Abstract
Previously we have shown that the improvement of cold tolerance by theophylline is due to antagonism at adenosine receptors rather than inhibition of phosphodiesterase. Since theophylline is a nonselective adenosine receptor antagonist for both A1 and A2 receptors, the present study investigated the adenosine receptor subtype involved in theophylline’s action. Acute systemic injection of selective A1 receptor antagonists (1,3-dialkyl-8-aryl or 1,3-dialkyl-8-cyclopentyl xanthine derivatives) significantly increased both the total and maximal heat production as well as cold tolerance. In contrast, injection of a relatively selective A2 receptor antagonist, 3,7-dimethyl-1-propargylxanthine (compound No. 19), failed to significantly alter the thermogenic response of the rat under cold exposure. Further, the relative effectiveness of these compounds in increasing total thermogenesis was positively correlated with their potency in blocking the A1 adenosine receptor (r= .52, p<0.01), but not in A2 adenosine receptor (r= .20, p<0.2). It is likely that the thermally beneficial effects of adenosine A1 antagonists are due to their attenuation of the inhibitory effects of endogenously released adenosine on lipolysis and glucose utilization, resulting in increased substrate mobilization and utilization for enhanced thermogenesis.
Keywords: Adenosine Thermogenesis; Cold tolerance; Adenosine receptors; 1,3,7-Substituted xanthine derivatives; 1,3,8-Substituted xanthine derivatives
IN quest of how cold tolerance can be improved to avoid life-threatening hypothermia, our animal (rat) studies have shown that a timely supply of metabolizable fuels is critical in eliciting the full aerobic capacity for heat production (22). Enhancing fuel availability by treatment with aminophylline (AMPY; 85% theophylline, 15% ethylenediamine) resulted in increased thermogenesis and significantly improved cold resistance and prevented the occurrence of hypothermia in severe cold (23,24). The effectiveness of AMPY in improving cold resistance has recently been extended to clinical trials. AMPY, alone or in combination with exogenous substrate (Ensure, a nutrition supplement containing glucose, protein and fat) supplies, also enhanced cold tolerance in man (27,28).
Although it has been clearly demonstrated that theophylline (THEO) is effective in improving cold tolerance in both animals and men, the precise cellular and molecular mechanisms via which its effect is manifested are currently unknown. Although classically THEO has been thought of as a phosphodiesterase inhibitor, this view has been seriously challenged recently (16). It is now recognized that THEO elicits many of its effects through antagonism of adenosine at A1 and A2 adenosine receptors (16). This scheme proposes that adenosine is formed as an end product of ATP hydrolysis following physiological stimulation (e.g., adrenergic or local hypoxia); it is released into the extracellular space and binds to adenosine receptors on the cell surface to modulate various physiological functions. Adenosine has been shown to be a potent antilipolytic agent both in vitro (10,12) and in vivo (11,18). In addition, this purine nucleoside has also been shown to reduce the sensitivity of insulin-stimulated glucose utilization in the soleus muscle (2). Therefore, the combined effects of endogenously produced adenosine could result in a decrease of both substrate mobilization and utilization, leading to reductions in both shivering and nonshivering thermogenesis and reduced cold tolerance. Conversely, the use of an adenosine antagonist, such as THEO, could result in improved cold tolerance. In support of this scheme, we have shown that 1) pretreatment with a specific adenosine receptor antagonist (8-phenyltheophylline), but not a phosphodiesterase inhibitor (enprofylline), significantly enhanced thermogensis and improved cold resistance similar to the improvement after THEO pretreatment (26); and 2) pretreatment with adenosine deaminase, which attenuates adenosine’s effects by converting adenosine into inosine, caused similar increases in heat production and cold tolerance (25).
Based on the above results, it is probable that the effectiveness of THEO in improving thermogenesis and cold tolerance is via the antagonism of adenosine. It has been well-documented that there are two different types of adenosine receptor, A1 and A2. Depending on the specificity and affinity of the agonist, different physiological responses can be elicited by the activation of different types of adenosine receptor [for reviews see (4,19)]. Since THEO is a competitive antagonist with about equal affinity for A1 and A2 receptors [Ki = 14 µM for both; (6)], it is, therefore, uncertain via which receptor type THEO elicits its beneficial effects. The answer to this is essential, since once realized, the design and selection of a specific compound for enhancing thermogenesis and cold tolerance may be greatly facilitated.
