 minutes followed by incubation in TBS blocking buffer solution (Tris 0.1 M, pH 7.4, 40 ml) containing bovine serum albumin (BSA 5%) applied at 2° to 3°C for 16 hours in combination with gentle horizontal agitation. Prior to further processing nitrocellulose membranes were vigorously rinsed in Tris buffered saline (Tris 0.1 M, pH 7.4, 40 ml, n = 3 rinses).
Rinsed BSA-blocked nitrocellulose membranes developed for Western-blot immunodetection analyses were incubated with biotinylated goat anti-murine IgG (1:10,000 dilution) at 4°C for 18 hours applied in combination with gentle horizontal agitation. Nitrocellulose membranes were then vigorously rinsed in TBS (pH 7.4, 4°C, 50 ml, n = 3) followed by incubation in blocking buffer (Tris 0.1 M, pH 7.4, with BSA 5%, 40 ml). Blocking buffer was decanted from nitrocellulose membrane blots which were then vigorously rinsed in TBS (pH 7.4, 4°C, 50 ml, n = 3 rinses) before incubation with strepavidin-[horseradish peroxidase] (strepavidin-HRPO 1:100,000 dilution) at 4°C for 2 hours applied in combination with gentle horizontal agitation. Prior to chemiluminescent autoradiography nitrocellulose membranes were vigorously rinsed in Tris buffered saline (Tris 0.1 M, pH 7.4, 40 ml, n = 3 rinses). Strepavidin-HRPO treated nitrocellulose membranes were then incubated in HRPO chemiluminescent substrate (25°C; 5-to-10 mins.). Autoradiography images were acquired by exposing radiographic film (Kodak BioMax XAR radiograph film) to nitrocellulose membranes sealed in transparent ultraclear re-sealable plastic bags.
2.2.3 Mammary Carcinoma Tissue Culture Cell Culture
Human chemotherapeutic-resistant human mammary adenocarcinoma (SKBr-3) cell line was utilized as an ex-vivo neoplasia model and was acquired directly from American Tissue Cell Culture (ATCC) within 24 months of investigation. Mammary adenocarcinoma (SKBr-3) has been the only cell line or cell type utilized, cultivated or preserved/stored frozen in the laboratory during a period of the past 6 years and during the conduction of research investigations currently described. Characteristically, mammary adenocarcinoma (SKBr-3) uniquely over-expresses epidermal growth factor receptor 1 (EGFR, ErbB-1, HER1) and highly over-expresses epidermal growth factor receptor 2 (EGFR2, HER2/neu, ErbB-2, CD340, p185) at 2.2 × 105/cell and 1 × 106/cell respectively.
Populations of the mammary adenocarcinoma (SKBr-3) cell line were propagated in 150-cc2 tissue culture flasks containing McCoy’s 5a Medium Modified supplemented with fetal bovine serum (10% v/v) and penicillin-streptomycin at a temperature of 37°C under a gas atmosphere of air (95%) and carbon dioxide (5% CO2). Tissue culture media was not supplemented with growth factors, growth hormones or other growth stimulants of any type. Investigations were all performed using mammary adenocarcinoma (SKBr-3) propagated to a ≥85% level of confluency.
2.2.4 Cell-ELISA IgG Binding Assay
Cell suspensions of mammary adenocarcinoma (SKBr-3) were seeded into 96-well microtiter plates in aliquots of 2 × 105 cells/well and allowed to form confluent adherent monolayers over a period of 48 hours. The growth media within individual wells was then removed manually by pipette and the adherent mammary adenocarcinoma (SKBr-3) monolayers serially rinsed (n = 3) with PBS followed by stabilization onto the plastic surfaces of microtiter plates with paraformaldehyde (4% in PBS, 15 minutes). Adherent mammary adenocarcinoma (SKBr-3) monolayers were then incubated with covalent epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] each formulated at gradient concentrations of 0.010, 0.025, 0.050, 0.250, and 0.500 μg/ml IgG (200 μl/well) in tissue culture growth media. Direct contact incubation of mammary adenocarcinoma (SKBr-3) with epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] was performed at 37°C over a period of 3 hours under a gas atmosphere of air (95%) and carbon dioxide (5% CO2). Residual non-cell bound epirubicin-(C3-amide)-[anti-HER2/neu] or epirubicin-(C3-amide)-SS-[anti-HER2/neu] was removed by serial rinsing with PBS (n=3). Development of mammary adenocarcinoma (SKBr-3) then entailed incubation with β-galactosidase-[goat anti-mouse IgG] (1:500 dilution) at 25°C for 20 hours followed by serial rinsing with PBS (n=3) to remove residual non-cell bound 2° immunoglobulin. In the final stage of cell-ELISA development mammary adenocarcinoma (SKBr-3) monolayers were incubated with the β-galactosidase substrate, nitrophenyl-β-D-galactopyranoside (100 μl/well of ONPG formulated fresh at 0.9 mg/ml in PBS pH 7.2 containing MgCl2 10 mM, and 2-mercaptoethanol 0.1 M). Absorbance within each individual well was then measured at 410 nm after incubation at 37°C for a period of 15 minutes (630 nm reference wavelength).
2.2.5 Cell Survival Assay for Measuring Covalent Epirubicin-Immunochemotherapeutic Cytotoxic Potency
Covalent epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutics were formulated in growth media at standardized epirubicin-equivalent concentrations of 10−10, 10−9, 10−8, 10−7, and 10−6 M (final concentration). Individual epirubicin-equivalent concentrations of covalent epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutics were then transferred in triplicate into 96-well microtiter plates containing chemotherapeutic-resistant mammary adenocarcinoma growth media 200 μl/well).
Contents within individual well of 96-well microtiter plates were removed manually by pipette at 72-hours and then the mammary adenocarcinoma (SKBr-3) monolayers were serially rinsed (n = 3) with PBS followed by incubation with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT 5 mg/ml in RPMI-1640 growth media devoid of pH indicator or bovine fetal calf serum). During a 3-to-4 hour incubation period at 37°C under a gas atmosphere of air (95%) and carbon dioxide (5% CO2) chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) populations were allowed to biochemically convert intracellular MTT to navy-blue formazone crystals by the endogenous enzyme, mitochondrial succinate dehydrogenase. Contents of 96-well microtiter plates were then removed, and serially rinsed with PBS (n = 3) followed by dissolving the resulting blue intracellular formazone crystals with DMSO (300 μl/well). Spectrophotometric absorbance of the blue-colored supernatant was then measured at 570 nm using a computer-integrated microtiter plate reader.
3. Results
3.1 Synthetic Chemistry
During the Phase-I synthesis scheme the ester sites of succinimidyl 4,4-azipentanoate or succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithioproprionate were reacted with epirubicin resulting in the creation of a covalent amide bond at the C3 monoamine on the anthracycline carbohydrate moiety (daunosamine-NH2−3′) (Figure 1). The resulting Phase-I products generated were UV-photoactivated epirubicin intermediates with or without an internal/integral disulfide bond accompanied simultaneously by the liberation of a succinimide “leaving” complex (Figure 1). In Phase-II of the synthesis scheme, the UV-photoactivated epirubicin-(C3-amide) intermediates non-specifically react with chemical groups to form covalent bonds within the anti-HER2/neu immunoglobulin sequence (Figure 1). Epirubicin was formulated in molar excess of succinimidyl 4,4-azipentanoate or succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithioproprionate and allowed to react over a prolonged period of time in order to maximize production of UV-photoactivated epirubicin-(C3-amide) and epirubicin-(C3-amide)-SS-intermediates and minimize concentrations of residual reagents.
Figure 1.
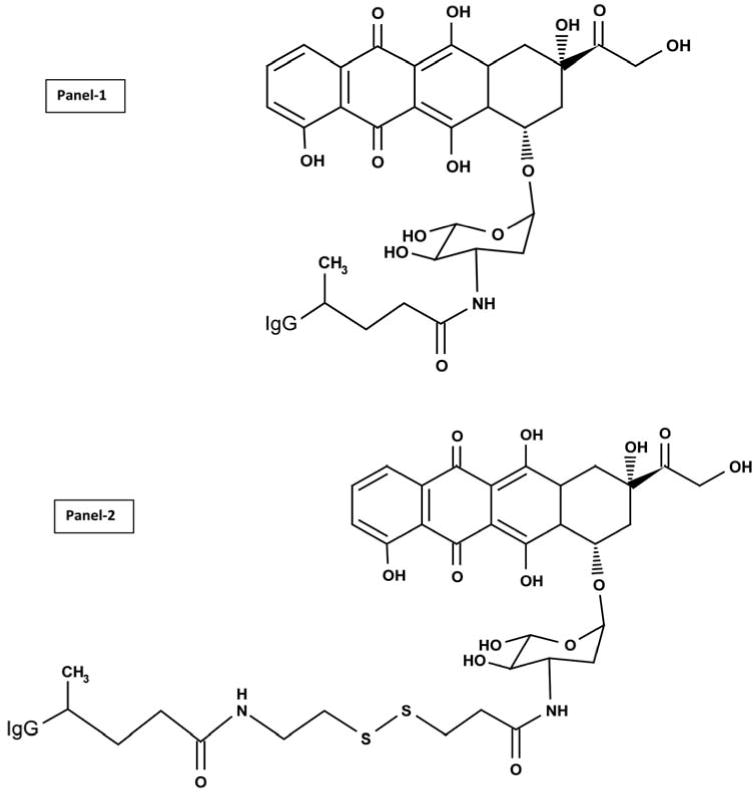
Molecular structure and chemical composition of two covalent epirubicin-(C3-amide)-[anti-HER2/neu] immunochemotherapeutics created with a 2-phase synthesis scheme using a epirubicin UV-photoactivated intermediate with and without the incorporation of an internal disulfide bond. Top Panel-1- covalent epirubicin-(C3-amide)-[anti-HER2/neu] immunochemotherapeutic synthesized utilizing succinimidyl 4,4-azipentanoate; Bottom Panel-2-covalent epirubicin-(C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutic created using succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithioproprionate which allows incorporation of an internal disulfide bond that is potentially cleavable intracellularly. Phase-I Synthesis Scheme: an amide bond is created at the C3 monoamino group of the anthracycline carbohydrate moiety through the amine-reactive N-hydroxysuccinimide (NHS ester) group of either succinimidyl 4,4-azipentanoate or succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithioproprionate. Phase-II Synthesis Scheme: epirubicin UV photoactivated intermediates create a covalent bond at amino acid residues within the amino acid sequence of anti-HER2/neu monoclonal immunoglobulin.
3.2 Molecular Properties
The percent of non-covalently bound anthracycline contained in covalent epirubicin immunochemotherapeutics following separation by micro-scale desalting/buffer exchange column chromatography was consistently < 4.0% of the total epirubicin content (Coyne, Jones, & Pharr 2011; Coyne et al., 2011; 2009). Residual non-covalently bound anthracycline is generally considered to not be available for further removal by serial/repeated column chromatography (Beyer et al., 2001). The anthracycline molar-incorporation-indexes for epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] were 40% (39.65%) and 47% (47.15%) respectively. Related preliminary analyses revealed that the epirubicin UV-photoactivated intermediates when synthesized in DMSO retained reactivity after freezing for at least 48 hours at −20°C based on an epirubicin molar-incorporation-index of 51% when the reaction mixture was combined with bovine serum albumin at a succinimidyl 4,4-azipentanoate-to-BSA molar-ratio of 7–9:1 in concert with subsequent UV-photoactivation (354 nm, 25°C, 15 minutes). Higher epirubicin molar-incorporation-indexes are possible to achieve with modifications in methodology but the harsher synthesis conditions required for such purposes are accompanied by substantial reductions in final covalent immunochemotherapeutic yield, (Greenfield et al., 1990) and declines in antigen-immunoglobulin binding-avidity (e.g. cell-ELISA parameters). Evaluation of covalent epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutics size-separated by SDS-PAGE and developed by Western blot analysis utilizing anti-murine IgG-strepavidin as a 1° immunoglobulin produced chemiluminescent autoradiography images that detected single (major) 150-kD band profiles for both the anti-HER2/neu reference control and each individual covalent epirubicin immunochemotherapeutic similar to results previously reported for other methodologies (Figure 2) (Coyne et al., 2011; Coyne et al., 2011b; Coyne et al., 2009; Di Stefano, Lanza, Kratz, Merina, & Fiume 2004; Sinkule, Rosen & Radosevich 1991).
Figure 2.
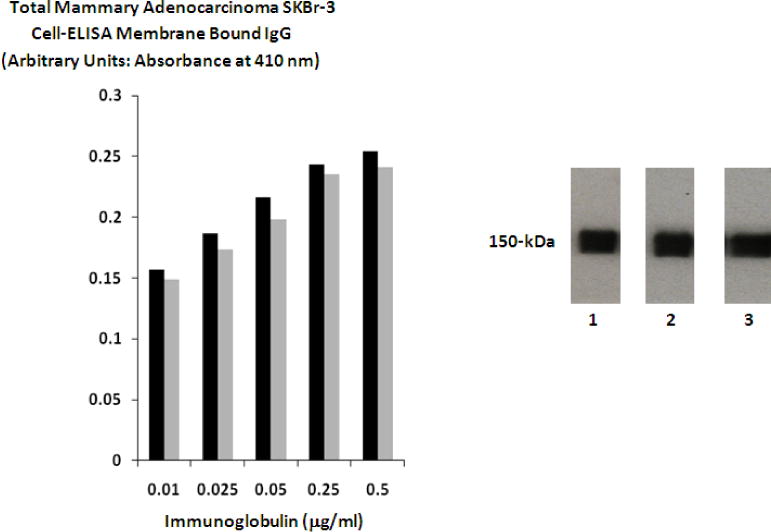
Biological and Physical Properties. Left Panel 1: Detection of total immunoglobulin bound to the exterior surface membrane of mammary adenocarcinoma in the form of covalent epirubicin immunochemotherapeutic. Legend: (■) covalent epirubicin-(C3-amide)-[anti-HER2/neu] immunochemotherapeutic; and (■) covalent epirubicin-(C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutic. Mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with the covalent epirubicin-(C3-amide)-[anti-HER2/neu] immunochemotherapeutics over a 4-hour period and total immunoglobulin bound on the exterior surface membrane was measured by cell-ELISA analysis. Right Panel 2: Western-blot chemiluminescent autoradiography of covalent epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutics. Legend: (Lane-1) murine anti-human HER2/neu immunoglobulin; (Lane-2) covalent epirubicin-(C3-amide)-[anti-HER2/neu] immunochemotherapeutic; and (Lane-3) covalent epirubicin-(C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutic. Immunoglobulin preparations were mass-separated by SDS-PAGE and transferred laterally onto sheets of nitrocellulose membrane to facilitate detection with biotinylated goat anti-mouse IgG. Subsequent analysis entailed incubation of nitrocellulose membranes with conjugated strepavidin-HRPO in combination with the use of an HRPO chemiluminescent substrate to facilitate the acquisition of autoradiography images.
3.3 Cell-ELISA Total Membrane IgG Binding Analyses
Epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] formulated at standardized immunoglobulin-equivalent concentrations of 0.010, 0.025, 0.050, 0.250, 0.500 μg/ml IgG produced cell-ELISA profiles that detected proportional increases in total immunoglobulin bound to mammary adenocarcinoma (SKBr-3) external surface membranes (Figure 2).
