Abstract
Cell-based therapies for global cerebral ischemia represent promising approaches for neuronal damage prevention and tissue repair promotion. We examined the potential of Marrow-Isolated Adult Multilineage Inducible (MIAMI) cells, a homogeneous subpopulation of immature human mesenchymal stromal cell, injected into the hippocampus to prevent neuronal damage induced by global ischemia using rat organotypic hippocampal slices exposed to oxygen-glucose deprivation (OGD) and rats subjected to asphyxial cardiac arrest (ACA). We next examined the value of combining fibronectin-coated biomimetic microcarriers (FN-BMMs) with EGF/bFGF pre-treated MIAMI compared to EGF/bFGF pre-treated MIAMI cells alone, for their in vitro and in vivo neuroprotective capacity. Naïve and EGF/bFGF pre-treated MIAMI cells significantly protected the Cornu Ammonis layer 1 (CA1) against ischemic death in hippocampal slices and increased CA1 survival in rats. MIAMI cells therapeutic value was significantly increased when delivering the cells complexed with FN-BMMs, probably by increasing stem cell survival and paracrine secretion of pro-survival and/or anti-inflammatory molecules as concluded from survival, differentiation and gene expression analysis. Four days after OGD and ACA, few transplanted cells administered alone survived in the brain whereas stem cell survival improved when injected complexed with FN-BMMs. Interestingly, a large fraction of the transplanted cells administered alone or in complexes expressed βIII-Tubulin suggesting that partial neuronal transdifferentiation may be a contributing factor to the neuroprotective mechanism of MIAMI cells.
Keywords: Cerebral ischemia, Neuroprotection, Gene expression, Marrow Isolated Adult Multilineage Inducible Cells, tissue engineering
INTRODUCTION
Global cerebral ischemia that usually results from cardiac arrest (CA) remains one of the leading causes of death and disability in the USA affecting 150,000 Americans each year (Noh et al. 2005). The chances of survival following CA are poor despite the fast emergency responses and improved techniques of defibrillation. During the ischemic insult all brain areas experience oxygen and glucose deprivation but only selected neuronal populations such as the Cornu Ammonis layer 1 (CA1) pyramidal neurons in the hippocampus degenerate and die days later (Noh et al. 2005). Current treatments, although helpful, fail to prevent cognitive, motor, and speech impairment due to brain damage caused by CA. Thus, the development of neuroprotective and neurorestorative therapies remains a major unfulfilled medical need. In this regard, a stem cell-based therapy provides a promising therapeutic approach for preventing neuronal damage and promoting tissue repair.
Among the different stem cell sources, adult multipotent mesenchymal stromal cells (MSCs) are good candidates for cell therapy studies due to their extensive differentiation potential (D'Ippolito et al. 2004), their immunomodulatory characteristics (Le Blanc 2003)(Maitra et al. 2004) and their ability to secrete a variety of growth factors and cytokines (Caplan & Dennis 2006). One goal is to isolate the ideal MSCs population from the patient's bone marrow, expand them in culture (or not) and transplant them to affected tissue for therapeutic benefit.
Evidence supporting MSC use in cerebral therapy is that when transplanted into adult rat brains, they respond to microenvironmental signals to differentiate into neural-like cells (Kopen et al. 1999)(Jendelova et al. 2004). In addition, MSCs, can migrate to the cerebral damage areas (Delcroix et al. 2009)(Jendelova et al. 2004)(Sykova & Jendelova 2007), and provide a functional improvement in animal models, either directly or by paracrine secretion of various growth factors (Zheng et al.)(Chen et al. 2002)(Zhang et al. 2005). Clinically, MSC administration into the central nervous system (CNS) is feasible, appears to be safe in human subjects (Bang et al. 2005) (Lee et al. 2010) and is not hindered by ethical and tissue rejection-related concerns. A significant problem with human (h)MSC is their heterogeneity during culture and their inconsistent effects (Li et al. 2008). The use of marrow-isolated adult multilineage inducible (MIAMI) cells could overcome this limitation.
MIAMI cells are a unique hMSC subpopulation exhibiting a homogeneous morphology and gene expression profile characterized by the increased expression of markers present in pluripotent embryonic stem cells, (Oct-4, hTeRT, Nanog, Rex-1, and SSEA-4 (D'Ippolito et al. 2006), and the potential to generate differentiated cells derived from all three embryonic germ layers (D'Ippolito et al. 2004)(D'Ippolito et al. 2006). MIAMI cells are capable of differentiating into immature neuron-like cells exhibiting neuronal ionic channel activity in vitro on a fibronectin substrate, in a neurotrophine-3 dependent manner (Tatard et al. 2007). We recently showed that the pre-treatment of MIAMI cells with epidermal growth factor (EGF) combined with basic fibroblast growth factor (bFGF) enhanced neural specification and the response to neuronal commitment of MIAMI cells in vitro (Delcroix et al.2010a).
Cell-based therapies for treating cerebral ischemia raised great interest. However, only few studies using rat umbilical matrix cells (Jomura et al. 2007) and hMSCs (Ohtaki et al. 2008)(Zheng et al. 2010) have been reported using global ischemia models. Further studies are necessary to understand the stem cell mode of action in preventing neuronal damage after an intrinsically disseminated insult. The neurological benefits are assumed to mainly derive from the production of growth factors and other paracrine factors from MSCs in the ischemic tissue (Caplan & Dennis 2006)(Chen et al. 2002)(Delcroix et al. 2010b)(Ohtaki et al. 2008). In these studies, cell survival and the number of cells expressing neuronal or glial markers in the brain was very low (Caplan & Dennis 2006). Studies with neural stem cells and neural precursors associated with biomaterial-based scaffolds in order to enhance their functionality have been reported (for review (Delcroix et al. 2010b)(Tatard et al. 2005a)). All this evidence strongly supports the need to implement strategies that will enhance MSC survival, engraftment, differentiation and contribution to functional recovery thus, enhancing post-injury repair after cerebral ischemia.
To this end, pharmacologically active microcarriers (PAMs) conveying stem cells, provide a powerful tissue engineering approach. PAMs are biodegradable, biocompatible poly(lactic-co-glycolic acid) microparticles that release therapeutic molecules in a controlled manner while providing a biomimetic 3D support of extracellular matrix molecules. These combined actions stimulate cell survival and differentiation (Tatard et al. 2005b). The utility of PAMs has been validated in a rat model of Parkinson's disease (Tatard et al. 2007, Tatard et al. 2004).
In the present study we used MIAMI cells alone or conveyed by biomimetic microcarriers (BMMs), a primary prototype model for PAMs that do not release therapeutic molecules and that have a fibronectin (FN) surface to promote MSC survival (Karoubi et al. 2009), in order to investigate any potential synergistic therapeutic effects in ex vivo and in vivo rat models of global cerebral ischemia. The first objective was to assess the capacity of naïve MIAMI cells and EGF/bFGF (E/F) pre-treated pro-neural MIAMI cells to prevent hippocampal neuronal damage induced by global ischemia using rat organotypic hippocampal slices exposed to oxygen-glucose deprivation. We then evaluated the potential mechanisms underlying any neuroprotective effects. This therapeutic strategy was further evaluated in rats subjected to global cerebral ischemia caused by asphyxial cardiac arrest. Finally, we examined the value of combining FN-BMMs with pre-treated MIAMI compared to pre-treated MIAMI cells alone, for their in vitro and in vivo neuroprotective capacity.
MATERIALS AND METHODS
CELL CULTURE
Isolation and culture of MIAMI cells
Whole bone marrow from the iliac crest of a 20-year-old male living donor was obtained commercially (Lonza Walkersville, Maryland; MIAMI #3515). As previously described (D'Ippolito et al. 2004), MIAMI cells were isolated from whole bone marrow. Briefly, cells were plated at a density of 105 cells/cm2 in DMEM-low glucose media (Gibco, Carlsbad, CA, USA), containing 3% fetal bovine serum (Hyclone, South Logan, Utah, USA) and antibiotics on a FN (Sigma) substrate, under low oxygen conditions (3% O2, 5% CO2 and 92% N2). Fourteen days after the initial plating, non-adherent cells were removed. Single-cell-derived and pooled colonies of adherent cells were rinsed and sub-cloned. These cells were selected and plated at low density for expansion (100 cells/cm2) on 1.25 ng/cm2 FN-coated vessels. Cells were expanded in DMEM-low glucose, 3% FBS, antibiotics, 20mM ascorbic acid (Fluka, Ronkonkoma, NY, USA), and an essential fatty acid solution in low oxygen conditions (3% oxygen). Culture medium was changed every 2-3 days and the cells were split at ~50-60% confluency.