METHOD
Male Sprague-Dawley rats, 4–5 months old, were used. The animals were housed individually in polycarbonate cages with wood shaving bedding at 22 ± 1°C in a walk-in environmental chamber under 12 L:12 D photoperiod. Water was made available at all times. In order to maintain a relatively constant body weight throughout the whole experimental period, food (Rodent Blox, consisting of 24% protein, 4% fat, 65% carbohydrate, 4.5% fiber and vitamins; Wayne Lab. Animal Diets, Chicago, IL) was rationed daily after the body weight had reached about 400 g. Rats were divided into groups and each group of 6–8 rats was assigned for one tested compound. Either vehicle or various doses of the tested compound was randomized in each animal in a self-control design.
The protocol for acute cold exposure was similar to that described earlier (22). Briefly, the animal was removed from its home cage, placed in a metal metabolism chamber and exposed to −10°C under helium-oxygen (79% He–21% O2) for 120 min. Helium-oxygen (1.5 l/min, STP) was used to facilitate heat loss. Exhaust gas from the metabolic chamber was divided into two streams; one stream was for oxygen measurement by an oxygen analyzer (Applied Electrochemistry Model S-3) following drying by Drierite and CO 2 removal by Ascarite. The second stream was only dried for measurement of CO2 by an Applied Electrochemistry CD-2 analyzer. Oxygen consumption and CO2 production were recorded simultaneously and continuously and integrated by an on-line computerized data acquisition system. Heat production (HP) was calculated from oxygen consumption and respiratory quotient using Kleiber’s equation (22). The integrated HP for each 15-min period was used as the rate of HP. The sum of HP from 7 consecutive 15-min periods (min 16–120) constituted the total HP and the highest rate of any one 15-min period was used as the maximum HP. The colonic temperature (6 cm from anus) (Tb) was measured with a thermocouple thermometer (BAT-12, Bailey Inst.) immediately before vehicle or tested compound injection and immediately after cold exposure. The change in Tb was used as an index for cold tolerance. Either vehicle or tested compound was administered IP in a volume of 1 ml/kg 15 min prior to cold exposure. To avoid habituation and possible acclimation, at least 2 weeks was allowed between successive cold exposures in the same animal.
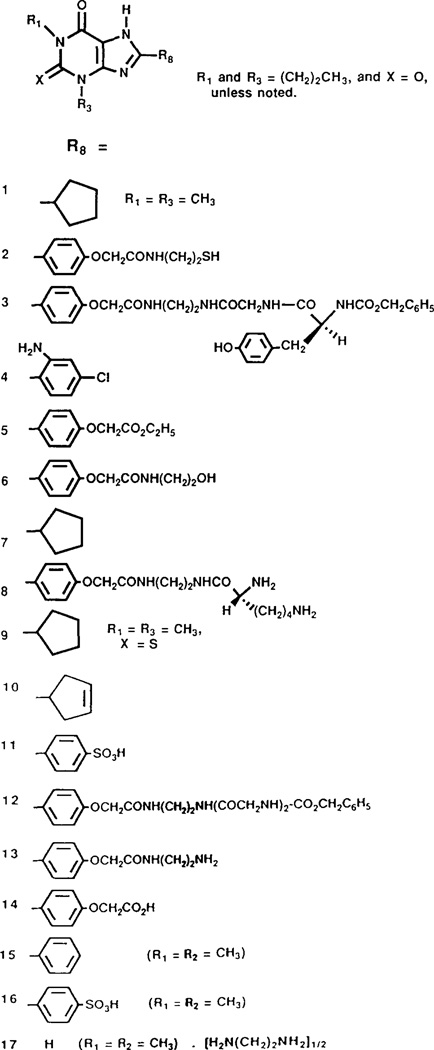
The compounds used in the present study are shown in Figs. 1 and 2. Most of the compounds were dissolved in dimethyl sulfoxide, except compounds No. 1 and No. 11 which were dissolved in physiological saline. The control animals received the same vehicle for comparison. Statistical analysis was by Wilcox-on’s Signed Ranks test for comparisons of treatment effect in individuals of the same group. Significance was set at p<0.05 unless otherwise stated.
FIG. 1.
Structures of 1,3,8-substituted xanthine derivatives used in the present study. No. 1: 1,3-cyclopentyl-theophylline, No. 2: 1,3-dipropyl-8-(4-((2-thioethyl)amino)-carbonylmethyloxyphenyl)xanthine, No. 3: Z-L-Tyr-Gly-XAC, No. 4: 1,3-dipropyl-8-(2-amino-4-chlorophenyl)-xanthine (PACPX), No. 5: 8-[4-[carboxymethyloxy]phenyl]-l,3-dipropylxanthine (XCC-OEt), No. 6: 1,3-dipropyl-8-(4-((2-hydroxyethyl)amino)-carbonyl-methyloxyphenyl) xanthine, No. 7: 1,3-dipropyl-8-cyclopentyl-xanthine, No. 8: D-Lys-XAC, No. 9: 8-cyclopentyl-2-thio-theophylline, No. 10: 8-(3,4-dehydro-cyclopentyl)-1,3-dipropylxanthine, No. 11 : 1,3-dipropyl-8-p-sulfophenyl-xanthine, No. 12: Z-GIy2-XAC, No. 13: 1,3-dipropyl-8-(4-((2-aminoethyl)amino)-carbonylmethyloxyphenyl)-xanthine (XAC), No. 14: 1,3-dipropyl-8-(4-carboxymethyloxyphenyl)-xanthine (XCC), No. 15: 8-phenyltheophylline, No. 16: 8-p-sulfophenyl-theophylline, No. 17: aminophylline.