3.4. Cytotoxic Anti-Neoplastic Potency
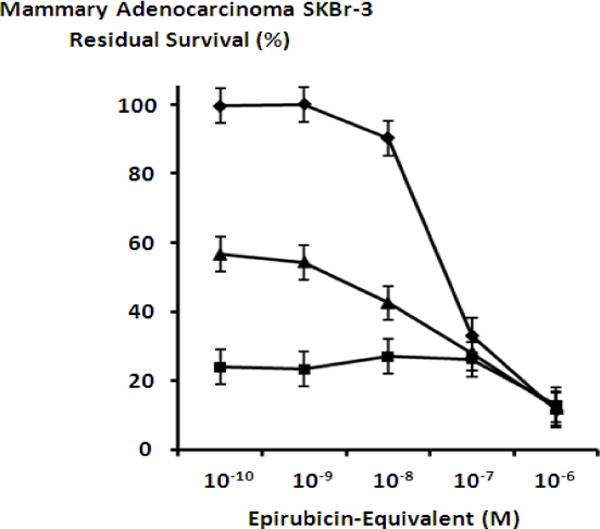
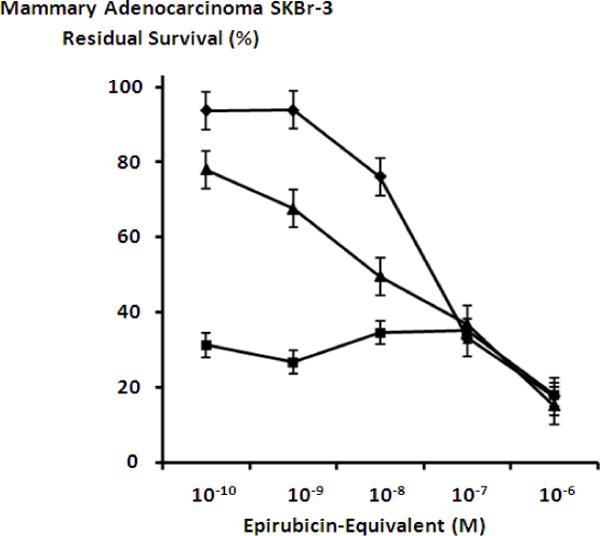
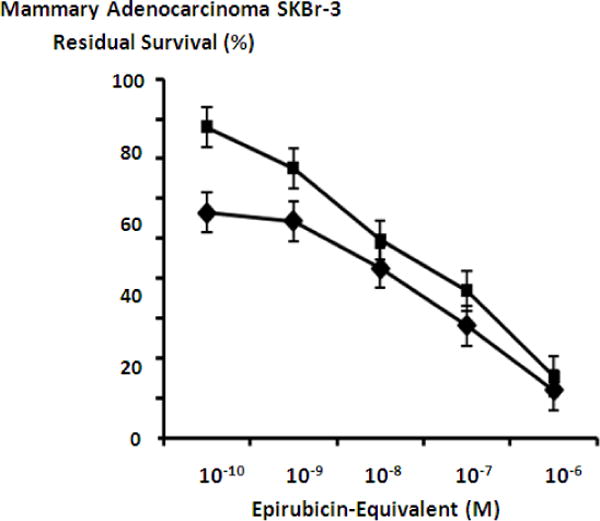
Mean maximum cytotoxic anti-neoplastic potencies of epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) were 88.5% and 82.4% (11.5% & 17.6% residual survival) respectively when assessed at an epirubicin-equivalent concentration of 10−6 M (Figure 3). Cytotoxic anti-neoplastic potencies of epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) were slight greater compared to epirubicin formulated at anthracycline-equivalent concentrations but the differences in mean values were not significant (Figure 3). The covalent epirubicin-(C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutic with an internal disulfide bond produced mean cytotoxic anti-neoplastic potency levels that were not substantially different from those measured for epirubicin-(C3-amide)-[anti-HER2/neu] (Figure 3). Individual fractions of anti-HER2/neu monoclonal immunoglobulin alone did not exert any detectable anti-neoplastic activity against mammary carcinoma (SKBr-3) at the end of a 72-hour incubation period which is in accord with observations reported in previous investigations (Figure 4) (Coyne et al., 2011; Coyne et al., 2011b; Coyne et al., 2009; Dillman et al., 1989; Sapra et al., 2005; Sinkule et al., 1991; Sivam, Martin, Reisfeld, & Mueller 1995; Yang & Reisfeld 1988a).
Figure 3.
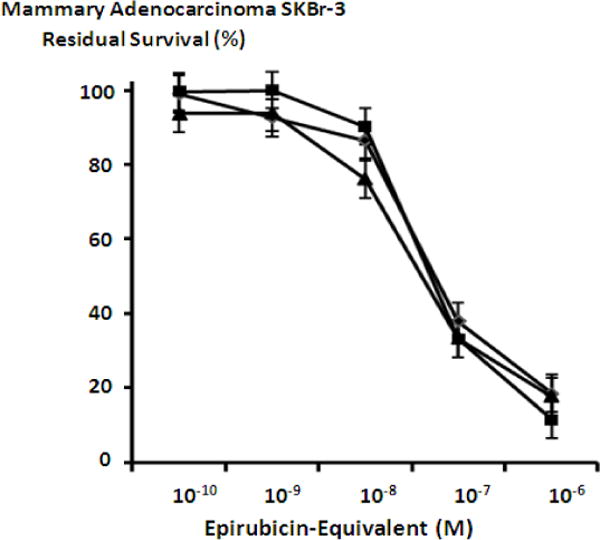
Influence of covalent bonding epirubicin to anti-HER2/neu monoclonal immunoglobulin on cytotoxic anti-neoplastic potency against chemotherapeutic-resistant mammary adenocarcinoma. Legend: (■) covalent epirubicin-(C3-amide)-[anti-HER2/neu] immunochemotherapeutic; (▲) covalent epirubicin-(C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutic; and (◆) epirubicin chemotherapeutic. Mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with epirubicin immunochemotherapeutic for 72-hours and cytotoxicity measured as a function (%) of cell MTT vitality stain intensity (absorbance at 570 nm) relative to matched negative reference controls.
Figure 4.
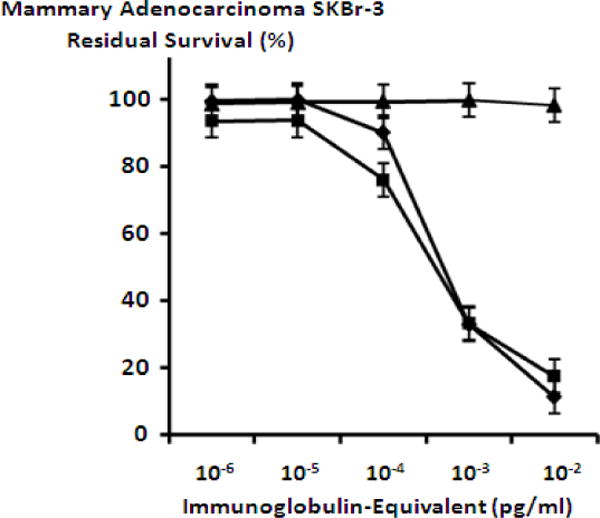
Relative cytotoxic anti-neoplastic potency of covalent epirubicin-immunochemotherapeutics compared to anti-HER2/neu monoclonal immunoglobulin against chemotherapeutic-resistant mammary adenocarcinoma. Legend: (◆) epirubicin-(C3-amide)-[anti-HER2/neu]; (■) epirubicin-(C3-amide)-SS-[anti-HER2/neu]; and (▲) anti-HER2/neu monoclonal immunoglobulin. Mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with immunochemotherapeutics or monoclonal immunoglobulin over a 72-hour period and cytotoxicity measured as a function (%) of MTT cell vitality stain intensity (absorbance at 570 nm) relative to matched negative reference controls.
The benzimadazole tubulin/microtubule inhibitors, albendazole, flubendazole and mebendazole exerted substantial cytotoxic anti-neoplastic potency against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) at final concentrations formulated between 0.05 μM to 2.5 μM (Figure 5). Mean cytotoxic anti-neoplastic potency profiles for both flubendazole and mebendazole revealed progressive increases from near 0% and 0% at 0.05 μM to 70.2% and 63.1% (29.8% and 36.9% residual survival) at the benzimidazole-equivalent concentration of 0.4 μM (Figure 5). Mean cytotoxic anti-neoplastic potency profiles for albendazole revealed a progressive increase in cytotoxic anti-neoplastic potency from 6.2% (93.8% residual survival) at a benzimidazole-equivalent concentration of 0.4 μM, to a near maximum of 65.4% (34.6% residual survival) at 2.0 mM (Figure 5). Mean maximum cytotoxic anti-neoplastic potencies for albendazole, flubendazole and mebendazole were 64.8%, 68.7% and 70.9% (35.2%, 31.3.% and 29.1% residual survival) at a benzimidazole-equivalent concentration of 2.5 μM (Figure 5).
Figure 5.
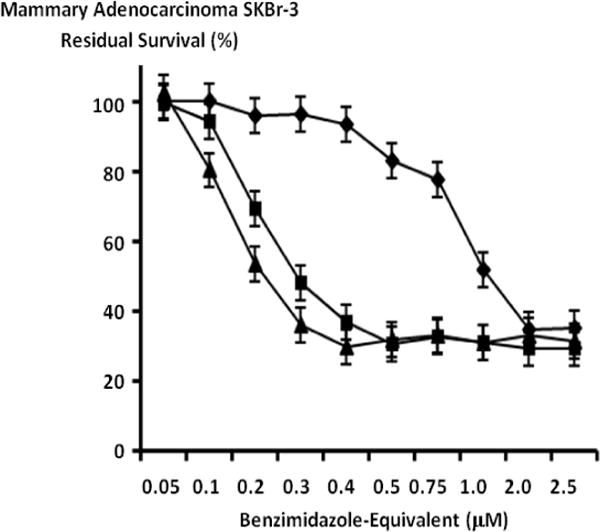
Relative cytotoxic anti-neoplastic potency of benzimidazole tubulin/microtubule inhibitors against chemotherapeutic-resistant mammary adenocarcinoma. Legend: (◆) albendazole; (▲) flubendazole; and (■) mebendazole. Mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with individual benzimidazole tubulin/microtubule inhibitors for 72-hours and cytotoxicity measured as a function of MTT cell vitality stain intensity (absorbance at 570 nm) relative to matched negative reference controls.
The anti-fungal tubulin/microtubule inhibitor griseofulvin, in addition to methylselenocysteine exerted effective levels of cytotoxic anti-neoplastic potency against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) when formulated at final concentrations between 10 μM to 100 μM (Figure 6). Increases in griseofulvin concentration to a level of 40 μM resulted in a progressive elevation in cytotoxic anti-neoplastic potency to 72.6% (27.4% residual survival) with a peak maximum level of 90.8% (9.2% residual survival) detected at 100 μM (Figure 6). Methylselenocysteine created progressive and rapid elevations in mean cytotoxic anti-neoplastic potency from 2.6% to 58.7% (97.4% to 41.3% residual survival) between the concentration range of 20 μM to 40 μM with peak levels of 85.2% (14.8% residual survival) detected at a final concentration of 100 μM (Figure 6).
Figure 6.
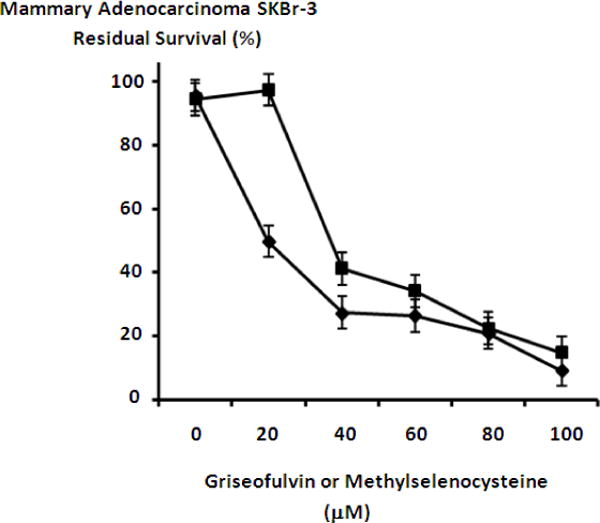
Relative cytotoxic anti-neoplastic potency of griseofulvin tubulin/microtubule inhibitor and methylselencysteine against chemotherapeutic-resistant mammary adenocarcinoma. Legend: (◆) griseofulvin; and (■) methyselenocysteine. Mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with griseofulvin or methylselenocysteine for 72-hours and cytotoxicity measured as a function of MTT cell vitality stain intensity (absorbance at 570 nm) relative to matched negative reference controls.
Mebendazole and griseofulvin applied in combination with either epirubicin-(C3-amide)-[anti-HER2/neu] or epirubicin-(C3-amide)-SS-[anti-HER2/neu] resulted in marked increase in cytotoxic anti-neoplastic potency against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) (Figures 7, 8 & 9). Mebendazole at a fixed final concentration of 0.35 μM applied in combination with epirubicin-(C3-amide)-[anti-HER2/neu] produced mean cytotoxic anti-neoplastic potency levels of 76.1% to 73% (23.9% to 27.0% residual survival), while mebendazole in combination with epirubicin-(C3-amide)-SS-[anti-HER2/neu] produced mean cytotoxic anti-neoplastic potency levels of 68.7% and 65.0% (31.3% to 35.0% residual survival) between the epirubicin-equivalent concentration range of 10−10 M to 10−7 M respectively (Figures 7, 8 & 9). Mean peak cytotoxic anti-neoplastic potency for mebendazole in combination with epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] was 87.1% and 81.9% (12.9% and 18.1% residual survival) at the epirubicin-equivalent concentration of 10−6 M (Figures 7, 8 & 9).
Figure 7.
Collective cytotoxic anti-neoplastic potency of epirubicin-(C3-amide)-[anti-HER2/neu] synthesized with an epirubicin UV-photoactivated intermediate when applied in combination with mebendazole or griseofulvin tubulin/microtubule inhibitors. Legend: (◆) epirubicin-(C3-amide)-[anti-HER2/neu]; (▲) epirubicin-(C3-amide)-[anti-HER2/neu] in combination with griseofulvin (15 μM fixed concentration); and (■) epirubicin-(C3-amide)-[anti-HER2/neu] in combination with mebendazole (0.35 μM fixed concentration). Mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with the covalent epirubicin-immunochemotherapeutic in combination with the tubulin/microtubule inhibitors for 72-hours and cytotoxicity measured as a function of MTT cell vitality stain intensity (absorbance at 570 nm) relative to matched negative reference controls.
Figure 8.
Collective cytotoxic anti-neoplastic potency of epirubicin-(C3-amide)-SS-[anti-HER2/neu] synthesized with an epirubicin UV-photoactivated intermediate when applied in combination with mebendazole or griseofulvin tubulin/microtubule inhibitors. Legend: (◆) epirubicin-(C3-amide)-SS-[anti-HER2/neu]; (▲) epirubicin-(C3-amide)-SS-[anti-HER2/neu] in combination with griseofulvin (15 μM fixed concentration); and (■) epirubicin-(C3-amide)-SS-[anti-HER2/neu] in combination with mebendazole (0.35 μM fixed concentration). Mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with the covalent epirubicin-immunochemotherapeutic in combination with the tubulin/microtubule inhibitors for 72-hours and cytotoxicity measured as a function of MTT cell vitality stain intensity (absorbance at 570 nm) relative to matched negative reference controls.
Figure 9.
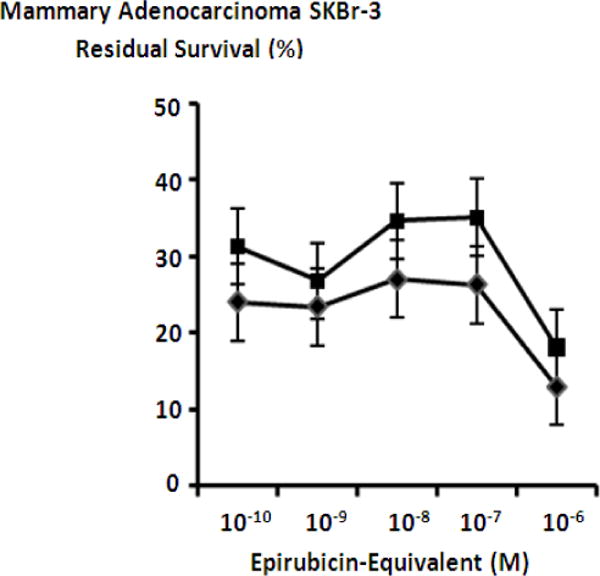
Collective cytotoxic anti-neoplastic potency of epirubicin-(C3-amide)-[anti-HER2/neu] or epirubicin-(C3-amide)-SS-[anti-HER2/neu] synthesized with an internal disulfide bond when each covalent anthracycline immunochemotherapeutic was applied in combination with mebendazole. Legend: (◆) epirubicin-(C3-amide)-[anti-HER2/neu] in combination with mebendazole (0.35 μM fixed concentration; and (■) epirubicin-(C3-amide)-SS-[anti-HER2/neu] in combination with mebendazole (0.35 μM fixed concentration). Mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with covalent epirubicin-immunochemotherapeutics in combination with benzimidazole for 72-hours and cytotoxicity measured as a function of MTT cell vitality stain intensity (absorbance at 570 nm) relative to matched negative reference controls.