Pre-treatment of MIAMI cells with EGF/bFGF
To enhance neuronal specification, MIAMI cells were pre-treated in vitro for 7 days concurrently with epidermal growth factor (EGF; (Peprotech, Rocky Hill, NJ, USA) and basic fibroblast growth factor (bFGF; Peprotech) under low oxygen tension (E/F-treatment; 50 ng/mL each). Prior to injection of MIAMI cells into rat hippocampal organotypic cultures or into CA1 rat hippocampus, the EGF/bFGF pre-treated cells were detached, washed twice with phosphate buffer saline (PBS) and resuspended in the above described expansion medium without growth factors for organotypic injection or in Hanks balanced salt solution (HBSS) for rat CA1 injection.
RNA isolation and mRNA quantitation
After E/F pre-treatment, MIAMI cells were harvested and RNA was isolated using the RNAqueous®-4PCR kit (Ambion Inc, Austin TX, USA). RNA reverse transcription to cDNA was done on the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time PCR (RT-qPCR) was done using 10μl of 1:20 diluted cDNA on the Mx3005P Multiplex Quantitative PCR System (Stratagen, La Jolla, CA, USA) using qPCR SYBR GREEN Reagents (Brilliant® II SYBR® Green QPCR Master Mix, Agilent Technologies) with ROX reference dye. All of the corresponding RT-qPCR data analyzed were normalized to housekeeping genes; eukaryotic translational elongation factor 1 alpha (EF1a, NM_001402), and ribosomal protein L13a (RPL13a, NM_01242), were used (Curtis et al. 2010). A list of primer pair sequences used for the in vitro studies are in Table 1.
Table 1.
A) List of primer pairs used for cell culture studies
| Gene | Full Name | Accession Number | Sequence |
|---|---|---|---|
| Normalization Genes | |||
| EF1a | Eukaryotic translational elongation factor 1 alpha | NM_001101 | F=5′-AGGTGATTATCCTGAACCATCC-3’ R=5′-AAAGGTGGATAGTCTGAGAAGC-3’ |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | NM_002046 | F=5′-TGCACCACCAACTGCTTAGC-3′ R=5′-GGCATGGACTGTGGTCATGAG-3′ |
| HPRT1 | Hypoxanthine phosphoribosyltransferase 1 | NM_000194 | F=5’-TGACACTGGCAAAACAATGCA-3’ R=5’GGTCCTTTTCACCAGCAAGCT-3’ |
| RPL13a | Ribosomal protein L13a | NM_01242 | F=5’-CCTGGAGGAGAAGAGGAAAGAGA-3’ R=5’-TTGAGGACCTCTGTGTATTTGTCAA-3’ |
| YWHAZ | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide variant 1 & 2 |
NM_003406 NM_145690 |
F=5’-ACTTTTGGTACATTGTGGCTTCAA-3’ R=5’-CCGCCAGGACAAACCAGTAT-3’ |
| UBC | Ubiquitin C | NM_021009 | F=5’-ATTTGGGTCGCGGTTCTTG-3’ R=5’-TGCCTTGACATTCTCGATGGT-3’ |
| Target Genes | |||
| CCNB1 | Cyclin B1 | NM_031966 | F=5’-TTGGGGACATTGGTAACAAAGTC-3’ R=5’-ATAGGCTCAGGCGAAAGTTTTT-3’ |
| CCND1 | Cyclin D1 | NM_053056 | F=5’-GTGCTGCGAAGTGGAAACC-3’ R=5’-ATCCAGGTGGCGACGATCT-3’ |
| HES1 | Hairy enhancer of split 1 | NM_005524 | F=5’-ATGGAGAAAAATTCCTCGTCCC-3’ R=5’-TTCAGAGCATCCAAAATCAGTGT-3’ |
| OCT4-A | POU class 5 homeobox 1 (POU5F1), transcript variant 1 | NM_002701 | F=5’-TGGAGAAGGAGAAGCTGGAGCAAAA-3’ R=5’-GGCAGATGGTCGTTTGGCTGAATA-3’ |
| MAP1b | Microtubule-associated protein 1B | NM_005909 | F=5’-CCTCGAGACGTGATGAGTGA-3’ R=5’-TTGGGCGTCAGAGAGAAGTT-3’ |
| MAP2 | Microtubule-associated protein 2 | NM_001039538 | F=5’-CTGCTTTACAGGGTAGCACAA-3’ R=5’-TTGAGTATGGCAAACGGTATG-3’ |
| MSI1 | Musashi homolog 1 | NM_002442 | F=5’-GACTCGAACGAAGAAGATCTTTG-3’ R=5’-TTCACACACTTTCTCCACGATG-3’ |
| NES | Nestin | NM_006617 | F=5’-AGAGGGGAATTCCTGGAG-3’ R=5’-CTGAGGACCAGGACTCTCTA-3’ |
| NFM | Neurofilament, medium polypeptide 150kDa | NM_005382 | F=5’-GATCCAGGCATCGCACATCA-3’ R=5’-CTGGTGCATATTCTGGTCTGA-3’ |
| NFH | Neurofilament, heavy polypeptide | NM_021076 | F=5’-GCAGTCCGAGGAGTGGTTC-3’ R=5’-TAGCGTCTGTGTTCACCTTGG-3’ |
| NEUROG2 | Neurogenin 2 | NM_024019 | F=5’-CGCATCAAGAAGACCCGTAG-3’ R=5’-GTGAGTGCCCAGATGTAGTTGTG-3’ |
| PITX3 | Paired-like homeodomain 3 | NM_005029 | F=5’-AGAGGACGGTTCGCTGAAAAA-3’ R=5’-AGCTGCCTTTGCATAGCTCG-3’ |
| Prox1 | Prospero homeobox 1 | NM_002763 | F=5’-GAGAGATTCCTGGAAGTTGCTC-3’ R=5’-CATATCCAGCTTGCAGATGAC-3’ |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21), transcript variant 1 & 2 |
NM_000389 NM_078467 |
F=5’-CCTGTCACTGTCTTGTACCCT-3’ R=5’-GGTTTGGAGTGGTAGAAATCT-3’ |
| TrkA | Neurotrophin tyrosine kinase, receptor, type 1, transcript variant 1-3 | NM_002529 | F=5’-CTCCAAGGCCACATCATCGAG-3’ R=5’-GAAGAAGCGCACGATGTGCTG-3’ |
| TrkB | Neurotrophin tyrosine kinase, receptor, type 2, transcript variant 1-5 |
NM_006180 NM_001007097 |
F=5’-TTCCCCTGGCAAACCTGCAG-3’ R=5’-TGGATGCAGCCGTGGTACTC-3’ |
| TrkC | Neurotrophin tyrosine kinase, receptor, type 3, transcript variant 1-3 |
NM_001012338 NM_002530 |
F=5’-GCCAACCAGACCATCAATGGCCAC-3’ R=5’-TGACAGCCACGGGACCCTTCATTC-3’ |
| TrkCc | Tyrosine kinase deficient-neurotrophin tyrosine kinase, receptor, type3, transcript variant 3 | NM_001007156 | F=5’-TACGAGGCAGCTCCTGCCACTATC-3’ R=5’-GGTGCCAATACTTGAGCCTGGCTC-3’ |
| B) List of primer pairs used for organotypic culture studies | |||
|---|---|---|---|
| Gene | Full Name | Accession Number | Sequence |
| Human Specific Normalization | |||
| hRPL13a | Ribosomal protein L13a | NM_01242 | F=5’-CATAGGAAGCTGGGAGCAAG-3’ R=5’-GCCCTCCAATCAGTCTTCTG-3’ |
| hYWHAZ | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide variant 1 & 2 |
NM_003406 NM_145690 |
F=5’- TGCTTGCATCCCACAGACTA-3’ R=5’- AGGCAGACAATGACAGACCA-3’ |
| Human Specific Target Genes | |||
| hIGF1 | Insulin-like growth factor 1 (somatomedin C) | NM_000618 | F=5’-CCTGCGCAATGGAATAAAGT-3’ R=5’-CAAGAAATCACAAAAGCAGCA-3’ |
| hIGFBP3 | Insulin-like growth factor binding protein 3, transcript variant 1 & 2 |
NM_001013398 NM_000598 |
F=5’-AATGGCACAATTCTTCGGAT-3’ R=5’-AAGCCATTCCTCCTTCCTGT-3’ |
| hIGFBP5 | Insulin-like growth factor binding protein 5 | NM_000599 | F=5’-CTTTGGAAACTTCTGCAGGG-3’ R=5’-GAAATTCGCAGGTTCTACGC-3’ |
| hSTC1 | Stanniocalcin 1 | NM_003155 | F=5’-AGGCAAGGCTGACTTCTCTG-3’ R=5’-AACTACTTGTCGCATTGGGG-3’ |
| hTSG6 | Tumor necrosis factor, alpha-induced protein 6 (TNFAIP6) | NM_007115 | F=5’-TCACATTTCAGCCACTGCTC-3’ R=5’-TGATCATATCGTCAGTTGTAGTGAA-3’ |
| hLTBP2 | Latent transforming growth factor binding protein 2 | NM_000428 | F=5’-GAGCCCAGCTGGAGTAGGA-3’ R=5’-AGCTTCTCTGAGTCTAGGGGG-3’ |
| Rat Specific Normalization | |||
| rRPL13a | Ribosomal protein L13a | NM_173340 | F=5’-GGCTGAAGCCTACCAGAAAG -3’ R=5’-CTTTGCCTTTTCCTTCCGTT-3’ |
| Rat Specific Target Genes | |||
| rIGFI | Insulin-like growth factor 1 (somatomedin C), transcript variant 1-4 |
NM_178866 NM_0010824779 |
F=5’- GCTGAAGCCGTTCATTTAGC -3’ R=5’- GAGGAGGCCAAATTCAACAA-3’ |
| rIGFBP3 | Insulin-like growth factor binding protein 3 | NM_012588 | F=5’- CTCCATGTGCAGAGATGTCG -3’ R=5’- CTCTTTTGAAAGCTGCTCC -3’ |
| rIGFBP5 | Insulin-like growth factor binding protein 5 | NM_012817 | F=5’-AAGGAGACACTCCCCATTCC-3’ R=5’-TTCCCTTCTCTGTCCGTTCA-3’ |
Fibroblast culture
Post-natal human foreskin fibroblast cells were obtained from ATCC (Manassas, VA, USA) and cultured in DMEM-high glucose, 10% fetal bovine serum plus antibiotics, in atmosphere O2 and 5% CO2.
