Abstract
Context
For adults with end-stage kidney disease, live donor kidney transplantation (LDKT) yields superior outcomes over long-term dialysis and deceased donor kidney transplantation. However, blacks receive LDKT at a much lower rate than adults of any other race or ethnicity.
Objective
To examine the LDKT readiness stage of blacks on the transplant waiting list and its association with LDKT knowledge, concerns, and willingness.
Design
Cross-sectional analysis of baseline data from a randomized controlled trial to improve knowledge and reduce concerns about LDKT.
Patients and Setting
One hundred fifty-two black patients on the kidney transplant waiting list at a single transplant center in the northeastern United States.
Main Outcomes
LDKT readiness stage, knowledge, concerns, and willingness to talk to others about living donation.
Results
Sixty percent of patients were not considering or not yet ready to pursue LDKT, while only 11% had taken action to talk to family members or friends about the possibility of living kidney donation. Patients in later stages of LDKT readiness (i.e., had talked to others about donation or were preparing to do so) had significantly more knowledge (p<0.001), fewer concerns (p=0.002), and more willingness (p=0.001) to talk to others about living donation than those in earlier readiness stages.
Conclusions
The large percentage of blacks who are in the earlier stages of LDKT readiness may account for the low rate of LDKT in this patient population at our transplant center. Innovative and tailored LDKT educational strategies for black patients are needed to help reduce racial disparities in LDKT.
Keywords: Kidney transplantation, living donation, live donor kidney transplant
INTRODUCTION
For adults in the late stages of chronic kidney disease, kidney transplantation provides the most optimal quality of life and long-term survival outcomes. [1,3] In addition to having disproportionately higher rates of chronic kidney disease relative to whites, blacks face numerous barriers along the clinical pathway to transplantation. These barriers include later diagnosis of kidney disease, longer dialysis exposure before referral for transplant evaluation, higher likelihood of not completing the complex transplant evaluation and being denied transplant listing, and longer waiting time and higher death rates once added to the transplant waiting list. [4–13]
Compared to a deceased donor kidney transplantation, live donor kidney transplantation (LDKT) has many advantages, including higher patient and graft survival rates, lower rates of acute rejection, reduction in overall dialysis exposure, more rapid improvement in functional health status, and more cost savings to the healthcare system. [12] However, the rate of LDKT for blacks is substantially lower than for any other racial or ethnic group in the United States. [14] In 2012, for instance, only 18% of the kidney transplants received by blacks were from living donors, a much lower percentage compared to Asians (26%), Hispanics (31%), and non-Hispanic whites (44%). There are many plausible explanations for the lower LDKT rate among blacks, including a higher likelihood of potential living donors being turned down due to disproportionately higher rates of diabetes, hypertension, and obesity in the black population and more concerns about their long-term outcomes following donor nephrectomy. [15–17] Others have noted that blacks may cope with the need for transplantation and the possibility of LDKT differently than whites, which may contribute to less discussion with family members and friends about possible living donation. [18] Moreover, some patients may have religious objections to transplantation and harbor mistrust about the healthcare system because of prior discrimination in dialysis units [11,19].
Despite progressing through transplant educational processes and being added to the transplant waiting list, patients may still be at different stages of readiness about pursuing LDKT and this readiness may be affected by many different variables. A better understanding of the factors associated with LDKT readiness may facilitate the development of innovative strategies to improve education and remove barriers for black patients. In the current study, we sought to characterize the readiness to pursue LDKT among a cohort of black patients wait-listed for kidney transplantation. Additionally, we examined whether readiness to pursue this transplant option was associated with LDKT knowledge, concerns about pursuing LDKT, willingness to talk to family members and friends about living donation, and medical and sociodemographic factors.
PARTICIPANTS AND METHODS
Study Population and Recruitment Procedures
Data presented in this study were collected as part of the baseline assessment for the House Calls Trial, which is a randomized controlled trial designed to evaluate the effectiveness of a home-based educational program on LDKT rates for black patients approved and listed for kidney transplantation. [20] Eligibility criteria for the House Calls Trial included self-identification as black race, meeting medical eligibility criteria for activation on the kidney transplant waiting list, at least 21 years old, and primary residence within 2½ hours driving time from the transplant center. Patients were excluded if they did not speak English or if they had severe cognitive limitations.
Eligible patients received a letter informing them about the study in the month preceding a scheduled transplant clinic appointment. At the time of their visit, a study nephrologist or other research team member discussed the study with the patient, confirmed eligibility, and obtained written informed consent. The baseline questionnaires, medical record data, and sociodemographic characteristics were obtained prior to randomization and study intervention. All study procedures were approved by the Committee on Clinical Investigations at Beth Israel Deaconess Medical Center (Protocol #2007P-000223).
