Abstract
Rhino-orbito-cerebral mucormycosis is an uncommon aggressive life-threatening opportunistic fungal infection that affects mainly the immunocompromised population with mortality rate up to 50%. Due to its aggressive nature, early detection and prompt management are of great importance for a good prognosis. Our report describes a fatal outcome of a case of rhino-orbito-cerebral mucormycosis following tooth extraction in an uncontrolled non-insulin-dependent diabetes mellitus patient after 14 days of admission.
Keywords: Fatal, fungal infection, mucormycosis, rhino-orbito-cerebral, tooth extraction
Introduction
Mucormycosis is an aggressive fatal fungal infection caused by fungi of the order Mucorales of the family zygomycetes, and the common causative species are Rhizopus, Rhizomucor, and Absidia.1,2 The fungus is normally found in soil, manure, bread mold, decaying fruits, and is commonly found in the nasal mucosa of the immune competent individual not causing any pathology.2-4 Infection usually occurs by inhalation through the nose and mouth, lacerations of the skin and extraction sites - as seen in our case - can be an entry site for the fungus.3 It affects mainly immunocompromised individuals at any age group. Most commonly affecting diabetic patients about 40-50%,5 as our case was an uncontrolled diabetic, and in recent medicine, the development of transplant technology and the increased incidence of cancer, the patient population started to shift toward the chronically immunosuppressed individuals.4 There were reports of affection of immunocompetent individuals.6,7
Case Report
A 57-year-old female presented to the Emergency Department at Nasser Institute Hospital complaining of pain and swelling of her right cheek following maxillary tooth extraction 2 weeks ago. Examination revealed facial cellulitis involving her right buccal, infraorbital and temporal areas, chemosis, dark red discoloration, and orbital apex syndrome of the right eye (Figure 1). Intraoral black discoloration of the mucosa of the right half of the palate was observed (Figure 2). She was vitally stable and afebrile with blood pressure 130/90 mmHg and a pulse rate of 85 beats/min, she was fully conscious with a Glasgow Coma Scale (GCS) 15/15, and gave history of uncontrolled non-insulin dependent diabetes mellitus and controlled hypertension. Laboratory investigations revealed leukocytosis (total leukocyte count [TLC]) 27.47 × 103 with absolute neutrophilia, and evidence of impaired kidney function; blood urea nitrogen (BUN) 32.0 mg/dl and serum creatinine 1.40 mg/dl, hemoglobin 10.5 g/dl, hematocrit 29.1%, platelet count 242 × 103, aspartate aminotransferase 52 U/L, alanine aminotransferase 52 U/L, albumin 2.2 g/dl, and random blood glucose 158 mg/dl. Facial and brain computed tomography (CT) scans revealed clouding of the right nasal cavity, maxillary, ethmoid (Figure 3a), frontal and sphenoid (Figure 3b) sinuses. Clinical and radiographic data were suggestive of late presentation of rhino-orbito-cerebral mucormycosis. Patient was admitted to our service for confirmation of the diagnosis followed by radical surgical debridement. During the preparation for surgery the patient’s level of consciousness started to deteriorate and was confused, a brain CT was ordered immediately and revealed no abnormalities. The patient was admitted to the operation room (OR) for surgical debridement and to obtain