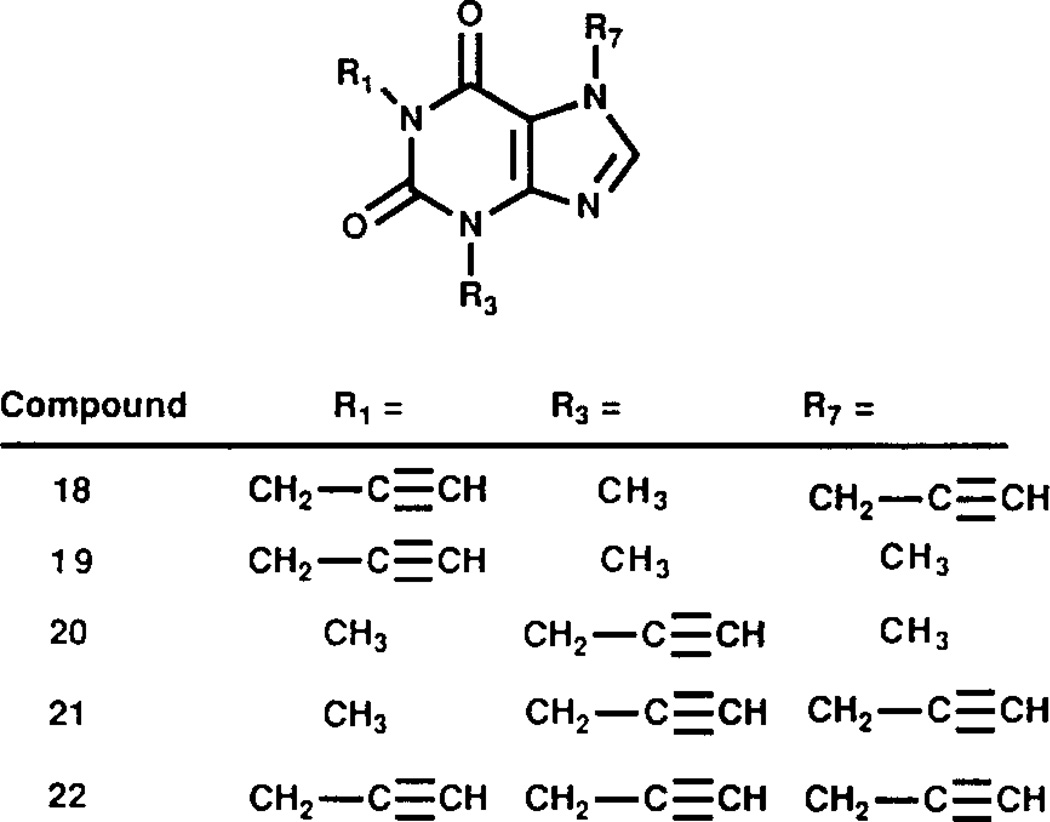
FIG. 2.
Structures of 1,3,7-substituted xanthine derivatives used in the present study. No. 18: 1,7-dipropargyl-3-methylxanthine, No. 19: 3,7-dimethyl-l-propargylxanthine, No. 20: 1,7-dimethyl-3-propargylxanthine, No. 21: 3,7-dipropargyl-l-methylxanthine, No. 22: 1,3,7-tripropargylxanthine.