Questionnaires and Other Variables
The self-report questionnaires were developed via formative research and have been described in prior publications. [20–22]
Readiness to pursue LDKT
Using an assessment model based, in part, on stages of change [23], we developed a brief tool to assess patients’ stage of readiness to pursue LDKT. This tool asks patients to select one of five statements that best reflects their stage of readiness: I am not thinking about or considering live donor kidney transplantation (Pre-contemplation); I am now beginning to think about or consider live donor kidney transplantation (Contemplation); I have thought about live donor kidney transplantation and I am seriously considering this possibility (Preparation); I have thought about live donor kidney transplantation, and I have talked to someone who is willing to be evaluated as a possible living donor (Action); and I have thought about live donor kidney transplantation and I have someone who has contacted the transplant center to be evaluated as a potential living donor (Maintenance).
LDKT knowledge (α = 0.79)
Patients responded to 16 true-false statements designed to assess their knowledge of LDKT and living donation (e.g., Kidneys from living donors usually last longer than kidneys from donors who have recently died; A living kidney donor must have his/her own health insurance to cover the costs of surgery). Scores can range from 0 to 16, with higher scores reflecting more knowledge.
LDKT concerns (α = 0.76)
Using a 5-point Likert-type scale, patients responded to 21 items reflecting possible concerns about pursuing LDKT (e.g., I am concerned that the donor would no longer be able to do activities that they enjoy; I am concerned that the surgery and recovery for the donor would be painful). Scores can range from 21 to 105, with higher scores reflecting more concerns about LDKT.
Willingness to discuss LDKT
Patients were asked to indicate their willingness to talk to family members or friends about possible living kidney donation, using a 1 (not at all willing) to 7 (extremely willing) rating scale.
Medical and sociodemographic characteristics
We collected the following information from the patient’s medical record: primary cause of renal failure; dialysis status (yes, no), type (hemodialysis, peritoneal dialysis), and duration (months); time on the transplant waiting list (months); and prior kidney transplant (yes, no, type). Additionally, we administered the SF-36 Health Survey [24] to obtain an estimate of the patient’s current health-related quality of life. The SF-36 is used extensively in clinical transplantation research and includes eight quality of life domains and two summary scores (Physical Component Summary, Mental Component Summary), with higher scores reflecting more favorable perceptions of quality of life. Finally, we recorded the patient’s age, gender, race/ethnicity, highest formal education completed, employment status, and marital status.
Statistical Analyses
First, descriptive analyses were calculated to characterize the medical and sociodemographic characteristics of the study sample, as well as the distributional properties of the questionnaires. Second, Pearson correlation coefficients were calculated to examine the relationships between LDKT knowledge, concerns, willingness and sociodemographic and medical characteristics. Third, analyses of variance (continuous variables) and chi square analyses (categorical variables) were used to examine the relationships between LDKT readiness stage and the other primary variables of interest (LDKT knowledge, concerns, and willingness to talk with others about LDKT) and sociodemographic and medical factors. The Action and Maintenance readiness stages were combined for this analysis due to the small cell size (n=3) for the Maintenance stage. Significant effects were followed by Tukey’s post hoc tests, adjusting for multiple comparisons, to identify significant group differences. PASW 17.0 (Chicago, IL) was used for all statistical analyses.
RESULTS
Sample Characteristics
One hundred fifty-two patients met study eligibility criteria and completed the baseline questionnaire assessments. Table 1 presents the sociodemographic and medical characteristics of the study sample, which was predominantly older than 50 years, male, not working, and not married or partnered. The majority had renal disease primarily from diabetes or hypertension, was receiving dialysis treatments, and had not previously received a kidney transplant. The study sample did not differ significantly from other non-study wait-listed patients based on sociodemographic or medical variables.
Table 1.
Sociodemographic and medical characteristics of the study sample (N = 152)
| Variables | Number (%) or Mean (± standard deviation) |
|---|---|
| Sociodemographic | |
| Age, yrs | 51.3 (±12.3) |
| 18 to 34 yrs | 18 (12) |
| 35 to 49 yrs | 45 (30) |
| 50 to 64 yrs | 70 (46) |
| 65 yrs and older | 19 (12) |
| Gender | |
| Female | 65 (43) |
| Male | 87 (57) |
| Education | |
| High school or less | 62 (41) |
| Some college | 41 (27) |
| College or professional degree | 49 (32) |
| Employment status | |
| Working for pay | 54 (36) |
| Not working | 98 (64) |
| Marital status | |
| Not married or partnered | 92 (60) |
| Married or partnered | 60 (40) |
| Medical | |
| Primary cause of renal disease | |
| Diabetes | 58 (38) |
| Hypertension | 44 (29) |
| Other | 50 (33) |
| Dialysis | |
| Hemodialysis | 108 (71) |
| Peritoneal | 18 (12) |
| Pre-dialysis | 26 (17) |
| Dialysis duration, mos. | 36.6 (±39.0) |
| Transplant waiting time, mos. | 16.9 (±25.1) |
| Prior kidney transplant | |
| None | 136 (89) |
| Deceased donor transplant | 10 (7) |
| Live donor transplant | 6 (4) |
| SF-36 (quality of life) | |
| Physical summary | 38.1 (±10.5) |
| Mental summary | 48.3 (±11.5) |
Stage of Readiness to Pursue LDKT
Forty-three patients (28%) reported that they were not currently thinking about LDKT (Pre-contemplation stage), 48 patients (32%) indicated that they were just beginning to consider the LDKT option (Contemplation stage), 44 patients (29%) stated that they were now seriously considering LDKT (Preparation stage), 14 patients (9%) had talked to someone who expressed willingness to be evaluated as a potential living donor (Action stage), and only 3 patients (2%) had someone who had recently had recently initiated a living donor evaluation (Maintenance stage).