Griseofulvin at a fixed final concentration of 35 μM applied in combination with epirubicin-(C3-amide)-[anti-HER2/neu] produced moderately progressive increases in mean cytotoxic anti-neoplastic potency from 45.7% to 88.0% (54.3% to 12.0% residual survival) between the epirubicin-equivalent concentration range of 10−9 M to 10−6 M respectively (Figure 7). Similarly, griseofulvin at a final fixed concentration of 35 μM applied in combination with epirubicin-(C3-amide)-SS-[anti-HER2/neu] produced a moderately progressive increase in mean cytotoxic anti-neoplastic potency from between 22.0% to 84.7% (78.0% to 15.3% residual survival) within the final epirubicin-equivalent concentration range of 10−10 M to 10−6 M respectively (Figure 8). Mean peak cytotoxic anti-neoplastic potency levels for griseofulvin in combination with epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] were 88.0% and 84.7% (12.0% and 15.3% residual survival) at the final epirubicin-equivalent concentration of 10−6 M (Figures 7 & 8). Both mebendazole and griseofulvin in dual combination with epirubicin-(C3-amide)-[anti-HER2/neu] evoked slightly greater cytotoxic anti-neoplastic potency than levels detected when they were applied in concert with epirubicin-(C3-amide)-SS-[anti- HER2/neu] but this consistent trend was not considered profound (Figures 9 & 10).
Figure 10.
Collective cytotoxic anti-neoplastic potency of epirubicin-(C3-amide)-[anti-HER2/neu] or epirubicin-(C3-amide)-SS-[anti-HER2/neu] synthesized with an internal disulfide bond when each covalent anthracycline immunochemotherapeutic was applied in combination with griseofulvin. Legend: (◆) epirubicin-(C3-amide)-[anti-HER2/neu] in combination with griseofulvin (15 μM fixed concentration; and (■) epirubicin-(C3-amide)-SS-[anti-HER2/neu] in combination with griseofulvin (15 μM fixed concentration). Mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with covalent epirubicin-immunochemotherapeutics in combination with benzimidazole for 72-hours and cytotoxicity measured as a function of MTT cell vitality stain intensity (absorbance at 570 nm) relative to matched negative reference controls.
4. Discussion
4.1 Immunochemotherapeutic
A number of methods have been described for synthesizing covalent anthracycline-immunochemotherapeutics, but most techniques have a lengthy duration (e.g. days), often afford low total yields, and employ dedicated organic chemistry reactions for the production of only a single immunochemotherapeutic. In this context, the heterobifunctional covalent bond forming reactants, succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) (Coyne et al., 2009; Frost, Jensen & Lindegren 2010; Karacay et al., 1997; Lau, Bérubé, & Ford 1995), N-ɛ-maleimidocaproic acid hydrazide (EMCH) (Coyne et al., 2011; Furgeson, Dreher, & Chilkoti 2006; Kruger et al., 1997), or N-[p-maleimidophenyl]-isocyanate (PMPI) (Annunziato, Patel, Ranade & Palumbo 1993; Coyne et al., 2011; Liu, de Wijn, & van Blitterswijk 1998; Wang, et al., 2009) require pre-thiolation of peptide/protein based molecular platforms (e.g. IgG, Fab’, receptor ligands). Alternatively, several distinct advantages can be realized when covalent immunochemotherapeutics are synthesized utilizing succinimidyl 4,4-azipentanoate or succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithioproprionate which most notably include the application of relatively mild reaction conditions (e.g. lower risk of inter-IgG and intra-IgG polymerization or fragmentation) (Di Stegano et al., 2004), lack of pre-thiolation requirements, implementation of fewer individual organic chemistry reactions, option of employing non-anthracycline chemotherapeutic moieties, flexibile convenience of temporary UV-photoactivated chemotherapeutic intermediate storage, higher retained biological activity, and greater end-product yield (Coyne, Jones, & Bear 2012).
4.2 Cell-ELISA IgG Membrane Binding Analysis
Profiles for total membrane-bound IgG on the exterior surface of mammary adenocarcinoma (SKBr-3) populations incubated with epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] increased proportionately with gradient elevations in total immunoglobulin content (Figure 2). Total cell membrane-bound epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] was higher than previously observed for [i] epirubicin-(C3-amide)-[anti-HER2/neu] synthesized with succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) in combination with N-succinimidyl-S-acetylthioacetate (SATA) (Coyne et al., 2009), [ii] epirubicin-(C13-keto)-[anti-HER2/neu] synthesized with N-ɛ-maleimidocaproic acid hydrazide (EMCH) in combination with 2-iminothiolane (2-IT) (Coyne et al., 2011b), or [iii] gemcitabine-(C5-carbamate)-[anti-HER2/neu] synthesized with N-[p-maleimidophenyl]-isocyanate (PMPI) in combination with 2-iminothiolane (2-IT) (Coyne et al., 2011). Presumably the difference in part reflects a higher degree of retained epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] binding-avidity for exterior surface membrane-associated HER2/neu because succinimidyl 4,4-azipentanoate and succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithioproprionate do not require immunoglobulin pre-thiolation prior to the Phase-II synthesis reaction.
In addition to protein (immunoglobulin) pre-thiolation that can under certain conditions result in intra-molecular and inter-molecular disulfide bond formation, the binding-avidity of epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] for membrane HER2/neu was also likely influenced by the covalent bonding of the anthracycline moiety to different amino acid residues within the Fab’ antigen binding region of anti-HER2/neu immunoglobulin. Seemingly modest alterations in synthetic chemistry reactions and elevations in chemotherapeutic molar-incorporation-index can profoundly influence immunoglobulin binding properties (Yang & Reisfeld 1988a). The relatively mild conditions employed during organic chemistry reaction schemes and the relatively low molar-incorporation-index of 40.0% collectively contribute to the high biological integrity of epirubicin (C3-amide)-[anti-HER2/neu] and epirubicin (C3-amide)-SS-[anti-HER2/neu] based on the collective interpretation of results from SDS-PAGE chemiluminescent autoradiography, cell-ELISA analyses and cytotoxic anti-neoplastic potency against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3).
4.3 Cytotoxic Potency of Covalent Epirubicin Immunochemotherapeutics
Creation of a synthetic covalent bond between epirubicin and anti-HER2/neu monoclonal immunoglobulin for the production of epirubicin-(C3-amide)-[anti-HER2/neu] or epirubicin-(C3-amide)-SS-[anti-HER2/neu] did not decrease the cytotoxic potency of the anthracycline against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) when assessed between the epirubicin-equivalent concentration range of 10−10 M to 10−6 M (Figures 3 & 4). Similar properties have been recognized previously for epirubicin-(C13-imino)-[anti-HER2/neu] (Coyne et al., 2011), epirubicin-(C3-amide)-[anti-HER2/neu] (Coyne et al., 2009), epirubicin-(C3-amide)-[anti-EGFR] (Coyne et al., 2009) and analogous covalent immunochemotherapeutics designed to selectively “target” anthracycline delivery (Dillman et al., 1989; Herbert, Norris, & Sauk 2003; Johnson, Briggs, Gutowski, & Barton 1995; King et al., 1999; Sivam et al., 1995; Stan, Radu, Casares, Bona, & Brumeanu 1999; Yang et al., 1988a).
Synthetic incorporation of an internal (integral) disulfide bond into the molecular structure of covalent immunochemotherapeutics has potential merit as a strategy for enhancing the intracellular bioavailability of chemotherapeutic moieties following internalization by mechanisms of receptor-mediated endocytosis. The presumption is largely based on the concept that glutathione (GSH) tri-peptide is found intracellularly at levels that are 100× to 100×(≅2-to-10 mM) higher than concentrations found within the extracellular fluid or plasma compartments (≅2-to-20 μM). Given this difference, disulfide bond structures have been synthetically introduced into maytansinoid-immunochemotherapeutics with the intent of improving their intracellular bioavailability following internalization by “targeted” neoplastic cell populations (Erickson et al., 2010; Kellogg et al., 2011). Due to these considerations epirubicin-(C3-amide)-SS-[anti-HER2/neu] was synthesized with an internal (integral) disulfide bond structure in order to determine if such a chemical modification influences cytotoxic anti-neoplastic potency against mammary adenocarcinoma (SKBr-3) presumably by promoting elevations in the intracellular bioavailability of the anthracycline moiety (Figure 1). In contrast to the cytotoxic anti-neoplastic potency of covalent maytansinoid-immunochemotherapeutics against various cancer cell types like human intestinal carcinoma (xenografts) (Erickson et al., 2010; Kellogg et al., 2011), the cytotoxic anti-neoplastic potency of epirubicin-(C3-amide)-SS-[anti-HER2/neu] was not significantly greater compared to epirubicin-(C3-amide)-[anti-HER2/neu] (Figure 3). Similar to results detected in comparisons between epirubicin-(C3-amide)-SS-[anti-HER2/neu] and epirubicin-(C3-amide)-[anti-HER2/neu] several other covalent immunochemotherapeutics with internal disulfide bonds incorporated into their molecular structure have also been found to not possess increased levels of cytotoxic anti-neoplastic potency (Erickson et al., 2006; Lewis Phillips et al., 2008; Sun et al., 2011). Certain maytansinoid-SS-[anti-HER2/neu] immunochemotherapeutics in this regard that contain a synthetically introduced disulfide bond do not exert higher planes of cytotoxic anti-neoplastic potency against HER2/neu positive breast cancer (Lewis Phillips et al., 2008). Potency of epirubicin-(C3-amide)-SS-[anti-HER2/neu] against mammary adenocarcinoma (SKBr-3) might have been improved if a modified analog of succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithioproprionate had been utilized to incorporate an internal disulfide bond structure at a physically different location or in a different molecular configuration (Kellogg et al., 2011). A supportive analogy is the observation that mytansinoid-immunochemotherapeutics that contain two methyl groups in close proximity to chemotherapeutic moieties and are devoid of methyl groups on the “linker side” exert only intermediate levels of plasma stability, but superior levels of cytotoxic anti-neoplastic potency against xenografts of human intestinal carcinoma (Kellogg et al., 2011). Synthetic introduction of disulfide bond structures into covalent mytansinoid-immunochemotherapeutics can therefore increase in-vivo susceptibility to premature enzymatic degradation but if they are located in a sterically hindered position they are less susceptible to enzyme-mediated liberation within the intravascular compartment (Kellogg et al., 2011). Unfortunately it was not possible to evaluate the influence of disulfide bond position on the cytotoxic potency of epirubicin-(C3-amide)-SS-[anti-HER2/neu] against mammary carcinoma due to a lack of available reagents.
Other biochemical and cell biology associated variables may also account for the lack of improved cytotoxic anti-neoplastic potency of epirubicin-(C3-amide)-SS-[anti-HER2/neu] against chemotherapeutic-resistant human mammary adenocarcinoma (SKBr-3) compared to epirubicin-(C3-amide)-[anti-HER2/neu] (Figure 3). Covalent epirubicin-(C13-imino)-[anti-HER2/neu] immunochemotherapeutic synthesized with an internal bond structure that reportedly has acid-labile properties does not exert significantly higher levels of cytotoxic anti-neoplastic potency against mammary adenocarcinoma (SKBr-3) than a non-acid-labile epirubicin-(C3-amide)-[anti-HER2/neu] immunochemotherapeutic (Coyne et al., 2011b). The fact that epirubicin-(C3-amide)-SS-[anti-HER2/neu] also did not exert an enhanced level of cytotoxic anti-neoplastic activity against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) may therefore reflect a cell biology related variable that explains a lack of enhanced efficacy. Interestingly, minimal or no correlation frequently exists between the in-vitro and in-vivo potency of covalent immunochemotherapeutics with synthetically introduced disulfide bond structures (Kellogg et al., 2011) which is in marked contrast to covalent immunochemotherapeutics devoid of this same internal chemical structure. Differences in cytotoxic anti-neoplastic potency to this degree in-vivo have been attributed to the influence of hepatic metabolization and variations in the creation of lipophilic and hydrophilic metabolites that determine the extent of distribution within fluid compartments and penetration across intact cancer cell membranes (Erickson et al., 2010).
The cytotoxic anti-neoplastic potency of the prototypic epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin (C3-amide)-SS-[anti-HER2/neu] immunochemotherapeutics can potentially be enhanced in-vivo through several molecular strategies that involve exploiting the over-expression of membrane HER2/neu and the molecular properties of anti-HER2/neu as a selective “targeted” delivery platform. Given this perspective, endogenous trophic receptor over-expression is a critical variable that influences the cytotoxic anti-neoplastic potency of anthracycline-[anti-HER2/neu], anthracycline-[anti-EGFR] and related covalent immunochemotherapeutics because it provides opportunities to [i] significantly suppress neoplastic cell growth for populations with proliferation rates heavily dependent on tropic receptor over-expression; [ii] promote continual and selective chemotherapeutic deposition on the external surface membrane of neoplastic cells; and [iii] induce progressive active chemotherapeutic internalization by mechanisms of receptor-mediated endocytosis in a manner that promotes escalating increases in cytosol chemotherapeutic accumulation (Pimm, Paul, Ogumuyiwa, & Baldwin 1988; Shih et al., 1994; Stan et al., 1999; Yang et al., 1988a). The latter consideration is important since receptor-mediated-endocytosis of epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] can lead to increases in cytosol anthracycline concentrations that are 8.5× (Stan et al., 1999) to >100× (Pimm et al., 1988) greater than those that are attainable by simple passive anthracycline diffusion from the plasma or extracellular fluid compartments (e.g. following intravenous injection). Although specific data for HER2/neu and EGFR receptor complexes in chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) is limited, metastatic multiple myeloma internalizes approximately 8 × 106 anti-CD74 monoclonal antibody molecules per day following binding to membrane CD74 sites (Hansen, Ong & Diril 1996). Following selective “targeted” delivery more than 50% of an anthracycline at 24-hours is retained intracellularly (Stan et al., 1999) where it is primarily associated with either internal membrane structures or it becomes distributed throughout the cytosol environment (Liu et al., 2010; Shih et al., 1994). Conversely, “free” non-conjugated anthracycline upon passive diffusion across intact cellular lipid bilayer membranes is detected predominately in complex with nuclear DNA less than 30 minutes following initial exposure (Shih et al., 1994) whereas anthracycline liberated from covalent immunochemotherapeutics ultimately distributes into, and accumulates within the nucleus, mitochondria and golgi apparatus (Beyer, Rothen-Rutishauser, Unger, Wunderli-Allenspach & Kratz 2001).
Covalent immunochemotherapeutics like epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin (C3-amide)-SS-[anti-HER2/neu] that posssess properties that include selective “targeted” delivery due to their binding avidity of anti-HER2/neu, anti-EGFR, anti-VEGR or other anti-trophic receptor monoclonal immunoglobulins also afford a therapeutic strategy for achieving additive and synergistic levels of cytotoxic anti-neoplastic potency. More specifically, their molecular design makes possible the simultaneous inhibition of over-expressed trophic receptor complexes in concert with the selective “targeted” delivery of conventional and non-conventional chemotherapeutics. Chemotherapeutic examples relevant anti-HER2/neu include cyclophosphamide, docetaxel, doxorubicin, etoposide, methotrexate, paclitaxel, and vinblastine (Pegram, Lopez, Konecny & Slamon 2000; Slamon et al., 2001). Similar to anti-HER2/neu (Boone, Bhosle, Tilby, Hartley & Hochhauser 2009; Meden, Beneke, Hesse, Novophashenny, & Wischnewsky 2001; Pegram et al., 2000; Slamon, & Pegram 2001; Slamon et al., 2001; Winer & Burstein 2001), anti-EGFR (Ciardiello et al., 1999; Kim et al., 2006; Landriscina et al., 2010) and anti-VEGF (García-Sáenz et al., 2008; Lynn et al., 2010; Zhang et al., 2002) can also evoke additive and synergistic levels of cytotoxic anti-neoplastic potency when applied in dual combination with conventional chemotherapeutics and other anti-cancer modalities. Variations in biological characteristics between and within different types of neoplastic cell populations likely accounts for differential planes of cytotoxic anti-neoplatic potency achieved with individual covalent immunochemotherapeutics and other forms of anti-cancer therapy. However, the potential opportunities that exist with many if not most covalent immunochemotherapeutics to achieve additive or synergistic levels of efficacy represent a distinctly attractive therapeutic advantage.