a biopsy for confirmation. Under general anesthesia, a right maxillary vestibular incision and local tissue debridement were done, and no pus discharge was seen, tissues were extremely friable with abnormally minimal bleeding. Irrigation was done using normal saline, and the friable tissue was taken for histopathological confirmation. The patient was discharged from the OR to the intensive care unit (ICU) intubated, ventilated, and sedated for risk of the airway embarrassment and for further monitoring of consciousness. Post-operative medications included IV insulin infusion for tight glycemic control, IV administration of amphotericin B 1 mg/Kg/day with daily monitoring of kidney functions, IV ampicillin/sulbactam 1 g/0.5 g and clindamycin 600 mg both twice daily. Post-operative care included local irrigation with normal saline and local packing of a piece of gauze soaked in 1 vial of amphotericin B 50 mg twice daily. Sedation was discontinued in the ICU a few hours postoperatively, and the patient’s conscious level was improving and was on spontaneous breathing (continuous positive airway pressure) mode on the ventilator machine. The following day the patient’s conscious level deteriorated again with GCS 10/15, patient was tachypneic and tachycardic, an arterial blood gas revealed metabolic acidosis with respiratory compensation, the patient was ventilated on assisted ventilation (spontaneous intermittent mandatory ventilation) mode. Chemosis and total ophthalmoplegia were noticed on the left eye which was an indication of cavernous sinus thrombosis and was managed by IV heparin. A brain CT revealed an occipital lobe infarction. Patient’s general condition was deteriorating over the next 10 days, she developed ventilator acquired pneumonia and was treated by IV piperacillin/tazobactam 4 g/0.5 g and vancomycin 1 g both twice daily, with continuous decrease in GCS until it reached 3/15 and another brain CT revealed a new frontal lobe infarction. Fluctuating TLC counts was noticed and continuous deterioration in liver and kidney functions and the decision was taken to gradually decrease the dose of IV amphotericin B to 0.8 mg/Kg/day then to 0.5 mg/Kg/day, then was discontinued on the 10th day when the serum creatinine and BUN reached 2.40 mg/dl and 92.0 mg/dl respectively, local packing with amphotericin B was continued. The patient suffered from acute renal shutdown and serum creatinine, and BUN reached 3.60 mg/dl and 112 mg/dl, respectively, on the 13th day of admission and underwent renal dialysis. On the 14th day of admission, the patient suffered from multi-organ system failure and succumbed.
Figure 1.
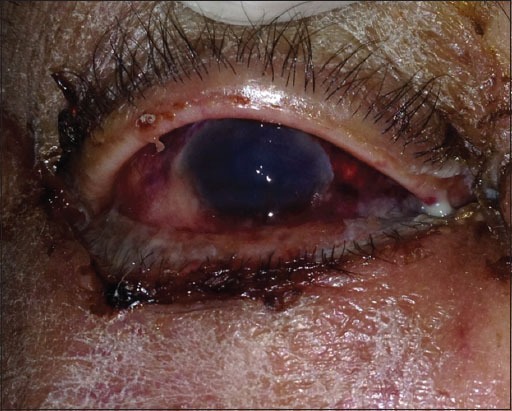
Dark red discoloration of the right eye.
Figure 2.
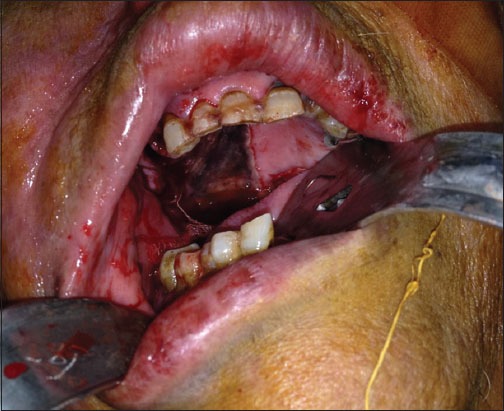
The black discoloration of the right half of the palate indicating palatal necrosis.
Figure 3.
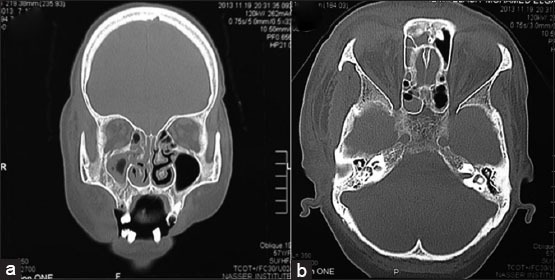
(a) Coronal cut of the patent’s computed tomography scan showing clouding of right maxillary sinus, ethmoid sinus, and nasal cavity. (b) Axial cut showing clouding of the sphenoid sinus, which is an indication of extensive spread of the fungi.
Histopathological examination of the specimens stained with H and E revealed pieces of the sinus mucosa showing inflammation and necrosis along with fungal colonies with large spores and hyphae consistent with the clinical diagnosis of mucormycosis (Figure 4).
Figure 4.