LDKT Knowledge, Concerns, and Willingness
The number of LDKT knowledge questions answered correctly ranged from 4 to 15 (mean = 9.7±2.4). The most common knowledge gaps were not knowing that: our transplant program will evaluate potential living donors with a history of hypertension (66%), most insurance companies will not pay for the living donor’s travel or lodging expenses (56%), the majority of living donors are in the hospital for only a few days after surgery (47%), neither the living donor nor their health insurance company must pay for the cost of surgery (40%), kidneys from a living donor usually lasts longer than a kidney from a deceased donor (40%), LDKT is still possible even if a potential donor does not have a compatible blood type (36%), and living donation does not adversely impact fertility (36%).
Scores on the LDKT concerns questionnaire ranged from 21 to 78 (mean = 39.2±9.6). Patients reported feeling most concerned (i.e., “somewhat” to “extremely”) about: psychological trauma for the living donor if the graft fails (66%), nobody volunteering to donate after initiating discussion (55%), a painful surgery and recovery for the living donor (50%), the donor’s family experiencing some hardship because of donor surgery or recovery (50%), it taking too long for the living donor to resume normal activities (37%), too many costly expenses for the living donor (37%), future health problems in the living donor due to surgery (37%), and the living donor would have problems getting or keeping health, life, or disability insurance (26%).
Mean rating of willingness to talk to others about possible living kidney donation was 3.7±1.8. Forty-three patients (28%) reported low (rating of 1 or 2), 88 patients (58%) reported moderate (rating of 3, 4, or 5), and 21 patients (14%) reported high (rating of 6 or 7) willingness to discuss LDKT.
More LDKT knowledge was associated with higher willingness to talk to others about living donation (r = 0.28, p=0.001) and having fewer LDKT concerns (r = −0.42, p<0.001). Also, patients with a high school education or less had significantly lower LDKT knowledge scores than those with at least some college education (F=3.2, p=0.04). Having more LDKT concerns was associated with lower willingness to discuss living donation with others (r = −0.27, p=0.001) and being unemployed (t=2.2, p=0.03).