Conceptually, there are at least five variables that can be modified to achieve higher total cytotoxic anti-neoplastic potency levels for epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu]. First, incubation times with mammary adenocarcinoma (SKBr-3) can be lengthened to periods >72 hours (Coyne et al., 2011) thereby allowing greater opportunity for larger amounts of epirubicin to be internalized by receptor-mediated-endocytosis and subsequent intracellular accumulation before and after liberation of the anthracycline moiety from epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu].
Second, cytotoxic anti-neoplastic potency can be evaluated utilizing neoplastic cell types that are not classified as chemotherapeutic-resistant analogous to those applied in majority of the investigations published to date that describe the efficacy of related covalent immunochemotherapeutics (Figures 3 thru 10). Few anthracycline-immunoconjugates have been reported to exert cytotoxic anti-neoplastic potency against chemotherapeutic (multi-drug) resistant neoplastic cell populations that is relatively greater than the same anthracycline in a “free” unbound form (Dillman et al., 1989). Relevant exceptions are; [i] covalent epirubicin-(C3-amide)-[anti-HER2/neu] (Coyne et al., 2009), epirubicin-(C3-amide)-[anti-EGFR] (Coyne et al., 2009) and epirubicin-(C13-imino)-[anti-HER2/neu] (Coyne et al., 2011b), against chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3); [ii] covalent anthracycline ligand-chemotherapeutics utilizing epidermal growth factor (EGF) or an EDF-fragment against chemotherapeutic-resistant mammary carcinoma MCF-7AdrR (Lutsenko Feldman & Severin 2002); and [iii] covalent daunorubicin immunochemotherapeutic synthesized using anti-chondroitin sulfate proteoglycan 9.2.27 surface marker evaluated for cytotoxic anti-neoplastic potency against the metastatic melanoma M21 cell type (Dillman et al., 1989; Yang et al., 1988a; Yang & Reisfeld 1988b).
Third, cytotoxic anti-neoplastic potency of covalent immunochemotherapeutics can be assessed by measuring cellular proliferation with either [H3]-thymidine, or an ATP-based assay method because of their reportedly ≥10-fold greater sensitivity in detecting early cell injury compared to MTT vitality stain based assay methods (Mueller, Kassack, & Wiese 2004; Ulukaya, Ozdikicioglu, Oral, & Dermirci 2008). Despite this consideration, MTT vitality stain continues to be extensively applied for the routine assessment of cytotoxic anti-neoplastic potency of chemotherapeutic agents in part because the detection of true cancer cell death is generally considered superior to the detection of reversible cellular injury (Dery, Van Themsche, Provencher, Mes-Masson, & Asselin 2007; Huang, Pierstorff, Osawa, & Ho 2007; Kars, Iseri, Gunduz, & Molnar 2008; Spee et al., 2006; Varache-Lembège, Larrouture, Montaudon, Robert, & Nuhrich 2008)
Fourth, cytotoxic anti-neoplastic potency can be delineated in-vivo utilizing human neoplastic xenographs in animal hosts as a neoplastic disease model where the efficacy of covalent immunochemotherapeutics frequently tends to be higher than in ex-vivo tissue culture models utilizing the same identical cancer cell type (Aboud-Pirak, Hurwitz, Bellot, Schlessinger, & Sela 1989; Johnson et al., 1995; Zhang, Wang, Li, Liu, & Dong 1992). Enhanced levels of covalent immunochemotherapeutic potency measured in-vivo that can not effectively be assessed in ex-vivo tissue culture is presumed to at least in part be dependent upon responses by the endogenous immune system through processes that include antibody-dependent cell cytotoxicity (ADCC) phenomenon in concert with complemented-mediated cytolysis activated by the formation of HER2/neu-immunoglobulin complexes on the exterior surface membrane of “targeted” neoplastic cells. Endogenous immune cell types involved in ADCC responses release cytotoxic mediators known to additively and synergistically enhance the cytotoxic anti-neoplastic activity of conventional chemotherapeutics (Coyne, Fenwick & Ainsworth 1997). The contributions of ADCC and complement-mediated cytolysis to the in-vivo cytotoxic anti-neoplastic potency of covalent anthracycline immunochemotherapeutics would be further complemented by the additive and synergistic properties attained with monoclonal immunoglobulin inhibitors of trophic receptors applied in dual combination with conventional chemotherapeutics (Ciardiello et al., 1999; Fry, Schilke, McGuire, & Bird 2010; García-Sáenz et al., 2008; Jin et al., 2010; Kim et al., 2006; Landriscina, Maddalena et al., 2010; Lynn et al., 2010; Pegram, Lopez, Konecny, & Slamon 2000; Slamon et al., 2001; Slamon & Pegram 2001; Winer & Burstein 2001; Zhang et al., 2002). Additive or synergistic interactions of this type have been detected with anti-HER2/neu in concert with cyclophosphamide, docetaxel, doxorubicin, etoposide, methotrexate, paclitaxel, or vinblastine (Pegram, Lopez, Konecny, & Slamon 2000; Slamon et al., 2001).
Fifth, the synthesis strategy for epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] could have been modified to increase the anthracycline molar-incorporation-index. Unfortunately, such modifications usually entail harsher synthesis conditions that impose a higher risk for declines in retained biological function and substantial reductions in final/total yield (Greenfield et al., 1990; Zhang et al., 1992). In addition to harsher synthesis conditions, excessively high molar incorporation indexes for anthracycline can (as previously discussed) also reduce the biological integrity of immunoglobulin fractions when the number of chemotherapeutic moieties covalently introduced into the Fab’ antigen-binding region becomes excessive. Such modifications can result in only modest declines in immunoreactivity (e.g. 86% for a 73:1 ratio) but disproportionate declines in anti-neoplastic potency with reductions in activity to levels that are substantially lower than those associated with non-conjugated “free” anthracycline (Zhang et al., 1992).
4.4 Cytotoxic Anti-Neoplastic Potency of Benzimidazoles
The benzimidazole anthelmintics exert a mechanism-of-action in neoplastic cells that is distinctly different, but similar to that of the vinca alkaloids (Spagnuolo et al., 2010) which involves binding to colchicine-sensitive sites on β-tubulin protein. Due to this mechanism-of-action, the benzimidazoles inhibit tubulin polymerization or tubulin de-polymerization resulting in a suppression of normal microtubule assembly and function necessary for mitosis (cell cycle M-phase). Coincident with a disruption of mitosis, benzimidazole tubulin/microtubule inhibitors are believed to induce apoptosis in neoplastic cells through a variety of pathways based upon detected elevations in Bcl-2 phosphorylation, caspase-3, caspase-8, caspase-9, cytochrome-C release, p53, DNA laddering phenomenon, and DNA fragmentation (TUNEL) (Doudican, Rodriguez, Osman, & Orlow 2008; Khalilzadeh, Wangoo, Morris, & Pourgholami 2007; Martarelli, Pompei, Baldi, & Mazzoni 2008; Pourgholami, Akhter, Wang, Lu, & Morris 2005; Sasaki et al., 2002). Declines in neoplastic cell growth and vitality induced by benzimidazole tubulin/microtubule inhibitors have been recognized as a function of alterations in parameters that reflect G2/M and G0–G1 arrest, decreased [3H]thymidine incorporation, spheroid cell formation, altered cell vitality staining intensity, and lower functional growth characteristics (Pourgholami et al., 2005; 2001; Sasaki et al., 2002; Martarelli et al., 2008). In neoplastic tissues, benzimidazole anthelmintics have been shown to reduce expression of CD31 (tumor angiogenesis biomarker), carcinoembryonic antigen (CEA: in-vivo), and α-feto protein (AFP: in-vivo); while also suppressing migration/invasion (in-vitro), metastasis (in-vivo), and tumor (in-vivo) growth kinetics (Martarelli et al., 2008; Mukhopadhyay, Sasaki, Ramesh & Roth 2002; Morris, Jourdan & Pourgholami 2001). Preliminary experimental investigations have detected adrenocortical carcinoma (xenographs), colorectal cancer, hepatocellular carcinoma, leukemia, lung cancer, (non-small cell), melanoma (chemo-resistant), myeloma, and ovarian cancer that are sensitive to benzimidazole tubulin/microtubule inhibitors (Martarelli et al., 2008; Morris, Jourdan & Pourgholami 2001; Khalilzadeh et al., 2007; Spagnuolo et al., 2010; Mukhopadhyay et al., 2002; Sasaki et al., 2002; Doudican et al., 2008; Pourgholami et al., 2001; 2005; 2009; 2010). The cytotoxic anti-neoplastic potency of the benzimidazole class of tubulin/microtubule inhibitors against breast cancer has previously remained largely unknown.
In chemotherapeutic-resistant human mammary adenocarcinoma (SKBr-3) the benzimidazoles, albendazole, mebendazole and flubendazole all demonstrated a degree of cytotoxic anti-neoplastic potency at a final concentration range between 0-to-2.5 μM (Figure 5). Previous descriptions have reported very similar results against other neoplastic cell types (Doudican et al., 2008; Khalilzadeh et al., 2007; Martarelli et al., 2008; Pourgholami et al 2005; Pourgholami et al., 2001; Sasaki et al., 2002; Spagnuolo et al., 2010). Flubendazole was the most potent benzimidazole while albendazole was substantially less potent than either flubendazole or mebendazole which closely correlates with their relative order of cytotoxic anti-neoplastic potency against leukemia and myeloma cell types (Figure 5) (Spagnuolo et al., 2010). In contrast to flubendazole, the creation of mammalian chromosomal aberrations has to date not been reported for either albendazole or mebendazole (Nianjun, Cerepnalkoski, Nwankwo, Dews, & Landolph 1994).
4.5 Cytotoxic Anti-Neoplastic Potency of Griseofulvin
The anti-fungal tubulin/microtubule inhibitor, griseofulfin and the organoselenium compound methylselenocysteine both exerted cytotoxic anti-neoplastic potency against mammary adenocarcinoma (SKBr-3) when formulated at final concentrations between 0-to-100 μM (Figure 6). Various organoselenium compounds including methylselenocysteine are known to exert cytotoxic anti-neoplastic properties against mammary adenocarcinoma/carcinoma (Coyne et al., 2011; Ip & Dong 2001; Johnson, Morrissey, Kapetanovic, Crowell, & McCormick 2008; Li et al., 2009; Medina, Thompson, Ganther, & Ip 2001) and other cancer cell types (Cao, Durrani, & Rustum 2004; Chintala et al., 2010) while also enhancing the potency of anthracyclines (Coyne et al., 2011; Juliger, Goenaga-Infante, Lister, Fitzgibbon, & Joel 2007; Li, Zhou, Dong & Ip C 2007; Li, Zhou, Wang, Zhang, Dong, & Ip 2007) and covalent epirubicin-immunochemotherapeutics (Coyne et al., 2011). Somewhat surprisingly the cytotoxic anti-neoplastic potency of griseofulvin against mammary adenocarcinoma (SKBr-3) was substantially greater than molar-equivalent (standardized) concentrations of methylselenocysteine (Figure 6). The final concentrations of 20 and 40 μM most prominently reflected this property but the difference was marked at 20 μM (Figure 7).
Similar to the benzimidazole tubulin/microtubule inhibitors, the mechanism-of-action for griseofulvin involves binding to tubulin protein and disruption of microtubule function resulting in an inhibition of normal mitosis (Rathinasamy et al., 2010). In neoplastic cells griseofulvin also promotes inhibition of centriole clustering, stabilization of microtubule dynamics, and G2/M arrest (Rathinasamy et al., 2010; Ho et al., 2001; Uen, Liu, Weng, Ho, & Lin, 2007). Failure of chromosomal division in turn induces tumor cell death but interestingly does not detectably influence normal healthy cell populations. Griseofulvin additionally stimulates p53 activation and induces apoptosis reflected by the detection of increases in DNA fragmentation (“laddering”), nuclear lamin alterations, accompanied by induced alterations in expression profiles for Cdc2 kinase, caspase-8, caspase-9, and changes in viability staining characteristics (Rathinasamy et al., 2010; Ho et al., 2001; Uen et al., 2007). Neoplastic cell types that clinical may be sensitive to griseofulvin include mammary carcinoma, cervical carcinoma, colorectal carcinoma, oral squamous cell carcinoma, hepatocellular carcinoma, osteosarcoma and myeloid leukemia (Panda, Rathinasamy, Santra, & Wilson, 2005; Rebacz et al., 2007; Ghadimi et al., 2000; Rønnest et al., 2009; Rathinasamy et al., 2010; Ho et al., 2001; Uen et al., 2007).
The benzimidazole anthelmintics and griseofulvin anti-fungal agent have a mechanism-of-action that is highly analogous to that of many conventional tubulin/microtubule inhibitor chemotherapeutics. Even though very little is known about the cytotoxic anti-neoplastic properties of benzimidazole anthelmintic and griseofulvin anti-fungal agents, their mechanism-of-action suggests that they can potentially suppress the growth and vitality of cancer cell populations both alone, and in multi-chemotherapeutic regimens similar to the vinca alkaloids, taxanes (e.g. paclitaxel), podophyllotoxins (e.g. etoposide) and monomethyl auristatin E (MMAE). The cytotoxic anti-neoplastic potency of epirubicin-(C3-amide)-[anti-HER2/neu] and epirubicin-(C3-amide)-SS-[anti-HER2/neu] formulated between the final epirubin-equivalent concentrations of 10−10 M to 10−6 M was markedly increased when applied in concert with mebendazole and griseofulvin (Figures 7 & 8). Mebendazole and griseofulvin evoked slightly higher levels of cytotoxic anti-neoplastic activity in dual combination with epirubicin-(C3-amide)-[anti-HER2/neu] than was detected with epirubicin-(C3-amide)-SS-[anti-HER2/neu] but the variable responsible for this trend remains unknown although it may possibly be related to variations in intracellular epirubicin bioavailability or differences in the lipophilic characteristics of different epirubicin metabolite analogs (Figures 7, 8, 9 & 10) (Erickson et al., 2010).
Capacity of the benzimidazole class of tubulin/microtubule inhibitors to evoke additive or synergistic levels of cytotoxic anti-neoplastic potency when applied in dual combination with conventional (e.g. vinblastine) (Spagnuolo et al., 2010) or selectively “targeted”/delivered chemotherapeutic agents has previously been very rarely delineated. Similarly, the potential for griseofulvin in combination conventional chemotherapeutics in multi-combination regimens to create additive or synergistic levels of cytotoxic anti-neoplastic potency has to date been investigated on a very limited scale. In preliminary investigatins, however, griseofulvin has been found to complement the anti-neoplastic properties of nocodazole (Ghadimi et al., 2000; Ho et al., 2001) and vinblastine (Rathinasamy et al., 2010).