RESULTS
Effects of 1,3,8-Xanthine Derivatives on Cold Tolerance
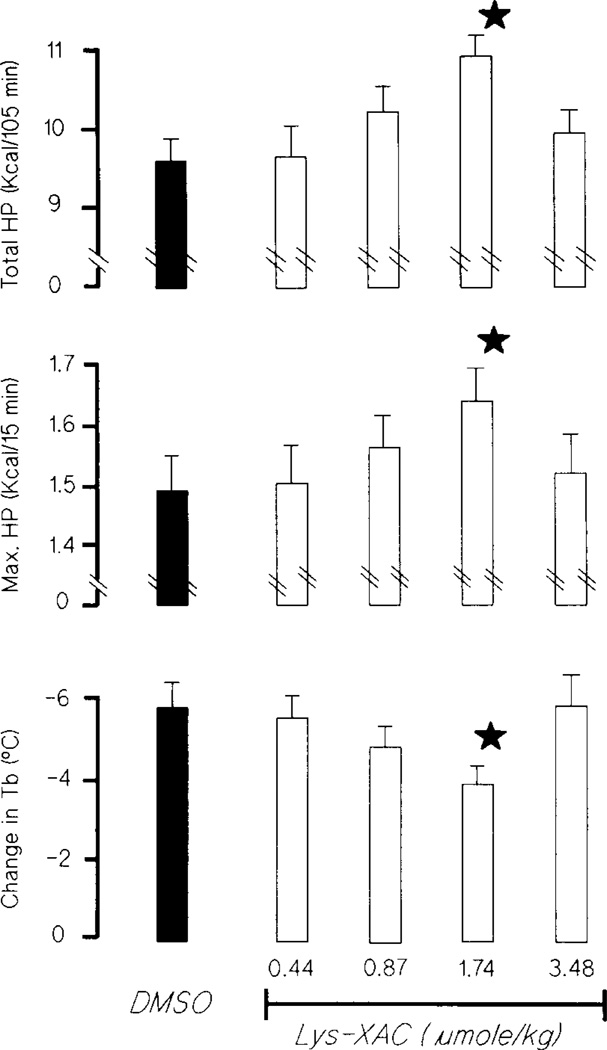
Most of the 1,3,8-xanthine derivatives tested, except compound Nos. 5, 10, 13, and 14, were found to significantly improve the cold tolerance of the rat under cold exposure. To illustrate this, the mean absolute increases in maximal and total HP and the changes of Tb in rats receiving vehicle or various doses of D-Lys-XAC (compound No. 8) are shown in Fig. 3. In comparison to vehicle control, both the total and maximal thermogenesis of the rats were significantly enhanced by 13.9 and 8.7% above control values, respectively, after receiving 1.74 µmole/kg D-Lys-XAC. The decrease of final Tb was also significantly less after this treatment. Further increasing the dose of D-Lys-XAC, up to 3.48 µmole/kg, caused a reversal of maximal and total HP as well as the final Tb towards the control values. This “nverted-U”-shape of dose-response curve on thermogenesis was observed for all the other adenosine receptor antagonists used in the present study.
FIG. 3.
Effects of systemic injections of either vehicle (closed columns) or various doses of D-Lys-XAC (open columns) on total and maximum heat production and final body temperature in rats exposed to HeO2 at −10°C. Vertical bars are mean±s.e., n=8 in all cases. ⋆Indicate p<0.05 by Wilcoxon’s Signed Rank test.
To evaluate the potency of different xanthine derivatives on improving cold tolerance, the optimal dose of each xanthine derivative in eliciting an increase in thermogenic response and the percentage of increase in total heat production above the control value were compared and the results are summarized in Table 1. When the optimal dose was compared, compound No. 1 appeared to be the most potent antagonist (0.004 µmole/kg), whereas compound No. 17 was the least potent (55.5 µmole/kg). However, compound No. 8 appeared to be the most effective antagonist in increasing total heat production (14%).
TABLE 1.
EFFECTS OF VARIOUS 1,3,8-XANTHINE DERIVATIVES IN INCREASING THE TOTAL HEAT PRODUCTION IN RATS UNDER COLD EXPOSURE
| Compound No. | Test Dose Range (µmole/kg) |
Most Effective Dose (µmole/kg) |
% Increase in HP Above Control |
|---|---|---|---|
| 1 | 0.0008–0.02 | 0.004 | 10* |
| 2 | 0.14–2.25 | 0.28 | 10* |
| 3 | 0.40–3.20 | 0.80 | 9* |
| 4 | 0.42–1.66 | 0.83 | 11* |
| 5 | 0.46–1.82 | 0.91 | 5 |
| 6 | 0.73–5.84 | 1.46 | 9* |
| 7 | 0.82–3.28 | 1.64 | 9* |
| 8 | 0.87–3.48 | 1.74 | 14* |
| 9 | 0.89–3.56 | 1.78 | 12* |
| 10 | 1.03–8.26 | 2.07 | 6 |
| 11 | 1.59–6.37 | 3.19 | 12* |
| 12 | 0.92–7.39 | 3.69 | 12* |
| 13 | 2.92–11.67 | 5.83 | 7 |
| 14 | 3.23–12.94 | 6.47 | 6 |
| 15 | 9.75–78.0 | 19.50 | 10* |
| 16 | 6.70–26.8 | 13.42 | 10* |
| 17 | 13.87–208.14 | 55.50 | 13* |
All compounds were tested at least with 3 different doses and with at least 6 animals per group. Compounds number are corresponding to those listed in Fig. 1.