FN-BMMs
FN-BMMs formulation and characterization
Microcarriers used herein are 30 μm biodegradable, biocompatible poly(lactic-co-glycolic acid) microparticles. Polymer used for microparticle formulation was a poly(lactic-co-glycolic acid) copolymer with a lactic:glycolic ratio of 37.5:25 (MW: 14,000 Da) (Phusis, Saint Ismier, France). Microparticles were prepared using a single emulsion solvent extraction-evaporation method described previously to obtain 60 μm microparticles with some modifications (Giteau et al. 2008). The organic solution (2 mL; 3:1 methylene chloride:acetone) containing poly(lactic-co-glycolic acid) (150 mg) was emulsified in an aqueous phase (90 mL; 6% poly(vinylalcohol)) (Mowiol® 4-88, Kuraray Specialities Europe, Frankfurt, Germany) maintained at 1°C and mechanically stirred at 1000 rpm for 1 min (Heidolph, RZR 2041, Merck Eurolab, Paris, France). After addition of 100 mL of deionized water and stirring for 10 min, the resulting emulsion was added to 500 mL deionized water and stirred for 20 min for organic solvent extraction. Microparticles were filtered on a 0.45 μm filter (HVLP type, Millipore SA, Guyancourt, France), washed and freeze-dried. Particle average volume diameter and size distribution were evaluated using a Multisizer™ Coulter Counter (Beckman Coulter, Roissy, France). FN-BMMs were prepared coating the obtained microcarriers with a combination of FN at 16 μg/mL and poly-D-lysine at 24 μg/mL (Sigma-Aldrich) to functionalize their surface and favour cell attachment. To this end, microcarriers were resuspended in PBS, sonicated for full dispersion and mixed with the coating molecule solution. For adsorption, the mixture “microcarrier/coating molecules” was placed under rotation at 15 rpm at 37 °C during 4 h. FN-BMMs were washed 3 times in distilled sterile water, lyophilized and kept at −20°C for long-term storage. The FN-BMMs electrical surface charge was determined by zeta potential measurements using a Zetasizer 2000 (Malvern Instruments, Orsay, France) operating at 150 V at room temperature. FN-BMMs were dispersed in 1 mM NaCl and sonicated prior to every measurement. Results are the average of 10 measurements. Experiments were performed in triplicate.
Formation of MIAMI/FN-BMM complexes
FN-BMMs (0.5mg) were resuspended in culture medium for 15 min in coated Eppendorf tubes (Sigmacote, Sigma). After sonication, the cell suspension was added (7×104 cells/0.5 mg FN-BMMs for in vitro experiments and 4×105 cells/0.5 mg FN-BMMs for in vivo experiments) and the mixture was then gently swirled and plated in 1.9 cm2 Costar ultra low cluster plate (Corning, Avon, France). Plates were incubated at 37°C for 4 h to allow cell attachment on FN-BMMs surfaces. Cells/FN-BMMs complexes were recovered, washed and pelleted by centrifugation at 200g for 2 min. Cell adhesion to FN-BMMs surfaces was assessed by microscopic observation and cells adhered to FN-BMMs were quantified using the Cyquant cell proliferation assay (Invitrogen, Cergy Pontoise, France), following the manufacturer's guidelines. MIAMI/FN-BMMs complexes were observed using light microscopy and scanning electron microscopy. Samples were prepared for scanning electron microscopy analysis as previously described (Tatard et al. 2005b).
ASSESMENT OF THE NEUROPROTECTIVE EFFECT OF MIAMI CELLS IN VITRO
Preparation of rat organotypic hippocampal slices cultures
All animal protocols, for the ex vivo and in vivo studies, were approved by the Animal Care and Use Committee of the University of Miami and carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and were approved by the Animal Care Committee of the University of Miami. Unless otherwise specified, all reagents were obtained from Sigma-Aldrich. Organotypic hippocampal slice cultures were prepared as described previously (Raval et al. 2003). Hippocampi from post-natal 9- to 11-day old Sprague-Dawley rat pups were dissected and transversally sliced to 400 μm thickness using a McIlwain tissue chopper. Slices were placed in ice-cold Gey's balanced salt solution supplemented with 6.5 mg/mL glucose for one hour and were next transferred to 30 mm diameter membrane inserts (Millicell-CM, Millipore, Bedford, MA, USA). The culture medium consisted of 50% MEM, 25% HBSS, 25% heat-inactivated horse serum (all from Gibco/Life Technologies, Carlsbad, CA, USA) supplemented with 6.5 mg/mL glucose and 1 mM glutamine. The culture plates were kept at 37°C in a humidified atmosphere with 5% CO2. Slices were kept in culture for 14-15 days before the experiments with media changes every three days.
Induction of ischemia by oxygen glucose deprivation (OGD)
Ex vivo ischemia was simulated using an established model consisting of oxygen and glucose deprivation (OGD) (Raval et al. 2003). In this model, oxygen is replaced with nitrogen and glucose with sucrose. Slices were washed three times with aglycemic Hanks’ balanced salt solution (pH 7.4). Subsequently, the slice cultures were transferred into an anaerobic chamber (PROOX model 110, BioSpherix, Ltd., Redfield, NY, USA) which was placed in a water-jacketed incubator containing 95% N2/5% CO2 at 37 °C. The chamber was sealed for 40 min of ischemic insult. Following OGD, slices were transferred to normal culture media and placed back into the incubator.
Ex vivo experimental groups
One hour after OGD, naïve MIAMI cells, E/F pre-treated MIAMI cells, naive MIAMI cells/FN-BMMs, E/F pre-treated MIAMI cells/FN-BMMs, human fibroblasts, FN-BMMs or culture medium (control) were injected at 3 sites in the CA1 cell body layer of hippocampal slices. Total injection volume consisted of 2 μl of culture media containing approximately 7,000 cells or 0.05 mg of FN-BMMs.
Assessment of neuronal cell death by propidium iodide (PI) staining technique
To determine the extent of neuronal damage in the organotypic slice culture, we used the propidium iodide method (Raval et al. 2003)(Xu et al. 2002). Slices were incubated in medium supplemented with 2 μg/mL propidium iodide (Sigma) for 1 h prior to imaging. Images were taken using a fluorescence microscope (Olympus IX 50), equipped with a light-intensifying SPOT CCD camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA), and SPOT Advanced software was used to assess the proportion of cell death. Images of the slices were taken (1) at baseline prior to OGD; (2) 24 h after OGD to assess ischemic damage; and (3) 24 h after NMDA treatment to assess maximum damage to neuronal cells. The hippocampal CA1 subfield was chosen as the region of interest, and quantification was performed using Scion Image software. The percentage of relative optical intensity served as an index of neuronal cell death. Relative cell death was calculated from each relative optical density as follows: Relative % cell death=(Fexp-Fmin)/(Fmax-Fmin)×100, where Fexp is the fluorescence of the test condition, Fmax is maximum fluorescence (100 μm NMDA treatment for 1-h), and Fmin is background fluorescence (prior to OGD). An investigator blinded to the experimental groups measured the propidium iodide intensity in slices.