Despite these voids in knowledge, multiple implications arise upon the acknowledgement that benzimidazole and griseofulvin tubulin/microtubule inhibitors can produce additive or synergistic cytotoxic anti-neoplastic potency when applied in dual combination with conventional or selectively “targeted” chemotherapeutics. Results observed with the benzimidazoles and griseofulvin tubulin/microtubule inhibitors indicate that they potentially have realistic utility as an alternative class of chemotherapeutic capable of providing “new” opportunities for achieving more potent long-term resolution of even the most resistant forms of breast cancer and other neoplastic disease states while simultaneously posing a lower risk of undesirable sequelae. In either a mono-therapy format or as a component of a combination multi-chemotherapeutic regimen these attributes can potentially be attained because benzimidazole and griseofulvin tubulin/microtubule inhibitors are apparently poor P-glycoprotein substrates (Khalilzadeh et al., 2007; Spagnuolo et al., 2010) and they have a relatively wider margin-of-safety than many if not most conventional chemotherapeutics (de Silva, Guyatt & Bundy 1997; Morris et al., 2001b; Pourgholami et al., 2005; 2010). The benzimidazole anthelmintics and griseofulvin when applied in additive and synergistic combinations with other chemotherapeutics or anti-cancer modalities can potentially add another level of safety because they are able to ultimately afford lower total dosage requirements. Presumably the highest levels of additive and synergistic cytotoxic anti-neoplastic potency and widest margin-of-safety can be attained when benzimidazole anthelmintics or griseofulvin are substituted for conventional tubulin/microtubule inhibitor chemotherapeutics in combination regimens that also apply covalent anthracyline-immunochemotherapeutics with properties of selective “targeted” delivery. Such considerations are critically important to the development of safer and more effective treatment regimens in order to reduce collateral cardiotoxicity (Danesi et al., 2006; Last et al., 2003; Nakano, Takeshige, Toshima, Tokunaga, & Minakami 1989) and nephroticity (Bulucu et al., 2008) that commonly limit systemic anthracycline administration.
5. Conclusion
Cardiotoxicity (doxorubicin ≫ epirubicin) (Danesi et al., 2006; Last et al., 2003; Nakano et al., 1989), nephroticity (Bulucu et al., 2008) and chemotherapeutic resistance represent complications that can commonly limit anthracycline administration in modern clinical oncology. The molecular design and methodology delineated for the synthetic production of covalent epirubicin-immunochemotherapeutics utilizing a UV-photoactivated anthracycline intermediate addresses a need to discover and optimize laboratory methods for the expedient production of anti-cancer therapies at higher end-product yields that possess properties of selective “targeted” delivery that complement the efficacy and potency of conventional and unconventional chemotherapeutics. In this context, selective “targeted” epirubicin delivery affords the opportunity to achieve cytosol concentrations that are greater than can be attained by simple passive diffusion, serve as a molecular mechanism for minimizing the impact of chemotherapeutic-resistance in many forms of neoplastic disease, and it serves as a way of minimizing chemotherapeutic diffusion into innocent tissues and organ systems (potential for a relatively wider margin-of-safety). Covalent anthracycline immunochemotherapeutics are relevant to the treatment of breast cancer and many other neoplastic conditions complicated by aggressive localized growth characteristics or have a high probability for metastasis and therapeutic resistance (Alexander, Greene, Torti & Kucera 2005). Preliminary laboratory analyses that detects and measures over-expression of trophic membrane receptors (e.g. HER2/neu, EGFR, VEGFR), proteins associated with chemotherapeutic resistance (e.g. P-glycorprotein, breast cancer type susceptibility protein/BRCA1 (Chekhun et al., 2009), and endocrine receptor profiles (e.g. estrogen, progesterone, testosterone) could be applied to account for biological variations and identify neoplastic conditions most effectively resolved with covalent immunochemotherapeutics like epirubicin-(C3-amide)-[anti-HER2/neu]. Base on these considerations, future investigations devoted to delineating the benefit of implementing epirubicin-(C3-amide)-[anti-HER2/neu] in the formulation of individualized treatment protocols is warranted.
Discovery of the cytotoxic anti-neoplastic potency of the benzimidazole anthelmintics and griseofulvin against mammary adenocarcinoma (SKBr-3) illustrates their potential applicability as an alternative class of tubulin/microtubule inhibitor chemotherapeutics and encourages support for future investigations devoted to determining their role in the development of new treatment regimens. Relevant attributes include a greater level of efficacy against certain chemotherapeutic-resistant neoplasias that over-express P-glycoprotein and the opportunity they provide to additively or synergistically complement the efficacy of conventional and selectively “targeted”/delivered chemotherapeutics. Such considerations are directly relevant to current trends and objectives in modern clinical oncology directed at identifying multi-treatment protocols with a wider margin-of-safety that more potently resolve locally aggressive, highly metastatic and resistant forms of cancer.
Acknowledgments
The authors would like to acknowledge Mr. Tom Thompson in the Office of Agriculture Communications for assistance with the autoradiography photographic illustration.
References
- Aboud-Pirak E, Hurwitz E, Bellot F, Schlessinger J, Sela M. Inhibition of human tumor growth in nude mice by a conjugate of doxorubicin with monoclonal antibodies to epidermal growth factor receptor. Proc Natl Acad Sci. 1989;86(10):3778–3781. doi: 10.1073/pnas.86.10.3778. http://dx.doi.org/10.1073/pnas.86.10.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RL, Greene BT, Torti SV, Kucera GL. A novel phospholipid gemcitabine conjugate is able to bypass three drug-resistance mechanisms. Cancer Chemother Pharmacol. 2005;56(1):15–21. doi: 10.1007/s00280-004-0949-0. http://dx.doi.org/10.1007/s00280-004-0949-0. [DOI] [PubMed] [Google Scholar]
- Annunziato ME, Patel US, Ranade M, Palumbo PS. p-Maleimidophenyl isocyanate: A novel heterbifunctional linker for hydroxyl to thiol coupling. Bioconjugate Chem. 1993;4:212–218. doi: 10.1021/bc00021a005. http://dx.doi.org/10.1021/bc00021a005. [DOI] [PubMed] [Google Scholar]
- Antón A, Barnadas A, Florián J, Ribelles N, Lomas M, Lao J, Ramos M. Biweekly vinorelbine and tegafur/uracil in patients with metastatic breast cancer previously treated with anthracyclines and taxanes: GEICAM 2000–02 phase II study. Clin Transl Oncol. 2011;13(4):281–286. doi: 10.1007/s12094-011-0654-5. http://dx.doi.org/10.1007/s12094-011-0654-5. [DOI] [PubMed] [Google Scholar]
- Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, Kohn EC. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26(22):3709–3714. doi: 10.1200/JCO.2007.10.8332. http://dx.doi.org/10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balayssac D, Ferrier J, Descoeur J, Ling B, Pezet D, Eschalier A, Authier N. Chemotherapy-induced peripheral neuropathies: from clinical relevance to preclinical evidence. Expert Opin Drug Saf. 2011;10(3):407–417. doi: 10.1517/14740338.2011.543417. http://dx.doi.org/10.1517/14740338.2011.543417. [DOI] [PubMed] [Google Scholar]
- Beyer U, Rothen-Rutishauser B, Unger C, Wunderli-Allenspach H, Kratz F. Difference in the intracellular distribution of acid-sensitive doxorubicin-protein conjugates in comparison to free and liposomal-formulated doxorubicin as shown by confocal microscopy. Pharm Res. 2001;18:29–38. doi: 10.1023/a:1011018525121. http://dx.doi.org/10.1023/A:1011018525121. [DOI] [PubMed] [Google Scholar]
- Boone JJ, Bhosle J, Tilby MJ, Hartley JA, Hochhauser D. Involvement of the HER2 pathway in repair of DNA damage produced by chemotherapeutic agents. Mol Cancer Ther. 2009;8(11):3015–3023. doi: 10.1158/1535-7163.MCT-09-0219. http://dx.doi.org/10.1158/1535-7163.MCT-09-0219. [DOI] [PubMed] [Google Scholar]
- Broyl A, Corthals SL, Jongen JL, van der Holt B, Kuiper R, de Knegt Y, Sonneveld P. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol. 2010;11(11):1057–1065. doi: 10.1016/S1470-2045(10)70206-0. http://dx.doi.org/10.1016/S1470-2045(10)70206-0. [DOI] [PubMed] [Google Scholar]
- Bulucu F, Oktenli C, Kenar L, Ocal R, Koc B, Inal V, Aydin A. Efficacy of deferoxamine, N-acetylcysteine and selenium treatments in rats with Adriamycin-induced nephrotic syndrome. J Nephrol. 2008;21(4):576–583. [PubMed] [Google Scholar]
- Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10(7):2561–2569. doi: 10.1158/1078-0432.ccr-03-0268. http://dx.doi.org/10.1158/1078-0432.CCR-03-0268. [DOI] [PubMed] [Google Scholar]
- Cameron DA, Stein S. Drug Insight: intracellular inhibitors of HER2–clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol. 2008;5(9):512–520. doi: 10.1038/ncponc1156. http://dx.doi.org/10.1038/ncponc1156. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, Tredici G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology. 2000;21(3):389–393. [PubMed] [Google Scholar]
- Chan A, Miles DW, Pivot X. Bevacizumab in combination with taxanes for the first-line treatment of metastatic breast cancer. Ann Oncol. 2010;21(12):2305–2315. doi: 10.1093/annonc/mdq122. http://dx.doi.org/10.1093/annonc/mdq122. [DOI] [PubMed] [Google Scholar]
- Chekhun VF, Zhylchuk VE, Lukyanova NY, Vorontsova AL, Kudryavets YI. Expression of drug resistance proteins in triple-receptor-negative tumors as the basis of individualized therapy of the breast cancer patients. Exp Oncol. 2009;31(2):123–124. [PubMed] [Google Scholar]
- Ceresa C, Cavaletti G. Drug transporters in chemotherapy induced peripheral neurotoxicity: current knowledge and clinical implications. Curr Med Chem. 2011;18(3):329–341. doi: 10.2174/092986711794839160. http://dx.doi.org/10.2174/092986711794839160. [DOI] [PubMed] [Google Scholar]
- Chang DZ, Olencki T, Budd GT, Peereboom D, Ganapathi R, Osterwalder B, Bukowski R. Phase I trial of capecitabine in combination with interferon alpha in patients with metastatic renal cancer: toxicity and pharmacokinetics. Cancer Chemother Pharmacol. 2001;48(6):493–498. doi: 10.1007/s002800100366. http://dx.doi.org/10.1007/s002800100366. [DOI] [PubMed] [Google Scholar]
- Chen FL, Xia W, Spector NL. Acquired resistance to small molecule ErbB2 tyrosine kinase inhibitors. Clin Cancer Res. 2008;14(21):6730–6734. doi: 10.1158/1078-0432.CCR-08-0581. http://dx.doi.org/10.1158/1078-0432.CCR-08-0581. [DOI] [PubMed] [Google Scholar]
- Chintala S, Tóth K, Cao S, Durrani FA, Vaughan MM, Jensen RL, Rustum YM. Se-methylselenocysteine sensitizes hypoxic tumor cells to irinotecan by targeting hypoxia-inducible factor 1alpha. Cancer Chemother Pharmacol. 2010;66(5):899–911. doi: 10.1007/s00280-009-1238-8. http://dx.doi.org/10.1007/s00280-009-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello F, Bianco R, Damiano V, De Lorenzo S, Pepe S, De Placido S, Tortora G. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5(4):909–916. [PubMed] [Google Scholar]
- Chamberlain MC, Tsao-Wei DD, Groshen S. Phase II trial of intracerebrospinal fluid etoposide in the treatment of neoplastic meningitis. Cancer. 2006;106(9):2021–2027. doi: 10.1002/cncr.21828. http://dx.doi.org/10.1002/cncr.21828. [DOI] [PubMed] [Google Scholar]
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- Coyne CP, Fenwick BW, Ainsworth J. Anti-neoplastic activity of chemotherapeutic “loaded” neutrophils against human mammary carcinoma. Biotherapy. 1997;10:145–159. doi: 10.1007/BF02678542. http://dx.doi.org/10.1007/BF02678542. [DOI] [PubMed] [Google Scholar]
- Coyne CP, Ross M, Bailey J, Jones T. Dual potency anti-HER2/neu and anti-EGFR anthracycline-immunoconjugates in chemotherapeutic-resistant mammary carcinoma combined with cyclosporin-A and verapamil P-glycoprotein inhibition. J Drug Targeting. 2009;17:474–489. doi: 10.1080/10611860903012802. http://dx.doi.org/10.1080/10611860903012802. [DOI] [PubMed] [Google Scholar]
- Coyne CP, Jones T, Pharr T. Synthesis of a covalent gemcitabine-(carbamate)-[anti-HER2/neu] immunochemotherapeutic and cytotoxic anti-neoplastic activity against chemotherapeutic-resistant SKBr-3 mammary carcinoma. Bioorganic and Medicinal Chemistry. 2011;19:67–76. doi: 10.1016/j.bmc.2010.11.046. http://dx.doi.org/10.1016/j.bmc.2010.11.046. [DOI] [PubMed] [Google Scholar]
- Coyne CP, Jones T, Sygula A, Bailey J, Pinchuk L. Epirubicin-[anti-HER2/neu] synthesized with an epirubicin-(C13-imino)-EMCS analog: Anti-neoplastic activity against chemotherapeutic-resistant SKBr-3 mammary carcinoma in combination with organic selenium. Journal of Cancer Therapy. 2011;2(1):22–39. doi: 10.4236/jct.2011.21004. http://dx.doi.org/10.4236/jct.2011.21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CP, Jones T, Bear R. Synthesis of epirubicin-(C3-amide)-[anti-HER2/neu] utilizing a UV-photoactivated epirubicin intermediate. Cancer Biotherapy and Radiopharmaceuticals. 2012;27:41–55. doi: 10.1089/cbr.2011.1097. http://dx.doi.org/10.1089/cbr.2011.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesi F, Malaguti M, Nunzio MD, Maranesi M, Biagi PL, Bordoni A. Counteraction of adriamycin-induced oxidative damage in rat heart by selenium dietary supplementation. J Agric Food Chem. 2006;54(4):1203–1208. doi: 10.1021/jf0518002. http://dx.doi.org/10.1021/jf0518002. [DOI] [PubMed] [Google Scholar]
- de Silva N, Guyatt H, Bundy D. Anthelmintics: a comparative review of their clinical pharmacology. Drugs. 1997;53(5):769–788. doi: 10.2165/00003495-199753050-00004. http://dx.doi.org/10.2165/00003495-199753050-00004. [DOI] [PubMed] [Google Scholar]
- Dempke W, Heinemann V. Ras mutational status is a biomarker for resistance to EGFR inhibitors in colorectal carcinoma. Anticancer Res. 2010;30(11):4673–4677. [PubMed] [Google Scholar]
- Dery MC, Van Themsche C, Provencher D, Mes-Masson AM, Asselin E. Characterization of EN-1078D, a poorly differentiated human endometrial carcinoma cell line: a novel tool to study endometrial invasion in-vitro. Reprod Biol Endocrinol. 2007;5:38–39. doi: 10.1186/1477-7827-5-38. http://dx.doi.org/10.1186/1477-7827-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbène S, Giorgi-Renault S. Drugs that inhibit tubulin polymerization: the particular case of podophyllotoxin and analogues. Curr Med Chem Anticancer Agents. 2002;2(1):71–90. doi: 10.2174/1568011023354353. http://dx.doi.org/10.2174/1568011023354353. [DOI] [PubMed] [Google Scholar]