Significantly different from appropriate vehicle control group. Statistical analysis was based on the absolute values of HP obtained after vehicle and drug treatment. As each compound was tested in self-control design, the effectiveness of each compound was thus compared in terms of % increase over control value.
Effects of 1,3, 7-Xanthine Derivatives on Cold Tolerance
Table 2 summarizes the effect of 1,3,7-xanthine derivatives on total HP of rats exposed to He-O2 and cold. After pretreating with a 1,3,7-xanthine derivative, the maximum increase in total heat production was only about 5–6%, which did not achieve any statistical significance beyond the control values. Consequently, the final Tb of the drug-treated rat was about the same as its control value. Further increasing the doses of compounds Nos. 21 and 22 beyond the highest dose listed in Table 2 markedly suppressed the thermogenic capacity of the rat and the experiment had to be terminated prematurely to avoid irreversible hypothermia (results not shown).
TABLE 2.
EFFECTS OF VARIOUS 1,3,7-XANTHINE DERIVATIVES IN INCREASING THE TOTAL HEAT PRODUCTION IN RATS UNDER COLD EXPOSURE
| Compound No. | Test Dose Range (µmole/kg) |
Most Effective Dose (µmole/kg) |
% Increase in HP Above Control |
|---|---|---|---|
| 18 | 1.28–5.12 | 2.56 | 6 |
| 19 | 2.86–22.91 | 5.72 | 5 |
| 20 | 5.73–22.92 | 11.46 | 5 |
| 21 | 2.56–10.24 | / | 0 |
| 22 | 1.16–18.60 | / | 0 |
All compounds were tested at least with 3 different doses and with at least 6 animals per group. Compound numbers are corresponding to those listed in Fig. 2.
DISCUSSION
It is well-known that adenosine can elicit different physiological responses depending upon whether it acts on the A1 or A2 type of adenosine receptor (4,19). Our attempts in trying to resolve which receptor subtype is involved in the inhibition of thermogenesis by the endogenous adenosine led to the use of more selective A1 and A2 receptor antagonists. It has been shown that replacement of the methyl groups of theophylline with n-propyl or larger alkyl groups yields xanthines with higher selectivity for A1 adenosine receptors, particularly when combined with an 8-phenyl moiety [for review see (7)]. To test if A1 receptor-mediated responses reduce maximum thermogenic capability, a series of 1,3,8-xanthine derivatives which are A1 selective antagonists were used. Pretreating the rats with most of the A1 selective antagonists caused a dose-related increase in both maximal and total HP (Table 1) and consequently higher final Tb than that of the control group. The marginal thermogenic enhancing effects of compound Nos. 5 and 14 may be due to their less selectivity as A1 antagonists (14). However, the lower than expected effectiveness of compound Nos.10 and 13 in improving cold tolerance cannot be readily explained as these compounds have been shown to be potent and selective A1 receptor antagonists (13,14). Since compound No. 16, another adenosine receptor antagonist like No. 13 which does not penetrate into brain (17), significantly increased heat production and cold tolerance, it is unlikely that the deficiency of compound No. 13 is due to its inability to cross the blood-brain barrier (8). Whether the discrepancy is due to in vivo drug absorption or metabolism remains a good possibility and needs to be resolved in future studies.
In contrast to those observed with 1,3,8-substituted xanthine derivatives, systemic injection with 1,3,7-trialkylxanthines at varying doses failed to alter the total HP and the final Tb (Table 2). The failure of these derivatives in increasing heat production can not be due to their relatively weak potency in blocking the adenosine receptor (3,21), as a marked suppression in thermogenesis was observed when higher doses of these compounds were administered. It is of interest to note that compound No. 19, which is about 4-fold more selective in blocking the A2 adenosine receptor (3,21), failed to produce the similar beneficial effect in improving cold tolerance as those observed with the selective A1 receptor antagonists. Therefore, it is unlikely that the thermogenic response can be altered by blocking the A2-selective functions elicited by endogenously released adenosine during cold exposure.