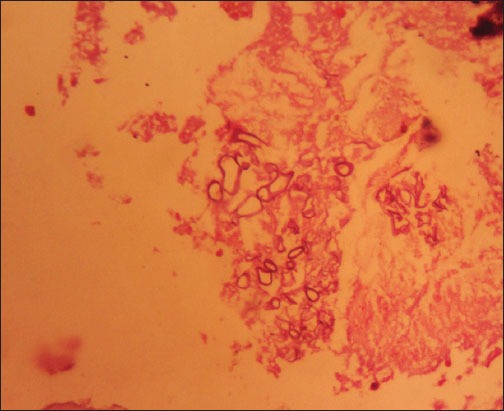
Histopathological specimen stained with H and E at 400 × magnification, showing colonies of fungal spores and hyphae.
Discussion
There are several forms of mucormycosis including cutaneous, rhino-orbito-cerebral, pulmonary, gastrointestinal, central nervous system, disseminated and miscellaneous.8 There were also reports of mucormycosis of the mandible and tongue.9,10 Clinical manifestations of rhino-orbito-cerebral mucormycosis include signs in the oral, nasal and paranasal sinus mucosa, orbital signs and symptoms and intracranial extension of the disease.1 In this report our patient presented with swelling of the temporal, buccal, and periorbital region, it was mentioned in the literature that the disease usually starts with pain and swelling of the cheeks and the periorbital region, where it invades the sinuses and nasal mucosa, then spreads to the orbit and extends intracranially.11 From the nasal mucosa and maxillary sinus, the fungi can spread to the oral cavity giving rise to black palatal discoloration that indicates necrosis of palatal mucosa and disease progression might lead to palatal necrosis and perforation as shown with our case.1,12 Black eschar nasal discharge with thickening of the nasal mucosa, necrosis of the turbinates and nasal septum are common findings in mucormycosis, black discoloration, and necrosis is an indication of disease progression with poor prognosis. Once the disease has reached the nasal cavity and maxillary sinus, it can easily reach the orbit through the nasolacrimal duct or the ethmoid air cells causing chemosis, proptosis, decreased visual acuity, external ophthalmoplegia and as the disease progresses orbital apex syndrome develops.4,13 Due to the disease’s high affinity to the arteries and its internal elastic lamina causing thrombosis and infarctions, the disease spreads from the orbit to the brain via the central retinal artery or ophthalmic artery or the carotid artery, the disease can also spread directly through the superior orbital fissure and cribriform plate of ethmoid. Once the brain is involved, the patient’s conscious level starts to deteriorate and plunders into a coma.1,4,14,15 The vascular spread can cause cavernous sinus thrombosis and brain infarctions as shown with our case and once the cavernous sinus is involved the patient has a poor prognosis.3,4 Definitive diagnosis of this disease is only through histopathological examination revealing non-septate hyphae with right angle branching seen with H and E stains and more specifically periodic acid-Schiff stains.4 Imaging of these cases is required to aid in diagnosis and detecting the extent of involvement of the anatomic structures and aid in surgical planning, while CT scans are great in detecting sinus mucosal thickening and bone destruction, magnetic resonance imagings are reliable for detecting the vascular infiltration of the disease and intracranial extensions without use of contrast.16,17
Management of these cases is done through early diagnosis, aggressive surgical debridement, correction of the underlying condition, systemic and local antifungal therapy and all should be done at the same time or within a short span of time.4,18,19 The earlier the diagnosis, the lesser the extent of infiltration of the disease and thus reduces the extent of surgical debridement and post resection defect and thus better prognosis. The surgical resection should be wide and aggressive to remove all the necrotic tissue as the disease is vaso-occlusive, and the systemic antifungals might not reach the entire necrotic area.1-4,11-15 Correction of the underlying condition is of utmost importance as it is what originally provoked the disease to infest the body.4,11,14,18,19 The most potent antifungal used for mucormycosis is amphotericin B and the recommended dose is 1 mg/Kg/day with a total dose of 3-4 g given over a period of 6-12 weeks,1 yet it has serious side effects including fever, chills, malaise, thrombophlebitis, azotemia, and renal tubular acidosis. Thus, its lipid formulations such as liposomal amphotericin B are more commonly used as they have much less side effects and are safer on the kidneys, and the recommended doses are 5-7.5 mg/Kg/day.1,4,14,15 There have been reports on successful outcomes from combination therapy of liposomal amphotericin B and other drugs such as micafungin and posaconazole.4,20-22 Furthermore, the use of amphotericin B and liposomal amphotericin B in local irrigation was supported in the literature as mentioned that the disease is vaso-occlusive, and the systemic doses might not reach the entire necrotic surface and local application might improve the prognosis.19