- Diener E, Diner U, Sinha A, Xie S, Vergidis R. Specific immunosuppression by immunotoxins containing daunomycin. Science. 1986;231(4734):148–150. doi: 10.1126/science.3484557. http://dx.doi.org/10.1126/science.3484557. [DOI] [PubMed] [Google Scholar]
- Dillman RO, Johnson DE, Ogden J, Beidler D. Significance of antigen, drug, and tumor cell targets in the preclinical evaluation of doxorubicin, daunorubicin, methotrexate, and mitomycin-C monoclonal antibody immunoconjugates. Mol Biotherapy. 1989;1:250–255. [PubMed] [Google Scholar]
- Di Stefano G, Lanza M, Kratz F, Merina L, Fiume L. A novel method for coupling doxorubicin to lactosaminated human albumin by an acid sensitive hydrazone bond: synthesis, characterization. and preliminary biological properties of the conjugate. Eur J Pharm Sci. 2004;23:393–397. doi: 10.1016/j.ejps.2004.09.005. http://dx.doi.org/10.1016/j.ejps.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Dorsey JF, Dowling ML, Kim M, Voong R, Solin LJ, Kao GD. Modulation of the anti-cancer efficacy of microtubule-targeting agents by cellular growth conditions. Cancer Biol Ther. 2010;9(10):809–818. doi: 10.4161/cbt.9.10.11453. http://dx.doi.org/10.4161/cbt.9.10.11453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudican N, Rodriguez A, Osman I, Orlow SJ. Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol Cancer Res. 2008;6(8):1308–1315. doi: 10.1158/1541-7786.MCR-07-2159. http://dx.doi.org/10.1158/1541-7786.MCR-07-2159. [DOI] [PubMed] [Google Scholar]
- Dumontet C, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol. 1999;17(3):1061–1070. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, Blättler WA. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66(8):4426–4433. doi: 10.1158/0008-5472.CAN-05-4489. http://dx.doi.org/10.1158/0008-5472.CAN-05-4489. [DOI] [PubMed] [Google Scholar]
- Erickson HK, Widdison WC, Mayo MF, Whiteman K, Audette C, Wilhelm SD, Singh R. Tumor delivery and in-vivo processing of disulfide-linked and thioether-linked antibody-maytansinoid conjugates. Bioconjug Chem. 2010;21(1):84–92. doi: 10.1021/bc900315y. http://dx.doi.org/10.1021/bc900315y. [DOI] [PubMed] [Google Scholar]
- Escuin D, Burke PA, McMahon-Tobin G, Hembrough T, Wang Y, Alcaraz AA, Giannakakou P. The hematopoietic-specific beta1-tubulin is naturally resistant to 2-methoxyestradiol and protects patients from drug-induced myelosuppression. Cell Cycle. 2011;8(23):3914–3924. doi: 10.4161/cc.8.23.10105. http://dx.doi.org/10.4161/cc.8.23.10105. [DOI] [PubMed] [Google Scholar]
- Etrych T, Mrkvan T, Ríhová B, Ulbrich K. Star-shaped immunoglobulin-containing HPMA-based conjugates with doxorubicin for cancer therapy. J Control Release. 2007;122:31–38. doi: 10.1016/j.jconrel.2007.06.007. http://dx.doi.org/10.1016/j.jconrel.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Feldweg AM, Lee CW, Matulonis UA, Castells M. Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol. 2005;96(3):824–829. doi: 10.1016/j.ygyno.2004.11.043. http://dx.doi.org/10.1016/j.ygyno.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Finkelstein Y, Aks S, Hutson J, Juurlink D, Nguyen P, Dubnov-Raz G, Bentur Y. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol. 2010;48(5):407–414. doi: 10.3109/15563650.2010.495348. http://dx.doi.org/10.3109/15563650.2010.495348. [DOI] [PubMed] [Google Scholar]
- Frost SH, Jensen H, Lindegren S. In-vitro evaluation of avidin antibody pretargeting using [211At]-labeled and biotinylated poly-L-lysine as effector molecule. Cancer. 2010;116(4 Suppl):1101–1110. doi: 10.1002/cncr.24798. http://dx.doi.org/10.1002/cncr.24798. [DOI] [PubMed] [Google Scholar]
- Fry AK, Schilke KF, McGuire J, Bird KE. Synthesis and anticoagulant activity of heparin immobilized “end-on” to polystyrene microspheres coated with end-group activated polyethylene oxide. J Biomed Mater Res B Appl Biomater. 2010;94(1):187–195. doi: 10.1002/jbm.b.31640. [DOI] [PubMed] [Google Scholar]
- Furgeson DY, Dreher MR, Chilkoti A. Structural optimization of a “smart” doxorubicin-polypeptide conjugate for thermally targeted delivery to solid tumors. J Controled Release. 2006;110:362–369. doi: 10.1016/j.jconrel.2005.10.006. http://dx.doi.org/10.1016/j.jconrel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- García-Sáenz JA, Martín M, Calles A, Bueno C, Rodríguez L, Bobokova J, Díaz-Rubio E. Bevacizumab in combination with metronomic chemotherapy in patients with anthracycline- and taxane-refractory breast cancer. J Chemother. 2008;20(5):632–639. doi: 10.1179/joc.2008.20.5.632. [DOI] [PubMed] [Google Scholar]
- Ghadimi BM, Sackett DL, Difilippantonio MJ, Schröck E, Neumann T, Jauho A, Ried T. Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations. Genes Chromosomes Cancer. 2000;27(2):183–190. http://dx.doi.org/10.1002/(SICI)1098-2264(200002)27:2<183::AID-GCC10>3.0.CO;2-P. [PMC free article] [PubMed] [Google Scholar]
- Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen J, Song E. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem. 2011;286(21):19127–19137. doi: 10.1074/jbc.M110.216887. http://dx.doi.org/10.1074/jbc.M110.216887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. http://dx.doi.org/10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- Greenfield RS, Kaneko T, Daues A, Edson MA, Fitzgerald KA, Olech LJ, Braslawsky GR. Evaluation in-vitro of adriamycin immunoconjugates synthesized using an acid-sensitive hydrazone linker. Cancer Res. 1990;50(20):6600–6607. [PubMed] [Google Scholar]
- Hansen HJ, Ong GL, Diril H. Internalization and catabolism of radiolabeled antibodies to the MHC class-II invariant chain by B-cell lymphomas. Biochem J. 1996;320:293–300. doi: 10.1042/bj3200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CA, Ward RL, Dobbins TA, Drew AK, Pearson S. The efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysis. Ann Oncol. 2011;22(6):1308–1317. doi: 10.1093/annonc/mdq593. http://dx.doi.org/10.1093/annonc/mdq593. [DOI] [PubMed] [Google Scholar]
- Harrison M, Swanton C. Epothilones and new analogues of the microtubule modulators in taxane-resistant disease. Expert Opin Investig Drugs. 2008;17(4):523–546. doi: 10.1517/13543784.17.4.523. http://dx.doi.org/10.1517/13543784.17.4.523. [DOI] [PubMed] [Google Scholar]
- Hatzis C, Pusztai L, Valero V, Booser DJ, Esserman L, Lluch A, Symmans WF. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA. 2011;305(18):1873–1881. doi: 10.1001/jama.2011.593. http://dx.doi.org/10.1001/jama.2011.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Li R, Kanwar J, Zhou S. Structural and functional properties of human multidrug resistance protein 1 (MRP1/ABCC1) Curr Med Chem. 2011;18(3):439–481. doi: 10.2174/092986711794839197. http://dx.doi.org/10.2174/092986711794839197. [DOI] [PubMed] [Google Scholar]
- Hempel G, Schulze-Westhoff P, Flege S, Laubrock N. Therapeutic drug monitoring of doxorubicin in paediatric oncology using capillary electrophoresis. J Electrophoresis. 1998;19(16–17):2939–2943. doi: 10.1002/elps.1150191624. http://dx.doi.org/10.1002/elps.1150191624. [DOI] [PubMed] [Google Scholar]
- Herbert C, Norris K, Sauk JJ. Targeting of human squamous carcinomas by SPA470-doxorubicin immunoconjugates. J Drug Target. 2003;11(2):101–107. doi: 10.1080/1061186031000121478. http://dx.doi.org/10.1080/1061186031000121478. [DOI] [PubMed] [Google Scholar]
- Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, Crew KD. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125(3):767–774. doi: 10.1007/s10549-010-1278-0. http://dx.doi.org/10.1007/s10549-010-1278-0. [DOI] [PubMed] [Google Scholar]
- Herzig RH. High-dose etoposide and marrow transplantation. Cancer. 1991;67(1 Suppl):292–298. doi: 10.1002/1097-0142(19910101)67:1+<292::aid-cncr2820671314>3.0.co;2-7. http://dx.doi.org/10.1002/1097-0142(19910101)67:1+<292::AID-CNCR2820671314>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ho YS, Duh JS, Jeng JH, Wang YJ, Liang YC, Lin CH, Lin JK. Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int J Cancer. 2001;91(3):393–401. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1070>3.0.co;2-#. http://dx.doi.org/10.1002/1097-0215(200002)9999:9999<::AID-IJC1070>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Honig A, Rieger L, Sutterlin A, Kapp M, Dietl J, Sutterlin MW, Kämmerer U. Brain metastases in breast cancer–an in-vitro study to evaluate new systemic chemotherapeutic options. Anticancer Res. 2005;25(3A):1531–1537. [PubMed] [Google Scholar]
- Huang H, Pierstorff E, Osawa E, Ho D. Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett. 2007;7(11):3305–3314. doi: 10.1021/nl071521o. http://dx.doi.org/10.1021/nl071521o. [DOI] [PubMed] [Google Scholar]
- Hudis CA, Schmitz N. Dose-dense chemotherapy in breast cancer and lymphoma. Semin Oncol. 2004;31(3 Suppl 8):19–26. doi: 10.1053/j.seminoncol.2004.04.004. http://dx.doi.org/10.1053/j.seminoncol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Iarussi D, Indolfi P, Galderisi M, Bossone E. Cardiac toxicity after anthracycline chemotherapy in childhood. Herz. 2000;25(7):676–688. doi: 10.1007/pl00001982. http://dx.doi.org/10.1007/PL00001982. [DOI] [PubMed] [Google Scholar]
- Ip C, Dong Y. Methylselenocysteine modulates proliferation and apoptosis biomarkers in premalignant lesions of the rat mammary gland. Anticancer Res. 2001;21(2A):863–867. [PubMed] [Google Scholar]
- Jin R, Moreira Teixeira LS, Krouwels A, Dijkstra PJ, van Blitterswijk CA, Karperien M, Feijen J. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 2010;6(6):1968–1977. doi: 10.1016/j.actbio.2009.12.024. http://dx.doi.org/10.1016/j.actbio.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Briggs SL, Gutowski MC, Barton R. Anti-tumor activity of CC49-doxorubicin immunoconjugates. Anticancer Res. 1995;15(4):1387–1393. [PubMed] [Google Scholar]
- Johnson WD, Morrissey RL, Kapetanovic I, Crowell JA, McCormick DL. Subchronic oral toxicity studies of Se-methylselenocysteine, an organoselenium compound for breast cancer prevention. Food Chem Toxicol. 2008;46(3):1068–1078. doi: 10.1016/j.fct.2007.11.001. http://dx.doi.org/10.1016/j.fct.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliger S, Goenaga-Infante H, Lister TA, Fitzgibbon J, Joel SP. Chemosensitization of B-cell lymphomas by methylseleninic acid involves nuclear factor-kB inhibition and the rapid generation of other selenium species. Cancer Res. 2007;67(22):10984–10992. doi: 10.1158/0008-5472.CAN-07-0519. http://dx.doi.org/10.1158/0008-5472.CAN-07-0519. [DOI] [PubMed] [Google Scholar]
- Karacan O, Eyüboglu FO, Akçay S, Ozyilkan O. Acute interstitial pneumopathy associated with docetaxel hypersensitivity. Onkologie. 2004;27(6):563–565. doi: 10.1159/000081339. http://dx.doi.org/10.1159/000081339. [DOI] [PubMed] [Google Scholar]
- Karacay H, Sharkey RM, Govindan SV, McBride WJ, Goldenberg DM, Hansen HJ, Griffiths GL. Development of a streptavidin-anti-carcinoembryonic antigen antibody, radiolabeled biotin pretargeting method for radioimmunotherapy of colorectal cancer. Reagent development. Bioconjug Chem. 1997;8(4):585–594. doi: 10.1021/bc970102n. http://dx.doi.org/10.1021/bc970102n. [DOI] [PubMed] [Google Scholar]
- Kars MD, Iseri OD, Gunduz U, Molnar J. Reversal of multidrug resistance by synthetic and natural compounds in drug-resistant MCF-7 cell lines. Chemotherapy. 2008;54(3):194–200. doi: 10.1159/000140462. http://dx.doi.org/10.1159/000140462. [DOI] [PubMed] [Google Scholar]
- Kars MD, Iseri OD, Gündüz U. A microarray based expression profiling of paclitaxel and vincristine resistant MCF-7 cells. Eur J Pharmacol. 2011;657(1–3):4–9. doi: 10.1016/j.ejphar.2011.02.001. http://dx.doi.org/10.1016/j.ejphar.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Kellogg BA, Garrett L, Kovtun Y, Lai KC, Leece B, Miller M, Lutz RJ. Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjug Chem. 2011;22(4):717–727. doi: 10.1021/bc100480a. http://dx.doi.org/10.1021/bc100480a. [DOI] [PubMed] [Google Scholar]
- Khalilzadeh A, Wangoo KT, Morris DL. Pourgholami, M. H. Epothilone-paclitaxel resistant leukemic cells CEM/dEpoB300 are sensitive to albendazole: Involvement of apoptotic pathways. Biochem Pharmacol. 2007;74(3):407–414. doi: 10.1016/j.bcp.2007.05.006. http://dx.doi.org/10.1016/j.bcp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Kim S, Prichard CN, Younes MN, Yazici YD, Jasser SA, Bekele BN, Myers JN. Cetuximab and irinotecan interact synergistically to inhibit the growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice. Clin Cancer Res. 2006;12(2):600–607. doi: 10.1158/1078-0432.CCR-05-1325. http://dx.doi.org/10.1158/1078-0432.CCR-05-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Kim MJ, Kim HB, Lee JW, Bae JH, Kim DW, Kim SH. Suppression of multidrug resistance by treatment with TRAIL in human ovarian and breast cancer cells with high level of c-Myc. Biochim Biophys Acta. 2011;1812(7):796–805. doi: 10.1016/j.bbadis.2011.04.004. http://dx.doi.org/10.1016/j.bbadis.2011.04.004. [DOI] [PubMed] [Google Scholar]
- King HD, Yurgaitis D, Willner D, Firestone RA, Yang MB, Lasch SJ, Trail PA. Monoclonal antibody conjugates of doxorubicin prepared with branched linkers: A novel method for increasing the potency of doxorubicin immunoconjugates. Bioconjug Chem. 1999;10(2):279–288. doi: 10.1021/bc980100i. http://dx.doi.org/10.1021/bc980100i. [DOI] [PubMed] [Google Scholar]
- Kratz F, Warnecke A, Scheuermann K, Stockmar C, Schwab J, Lazar P, Unger C. Probing the cysteine-34 position of endogenous serum albumin with thiol-binding doxorubicin derivatives. Improved efficacy of an acid-sensitive doxorubicin derivative with specific albumin-binding properties compared to that of the parent compound. J Med Chem. 2002;45:5523–5533. doi: 10.1021/jm020276c. http://dx.doi.org/10.1021/jm020276c. [DOI] [PubMed] [Google Scholar]
- Kruger M, Beyer U, Schumacher P, Unger C, Zahn H, Kratz F. Synthesis and stability of four maleimide derivatives of the anti-cancer drug doxorubicin for the preparation of chemoimmunoconjugates. Chem Pharm Bull. 1997;45(2):399–401. http://dx.doi.org/10.1248/cpb.45.399. [Google Scholar]
- Kute TE, Savage L, Stehle JR, Kim-Shapiro JW, Blanks MJ, Wood J, Vaughn JP. Breast tumor cells isolated from in vitro resistance to trastuzumab remain sensitive to trastuzumab anti-tumor effects in vivo and to ADCC killing. Cancer Immunol Immunother. 2009;58(11):1887–1896. doi: 10.1007/s00262-009-0700-0. http://dx.doi.org/10.1007/s00262-009-0700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landriscina M, Maddalena F, Fabiano A, Piscazzi A, La Macchia O, Cignarelli M. Erlotinib enhances the proapoptotic activity of cytotoxic agents and synergizes with paclitaxel in poorly-differentiated thyroid carcinoma cells. Anticancer Res. 2010;30(2):473–480. [PubMed] [Google Scholar]