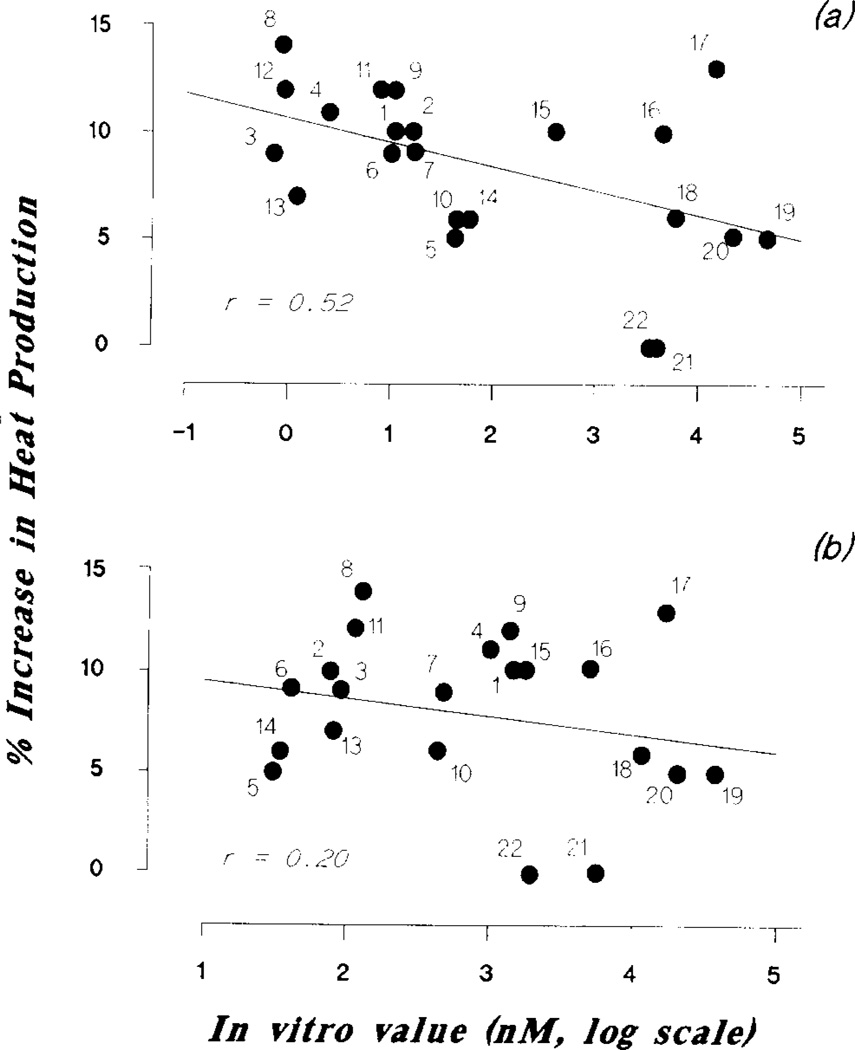
This suggestion is further supported by comparing the effectiveness of all antagonists tested in the present study in increasing total heat production of the animal under cold exposure. As shown in Fig. 4, there was a correlation (r= .52, 0.02<p<0.01) between the order of potency of these compounds in increasing heat production and as A1 receptor antagonists (determined by their ability to inhibit the binding of [3H]-CHA or [3H]-L-PIA to rat brain membranes) (7, 14, 20), but not as A2 receptor antagonists [determined either by their ability to inhibit NECA-stimulated adenylate cyclase in membrane of rat pheochromocytoma PC12 cells (14,20) or 2-chloroadenosine-stimulated cyclic AMP formation in guinea pig brain slices (7)] (r= .20, 0.5<p<0.2). The discrepancy between the binding data for some of the xanthines and the results obtained from the present study cannot be easily explained. Other than the possibility of differences in the fate of absorption and metabolism of these compounds after IP injection, the variation of this correlation may also be due to the assumption that a direct extrapolation of in vitro potency to in vivo potency can be made. It has been shown recently that the activity of a given xanthine in vivo is affected by its water solubility and bioavailability (1). This may explain why compound No. 17 is so effective in increasing total thermogenesis of the animal in spite of its lower potency as A1 receptor antagonist. In spite of these inherent problems currently existing in adenosine receptor pharmacology, it is nevertheless clear that xanthine antagonists elicit their thermogehic effects via blocking the A1, rather than the A2, adenosine receptor.
FIG. 4.
Scatter diagrams comparing the effectiveness of various adenosine receptor antagonists in enhancing thermogenesis and for either (a) the A1 adenosine receptor in rat brain membranes (the correlation coefficient was r = .52, 0.02<p<0.01); or (b) the A2 adenosine receptor in rat pheochromocytoma PC12 cells or 2-chloroadenosine-stimulated cyclic AMP formation in guinea pig brain slices (the correlation coefficient was r= .20, 0.5<p<0.2). Compound numbers are corresponding to structure and name in Figs. 1 and 2. In vitro data are obtained from Daly et al. (7), Jacobson et al. (14) and Ukena et al. (20).