NeuN immunohistochemistry staining
Seven days after injections, OGD slices were washed with PBS, fixed with 4% paraformaldehide for 4 h and washed with PBS. Slices were removed from membrane inserts and incubated free-floating in PBS containing 0.8% Triton X-100 (PBST). After pre-blocking with 10% goat serum, slices were incubated for 24-h at 4 °C with mouse monoclonal anti-NeuN (1:500 in PBST overnight at 4 °C; Chemicon, CA, USA). After overnight washing with PBST, the sections were then incubated with rhodamine-labeled anti-mouse secondary antibody (Santa Cruz Biotechnology, CA, USA), for 24-h at 4 °C temperature. Finally, the sections were rinsed, mounted using a Prolong Antifade kit (Molecular Probes, Inc., OR, USA), and then viewed on a Carl Zeiss Confocal Laser Scanning Microscope 510. The images of the sections were analyzed using LSM 5 image browser.
Human mitochondria and βIII-tubulin double immunofluorescen
Four days after injections, OGD slices were fixed with 4% paraformaldehyde at 4°C for 2-h. Carefully removed from inserts, permeabilized and blocked with 0.8% Triton X-100 at 4°C overnight. Blocking and diluent solutions consisted of PBST and 10% normal goat serum. Organotypic slices were incubated for 8-h with the primary antibodies βIII-tubulin and anti-human mithocondria (βIII-tubulin [TuJ1] Covance/mouse anti-human mithocondria MAB1273 Millipore), followed by 8-h incubation with the specific fluorescent secondary antibodies goat anti-rabbit IgG Alexa fluor 594 and goat anti-mouse IgG-FITC. DAPI (DAPI Nucleic Acid Stain D-1306 Molecular Probes) staining was performed as the final step for 5 min. PBST was used for the washes between each step and ProLong antifade kit to mount the samples (ProLong antifade kit P7481 Molecular Probes). Specific immunostaining was demonstrated in control experiments in which cells were exposed to primary isotypic antibodies and then incubated with conjugated antibodies. Color images were captured using a Nikon fluorescence microscope with FITC/Texas Red filters and merged using Adobe Photoshop 7. Confocal imaging of stained of brain organotypic slices was performed using a LEICA confocal microscope using 1-micron z-sections.
mRNA isolation and tissue species-specific RT-qPCR
Rat hippocampal organotypic slices with and without injected E/F pre-treated MIAMI cells were detached from insert membrane using a brush and washed in PBS prior to pelleting. The QIAshredder™ (Qiagen, Valencia, CA, USA) was used to disrupt the tissue prior to RNA isolation and cDNA synthesis as described above. Human and rat specific primer pair sequences were constructed for each gene analyzed (Curtis et al.). All human and rat species-specific primer pairs were validated with RT-qPCR using cDNA from human MIAMI cells H3515 or rat hippocampal organotypic slices either separately or in combination. All RT-qPCR results were normalized against a negative control, and a housekeeping gene. Ribosomal protein L13a primer pair sequences specific for human (hRPL13a, NM_01242) and rat (rRPL13a, NM_173340) were optimized and used for all normalization of RT-qPCR results. Zeta polypeptide variant 1 & 2 primer pairs specific for human were used as a second normalization genes (hYWHAZ, NM_003406 & NM_145690). Human and rat species specific primer pair sequences used are listed in Tables 1.
ASSESMENT OF THE NEUROPROTECTIVE EFFECT OF MIAMI CELLS IN VIVO
In vivo experimental groups
A total number of thirty male Sprague-Dawley rats weighing 250-350 g were used in this study. Animals that survived cardiac arrest were divided into the following groups: (Group 1) Sham asphyxial cardiac arrest (ACA). Sham surgery was performed. (Group 2) Ischemia + vehicle control. 8 min of ACA followed by the injection of 10-μl of HBSS 1h after ischemia onset. (Group 3) Ischemia + FN-BMMs. 8 min of ACA followed by the injection of 0.5 mg of FN-BMMs in 10-μl of HBSS 1h after ischemia onset. (Group 4) Ischemia + naive MIAMI cells. 8 min of ACA followed by the injection of 4 × 105 naive MIAMI cells in 10-μl of HBSS 1h after ischemia onset. (Group 5) Ischemia + E/F pre-treated MIAMI cells. 8 min of ACA followed by the injection of 4 × 105 E/F pre-treated MIAMI cells in 10-μl of HBSS 1h after ischemia onset. (Group 6) Ischemia + E/F pre-treated MIAMI cells-FN-BMMs complexes. 8 min of ACA followed by the injection of 4 × 105 E/F pre-treated MIAMI cells–FN-BMMs (0.5 mg) complexes in 10-μl of HBSS 1h after ischemia onset.
In vivo model of global cerebral ischemia, (ACA model)
The ACA model was performed as described previously (Della-Morte et al. 2009). Rats were fasted overnight and then anesthetized with 4% isoflurane and 70% nitrous oxide (in a balance of oxygen) followed by endotracheal intubation. Isoflurane was subsequently lowered to 1.5% to 2% for endovascular access. The femoral vein was cannulated and advanced 8-cm towards the heart and the femoral artery was cannulated for continuous blood pressure monitoring and blood gas analysis. Electrocardiographic leads were attached to the limbs. Vecuronium (2 mg/kg) (Gensia Sicor Pharmaceuticals, Irvine, CA, USA) was injected intravenously followed by mechanical ventilation (60-breaths/min) and lowering of isoflurane to 0.5%. Physiological variables, including, pCO2, pO2, pH, HCO3− and arterial base excess (ABL50, Radiometer Copenhagen, Westlake, OH, USA), were maintained within normal limits by adjusting the ventilator (UGO biological research apparatus, Comerio, Italy) volume settings. Mean arterial blood pressure (AMP 6600 Blood pressure amplifier, Gould Instrument Systems, Valley View, OH, USA) and electrocardiogram (ECG) (AMP 6600 Bioelectric amplifier, Gould Instrument Systems) were continuously monitored. The data was recorded using iWorx 118 Research Grade Data Recorder and Labscribe Data Acquisition Software (iWorx, Dover, NH, USA). The head and body temperatures were maintained at 36.5–37.0 °C using heating lamps. To induce ACA, apnea was induced by disconnecting the ventilator from the endotracheal tube. Eight min after asphyxia, resuscitation was initiated by administering a bolus injection of epinephrine (Sigma) (0.005 mg/kg, i.v.) and sodium bicarbonate (Sigma) (1 mq/kg, i.v.) followed by mechanical ventilation with 100% oxygen at a rate of 80 breaths/min and manual chest compressions at a rate of 200 min until mean arterial blood pressure reached 60 mm Hg and was maintained by a spontaneously beating heart for more than 10 s. 10 min after the restoration of spontaneous circulation, the ventilator rate was decreased to 60 breaths/min and the oxygen lowered to 30% in a mixture with N2. Arterial blood gases were then measured. If any corrections in acid–base status were necessary, sodium bicarbonate was administered and/or the ventilator settings were adjusted.
MIAMI cells and MIAMI/FN-BMM complexes grafting in ischemic model rats
Once the rats were hemodynamically stable and spontaneously breathing they were placed under isoflurane anaesthesia on a stereotactic frame. The stereotaxic coordinates used for cell injection into the left CA1 hippocampus were −3.6 mm rostral to Bregma, 2 mm lateral from the midline and −2.6 mm ventral from the dura (Paxinos & Watson 1996). One hour after CA onset, the naïve or E/F pre-treated MIAMI cells, FN-BMMs alone or the E/F pre-treated MIAMI/FN-BMMs complexes were injected using a 10-μl Hamilton microsyringe connected to a programmable infusion pump at 1 μl/minute infusion rate. The needle was left in place for 10 min to avoid the cells being expelled from the brain. After the needle was withdrawn the skin was sutured closed. Catheters were removed, the animal was extubated, 100% O2 was delivered via face mask for 30 min and then animal was placed overnight in a humidified incubator that maintained an ambient temperature of 29 °C.
Histopathology
Rats were anesthetized with isoflurane 4 days after ACA and then perfused with a mixture of 40% formaldehyde, glacial acetic acid, and methanol, 1:1:8 by volume (Perez-Pinzon et al. 1997). Brains were removed, and coronal brain blocks were embedded in paraffin; coronal sections of 10-μm thickness were cut and stained with hematoxylin and eosin. The entire hippocampus (anterior to posterior) was examined. For each animal, normal neurons were counted in the CA1 region of each hippocampus by an investigator blinded to the experimental conditions. Coronal brain sections were made at the level of 3.8 mm from posterior to Bregma. For each section, 18 fields per sections were obtained along the medial to lateral extent of the CA1 region of the hippocampus. Neurons exhibiting ischemic cell change were identified by (1) eosinophilic cytoplasm, (2) dark-staining triangular shaped nuclei, and (3) eosinophilic-staining nucleolus. Three slides per rat were counted. The data are presented as the mean count from three slides.
h-Mitochondria and βIII-Tubulin double immunofluorescen
Endogeneous peroxidase was blocked using 3% H2O2 in methanol for 5 min. For antigen retrieval, sections were incubated for 20 min hot 10 mM citrate buffer (pH 6.0). Immunofluorescence of MIAMI cells was done using mouse monoclonal anti-human-mitochondria antibodies (1:100) (Millipore) for 30 min at room temperature. Antibody binding was detected with biotinylated anti-mouse IgG (1/200) for 20 min at room temperature, followed by FITC-avidin DCS (1/300, Vector laboratories) for 5 min, and by avidin/biotin blocking for 15 min. Then, immunostaining with a rabbit polyclonal anti-βIII-Tubulin (TuJ1, 1:1000) (Covance, Denver, PA, USA), detection with biotinylated anti-rabbit IgG and Texas Red DCS for 5 min. Color images were captured using a Nikon Eclipse 90i fluorescence microscope with FITC/Texas Red filters and merged using Adobe Photoshop 7. For negative controls, the same concentration of mouse or rabbit pre-immune IgGs (Santa Cruz Biotechnologies) were used resulting in lack of immunostaining.