- Last KW, Cornelius V, Delves T, Sieniawska C, Fitzgibbon J, Norton A, Lister TA. Presentation serum selenium predicts for overall survival, dose delivery, and first treatment response in aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21(12):2335–2341. doi: 10.1200/JCO.2003.06.145. http://dx.doi.org/10.1200/JCO.2003.06.145. [DOI] [PubMed] [Google Scholar]
- Lau A, Bérubé G, Ford CH. Conjugation of doxorubicin to monoclonal anti-carcinoembryonic antigen antibody via novel thiol-directed cross-linking reagents. Bioorg Med Chem. 1995;3(10):1299–1304. doi: 10.1016/0968-0896(95)00125-z. http://dx.doi.org/10.1016/0968-0896(95)00125-Z. [DOI] [PubMed] [Google Scholar]
- Lau A, Berube G, Ford CHJ, Gallant M. Novel doxorubicin-monoclonal anti-carcinoembryonic antigen antibody immunoconjugate activity in-vivo. Bioorganic and Medicinal Chemistry. 1995;3:1305–1312. doi: 10.1016/0968-0896(95)00126-2. http://dx.doi.org/10.1016/0968-0896(95)00126-2. [DOI] [PubMed] [Google Scholar]
- Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol. 2009;102(3):179–187. doi: 10.1016/S1081-1206(10)60078-6. http://dx.doi.org/10.1016/S1081-1206(10)60078-6. [DOI] [PubMed] [Google Scholar]
- Levy M, Spino M, Read SE. Colchicine: a state-of-the-art review. Pharmacotherapy. 1991;11(3):196–211. [PubMed] [Google Scholar]
- Li S, Zhou Y, Wang R, Zhang H, Dong Y, Ip C. Selenium sensitizes MCF-7 breast cancer cells to doxorubicin-induced apoptosis through modulation of phospho-Akt and its downstream substrates. Mol Cancer Ther. 2007;6(3):1031–1038. doi: 10.1158/1535-7163.MCT-06-0643. http://dx.doi.org/10.1158/1535-7163.MCT-06-0643. [DOI] [PubMed] [Google Scholar]
- Li S, Zhou Y, Dong Y, Ip C. Doxorubicin and selenium cooperative induce Fas signaling in the absence of Fas/Fas ligand interaction. Anticancer Research. 2007;27:3075–3082. [PubMed] [Google Scholar]
- Li Z, Carrier L, Belame A, Thiyagarajah A, Salvo VA, Burow ME, Rowan BG. Combination of methylselenocysteine with tamoxifen inhibits MCF-7 breast cancer xenografts in nude mice through elevated apoptosis and reduced angiogenesis. Breast Cancer Res Treat. 2009;118(1):33–43. doi: 10.1007/s10549-008-0216-x. http://dx.doi.org/10.1007/s10549-008-0216-x. [DOI] [PubMed] [Google Scholar]
- Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, Winer EP. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26(12):1993–1999. doi: 10.1200/JCO.2007.12.3588. http://dx.doi.org/10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao H, Volk KJ, Klohr SE, Kerns EH, Lee MS. Analysis of monoclonal antibody and immunoconjugate digests by capillary electrophoresis and capillary liquid chromatography. J Chromatogr A. 1996;735:357–366. doi: 10.1016/0021-9673(95)01054-8. http://dx.doi.org/10.1016/0021-9673(95)01054-8. [DOI] [PubMed] [Google Scholar]
- Liu Q, de Wijn JR, van Blitterswijk CA. Covalent bonding of PMMA, PBMA, and poly(HEMA) to hydroxyapatite particles. J Biomed Mater Res. 1998;40(2):257–263. doi: 10.1002/(sici)1097-4636(199805)40:2<257::aid-jbm10>3.0.co;2-j. http://dx.doi.org/10.1002/(SICI)1097-4636(199805)40:2<257::AID-JBM10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Liu JH, Cao L, Luo PG, Yang ST, Lu F, Wang H, Sun YP. Fullerene-conjugated doxorubicin in cells. ACS Appl Mater Interfaces. 2010;2(5):1384–1389. doi: 10.1021/am100037y. http://dx.doi.org/10.1021/am100037y. [DOI] [PubMed] [Google Scholar]
- Liu F, Liu S, He S, Xie Z, Zu X, Jiang Y. Survivin transcription is associated with P-glycoprotein/MDR1 overexpression in the multidrug resistance of MCF-7 breast cancer cells. Oncol Rep. 2010;23(5):1469–1475. doi: 10.3892/or_00000786. [DOI] [PubMed] [Google Scholar]
- Loew S, Schmidt U, Unterberg A, Halatsch ME. The epidermal growth factor receptor as a therapeutic target in glioblastoma multiforme and other malignant neoplasms. Anticancer Agents Med Chem. 2009;9(6):703–715. doi: 10.2174/187152009788680019. http://dx.doi.org/10.2174/187152009788680019. [DOI] [PubMed] [Google Scholar]
- Lowe EJ, Sposto R, Perkins SL, Gross TG, Finlay J, Zwick D, Abromowitch M. Children’s Cancer Group Study 5941 Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: final results of Children’s Cancer Group Study 5941. Pediatr Blood Cancer. 2009;52(3):335–339. doi: 10.1002/pbc.21817. http://dx.doi.org/10.1002/pbc.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko SV, Feldman NB, Severin SE. Cytotoxic and antitumor activities of doxorubicin conjugates with the epidermal growth factor and its receptor-binding fragment. J Drug Target. 2002;10(7):567–571. doi: 10.1080/1061186021000038058. http://dx.doi.org/10.1080/1061186021000038058. [DOI] [PubMed] [Google Scholar]
- Lynn KD, Udugamasooriya DG, Roland CL, Castrillon DH, Kodadek TJ, Brekken RA. GU81, a VEGFR2 antagonist peptoid, enhances the anti-tumor activity of doxorubicin in the murine MMTV-PyMT transgenic model of breast cancer. BMC Cancer. 2010;10(397) doi: 10.1186/1471-2407-10-397. http://dx.doi.org/10.1186/1471-2407-10-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni M, Delfanti S, Rovati B, Grasso D, Mariucci S, Bencardino K, Tinelli C, Danova M. Chemotherapy-induced anemia in breast cancer patients treated with pegfilgrastim-supported dose-dense regimens. Clin Exp Med. 2010;10(2):135–138. doi: 10.1007/s10238-009-0072-y. http://dx.doi.org/10.1007/s10238-009-0072-y. [DOI] [PubMed] [Google Scholar]
- Marches R, Uhr JW. Enhancement of the p27Kip1-mediated antiproliferative effect of trastuzumab (Herceptin) on HER2-overexpressing tumor cells. Int J Cancer. 2004;112(3):492–501. doi: 10.1002/ijc.20378. http://dx.doi.org/10.1002/ijc.20378. [DOI] [PubMed] [Google Scholar]
- Martarelli D, Pompei P, Baldi C, Mazzoni G. Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice. Cancer Chemotherapy and Pharmacology. 2008;61(5):809–817. doi: 10.1007/s00280-007-0538-0. http://dx.doi.org/10.1007/s00280-007-0538-0. [DOI] [PubMed] [Google Scholar]
- Meden H, Beneke A, Hesse T, Novophashenny I, Wischnewsky M. Weekly intravenous recombinant humanized anti-P185HER2 monoclonal antibody (herceptin) plus docetaxel in patients with metastatic breast cancer: a pilot study. Anticancer Res. 2001;21(2B):1301–1305. [PubMed] [Google Scholar]
- Medina D, Thompson H, Ganther H, Ip C. Se-methylselenocysteine: a new compound for chemoprevention of breast cancer. Nutr Cancer. 2001;40(1):12–17. doi: 10.1207/S15327914NC401_5. http://dx.doi.org/10.1207/S15327914NC401_5. [DOI] [PubMed] [Google Scholar]
- Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30(8):1426–1447. doi: 10.1016/j.clinthera.2008.08.008. http://dx.doi.org/10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Meyer L, Patte-Mensah C, Taleb O, Mensah-Nyagan AG. Cellular and functional evidence for a protective action of neurosteroids against vincristine chemotherapy-induced painful neuropathy. Cell Mol Life Sci. 2010;67(17):3017–3034. doi: 10.1007/s00018-010-0372-0. http://dx.doi.org/10.1007/s00018-010-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra D, Brumlik MJ, Okamgba SU, Zhu Y, Duplessis TT, Parvani JG, Jones FE. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol Cancer Ther. 2009;8(8):2152–2162. doi: 10.1158/1535-7163.MCT-09-0295. http://dx.doi.org/10.1158/1535-7163.MCT-09-0295. [DOI] [PubMed] [Google Scholar]
- Modjtahedi H, Essapen S. Epidermal growth factor receptor inhibitors in cancer treatment: advances, challenges and opportunities. Anticancer Drugs. 2009;20(10):851–855. doi: 10.1097/CAD.0b013e3283330590. http://dx.doi.org/10.1097/CAD.0b013e3283330590. [DOI] [PubMed] [Google Scholar]
- Morgillo F, Kim W, Kim E, Ciardiello F, Hong W, Lee H. Implications of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13(9):2795–2803. doi: 10.1158/1078-0432.CCR-06-2077. http://dx.doi.org/10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of surviving expression counteract the anti-tumor action of Erlotinib. Cancer Res. 2006;66(20):10100–10111. doi: 10.1158/0008-5472.CAN-06-1684. http://dx.doi.org/10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- Morris DL, Jourdan JL, Pourgholami MH. Pilot study of albendazole in patients with advanced malignancy. Effect on serum tumor markers/high incidence of neutropenia. Oncology. 2001;61(1):42–46. doi: 10.1159/000055351. http://dx.doi.org/10.1159/000055351. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay T, Sasaki J, Ramesh R, Roth JA. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in-vitro and in-vivo. Clin Cancer Res. 2002;8(9):2963–2969. [PubMed] [Google Scholar]
- Mueller H, Kassack MU, Wiese M. Comparison of the usefulness of the MTT, ATP and calcein assays to predict the potency of cytotoxic agents in various human cancer cell lines. Biomolecular Screening. 2004;9(6):506–515. doi: 10.1177/1087057104265386. http://dx.doi.org/10.1177/1087057104265386. [DOI] [PubMed] [Google Scholar]
- Muldoon LL, Neuwelt EA. BR96-DOX immunoconjugate targeting of chemotherapy in brain tumor models. J Neurooncol. 2003;65(11):49–62. doi: 10.1023/a:1026234130830. http://dx.doi.org/10.1023/A:1026234130830. [DOI] [PubMed] [Google Scholar]
- Nakano E, Takeshige K, Toshima Y, Tokunaga K, Minakami S. Oxidative damage in selenium deficient hearts on perfusion with adriamycin: protective role of glutathione peroxidase system. Cardiovasc Res. 1989;23(6):498–504. doi: 10.1093/cvr/23.6.498. http://dx.doi.org/10.1093/cvr/23.6.498. [DOI] [PubMed] [Google Scholar]
- Nanda R. Targeting the human epidermal growth factor receptor 2 (HER2) in the treatment of breast cancer: recent advances and future directions. Rev Recent Clin Trials. 2007;2(2):111–116. doi: 10.2174/157488707780599375. http://dx.doi.org/10.2174/157488707780599375. [DOI] [PubMed] [Google Scholar]
- Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD. Maihle, N. J. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res. 2009;69(6):2191–2194. doi: 10.1158/0008-5472.CAN-08-1056. http://dx.doi.org/10.1158/0008-5472.CAN-08-1056. [DOI] [PubMed] [Google Scholar]
- Naumovski L, Junutula J. Glembatumumab vedotin, a conjugate of an anti-glycoprotein non-metastatic melanoma protein B mAb and monomethyl auristatin E for the treatment of melanoma and breast cancer. Curr Opin Mol Ther. 2010;12(2):248–257. [PubMed] [Google Scholar]
- Neskoviæ-Konstantinoviæ ZB, Bosnjak SM, Raduloviæ SS, Mitroviæ LB. Daily oral etoposide in metastatic breast cancer. Anticancer Drugs. 1996;7(5):543–547. doi: 10.1097/00001813-199607000-00009. http://dx.doi.org/10.1097/00001813-199607000-00009. [DOI] [PubMed] [Google Scholar]
- Nianjun H, Cerepnalkoski L, Nwankwo JO, Dews M, Landolph JR. Induction of chromosomal aberrations, cytotoxicity, and morphological transformation in mammalian cells by the antiparasitic drug flubendazole and the antineoplastic drug harringtonine. Fundam Appl Toxicol. 1994;22(2):304–313. http://dx.doi.org/10.1006/faat.1994.1034. [PubMed] [Google Scholar]
- O’Brien C, Cavet G, Pandita A, Hu X, Haydu L, Mohan S, Lackner MR. Functional genomics identifies ABCC3 as a mediator of taxane resistance in HER2-amplified breast cancer. Cancer Res. 2008;68(13):5380–5389. doi: 10.1158/0008-5472.CAN-08-0234. http://dx.doi.org/10.1158/0008-5472.CAN-08-0234. [DOI] [PubMed] [Google Scholar]
- Overmoyer B. Options for the treatment of patients with taxane-refractory metastatic breast cancer. Clin Breast Cancer. 2008;8(2):S61–S70. doi: 10.3816/cbc.2008.s.002. http://dx.doi.org/10.3816/CBC.2008.s.002. [DOI] [PubMed] [Google Scholar]
- Page M, Thibeault D, Noel C, Dumas L. Coupling a preactivated daunorubicin derivative to antibody. A new approach. Anticancer Research. 1990;10:353–357. [PubMed] [Google Scholar]
- Panda D, Rathinasamy K, Santra MK, Wilson L. Kinetic suppression of microtubule dynamic instability by griseofulvin: implications for its possible use in the treatment of cancer. Proc Natl Acad Sci USA. 2005;102(28):9878–9883. doi: 10.1073/pnas.0501821102. http://dx.doi.org/10.1073/pnas.0501821102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya K, Meeke K, Clementz A, Rogowski A, Roberts J, Miele L, Osipo C. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br J Cancer. 2011;105(6):796–806. doi: 10.1038/bjc.2011.321. http://dx.doi.org/10.1038/bjc.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan G, Gupta V, Huang J, Gu X, Liu YY. Direct assessment of P-glycoprotein efflux to determine tumor response to chemotherapy. Biochem Pharmacol. 2010;80(1):72–79. doi: 10.1016/j.bcp.2010.03.010. http://dx.doi.org/10.1016/j.bcp.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier J, Magal P, Boulangé-Lecomte C, Webb G, Foll F. Consequences of cell-to-cell P-glycoprotein transfer on acquired multidrug resistance in breast cancer: a cell population dynamics model. Biol Direct. 2011;(6):5. doi: 10.1186/1745-6150-6-5. http://dx.doi.org/10.1186/1745-6150-6-5. [DOI] [PMC free article] [PubMed]
- Pegram MD, Lopez A, Konecny G, Slamon DJ. Trastuzumab and chemotherapeutics: drug interactions and synergies. Semin Oncol. 2000;27(6 Suppl 11):21–25. (discussion 92-100) [PubMed] [Google Scholar]
- Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17(17):2235–2249. doi: 10.1038/sj.onc.1202132. http://dx.doi.org/10.1038/sj.onc.1202132. [DOI] [PubMed] [Google Scholar]
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Sliwkowski MX. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. http://dx.doi.org/10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- Pimm MV, Paul MA, Ogumuyiwa T, Baldwin RW. Biodistribution and tumour localization of a daunomycin-monoclonal antibody conjugate in nude mice and human tumour xenografts. Cancer Immunol Immunother. 1988;27(3):267–271. doi: 10.1007/BF00205450. http://dx.doi.org/10.1007/BF00205450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourgholami MH, Woon L, Almajd R, Akhter J, Bowery P, Morris DL. In-vitro and in-vivo suppression of growth of hepatocellular carcinoma cells by albendazole. Cancer Lett. 2001;165(1):43–49. doi: 10.1016/s0304-3835(01)00382-2. http://dx.doi.org/10.1016/S0304-3835(01)00382-2. [DOI] [PubMed] [Google Scholar]
- Pourgholami MH, Akhter J, Wang L, Lu Y, Morris DL. Antitumor activity of albendazole against the human colorectal cancer cell line HT-29: in-vitro and in a xenograft model of peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2005;55(5):425–432. doi: 10.1007/s00280-004-0927-6. http://dx.doi.org/10.1007/s00280-004-0927-6. [DOI] [PubMed] [Google Scholar]