Based on present and previous results, it may be reasonable to suggest that the thermogehic capacity of rats can not be fully elicited unless the A1 selective effects of endogenously released adenosine are reduced. This suggestion is supported by the finding that both the antilipolytic effect (10–12, 18) and the inhibition of insulin-stimulated glucose uptake in the muscle (2) exerted by adenosine are mediated by the At receptor. These combined effects can conceivably reduce substrate mobilization and utilization, processes required for both shivering and nonshivering thermogenesis. Consequently, heat production and cold resistance are reduced. In addition, numerous studies have indicated that adenosine can presynaptically inhibit neurotransmitter release via the A1 receptor subtype (9,29); this could lead to reduced neural stimulation of muscles involved in shivering muscle and a reduced capacity in heat production. Peripheral injection of selective A1 adenosine antagonists could thus enhance thermogenesis by attenuating the inhibitory effect of adenosine in neurotransmission. Even though the quantitative contributions adenosine antagonists may exert to each of the above mechanism(s) are currently unknown, we have identified xanthine analogs that are considerably more potent than theophylline and which may provide important structure-activity leads for the development of therapeutic agents for improving cold resistance and for lessening the incidence and severity of accidental hypothermia.
ACKNOWLEDGEMENTS
The present study was supported by a Defence and Civil Institute of Environmental Medicine (Canada) research contract to L. Wang. The authors wish to thank Research Biochemicals Inc. for gifts of most of the antagonists.
REFERENCES
- 1.Bruns RF, Fergus JH. Solubilities of adenosine antagonists determined by radioreceptor assay. J. Pharm. Pharmacol. 1989;41:590–594. doi: 10.1111/j.2042-7158.1989.tb06537.x. [DOI] [PubMed] [Google Scholar]
- 2.Challiss RAJ, Leighton B, Lozeman FJ, Newsholme EA. The hormone-modulatory effects of adenosine in skeletal muscle. In: Gerlach E, Becker BF, editors. Topics and perspectives in adenosine research. Berlin: Springer-Verlag; 1987. pp. 275–285. [Google Scholar]
- 3.Choi OH, Shamim MT, Padgett WL, Daly JW. Caffeine and theophylline analogues: correlation of behavioral effects with activity as adenosine receptor antagonists and as phosphodiesterase inhibitors. Life Sci. 1988;43:387–398. doi: 10.1016/0024-3205(88)90517-6. [DOI] [PubMed] [Google Scholar]
- 4.Daly JW. Role of ATP and adenosine receptors in physiologic processes: Summary and prospectus. In: Daly JW, Kuroda Y, Phillis JW, Shimizu H, Ui M, editors. Physiology and pharmacology of adenosine derivatives. New York: Raven Press; 1983. pp. 275–290. [Google Scholar]
- 5.Daly JW, Padgett WL, Shamim MT. Analogs of caffeine and theophylline: Effect of structural alterations on affinity at adenosine receptors. J. Med. Chem. 1986;29:1305–1308. doi: 10.1021/jm00157a035. [DOI] [PubMed] [Google Scholar]
- 6.Daly JW, Padgett WL, Shamim MT, Butts-Lamb P, Waters J. 1,3-dialkyl-8-(p-sulfophenyl)xanthines: potent water-soluble antagonist for A1- and A2-adenosine receptors. J. Med. Chem. 1985;28:487–492. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- 7.Daly JW, Ukena D, Jacobson KA. Analogues of adenosine, theophylline, and caffeine: Selective interactions with A1 and A2 adenosine receptors. In: Gerlach E, Becker BF, editors. Topics and perspectives in adenosine research. Berlin: Springer-Verlag; 1987. pp. 23–36. [Google Scholar]
- 8.Evoniuk G, Jacobson KA, Shamim MT, Daly JW, Wurtman RJ. A1- and A2-selective adenosine antagonists: in vivo characterization of cardiovascular effects. J. Pharmacol. Exp. Ther. 1987;242:882–887. [PMC free article] [PubMed] [Google Scholar]
- 9.Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol. Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- 10.Fredholm BB, Lindgren E. The effect of alkylxanthines and other phosphodiesterase inhibitors on adenosine-receptor mediated decrease in lipolysis and cyclic AMP accumulation in rat fat cells. Acta Pharmacol. Toxicol. 1984;54:64–71. doi: 10.1111/j.1600-0773.1984.tb01896.x. [DOI] [PubMed] [Google Scholar]