STATISTICAL ANALYSES
Data are presented as the mean value of three independent experiments ± standard error of the mean (SEM), unless otherwise stated. Results are expressed as mean±SEM. Statistics were calculated with SPSS computer software for Windows (version 15.0, SPSS Inc, Chicago, Ill). For in vitro experiments non-parametric statistical analyses were used when values were not normally distributed. The differences among the groups were first evaluated using the Kruskal–Wallis Test, followed by Mann–Whitney U-test comparing individual groups where necessary. For in vivo experiments, statistical evaluation was performed using ANOVA test, followed by Tukey's post hoc test. P<0.05 were considered significant.
RESULTS
E/F pre-treatment promotes the neural specification of MIAMI cells
MIAMI cells respond to E/F pre-treatment by decreasing proliferation and acquiring a more neural phenotype characterized by the expression of genes typical of neural progenitor cells. A representative quantitative result of E/F pre-treatment on the proliferation rate of the cells is: MIAMI cells expanded with 50ng/ml EGF/bFGF for 2 five-day periods had a decreased growth rate. The doubling time increased from 28.65±0.43 to 30.81±0.64 comparing normal to E/F pre-treated MIAMI cells respectively. (n=3 independent experiments in triplicate). The major changes induced at the mRNA level in MIAMI cells by E/F pre-treatment are summarized in Table 2. Briefly, E/F pre-treatment decreased cyclinB1, involved in cellular proliferation, and increased the anti-proliferative gene p21, which is consistent with the observed decreased proliferation rate. E/F pre-treatment of MIAMI cells also increased mRNA of neural/neuronal cytoskeletal proteins such as neurofilament medium polypeptide and neurofilament heavy polypeptide. Microtubule-associated protein 2 and microtubule-associated protein 1b also tended to increase but with no statistical significance. The TrkB neurotrophin receptor was stimulated but the TrkA, TrkC, and TrkC-c mRNA levels did not change significantly. E/F pre-treatment of MIAMI cells also increased the expression of the pro-survival molecule stanniocalcin (STC-1) and decreased the expression of the anti-inflammatory molecule latent transforming growth factor binding protein 2 (LTBP2). These results confirm and extend our previous observations and illustrate an E/F-induced specification of MIAMI cells towards a neural phenotype and a different paracrine profile for naïve and E/F pre-treated cells. Thus, we decided to compare the neuroprotective/reparative capacity of immature naïve MIAMI cells (Fig 1A) with pre-neuronal E/F pre-treated MIAMI cells (Fig 1B) on brain injury caused by CA mediated ischemia.
Table 2.
Effect of EGF/bFGF treatment on MIAMI cell mRNA levels
| Transcription factors | Fold Change (SD) | Significance* | |
|---|---|---|---|
| Oct4a | ↓ | 1.26±0.40 | NO |
| NGN2 | ↑ | 1.62±1.36 | NO |
| Prox1 | ↔ | 1.02±0.20 | NO |
| Pitx3 | ↑ | 1.49±0.62 | NO |
| Hes1 | ↓ | 0.57±0.09 | p=0.0137 |
| Cell cycle | |||
| Cyclin B1 | ↓ | 0.54±0.13 | p=0.0144 |
| p21 | ↑ | 3.23±0.24 | p=0.0001 |
| Neuronal cytoskeletal proteins | |||
| Nestin | ↑ | 1.28±0.38 | NO |
| NFM | ↑ | 1.76±0.58 | p=0.0492 |
| NFH | ↑ | 1.96±0.48 | p=0.0165 |
| Map2 | ↑ | 1.64±0.98 | NO |
| Map1b | ↑ | 1.31±0.29 | NO |
| Neurotrophic receptors | |||
| TrkA | ↔ | 0.96±0.08 | NO |
| TrkB | ↑ | 1.35±0.20 | p=0.0494 |
| TrkC | ↔ | 1.09±0.17 | NO |
| TrkCc | ↑ | 1.42±0.74 | NO |
| Others | |||
| STC1 | ↑ | 4.21±0.18 | p<0.0001 |
| TSG6 | ↔ | 0.98±0.24 | NO |
| LTBP2 | ↓ | 0.23±0.18 | p=0.0039 |
p values were calculated using one-tailed student's t-test. p values <0.05 are considered significant
Figure 1.
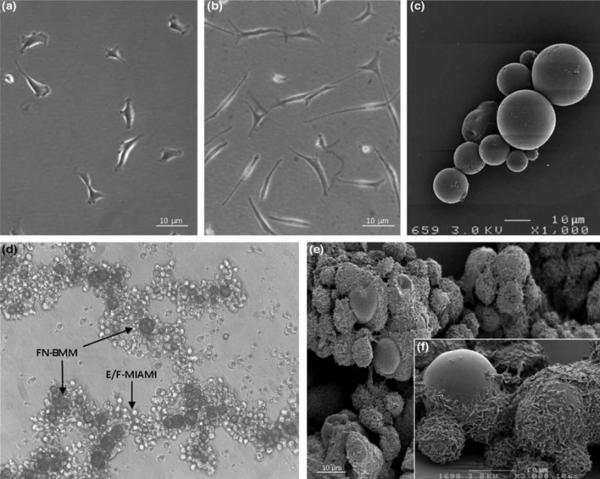
Representative images showing bright field images of näive MIAMI cells (A) and E/F pre-treated MIAMI cells (B), scanning electron microscopy images of FN-BMM (C), bright field (D) and scanning electron microscopy images of E/F pre-treated MIAMI/FN-BMM complexes (E) higher magnification of scanning electron microscopy of E/F pre-treated MIAMI/FN-BMM complexes (F). Images D, E and F show the 3 dimensional structures of the cells/FN-BMM complexes.
MIAMI/FN-BMMs complex characterization
Mean particle size of the microcarriers was 25.8 ± 8.8 μm (Fig 1C). The FN biomimetic surface was homogeneous as confirmed by confocal microscopy (data not shown). FN-BMMs have a zeta potential of 40.75 +/− 2.92 which is satisfactory for cell adhesion since positively charged surface promotes adhesion of the cells. E/F pre-treated MIAMI cells adhered well to FN-BMMs and formed 3D complexes at the end of the cell attachment protocol as observed by optical and scanning electron microscopy (Fig 1D-1E-1F). Moreover, viable cell quantification measures showed that 80% of both types of cells were adhered to the FN-BMMs.
MIAMI cells induce neuroprotection in the hippocampal organotypic slices OGD model and FN-BMMs enhance their therapeutic effect
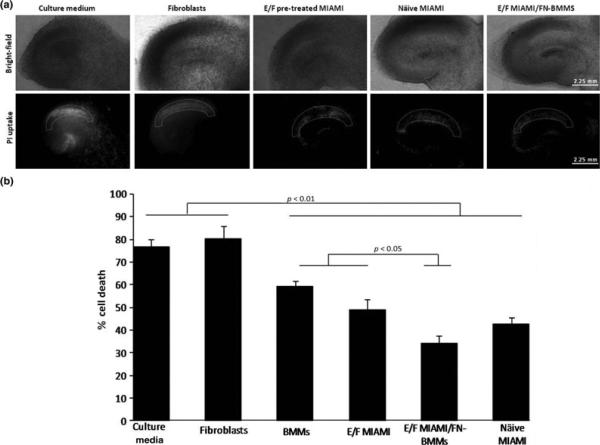
We examined the neuroprotective effect of naive and E/F pre-treated MIAMI cells injected alone or in complexes with FN-BMMs using an ischemia ex vivo model. Propidium fluorescence values (mean ± SEM) were: culture media, 76.68 ± 3.41 (n=19); naive MIAMI cells, 42.70 ± 3.1 (n=7); E/F pre-treated MIAMI cells, 48.97 ± 4.5 (n=10); E/F pre-treated MIAMI cells/FN-BMMs, 33.99 ± 3.33 (n=10); fibroblast, 80.27 ± 5.46 (n=6); BMM, 59.23 ± 2.60 (n=14). Both naïve and E/F pre-treated MIAMI cells alone or complexed with FN-BMMs significantly protected the hippocampus CA1 region compared to no protection with the culture media- or fibroblast-injected groups (p<0.01 for all the groups; non-parametric Kruskal-Wallis followed by Mann-Whitney-U test) (Fig 2B). The injection of E/F pre-treated MIAMI cells/FN-BMMs were significantly more neuroprotective than the injection of E/F pre-treated MIAMI cells alone demonstrating that the therapeutic value of these cells can be enhanced by delivering the cells in complexes with FN-BMMs (p<0.05; non-parametric Kruskal-Wallis followed by Mann-Whitney-U test). Näive MIAMI cells/FN-BMMs neuroprotective effect was similar that of E/F-pre-treated MIAMI cells/FN-BMMs (data not shown). Slices without ischemia exposed to FN-BMMs did not show cell death confirming their biocompatibility. Representative bright-field and PI fluorescent images for CA1 cell death quantification are shown in Fig 2A. Immunoreactivity against the neuronal marker NeuN 7-days after OGD showed abundant positive pyramidal neurons in the CA1 region of slices injected with naïve and E/F pre-treated administered alone or in complexes with FN-BMMs whereas the neuron numbers was dramatically reduced in the culture media or fibroblast-injected group (data not shown).