Adjunctive therapies for mucormycosis in the literature suggests the use of hyperbaric oxygen therapy and iron chelating agents that improve the prognosis of this aggressive disease.1,4 Hyperbaric oxygen inhibits fungal growth and promotes the action of amphotericin B. It also promotes tissue healing by enhancing the immune response and improves tissue oxygenation. A review of the literature showed that patients receiving hyperbaric oxygen therapy had higher survival rates provided that the underlying condition is reversed.23 Iron chelation is reported to improve the prognosis, as iron is important for fungal growth. Serum iron is almost unavailable to pathogens as it is highly bound to carrier proteins. Thus, patients with high levels of serum iron such as patients suffering from diabetic ketoacidosis are highly susceptible to mucormycosis infection. Yet patients being treated with iron chelating agent deferoxamine are more susceptible to mucormycosis especially by Rhizopus as it obtains its iron from the iron-deferoxamine complex. The agent recommended for iron chelation without aggravation of the disease is deferiprone; also deferasirox was effective in reducing iron concentration without aggravating the disease and had another advantage in that it acts synergistically with amphotericin B.24
Conclusion
We conclude that early diagnosis and management of rhino-orbito-cerebral mucormycosis is of great importance to improve survivability, once the patient develops cavernous sinus thrombosis and brain infarctions the prognosis is very poor. We recommend administration of systemic and local antifungals even when mucormycosis is suspected, this will not do harm but will benefit the patient once the disease is confirmed, aggressive surgical debridement when possible, management and control of the underlying condition. The adjunctive therapies supported by literature will aid in the resolution of the disease and improve the patient’s prognosis. Due to the scarcity of the disease, there are no randomized control trials on the adjunctive therapies but due to the aggressive nature of the disease, the authors of this article recommend the use of hyperbaric oxygen as the patient might benefit from it.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Prasad K, Lalitha RM, Reddy EK, Ranganath K, Srinivas DR, Singh J. Role of early diagnosis and multimodal treatment in rhinocerebral mucormycosis: Experience of 4 cases. J Oral Maxillofac Surg. 2012;70(2):354–62. doi: 10.1016/j.joms.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE, Stern D. 1st ed. Chicago, IL: Quintessence Publishing; 2003. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment; pp. 104–6. [Google Scholar]
- 3.Kim J, Fortson JK, Cook HE. A fatal outcome from rhinocerebralm mucormycosis after dental extractions: A case report. J Oral Maxillofac Surg. 2001;59(6):693–7. doi: 10.1053/joms.2001.23407. [DOI] [PubMed] [Google Scholar]
- 4.Wali U, Balkhair A, Al-Mujaini A. Cerebro-rhino orbital mucormycosis: An update. J Infect Public Health. 2012;5(2):116–26. doi: 10.1016/j.jiph.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Tierney MR, Baker AS. Infections of the head and neck in diabetes mellitus. Infect Dis Clin North Am. 1995;9(1):195–216. [PubMed] [Google Scholar]
- 6.Fairley C, Sullivan TJ, Bartley P, Allworth T, Lewandowski R. Survival after rhino-orbital-cerebral mucormycosis in an immunocompetent patient. Ophthalmology. 2000;107:555–8. doi: 10.1016/s0161-6420(99)00142-6. [DOI] [PubMed] [Google Scholar]