- 11.Fredholm BB, Sollevi A. Regulation of lipolysis and circulation in adipose tissue. In: Stone TW, editor. Purines: Pharmacology and physiological roles. London: Macmillan; 1985. pp. 223–232. [Google Scholar]
- 12.Hoffman BB, Chang H, Farahbakhsh Z, Reaven G. Inhibition of lipolysis by adenosine is potentiated with age. J. Clin. Invest. 1984;74:1750–1755. doi: 10.1172/JCI111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson KA, Kirk KL, Padgett WL, Daly JW. Functionalized congeners of adenosine: preparation of analogues with nigh affinity for A1-adenosine receptors. J. Med. Chem. 1985;28:1341–1346. doi: 10.1021/jm00147a039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson KA, De la Cruz R, Schulick R, Kiriasis L, Padgett W, Pfleiderer W, Kirk KL, Neumeyer JL, Daly JW. 8-Substituted xanthines as antagonists at A1- and A2-adenosine receptors. Biochem. Pharmacol. 1988;37:3653–3661. doi: 10.1016/0006-2952(88)90398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson KA, Ukena D, Padgett W, Daly JW, Kirk KL. Xanthine functionalized congeners as potent ligands at A2-adenosine receptors. J. Med. Chem. 1987;30:211–214. doi: 10.1021/jm00384a037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rall TW. Central nervous system stimulants: The methylxanthines. In: Gilman AG, Goodman LS, Rall TW, Murad F, editors. The pharmacological basis of therapeutics. 7th ed. New York: Macmillan; 1985. pp. 589–603. [Google Scholar]
- 17.Seale TW, Abla KA, Shamim MT, Carney JM, Daly JW. 3,7- dimethyl-l-propargylxanthine: a potent and selective in vivo antagonist of adenosine analogs. Life Sci. 1988;43:1671–1684. doi: 10.1016/0024-3205(88)90478-x. [DOI] [PubMed] [Google Scholar]
- 18.Sollevi A, Hjemdahl P, Fredholm BB. Endogenous adenosine inhibits lipolysis induced by nerve stimulation without inhibiting noradrenaline release in canine subcutaneous adipose tissue in vivo. Arch. Pharmacol. 1981;316:112–119. doi: 10.1007/BF00505303. [DOI] [PubMed] [Google Scholar]
- 19.Stiles GL. Adenosine receptors: Structure, function and regulation. Trends Pharmacol. Sci. 1986;7:486–490. [Google Scholar]
- 20.Ukena D, Daly JW, Kirk KL, Jacobson KA. Functionalized congeners of 1,3-dipropyl-8-phenylxanthine: potent antagonists for adenosine receptors that modulate membrane adenylate cyclase in pheochromocytoma cells, platelets and fat cells. Life Sci. 1986;38:797–807. doi: 10.1016/0024-3205(86)90596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ukena D, Shamim MT, Padgett W, Daly JW. Analogs of caffeine: antagonists with selectivity for A2 adenosine receptors. Life Sci. 1986;39:743–750. doi: 10.1016/0024-3205(86)90023-8. [DOI] [PubMed] [Google Scholar]
- 22.Wang LCH. Modulation of maximum thermogenesis by feeding in the white rat. J. Appl. Physiol. 1980;49:975–978. doi: 10.1152/jappl.1980.49.6.975. [DOI] [PubMed] [Google Scholar]
- 23.Wang LCH. Effects of feeding on aminophylline induced supra-maximal thermogenesis. Life Sci. 1981;29:2459–2466. doi: 10.1016/0024-3205(81)90700-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang LCH, Anholt EC. Elicitation of supramaximal thermogenesis by aminophylline in the rat. J. Appl. Physiol. 1982;53:16–20. doi: 10.1152/jappl.1982.53.1.16. [DOI] [PubMed] [Google Scholar]
- 25.Wang LCH, Lee TF. Enhancement of maximal thermogenesis by reducing endogenous adenosine activity in the rat. J. Appl. Physiol. 1990;68:580–585. doi: 10.1152/jappl.1990.68.2.580. [DOI] [PubMed] [Google Scholar]
- 26.Wang LCH, Jourdan ML, Lee TF. Mechanisms underlying the supramaximal thermogenesis elicited by aminophylline in rats. Life Sci. 1989;44:927–934. doi: 10.1016/0024-3205(89)90491-8. [DOI] [PubMed] [Google Scholar]
- 27.Wang LCH, Man SFP, Belcastro AN. Improving cold tolerance in the exercising men: Effects of substrate and theophylline. In: Heller HC, Musacchia XJ, Wang LCH, editors. Living in the cold. New York: Elsevier; 1986. pp. 539–547. [Google Scholar]
- 28.Wang LCH, Man SFP, Belcastro AN. Metabolic and hormonal responses in theophylline-increased cold resistance in males. J. Appl. Physiol. 1987;63:589–596. doi: 10.1152/jappl.1987.63.2.589. [DOI] [PubMed] [Google Scholar]
- 29.White TD. Role of adenine compounds in autonomic neurotransmission. Pharmacol. Ther. 1988;38:129–168. doi: 10.1016/0163-7258(88)90095-2. [DOI] [PubMed] [Google Scholar]