LDKT Readiness Stage and Associations with Knowledge, Concerns, and Willingness
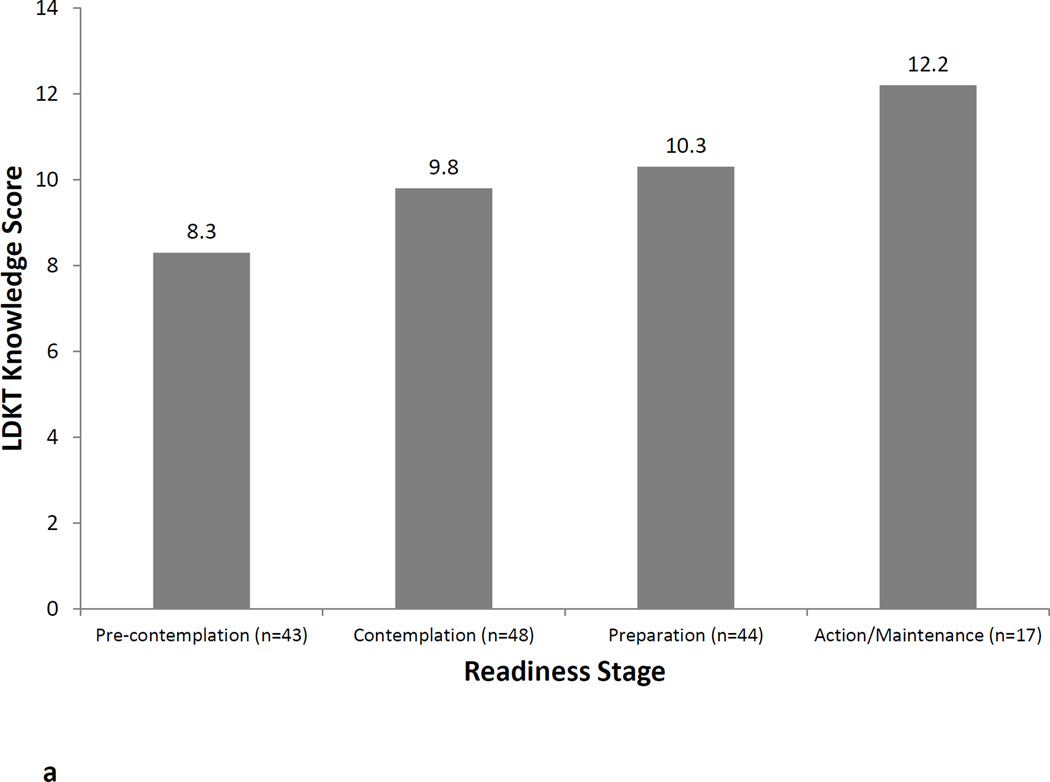
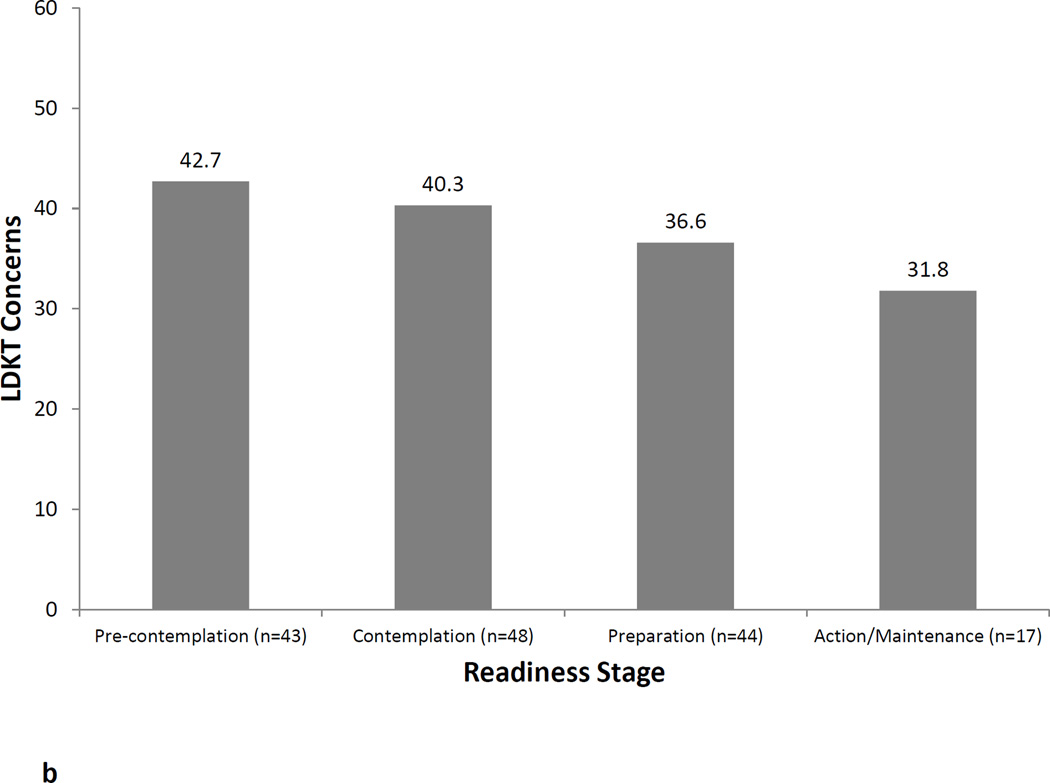
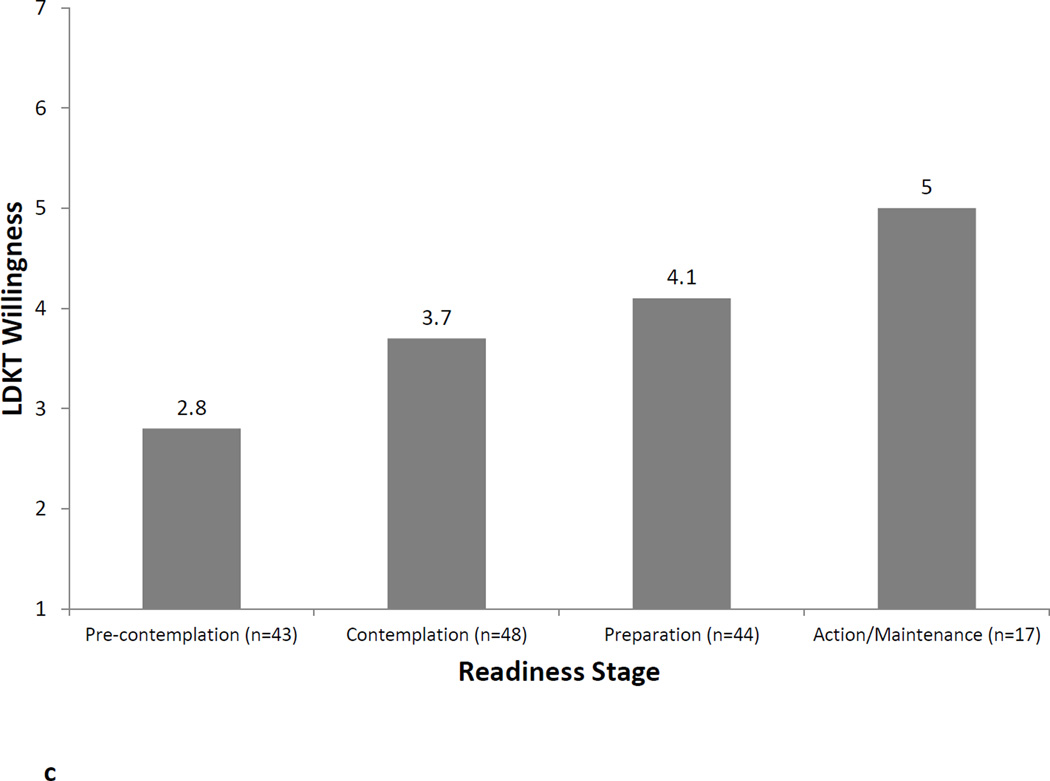
Figures 1a, 1b, and 1c illustrate the relationship between LDKT readiness stage and LDKT knowledge, concerns, and willingness, respectively. Analyses showed that patients in different LDKT readiness stages differed significantly on their knowledge levels (F=10.2, p<0.001), concerns (F=5.4, p=0.002), and willingness to talk to others about living donation (F=6.2, p=0.001). In all instances, post-hoc tests showed that patients in the Preparation or Action/Maintenance readiness stages had significantly more knowledge, fewer concerns, and more willingness to talk to others about living donation than those in the Pre-Contemplation and Contemplation readiness stages (all p-values<0.05). LDKT readiness stages was not significantly associated with any sociodemographic or medical variables (all p-values>0.05).
Figure 1.
a. LDKT knowledge by readiness stage.
b. LDKT concerns by readiness stage.
c. Willingness (1 = not at all willing to 7 = extremely willing) to talk to others about living donation by readiness stage.
DISCUSSION
While LDKT yields better outcomes in comparison to long-term dialysis and deceased donor kidney transplantation, this treatment option remains elusive for a majority of black patients on the transplant waiting list. [5,20,25] During the study enrollment period in our program, only 27% of the kidney transplants for blacks were from living donors, compared to 55% for Whites. The barriers to LDKT for blacks likely are multi-faceted, involving numerous factors at the patient, social network, healthcare setting, and societal levels. [5,26,27] In this study, we focused on the assessment of patient-level factors and found that 60% of black patients already evaluated and on the transplant waiting list were not considering or not yet ready to pursue LDKT. Strikingly, only 11% of patients had taken any action to talk to family members or friends about the possibility of living kidney donation. More transplant or LDKT knowledge and fewer LDKT concerns were both strongly associated with higher likelihood of being in a later stage of LDKT readiness (i.e., had talked to someone who was willing to be evaluated as a potential living donor).