Figure 2.
A) Representative images of hippocampal slice cultures. Bright-field and propidium iodide fluorescence images taken 24h after the lethal ischemic (40 min of OGD) insult of culture medium, fibroblasts, EGF/bFGF-pretreated MIAMI cells, naïve MIAMI and EGF/bFGF pre-treated MIAMI/FN-BMMs, respectively. B) Propidium fluorescence values measured in the CA1 pyramidal cells in rat organotypic slices one day after ischemia. BMMs, E/F pre-treated MIAMI cells, E/F pre-treated MIAMI cells/FN-BMMs and näive MIAMI cells were neuroprotective as compared with culture medium and human fibroblasts-injected group (p<0.01). E/F pre-treated MIAMI cells/FN-BMMs were more neuroprotective as compared to BMMs and E/F pre-treated MIAMI (p<0.05). % Cell death as defined in the methods, reflects the ratio of propidium iodide staining 24h after lethal ischemia (OGD) and propidium iodide staining 24 h after 100 μm/L NMDA treatment (total cell death).
In vitro detection of donor cells, cell viability estimation and neuron-like differentiation analysis
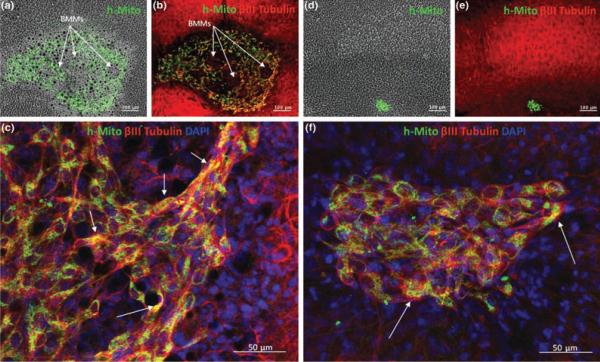
Human specific mitochondria antibody was used to detect the survival of MIAMI (näive and E/F-pre-treated) cells injected in hippocampal organotypic slices. Four days post-implantation, only some naive and E/F-pre-treated MIAMI cells were found directly in the CA1 region of the hippocampal slices as seen in Figure 3. FN-BMMs dramatically increased the number of MIAMI cells detected in the brain slices. A semi-quantitative analysis of stem cell survival showed 5- to 10-fold higher number of cells when implanted in complexes with FN-BMMs (Fig 3). As observe in Figure 3A and 3B, cells remained adhered to particles through the transplantation process. The structural support provided for the FN-BMMS to the cells is observed in Figures 3A and B. This support might contribute to enable MIAMI cells to survive and differentiate. Confocal imaging analysis of neuron-like differentiation analysis showed that a large fraction (40-60%) of the h-mitochondria positive cells were BIII-tubulin positive (Fig 3C and 3F). The neuron-like differentiation was similar for MIAMI cells injected alone or forming complexes with the FN-BMMs on day 4 after OGD.
Figure 3.
Representative images of survival and neuron-like differentiation studies showing organotypic slices injected with E/F pre-treated MIAMI cells/FN-BMMs (A, B and C) or with näive MIAMI cells (D, E and F) 4 days after OGD (10×). MIAMI cells are stained with anti-human mitochondria (green) and βIII tubulin (red). C) 20× confocal images showing the colocalization of human mitochondria positive cells with βIII tubulin for E/F pre-treated MIAMI cells/FN-BMMs injected group. F) 20× confocal images showing the colocalization of human mitochondria positive cells with βIII tubulin for naive MIAMI injected group. Stem cell survival rate is clearly increased by delivering the cells complexed with FN-BMMs. A large fraction (40-60%) of the transplanted cells are positive for βIII tubulin (yellow cells). The complexes between E/F pre-treated MIAMI cells and FN-BMMs 4 days after injection are clearly visible in A and B. Cells remained adhered to particles through the implantation process. The structural support provided for the FN-BMMS to the cells is shown in A and B.
OGD stimulates human STC1, LTBP2, tumor necrosis factor alpha-induced protein 6 (TSG6) and rat insulin-like growth factor binding protein 3 (IGFBP3) mRNA expression in rat hippocampal slices injected with E/F-MIAMI cells
To assess potential mechanisms by which E/F pre-treated MIAMI cells induced CA1 neuroprotection after ischemia/OGD we analyzed, by tissue species–specific RT-qPCR, changes in the expression of gene products previously implicated in MSC-mediated tissue repair (Curtis et al 2010). We quantified changes in human insulin-like growth factor 1 (IGF-1), hIGFBP-3, human insulin-like growth factor binding protein 5 (IGFBP-5), hLTBP2, hTSG-6, and hSTC1, as well as rIGF-1, rIGFBP-3, and rIGFBP-5, in rat hippocampal slices that had been injected with E/F pre-treated MIAMI cells before and after the ischemic insult. The hSTC1 mRNA, a pro-survival molecule (Block et al. 2009), increased 2-fold, the anti-inflammatory protein hTSG-6 mRNA (Lee et al. 2009) increased 2.74-fold, and hLTBP2 (Ohtaki et al. 2008) was increased 1.62-fold in E/F pre-treated MIAMI cells in response to the ischemic insult. In contrast, hIGF-1, hIGFBP-3, and hIGFBP-5 levels were unaffected. Analysis of rat specific mRNA transcripts, normalized against rRPL13a, detected no change in rIGF1, while rIGFBP3 increased (1.55±0.08) and rIGFBP5 decreased (−0.55±0.26) after induction of OGD, with no change after MIAMI cells injection.
FN-BMMs enhance the E/F pre-treated MIAMI cell-induced neuroprotection against cerebral ischemia in vivo
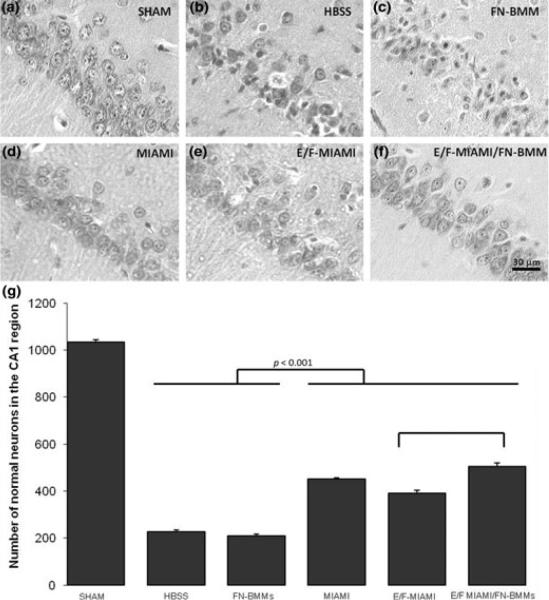
To further evaluate the therapeutic capacity of naïve and E/F pre-treated MIAMI cells forming complex or not with FN-BMMs in a more clinically relevant model, we used an in vivo model of ACA. Stem cells were injected by stereotaxic surgery 1h after ACA. Before and after the induction of ACA or sham ACA, physiological parameters including pCO2, pO2, HCO3− and plasma glucose concentration were similar among all experimental groups. During the induction of ACA, all cardiac-arrest groups showed immediate bradycardia when apnea was induced followed by hypotension to 50 mm Hg. The electrocardiogram pattern returned to normal within 5 min after return of spontaneous circulation (data not shown). No significant differences in physiological parameters were found between all groups. The mortality rate during and after the ACA procedure was 20%. Animals that survived ACA were injected 1 h after CA with the stem cells using a stereotaxic procedure. None of the animals receiving the different treatments died before the end of the planned recovery period. The number of normal neurons in the CA1 hippocampal region of sham operated rats (n=6) was 1034±11. Four days after CA, the number of normal neurons decreased to 193±8 in the saline treated group (n=4). All groups of rats treated with naïve MIAMI cells (n=3), E/F pre-treated MIAMI cells (n=4), or E/F pre-treated MIAMI/FN-BMMs complexes (n=3) significantly increased the number of normal neurons by 25.05% (452±6), 19.14% (391±13) and 30.07% (504±16) respectively compared with the saline treated group (P<0.0001). Interestingly, E/F pre-treated MIAMI/FN-BMMs complexes were significantly more neuroprotective than E/F pre-treated MIAMI cells injected alone (P<0.0001) (Fig. 4G). The injection of FN-BMMs without cells (n=4) (207±7) did not induce neuroprotection compared with the saline treated group.