- Pourgholami MH, Cai ZY, Wang L, Badar S, Links M, Morris DL. Inhibition of cell proliferation, vascular endothelial growth factor and tumor growth by albendazole. Cancer Invest. 2009;27(2):171–177. doi: 10.1080/07357900802210752. http://dx.doi.org/10.1080/07357900802210752. [DOI] [PubMed] [Google Scholar]
- Pourgholami MH, Cai ZY, Badar S, Wangoo K, Poruchynsky MS, Morris DL. Potent inhibition of tumoral hypoxia-inducible factor 1alpha by albendazole. BMC Cancer. 2010;(10):143. doi: 10.1186/1471-2407-10-143. http://dx.doi.org/10.1186/1471-2407-10-143. [DOI] [PMC free article] [PubMed]
- Pullarkat V, Slovak ML, Dagis A, Bedell V, Somlo G, Nakamura R, Forman SJ. Acute leukemia and myelodysplasia after adjuvant chemotherapy for breast cancer: durable remissions after hematopoietic stem cell transplantation. Ann Oncol. 2009;20(12):2000–2006. doi: 10.1093/annonc/mdp232. http://dx.doi.org/10.1093/annonc/mdp232. [DOI] [PubMed] [Google Scholar]
- Rak Tkaczuk KH. Ixabepilone as monotherapy or in combination with capecitabine for the treatment of advanced breast cancer. Breast Cancer: Basic and Clinical Research. 2011:1–14. doi: 10.4137/BCBCR.S5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschi E, Vasina V, Ursino MG, Boriani G, Martoni A, De Ponti F. Anticancer drugs and cardiotoxicity: Insights and perspectives in the era of targeted therapy. Pharmacol Ther. 2010;125(2):196–218. doi: 10.1016/j.pharmthera.2009.10.002. http://dx.doi.org/10.1016/j.pharmthera.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Rathinasamy K, Jindal B, Asthana J, Singh P, Balaji PV, Panda D. Griseofulvin stabilizes microtubule dynamics, activates p53 and inhibits the proliferation of MCF-7 cells synergistically with vinblastine. BMC Cancer. 2010;10:213. doi: 10.1186/1471-2407-10-213. http://dx.doi.org/10.1186/1471-2407-10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebacz B, Larsen TO, Clausen MH, Rønnest MH, Löffler H, Ho AD, Krämer A. Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer Res. 2007;67(13):6342–6350. doi: 10.1158/0008-5472.CAN-07-0663. http://dx.doi.org/10.1158/0008-5472.CAN-07-0663. [DOI] [PubMed] [Google Scholar]
- Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13(16):4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. http://dx.doi.org/10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- Rizzo R, Spaggiari F, Indelli M, Lelli G, Baricordi OR, Rimessi P, Ferlini A. Association of CYP1B1 with hypersensitivity induced by taxane therapy in breast cancer patients. Breast Cancer Res Treat. 2010;124(2):593–598. doi: 10.1007/s10549-010-1034-5. http://dx.doi.org/10.1007/s10549-010-1034-5. [DOI] [PubMed] [Google Scholar]
- Rønnest MH, Rebacz B, Markworth L, Terp AH, Larsen TO, Krämer A, Clausen MH. Synthesis and structure-activity relationship of griseofulvin analogues as inhibitors of centrosomal clustering in cancer cells. J Med Chem. 2009;52:3342–3347. doi: 10.1021/jm801517j. http://dx.doi.org/10.1021/jm801517j. [DOI] [PubMed] [Google Scholar]
- Sapra P, Stein R, Pickett J, Qu Z, Govindan SV, Cardillo TM, Goldenberg DM. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograph and in monkeys. Clin Cancer Res. 2005;11(14):5257–5264. doi: 10.1158/1078-0432.CCR-05-0204. http://dx.doi.org/10.1158/1078-0432.CCR-05-0204. [DOI] [PubMed] [Google Scholar]
- Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, Molinari F, De Dosso S, Saletti P, Siena S. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS ONE. 2009;4(10):e7287. doi: 10.1371/journal.pone.0007287. http://dx.doi.org/10.1371/journal.pone.0007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J, Ramesh R, Chada S, Gomyo Y, Roth JA, Mukhopadhyay T. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Mol Cancer Ther. 2002;1(13):1201–1209. [PubMed] [Google Scholar]
- Scaltriti M, Eichhorn PJ, Cortés J, Prudkin L, Aura C, Jiménez J, Baselga J. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci. 2011;108(9):3761–3766. doi: 10.1073/pnas.1014835108. http://dx.doi.org/10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully RE, Lipshultz SE. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc Toxicol. 2007;7(2):122–128. doi: 10.1007/s12012-007-0006-4. http://dx.doi.org/10.1007/s12012-007-0006-4. [DOI] [PubMed] [Google Scholar]
- Shen H, Lee FY, Gan J. Ixabepilone, a novel microtubule-targeting agent for breast cancer, is a substrate for P-glycoprotein (P-gp/MDR1/ABCB1) but not breast cancer resistance protein (BCRP/ABCG2) J Pharmacol Exp Ther. 2011;337(2):423–432. doi: 10.1124/jpet.110.175604. http://dx.doi.org/10.1124/jpet.110.175604. [DOI] [PubMed] [Google Scholar]
- Shih LB, Goldenberg DM, Xuan H, Lu HW, Mattes MJ, Hall TC. Internalization of an intact doxorubicin immunoconjugate. Cancer Immunol Immunother. 1994;38(2):92–98. doi: 10.1007/BF01526203. http://dx.doi.org/10.1007/BF01526203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkule JA, Rosen ST, Radosevich JA. Monoclonal antibody 44-3A6 doxorubicin immunoconjugates: comparative in vitro anti-tumor efficacy of different conjugation methods. Tumour Biol. 1991;12(4):198–206. doi: 10.1159/000217705. http://dx.doi.org/10.1159/000217705. [DOI] [PubMed] [Google Scholar]
- Sivam GP, Martin PJ, Reisfeld RA, Mueller BM. Therapeutic efficacy of a doxorubicin immunoconjugate in a preclinical model of spontaneous metastatic human melanoma. Cancer Res. 1995;55(11):2352–2356. [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. http://dx.doi.org/10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jone B, Shak S, Fuchs H, Paton V, Bajamonde A, Pegram M. Use of chemotherapy plus monoclonal antibody against HER2 for metastatic breast cancer that overexpress HER2. N Engl J Med. 2001;344(11):786–792. doi: 10.1056/NEJM200103153441101. http://dx.doi.org/10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Slamon D, Pegram M. Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol. 2001;28(1 Suppl 3):13–19. doi: 10.1016/s0093-7754(01)90188-5. http://dx.doi.org/10.1053/sonc.2001.22812. [DOI] [PubMed] [Google Scholar]
- Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26(4 Suppl 12):60–70. [PubMed] [Google Scholar]
- Spagnuolo PA, Hu J, Hurren R, Wang X, Gronda M, Sukhai MA, Schimmer AD. The antihelmintic flubendazole inhibits microtubule function through a mechanism distinct from Vinca alkaloids and displays preclinical activity in leukemia and myeloma. Blood. 2010;115(23):4824–4833. doi: 10.1182/blood-2009-09-243055. http://dx.doi.org/10.1182/blood-2009-09-243055. [DOI] [PubMed] [Google Scholar]
- Spee B, Jonkers MD, Arends B, Rutteman GR, Rothuizen J, Penning LC. Specific down-regulation of XIAP with RNA interference enhances the sensitivity of canine tumor cell-lines to TRAIL and doxorubicin. Mol Cancer. 2006;5:34. doi: 10.1186/1476-4598-5-34. http://dx.doi.org/10.1186/1476-4598-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan AC, Radu DL, Casares S, Bona CA, Brumeanu TD. Antineoplastic efficacy of doxorubicin enzymatically assembled on galactose residues of a monoclonal antibody specific for the carcinoembryonic antigen. Cancer Res. 1999;59:115–121. [PubMed] [Google Scholar]
- Stavridi F, Palmieri C. Efficacy and toxicity of nonpegylated liposomal doxorubicin in breast cancer. Expert Rev Anticancer Ther. 2008;8(12):1859–1869. doi: 10.1586/14737140.8.12.1859. http://dx.doi.org/10.1586/14737140.8.12.1859. [DOI] [PubMed] [Google Scholar]
- Sun X, Widdison W, Mayo M, Wilhelm S, Leece B, Chari R, Singh R, Erickson H. Design of antibody-maytansinoid conjugates allows for efficient detoxification via liver metabolism. Bioconjug Chem. 2011;22(4):728–735. doi: 10.1021/bc100498q. http://dx.doi.org/10.1021/bc100498q. [DOI] [PubMed] [Google Scholar]
- Svirnovski A, Shman T, Serhiyenka T, Savitski V, Smolnikova V, Fedasenka U. ABCB1 and ABCG2 proteins, their functional activity and gene expression in concert with drug sensitivity of leukemia cells. Hematology. 2009;14(4):204–212. doi: 10.1179/102453309X426218. http://dx.doi.org/10.1179/102453309X426218. [DOI] [PubMed] [Google Scholar]
- Tang SC. Strategies to decrease taxanes toxicities in the adjuvant treatment of early breast cancer. Cancer Invest. 2009;27(2):206–214. doi: 10.1080/07357900802178520. http://dx.doi.org/10.1080/07357900802178520. [DOI] [PubMed] [Google Scholar]
- Tang SC. Taxanes in the adjuvant treatment of early breast cancer, emerging consensus and unanswered questions. Cancer Invest. 2009;27(5):489–495. doi: 10.1080/07357900802427943. http://dx.doi.org/10.1080/07357900802427943. [DOI] [PubMed] [Google Scholar]
- Terkeltaub RA. Colchicine update: 2008. Semin Arthritis Rheum. 2009;38(6):411–419. doi: 10.1016/j.semarthrit.2008.08.006. http://dx.doi.org/10.1016/j.semarthrit.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Thorpe PE, Wallace PM, Knowles PP, Relf MG, Brown AN, Watson GJ, Blakey DC. New coupling agents for the synthesis of immunotoxins containing a hindered disulfide bond with improved stability in vivo. Cancer Res. 1987;47:5924–5931. [PubMed] [Google Scholar]
- Uen YH, Liu DZ, Weng MS, Ho YS, Lin SY. NF-kappaB pathway is involved in griseofulvin-induced G2/M arrest and apoptosis in HL-60 cells. J Cell Biochem. 2007;101(5):1165–1175. doi: 10.1002/jcb.21240. http://dx.doi.org/10.1002/jcb.21240. [DOI] [PubMed] [Google Scholar]
- Ulbrich K, Etrych T, Chytil P, Jelinkova M, Rihova B. HPMA copolymers with pH-controlled release of doxorubicin: In-vitro cytotoxicity and in-vivo antitumor activity. J Controlled Release. 2003;87:33–47. doi: 10.1016/s0168-3659(02)00348-6. http://dx.doi.org/10.1016/S0168-3659(02)00348-6. [DOI] [PubMed] [Google Scholar]
- Ulukaya E, Ozdikicioglu F, Oral AY, Dermirci M. The MTT assay yields a relatively lower result of growth inhibition than the ATP assay depending on the chemotherapeutic drug tested. Toxicol In Vitro. 2008;22(1):232–239. doi: 10.1016/j.tiv.2007.08.006. http://dx.doi.org/10.1016/j.tiv.2007.08.006. [DOI] [PubMed] [Google Scholar]
- VanderWeele D, Zhou R, Rudin C. Akt up-regulation increases resistance to microtubule-directed chemotherapeutic agents through mammalian target of rapamycin. Mol Cancer Ther. 2004;3(12):1605–1613. [PubMed] [Google Scholar]
- van Tellingen O, Buckle T, Jonker J, van der Valk M, Beijnen J. P-glycoprotein and Mrp1 collectively protect the bone marrow from vincristine-induced toxicity in-vivo. Br J Cancer. 2003;89(9):1776–1782. doi: 10.1038/sj.bjc.6601363. http://dx.doi.org/10.1038/sj.bjc.6601363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantelon JM, Munck JN, Bourhis JH, Pico JL, Fadel C, Ulusakarya A, Ribrag V. Thrombotic microangiopathy: a new dose-limiting toxicity of high-dose sequential chemotherapy. Bone Marrow Transplant. 2001;27(5):531–536. doi: 10.1038/sj.bmt.1702812. http://dx.doi.org/10.1038/sj.bmt.1702812. [DOI] [PubMed] [Google Scholar]
- Varache-Lembège M, Larrouture S, Montaudon D, Robert J, Nuhrich A. Synthesis and antiproliferative activity of aryl- and heteroaryl-hydrazones derived from xanthone carbaldehydes. Eur J Med Chem. 2008;43(6):1336–1343. doi: 10.1016/j.ejmech.2007.09.003. http://dx.doi.org/10.1016/j.ejmech.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. http://dx.doi.org/10.1200/JCO.20.3.719. [DOI] [PubMed] [Google Scholar]
- Wachters FM, Van Der Graaf WT, Groen HJ. Cardiotoxicity in advanced non-small cell lung cancer patients treated with platinum and non-platinum based combinations as first-line treatment. Anticancer Res. 2004;24(3b):2079–2083. [PubMed] [Google Scholar]
- Wang XL, Huang Y, Zhu J, Pan YB, He R, Wang YZ. Chitosan-graft poly(p-dioxanone) copolymers: preparation, characterization, and properties. Carbohydrate Res. 2009;344(6):801–807. doi: 10.1016/j.carres.2009.02.009. http://dx.doi.org/10.1016/j.carres.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Weickhardt A, Tebbutt N, Mariadason J. Strategies for overcoming inherent and acquired resistance to EGFR inhibitors by targeting downstream effectors in the RAS/PI3K pathway. Curr Cancer Drug. 2010;10(8):824–833. doi: 10.2174/156800910793357961. http://dx.doi.org/10.2174/156800910793357961. [DOI] [PubMed] [Google Scholar]
- Widakowich C, Dinh P, de Azambuja E, Awada A, Piccart-Gebhart M. HER-2 positive breast cancer: what else beyond trastuzumab-based therapy? Anticancer Agents Med Chem. 2008;8(5):488–496. doi: 10.2174/187152008784533062. [DOI] [PubMed] [Google Scholar]
- Windebank AJ. Chemotherapeutic neuropathy. Curr Opin Neurol. 1999;12(5):565–571. doi: 10.1097/00019052-199910000-00010. http://dx.doi.org/10.1097/00019052-199910000-00010. [DOI] [PubMed] [Google Scholar]
- Winer EP, Burstein HJ. New combinations with Herceptin in metastatic breast cancer. Oncology. 2001;61(Suppl 2):50–57. doi: 10.1159/000055402. http://dx.doi.org/10.1159/000055402. [DOI] [PubMed] [Google Scholar]
- Wong MY, Chiu GN. Simultaneous liposomal delivery of quercetin and vincristine for enhanced estrogen-receptor-negative breast cancer treatment. Anti-Cancer Drugs. 2010;21(4):401–410. doi: 10.1097/CAD.0b013e328336e940. http://dx.doi.org/10.1097/CAD.0b013e328336e940. [DOI] [PubMed] [Google Scholar]
- Worrell NR, Cumber AJ, Parnell GD, Mirza A, Forrester J, Ross WC. Effect of linkage variation on pharmacokinetics of ricin A chain-antibody conjugates in normal rats. Anticancer Drug Des. 1986;1:179–188. [PubMed] [Google Scholar]
- Yang HM, Reisfeld RA. Doxorubicin conjugated with monoclonal antibody directed to a human melanoma-associated proteoglycan suppresses growth of established tumor xenografts in nude mice. Proc Natl Acad Sci. 1988a;85:1189–1193. doi: 10.1073/pnas.85.4.1189. http://dx.doi.org/10.1073/pnas.85.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HM, Reisfeld RA. Pharmacokinetics and mechanism of action of a doxorubicin-monoclonal antibody 9.2.27 conjugate directed to a human melanoma proteoglycan.Preview. J Natl Cancer Inst. 1988b;80(14):1154–1159. doi: 10.1093/jnci/80.14.1154. http://dx.doi.org/10.1093/jnci/80.14.1154. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang N, Li N, Liu T, Dong Z. The antitumor effect of adriamycin conjugated with monoclonal antibody against gastric cancer in-vitro and in-vivo. Acta Pharmaceutica Sinica. 1992;27:325–330. [PubMed] [Google Scholar]
- Zhang L, Yu D, Hicklin DJ, Hannay JA, Ellis LM, Pollock RE. Combined anti-fetal liver kinase 1 monoclonal antibody (anti-VEGFR) and continuous low-dose doxorubicin inhibits angiogenesis and growth of human soft tissue sarcoma xenografts by induction of endothelial cell apoptosis. Cancer Res. 2002;62(7):2034–2042. [PubMed] [Google Scholar]