The large percentage of blacks who are in the earlier stages of LDKT readiness may account, at least in part, for the low rate of LDKT in this patient population at our transplant center. Why are so few patients in the later stages of behavioral action when it comes to LDKT? Our findings suggest that less knowledge and more concerns about LDKT may be more important determinants of LDKT readiness than sociodemographic or medical factors. Others have also found a relationship between knowledge deficits and lower rates of LDKT among blacks. [21,25,29] On average, patients in this study answered only slightly more than half of the knowledge questions correctly. This is surprising considering the questions were taken directly from the educational materials given to patients at the time of initial evaluation and are routinely discussed with them during the initial clinic visit with the transplant nephrologist, surgeon, and social worker. In combination with common concerns about the recovery and outcomes of potential living donors, some of the knowledge gaps observed in our patients may be deterrents to more active consideration of LDKT as a viable transplant option.
One might reasonably expect patients with longer transplant waiting time, more dialysis exposure, and lower physical quality of life to be in later stages of LDKT readiness. However, we found that these variables were not significantly associated with LDKT readiness stage, knowledge, concerns, or willingness. This finding suggests that transplant professionals should not simply assume that patients will more actively consider the LDKT option as their health deteriorates, or as they languish for several years on the waiting list. Like ours, many transplant programs provide an initial bolus of LDKT education in the early phases of evaluation and initial transplant listing, but then may have minimal contact with patients while on the waiting list. Our data suggests that the readiness stage of patients should be assessed throughout the transplant waiting period. We have developed a brief tool for assessing readiness and this can easily be integrated into the clinical care of the transplant patient.