Figure 4.
(A-F) Representative hematoxyline and eosin staining of the CA1 hippocampus on day 4 after ACA showing the predetermined CA1 areas where neuronal counting was performed as a measure of neuroprotection. The number of injured neurons was significantly reduced in groups MIAMI (n=3) (D) E/F pre-treated MIAMI (n=3) (E) and E/F pre-treated MIAMI/FN-BMMs (n=3) (F) compared with groups HBSS (n=4) (B) or FN-BMMs (n=4) (C) (p<0.001). (G) Treatment with naïve, E/F-treated MIAMI cells or with E/F-treated MIAMI/FN-BMM complexes significantly increased the number of normal neurons in the CA1 region compared to HBSS or FN-BMMs (p<0.001). E/F-MIAMI/FN-BMM complexes were significantly more neuroprotective than E/F-MIAMI cells injected alone (p<0.001). Results from one-way analysis of variance followed by Tukey's post hoc test. Scale bar 30 μm.
In vivo detection of donor cells, cell viability estimation and neuron-like differentiation analysis
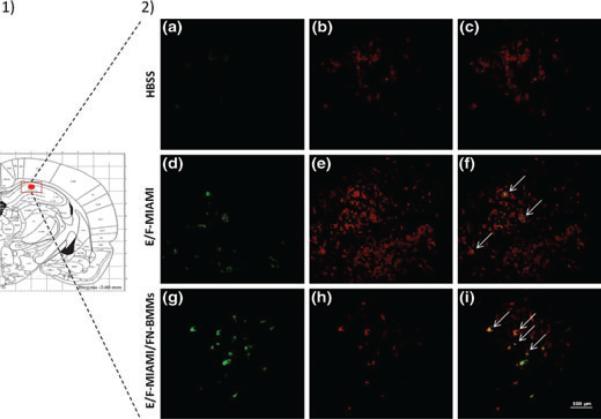
To detect the survival of MIAMI cells injected in rat brains, we used a human-specific mitochondrial antibody. Four days after injection, human-mitochondria labeled cells were present in the CA1 hippocampal region of animals injected with cells alone or cells combined with FN-BMMs (Fig 5). Cell engraftment estimation via observation of human-mitochondria positive cells suggested a higher number of E/F pre-treated MIAMI cells in cell/FN-BMMs complex injected animals. Furthermore, human cells remained close to the injection site without evidence of migration toward other brain regions. Double-immunofluorescence demonstrated co-localization of h-mitochondria and the neural marker βIII-tubulin in some cells (Fig 5).
Figure 5.
1) Anatomic references. The microinjection site as marked by the red dot for stem cell transplantation. The red square shows the area were images of h-mitochondria and β-III tubulin double immunofluorescent (DIF) were taken. 2) Representative fluorescence images of h-mitochondria and β-III tubulin DIF. Images are of brain sections from animals injected with HBSS (A-C), or E/F-treated MIAMI cells alone (D-F) or in complexes with FN-BMMs (G-I). Human cells were stained with anti-hu-Mito antibodies followed FITC-labeled secondary antibodies (green cells in left column). Neuronal cells in the hippocampus (red) were detected by staining with anti-β-III-tubulin antibodies (B, E and H). The brain of animals injected with HBSS show the absence of human cells, those injected with naïve (not shown) and E/F-treated MIAMI cells alone or in complexes with FN-BMMs show that a fraction of them developed features of neurons, characterized by the expression of β-III-tubulin (yellow cells in F and I) while others did not (green cells in F and I).
DISCUSSION
The present study demonstrates the neuroprotective effect of MIAMI cells alone or in combination with FN-BMMs on ameliorating hippocampal CA1 neuronal death due to cardiac arrest. Ex vivo experiments using ischemic organotypic slices showed that naïve and E/F pre-treated MIAMI cells were able to protect CA1 neurons from ischemic death, while fibroblasts did not. MIAMI cells therapeutic value was significantly enhanced when delivering the cells forming complexes with FN-BMMs. Neural cell protection might be attributed to MIAMI cell-specific paracrine effects. In vivo, the intra-hippocampal injection of the cells alone or combined with FN-BMMs 1 hour after 8 min ACA increased CA1 hippocampus neuronal survival. Moreover, FN-BMMs effectively enhanced E/F pre-treated MIAMI cells neuroprotective effect.
In this study, we chose a post-ischemia strategy to evaluate stem cell ability to promote neuroprotection after severe global cerebral ischemia. Experiments to test neuroprotective strategies become more relevant when treatments are administered after the injury since this is the most desirable intervention for patients. Our rationale was to administer stem cells in the early phases of the cell death process when neuroprotective strategies should be theoretically more useful. Therefore, we administered stem cells 1 hour after ischemia initiation both ex vivo and in vivo. It has been shown that the process of neuronal cell death is initiated within an hour of the ischemic insult in these two models of cerebral ischemia, which was identified by release of hippocampal mitochondrial cytochrome C into the cytoplasm (Raval et al. 2005). At this time point, naïve and E/F pre-treated MIAMI cells prevented cell death post-ischemia in both models. We considered appropriate the timing and delivery strategy in order to demonstrate the proof-of-principle in the described experiments. Based on the current results, additional time settings and delivery strategies will be examined in future studies in order to develop and approach suitable for clinical intervention.
We have completed previous studies (Jomura et al. 2007)(Ohtaki et al. 2008)(Zheng et al.) by using a highly homogeneous hMSC subpopulation, which is important for future clinical applications. MIAMI cells used in this study are characterized by their homogeneous morphology, molecular profile, and sustained and uniform expression of distinctive stem cells markers; which distinguish them from the more heterogeneous MSCs used in published studies on global cerebral ischemia (Ohtaki et al. 2008)(Zheng et al. 2010). MIAMI cells maintain a remarkably consistent molecular profile independent of age and gender which is achieved using culture conditions that mimic the niche where these cells are predicted to reside in vivo (D'Ippolito et al. 2004, D'Ippolito et al. 2006).
The survival of human cells transplanted into the rat brain could be reduced due to immune rejection. However, several studies have shown that human cells xenotranplanted into rodent brains (Ohtaki et al. 2008) and murine cells xenotransplanted into rat brains (Bible et al. 2009) survive, suggesting the immunoprivileged property of the rodent brain. Nevertheless, differentiation of progenitor cells into mature neural cells accompanied by the expression of neural/neuronal markers can be recognized as non-self and their survival compromised by immune responses. Thus the number of “differentiated” cells with neural/neuronal features may be compromised in the absence of immunosupression. Since the primary goal of the current studies was to assess neuroprotection by paracrine effects of undifferentiated cells we decided not to use immunosuppresants, which may impair the secretory capacity of the implanted cells. Additionally, Ohtaki et al., used human MSC injected into mice immunocompetent and immunosuppressed and they did not find significant differences in hMSC survival (Ohtaki et al., 2008). Moreover, taking into consideration the short duration/length of the in vivo experiments (4-days), we would not expect to see human cell rejection at this time point, or detect if they are rejected upon differentiation.