- 7.Singh V, Sharma B, Sen R, Agrawal S, Bhagol A, Bali R. Rhinocerebral mucormycosis: A diagnostic challenge and therapeutic dilemma in immunocompetent host. J Oral Maxillofac Surg. 2012;70(6):1369–75. doi: 10.1016/j.joms.2011.06.209. [DOI] [PubMed] [Google Scholar]
- 8.Topazian RG, Goldberg MH, Hupp JR. 4th ed. Philadelphia, PA: W. B. Saunders Company; 2002. Oral and Maxillofacial Infections; pp. 253–5. [Google Scholar]
- 9.Aras MH, Kara MI, Erkiliç S, Ay S. Mandibular mucormycosis in immunocompromised patients: Report of 2 cases and review of the literature. J Oral Maxillofac Surg. 2012;70(6):1362–8. doi: 10.1016/j.joms.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Pajpani M, Webb R. Lingual necrosis caused by mucormycosis in a patient with aplastic anaemia: Case report. Br J Oral Maxillofac Surg. 2014;52:e144–6. doi: 10.1016/j.bjoms.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Abedi E, Sismanis A, Choi K, Pastore P. Twenty-five years’ experience treating cerebro-rhino-orbital mucormycosis. Laryngoscope. 1984;94(8):1060–2. doi: 10.1288/00005537-198408000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Economopoulou P, Laskaris G, Ferekidis E, Kanelis N. Rhinocerebral mucormycosis with severe oral lesions: A case report. J Oral Maxillofac Surg. 1995;53(2):215–7. doi: 10.1016/0278-2391(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill BM, Alessi AS, George EB, Piro J. Disseminated rhinocerebral mucormycosis: A case report and review of the literature. J Oral Maxillofac Surg. 2006;64(2):326–33. doi: 10.1016/j.joms.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Talmi YP, Goldschmied-Reouven A, Bakon M, Barshack I, Wolf M, Horowitz Z, et al. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol Head Neck Surg. 2002;127(1):22–31. doi: 10.1067/mhn.2002.126587. [DOI] [PubMed] [Google Scholar]
- 15.Metzen D, Böhm H, Zimmermann M, Reuther T, Kübler AC, Müller-Richter UD. Mucormycosis of the head and neck. J Craniomaxillofac Surg. 2012;40:e321–7. doi: 10.1016/j.jcms.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Herrera DA, Dublin AB, Ormsby EL, Aminpour S, Howell LP. Imaging findings of rhinocerebral mucormycosis. Skull Base. 2009;19(2):117–25. doi: 10.1055/s-0028-1096209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terk MR, Underwood DJ, Zee CS, Colletti PM. MR imaging in rhinocerebral and intracranial mucormycosis with CT and pathologic correlation. Magn Reson Imaging. 1992;10(1):81–7. doi: 10.1016/0730-725x(92)90376-b. [DOI] [PubMed] [Google Scholar]
- 18.Pinto ME, Manrique HA, Guevara X, Acosta M, Villena JE, Solís J. Hyperglycemic hyperosmolar state and rhino-orbital mucormycosis. Diabetes Res Clin Pract. 2011;91:e37–9. doi: 10.1016/j.diabres.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Dökmetas HS, Canbay E, Yilmaz S, Elaldi N, Topalkara A, Oztoprak I, et al. Diabetic ketoacidosis and rhino-orbital mucormycosis. Diabetes Res Clin Pract. 2002;57:139–42. doi: 10.1016/s0168-8227(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa T, Takezawa K, Tojima I, Shibayama M, Kouzaki H, Ishida M, et al. Successful treatment of rhino-orbital mucormycosis by a new combination therapy with liposomal amphotericin B and micafungin. Auris Nasus Larynx. 2012;39(2):224–8. doi: 10.1016/j.anl.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Almannai M, Imran H, Estrada B, Siddiqui AH. Successful treatment of rhino-orbital mucormycosis with posaconazole and hyperbaric oxygen therapy. Pediatr Hematol Oncol. 2013;30(3):184–6. doi: 10.3109/08880018.2013.770587. [DOI] [PubMed] [Google Scholar]
- 22.Kok J, Gilroy N, Halliday C, Lee OC, Novakovic D, Kevin P, et al. Early use of posaconazole in the successful treatment of rhino-orbital mucormycosis caused by Rhizopus oryzae. J Infect. 2007;55(3):e33–6. doi: 10.1016/j.jinf.2007.05.178. [DOI] [PubMed] [Google Scholar]
- 23.John BV, Chamilos G, Kontoyiannis DP. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect. 2005;11(7):515–7. doi: 10.1111/j.1469-0691.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim AS, Spellberg B, Edwards J., Jr Iron acquisition: A novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21(6):620–5. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]