An important question that arises from these data is how best to help patients progress from not considering LDKT to eventually engaging others in a discussion about possible living donation. An educational approach that is tailored to the patient’s readiness stage may be necessary. [25,26,29] For those in the early stages of Pre-contemplation or Contemplation may need an approach that is focused on overcoming specific misunderstandings or knowledge deficits about LDKT benefits, e.g., that kidneys from living donors generally last longer than ones from deceased donors and that there are other viable options for incompatible donor-recipient pairs. Patients in the Preparation stage of readiness may require more of a focus on overcoming knowledge deficits that are specific to living donation (e.g., living donors are not held financially responsible for the cost of surgery). Also in this stage, patients may be more willing to discuss living donation with others, but translating that into behavioral action (i.e., talking to others) may necessitate addressing their specific concerns, particularly about donor costs and outcomes. In addition to the potential benefits associated with LDKT, it is essential that educational efforts openly address the risks of LDKT and living donation, as well as discuss what is known and unknown about living donation outcomes. Discussions that openly acknowledge both risks and benefits of LDKT and living donation may do more to engender trust among patients, particularly those at potentially higher risk of negative long-term outcomes. [37,38]
Many of the most common LDKT concerns endorsed by patients likely are applicable to transplant candidates of all races or ethnicities. However, concerns about donor evaluation, indirect costs, and outcomes may be heightened in black patients. Relative to whites, blacks who come forward as potential living donors are more likely to have medical conditions (e.g., diabetes, hypertension, obesity) that disqualify them as donors [15–17], incompatible blood type with the intended recipient [30], higher indirect cost burden [31–33], and less optimal long-term outcomes following donation [34–39]. Additionally, the recent discovery and media coverage of a genetic marker (i.e., apolipoprotein L1, or APOL1) to predict risk of future kidney disease in blacks may raise some questions from potential donors and their intended recipients about its utility in the donation decision-making process. [37,40,41] An open and direct discussion of these race-specific issues may attenuate some of the ambivalence or concern that black patients may have about pursing LDKT and help them to make a more informed decision that adheres to their personal values and preferences.
These study findings should be considered within the context of a few important study limitations. First, this study focused on the LDKT readiness of black patients in the Boston metropolitan area. It is unknown whether these study findings can be generalized to other minority populations or to blacks residing in other distinct geographic regions of the United States. Second, our kidney program’s patient education processes may differ from those of other programs, which may yield different findings regarding LDKT readiness, knowledge, concerns, and willingness. Third, findings may have been affected by selection bias, since patients who chose to participate in a clinical trial on LDKT educational strategies may differ on LDKT parameters than patients not participating in the trial. Fourth, we may have overlooked other variables that may be important to consider in assessing LDKT readiness stage, including self-efficacy about talking to others, the patient’s assessment of the pros and cons of this transplant option, medical distrust, insurability, and the size of the patient’s social network. [5,29,42–44] Finally, we did not examine the relationship between LDKT readiness stage and eventual receipt of LDKT, although this is being examined in the context of the parent House Calls Trial. [20]
In conclusion, LDKT yields superior outcomes over long-term dialysis and deceased donor kidney transplantation, yet blacks have a much lower LDKT rate compared to Whites. Few wait-listed black patients in our study had discussed LDKT with potential living donors and many were not actively considering this transplant option, perhaps because of knowledge deficits and heightened concerns about poor donor outcomes and disruptions to the lives of potential donors and their families. The development and evaluation of innovative and tailored LDKT educational strategies for black patients are needed to help reduce racial disparities in LDKT. [20,45,46]
ACKNOWLEDGMENTS
The project described is supported by Award Number R01DK079665 from the National Institute of Diabetes and Digestive and Kidney Diseases (JRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. This research was also supported, in part, by the Julie Henry Research Fund and the Center for Transplant Outcomes and Quality Improvement, The Transplant Institute, Beth Israel Deaconess Medical Center, Boston, MA.
We are thankful for the data collection and entry assistance we received from Timothy Antonellis, Tracy Brann, Ariel Hodara, Richard McCartney, Colleen Morse, Matthew Paek, Stacey Senat, Hongying Tang, and Denny Tsai.
Dr. Egbuna was a faculty member and transplant nephrologist at Beth Israel Deaconess Medical Center and Harvard Medical School during the development and initial implementation of the study. He is now employed as Clinical Research Medical Director for Amgen Inc., although Amgen has not been involved in any way with the study reported in this manuscript.
Footnotes
Conflicts: There are no conflicts of interest to report.
Contributor Information
James R. Rodrigue, Email: jrrodrig@bidmc.harvard.edu.
Matthew J. Paek, Email: matthewjared07@gmail.com.
Ogo Egbuna, Email: ogoegbuna@gmail.com.
Amy D. Waterman, Email: awaterman@mednet.ucla.edu.
Jesse D. Schold, Email: scholdj@ccf.org.
Martha Pavlakis, Email: mpavlaki@bidmc.harvard.edu.
Didier A. Mandelbrot, Email: dmandelb@bidmc.harvard.edu.
REFERENCES
- 1.Kovacs AZ, Molnar MZ, Szeifert L, Ambrus C, Molnar-Varga M, Szentkiralyi A, et al. Sleep disorders, depressive symptoms and health-related quality of life – a cross-sectional comparison between kidney transplant recipients and waitlisted patients on maintenance dialysis. Nephrol Dial Transplant. 2011;26:1058–1065. doi: 10.1093/ndt/gfq476. [DOI] [PubMed] [Google Scholar]
- 2.McDonald SP, Russ GR. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrol Dial Transplant. 2002;17:2212–2219. doi: 10.1093/ndt/17.12.2212. [DOI] [PubMed] [Google Scholar]
- 3.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11:917–922. doi: 10.1681/ASN.V115917. [DOI] [PubMed] [Google Scholar]
- 4.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343:1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 5.Ladin K, Rodrigue JR, Hanto DW. Framing disparities along the continuum of care from chronic kidney disease to transplantation: barriers and interventions. Am J Transplant. 2009;9:669–674. doi: 10.1111/j.1600-6143.2009.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powe NR, Melamed ML. Racial disparities in the optimal delivery of chronic kidney disease care. Med Clin North Am. 2005;89:475–488. doi: 10.1016/j.mcna.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68:914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation–clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343:1537–1544. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young CJ, Kew C. Health disparities in transplantation: focus on the complexity and challenge of renal transplantation in African Americans. Med Clin North Am. 2005;89:1003–1031. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Fan PY, Ashby VB, Fuller DS, Boulware LE, Kao A, Norman SP, et al. Access and outcomes among minority transplant patients, 1999–2008, with a focus on determinants of kidney graft survival. Am J Transplant. 2010;10:1090–1107. doi: 10.1111/j.1600-6143.2009.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navaneethan SD, Singh S. A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplant. 2006;20:769–775. doi: 10.1111/j.1399-0012.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 12.Young CJ, Gaston RS. African Americans and renal transplantation: disproportionate need, limited access, and impaired outcomes. Am J Med Sci. 2002;323:94–99. doi: 10.1097/00000441-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Joshi S, Gaynor JJ, Bayers S, Guerra G, Eldefrawy A, Chediak Z, et al. Disparities among Blacks, Hispanics, and Whites in time from starting dialysis to kidney transplant wait listing. Transplantation. 2013;95:309–318. doi: 10.1097/TP.0b013e31827191d4. [DOI] [PubMed] [Google Scholar]
- 14.United States Department of Health and Human Services, Health Resources and Services Administration. Scientific Registry of Transplant Recipients. 2013 Sep 25; www.optn.transplant.hrsa.gov. Website accessed.
- 15.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–152. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Differences in prevalence of obesity among black, white, and Hispanic adults - United States, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58:740–744. [PubMed] [Google Scholar]
- 17.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17:963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Lunsford SL, Simpson KS, Chavin KD, Hildebrand LG, Miles LG, Shilling LM, et al. Racial differences in coping with the need for kidney transplantation and willingness to ask for live organ donation. Am J Kidney Dis. 2006;47:324–331. doi: 10.1053/j.ajkd.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Klassen AC, Hall AG, Saksvig B, Curbow B, Klassen DK. Relationship between patients’ perceptions of disadvantage and discrimination and listing for kidney transplantation. Am J Public Health. 2002;92:811–817. doi: 10.2105/ajph.92.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigue JR, Pavlakis M, Egbuna O, Paek M, Waterman AD, Mandelbrot DA. The "House Calls" trial: a randomized controlled trial to reduce racial disparities in live donor kidney transplantation: rationale and design. Contemp Clin Trials. 2012;33:811–818. doi: 10.1016/j.cct.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigue JR, Cornell DL, Kaplan B, Howard RJ. Patients' willingness to talk to others about living kidney donation. Prog Transplant. 2008;18:25–31. doi: 10.1177/152692480801800107. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigue JR, Cornell DL, Lin JK, Kaplan B, Howard RJ. Increasing live donor kidney transplantation: a randomized controlled trial of a home-based educational intervention. Am J Transplant. 2007;7:394–401. doi: 10.1111/j.1600-6143.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 23.Prochaska J, DiClemente C, Norcross J. In search of how people change: Applications to addictive behaviors. Am Psychol. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Kosinki M, Dewey JE. How to score version 2 of the SF-36® health survey (standard & acute forms) Lincoln, RI: Quality Metric; 2000. [Google Scholar]
- 25.Waterman AD, Peipert JD, Hyland SS, McCabe MS, Schenk EA, Liu J. Modifiable patient characteristics and racial disparities in evaluation completion and living donor transplant. Clin J Am Soc Nephrol. 2013;8:995–1002. doi: 10.2215/CJN.08880812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterman AD, Rodrigue JR, Purnell TS, Ladin K, Boulware LE. Addressing racial and ethnic disparities in live donor kidney transplantation: priorities for research and intervention. Semin Nephrol. 2010;30:90–98. doi: 10.1016/j.semnephrol.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bratton C, Chavin K, Baliga P. Racial disparities in organ donation and why. Curr Opin Organ Transplant. 2011;16:243–249. doi: 10.1097/MOT.0b013e3283447b1c. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigue JR, Cornell DL, Kaplan B, Howard RJ. A randomized trial of a home-based educational approach to increase live donor kidney transplantation: effects in blacks and whites. Am J Kidney Dis. 2008;51:663–670. doi: 10.1053/j.ajkd.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Waterman AD, Robbins ML, Paiva AL, Hyland SS. Kidney patients' intention to receive a deceased donor transplant: development of stage of change, decisional balance and self-efficacy measures. J Health Psychol. 2010;15:436–445. doi: 10.1177/1359105309351248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunsford SL, Simpson KS, Chavin KD, Menching KJ, Miles LG, Shilling LM, et al. Racial disparities in living kidney donation: Is there a lack of willing donors or an excess of medically unsuitable candidates? Transplantation. 2006;82:876–881. doi: 10.1097/01.tp.0000232693.69773.42. [DOI] [PubMed] [Google Scholar]
- 31.Gill JS, Gill J, Barnieh L, et al. Income of living kidney donors and the income difference between living kidney donors and their recipients in the United States. Am J Transplant. 2012;12:3111–3118. doi: 10.1111/j.1600-6143.2012.04211.x. [DOI] [PubMed] [Google Scholar]
- 32.Gill J, Dong J, Rose C, Johnston O, Landsberg D, Gill J. The effect of race and income on living kidney donation in the United States. J Am Soc Nephrol. doi: 10.1681/ASN.2013010049. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schold JD, Goldfarb DA, Buccini LD, et al. Hospitalizations following living donor nephrectomy in the United States. Clin J Am Soc Nephrol. doi: 10.2215/CJN.03820413. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis CL. Living kidney donors: current state of affairs. Adv Chronic Kidney Dis. 2009;16:242–249. doi: 10.1053/j.ackd.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Gross CR, Messersmith EE, Hong BA, et al. Health-related quality of life in kidney donors from the last five decades: results from the RELIVE study. Am J Transplant. doi: 10.1111/ajt.12434. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibney EM, King AL, Maluf DG, Garg AX, Parikh CR. Living kidney donors requiring transplantation: focus on African Americans. Transplantation. 2007;84:647–649. doi: 10.1097/01.tp.0000277288.78771.c2. [DOI] [PubMed] [Google Scholar]
- 37.Lentine KL, Segev DL. Health outcomes among non-Caucasian living kidney donors: knowns and unknowns. Transpl Int. 2013;26:853–864. doi: 10.1111/tri.12088. [DOI] [PubMed] [Google Scholar]
- 38.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363:724–732. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan BR, Ibrahim HN. Long-term outcomes of kidney donors. Curr Opin Nephrol Hypertens. 2011;20:605–609. doi: 10.1097/MNH.0b013e32834bd72b. [DOI] [PubMed] [Google Scholar]
- 40.Pollak MR, Genovese G, Friedman DJ. APOL1 and kidney disease. Curr Opin Nephrol Hypertens. 2012;21:179–182. doi: 10.1097/MNH.0b013e32835012ab. [DOI] [PubMed] [Google Scholar]
- 41.Cohen DM, Mittalhenkle A, Scott DL, Young CJ, Norman DJ. African American living-kidney donors should be screened for APOL1 risk alleles. Transplantation. 2011;92:722–725. doi: 10.1097/TP.0b013e31822eec39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ladin K, Hanto DW. Understanding disparities in transplantation: do social networks provide the missing clue? Am J Transplant. 2010;10:472–476. doi: 10.1111/j.1600-6143.2009.02963.x. [DOI] [PubMed] [Google Scholar]
- 43.McDonald EL, Powell CL, Perryman JP, Thompson NJ, Arriola KR. Understanding the relationship between trust in health care and attitudes toward living donor transplant among African Americans with end-stage renal disease. Clin Transplant. 2013;27:619–626. doi: 10.1111/ctr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeves-Daniel AM, Farney AC, Fletcher AJ, et al. Ethnicity, medical insurance, and living kidney donation. Clin Transplant. 2013;27:E498–E503. doi: 10.1111/ctr.12168. [DOI] [PubMed] [Google Scholar]
- 45.Boulware LE, Hill-Briggs F, Kraus ES, et al. Protocol of a randomized controlled trial of culturally sensitive interventions to improve African Americans' and non-African Americans' early, shared, and informed consideration of live kidney transplantation: the Talking About Live Kidney Donation (TALK) Study. BMC Nephrology. 2011;12:34. doi: 10.1186/1471-2369-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ismail SY, Luchtenburg AE, Zuidema WC, et al. Multisystemic engagement and nephrology based educational intervention: a randomized controlled trial protocol on the Kidney Team At Home study. BMC Nephrol. 2012;13:62. doi: 10.1186/1471-2369-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]