Recent investigations with neural stem cells reported that EGF, bFGF and/or leukemia inhibitory factor treatment of the cells before brain implantation directed their proliferation and differentiation potential toward different neuronal phenotypes (Tarasenko et al. 2004). More recently, EGF-pretreatment was used to modify the paracrine secretions of MSCs (Tamama et al., 2010) Thus, we chose to investigate the neuroprotective potential of untreated näive and EGF/bFGF pre-treated MIAMI cells in global cerebral ischemia. After E/F pre-treatment, MIAMI cells initiated their cell cycle exit and directed their gene expression pattern toward a neural/neuronal phenotype, consistent with recent demonstrations with neural stem cells (Tarasenko et al. 2004) and further confirming our previous results (Delcroix et al. 2010a). E/F pre-treatment also increased the expression of STC-1, a pro-survival molecule that plays an important role during cerebral ischemia (Block et al. 2009) (Zhang et al. 2000) and decreased the expression of the anti-inflammatory molecule LTBP2 suggesting that E/F-treated may have a distinct paracrine profile when compared to naïve cells and confirming that E/F pre-treatment modify the paracrine secretions of MSCs. Previous observations indicated that MSCs repair tissues by their stem cell-like ability to differentiate and by the secretion of cytokines, chemokines and growth factors including EGF, FGF, vascular endothelial growth factor A, insulin-like growth factor 1, nerve growth factor beta, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor; (Crisostomo et al. 2008)(Prockop et al. 2007)(Rios et al. 2010)(Li & Chopp 2009). These results suggest that transplanted MSCs work as “small molecular factories” providing trophic support in response to the local environment which may produce therapeutic benefits in cell survival, tissue repair and functional recovery (Li & Chopp 2009). The acute effect observed and the low number of cells found in the rat brain 4 days after implantation suggest that cell transdifferentiation toward a neuronal phenotype and potential cell replacement cannot be the predominant neuroprotective mechanism. However, the finding that a large fraction (40-60%) of the implanted cells acquired features of neuronal cells (i.e., βIII-tubulin) opens the possibility that development of a pro-neuronal phenotype may be a contributing factor to the mechanisms mediating the neuroprotective effect of these cells. Neuroprotection by MIAMI cells might be mediated by complex paracrine actions, in agreement with previous studies with MSCs (Crisostomo et al. 2008)(Prockop et al. 2007)(Rios et al. 2010)(Li & Chopp 2009). In this sense, naïve and E/F pre-treated MIAMI cells secrete several pro-survival (fracktalkine, growth related oncogene protein, interleukine-8) and angiogenic cytokines (including vascular endothelial growth factor and monocyte chemotactic protein-1) (Rahnemai-Azar et al. 2010) that may be involved in neuronal protection during ischemia. In this study we also demonstrate that ischemia/OGD increases the expression of anti-inflammatory molecules hTSG-6 and hLTBP2, and the pro-survival molecule STC1 by E/F pre-treated MIAMI cells after their injection into rat hippocampal slices. These results show that E/F pre-treated MIAMI cells were activated in response to the ischemic environment and may cross-talk with ischemic rat cells as was seen in other systems (Ohtaki et al. 2008). Gene expression results reported here are also consistent with previous reports with MSCs in a mouse model of global cerebral ischemia (Ohtaki et al. 2008) and in a myocardial infarcted mouse model (Lee et al. 2009) suggesting a mechanism of action with some points in common for MSCs in general in response to an ischemic insult.
Another important contribution of the current study is the combination of adult stem cells and FN-BMMs for a central nervous system application. To our knowledge this is the first brain tissue engineering approach for a global cerebral ischemia application. Previous central nervous system studies combining scaffolds and stem cells were mainly focused on Parkinson's disease, Huntington's disease or stroke (review in (Delcroix et al. 2010b)). Results emphasized the importance of biomimetic 3D scaffold approaches in brain tissue engineering. Many of these studies proved not only that biomimetic scaffolds are not just space fillers but also that they have the potential to influence cell behavior in terms of survival, proliferation or differentiation toward tissue repair and regeneration (Delcroix et al. 2010b). FN-BMMs used in this paper are made of PLGA, a polymer totally biodegradable and biocompatible with the brain, which is of tremendous importance for a cerebral application to minimize glial scar and inflammation after brain implantation. These FN-BMMs also provide a small 3D structure for implantation by stereotaxic surgery in a precise area of the brain. For a clinical application, they could be produced in advance in cGMP conditions and then stored for months as a freeze-dried powder, needing only a few hours for cell adhesion before transplantation.
Although poly(lactic-co-glycolic acid) particulate scaffolds combined with many cell types have been previously studied (Bible et al. 2009)(Newman & McBurney 2004), the carriers used in this study represent a more advanced approach due to their biomimetic surface that can regulate cell behaviour. In the current work, FN-BMMs were specifically customized for a brain ischemic application combining the cell adhesion molecule FN on its surface as it has been shown to facilitate MSC survival (Karoubi et al. 2009). It is well-known that extracellular matrix molecules like FN may affect proliferation and life span of the cells. Karoubi et al recently investigated hMSC viability in a single-cell hydrogel capsule containing immobilized FN and fibrinogen (Karoubi et al. 2009). They found that the incorporation of these matrix molecules enhanced cell viability and metabolic activity among others. Results from our work showed that E/F pre-treated MIAMI cells attached to FN-BMMs were more neuroprotective than naïve or E/F pre-treated cells injected alone. Our results suggest that the biomimetic surface and 3D polymeric support that FN-BMMs provide might have increased E/F pre-treated MIAMI cell survival leading to augmented paracrine secretion and actions over time. Moreover, recent studies of our group showed that laminine coated-PAMs (60 μm particle size) increased the relative expression levels of vascular endothelial growth factor 24h after adhesion to FN-BMMs with respect to cells alone demonstrating that the 3D environment as well as the mechanical and signalling cues provided by the extracellular matrix molecule enhanced the paracrine secretion of E/F pre-treated MIAMI cells (Garbayo et al., In preparation). Given the short duration (4 days), there may be greater therapeutic benefit from MIAMI/FN-BMMs injected rats after a longer time period. These studies using FN-BMMs set the stage for future studies in which the microcarriers will be able to release bioactive molecules in a controlled fashion over extended periods of time, a notable characteristic of PAMs. Future studies will also include the use of PAMs loaded with molecules that prevent neuronal apoptosis, or promote MIAMI cell survival and neuronal differentiation after cerebral ischemia while conveying naïve or E/F pre-treated MIAMI cells on their surface. In this context, laminin-coated PAMs secreting neurotrophin-3 conveying MIAMI cells were evaluated in a hemi-parkinsonian rat model of Parkinson's disease. In this model, both key aspects of the PAMs, conveying cells in a biomimetic surface and controlled release of a bioactive molecule, had additive effects on the engraftment and functional outcomes of the therapeutic benefit of MIAMI cells (Delcroix et al. 2011). Thus, it would be reasonable to assume that PAMs loaded with neurotrophin-3 or brain-derived neurotrophic factor would further enhance the engraftment and functional outcomes of MIAMI cells in models of global ischemia.
In summary, we provided evidence that naïve and E/F pre-treated MIAMI cells protected CA1 hippocampal neurons from global cerebral ischemia and that FN-BMMs enhanced E/F pre-treated MIAMI cells therapeutic effect. Further studies are warranted to explore the optimal therapeutic window, route of delivery and long-term safety and efficacy of MIAMI cells and PAMs for the treatment of neurological conditions.
ACKNOWLEDGEMENTS
This work was supported by Merit Review awards from the Department of Veterans Affairs, USA to (PCS), by the National Institutes of Health Grants NS45676, NS054147 and NS34773 (MAPP), by Région des pays de La Loire and by the INSERM. We thank the Service Commun d'Imagerie et d'Analyse Microscopique of Angers for SEM images.
Abbreviations used
- MIAMI
Marrow-Isolated Adult Multilineage Inducible
- BMMs
Biomimetic Microcarriers
- CA1
Cornu Ammonis layer 1
- FN
Fibronectin
- ACA
Axphyxial cardiac arrest
- EGF
Epidermal growth factor
- bFGF
Basic fibroblast growth factor
- E/F
EGF/bFGF pre-treatment
- MSCs
Mesenchymal stromal cells
- PAMs
Pharmacologically Active Microcarriers
- PBS
Phosphate buffer saline
- HBSS
Hanks balanced salt solution
- RT-qPCR
Reverse Transcription-quantitative real-time PCR
- OGD
Oxygen and glucose deprivation
- STC1
Stanniocalcin
- TSG6
Tumor necrosis factor alpha-induced protein 6
- LTBP2
Latent transforming growth factor binding protein 2
- IGFBP
Insulin-like growth factor binding protein
- IGF-1
Insulin-like growth factor 1
Footnotes
DISCLOSURES/ CONFLICT OF INTEREST
The authors declare no conflict of interest
REFERENCES
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, Modo M. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials. 2009;30:2985–2994. [Google Scholar]
- Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, DiMattia G, Sullivan DE, Prockop DJ. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009;27:670–681. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Surgically relevant aspects of stem cell paracrine effects. Surgery. 2008;143:577–581. doi: 10.1016/j.surg.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis KM, Gomez LA, Rios C, Garbayo E, Raval AP, Perez-Pinzon MA, Schiller PC. EF1alpha and RPL13a represent normalization genes suitable for RT-qPCR analysis of bone marrow derived mesenchymal stem cells. BMC Mol Biol. 11:61. doi: 10.1186/1471-2199-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- D'Ippolito G, Howard GA, Roos BA, Schiller PC. Isolation and characterization of marrow-isolated adult multilineage inducible (MIAMI) cells. Exp Hematol. 2006;34:1608–1610. doi: 10.1016/j.exphem.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Delcroix GJ, Curtis KM, Schiller PC, Montero-Menei CN. EGF and bFGF pre-treatment enhances neural specification and the response to neuronal commitment of MIAMI cells. Differentiation. 2010a;80(4-5):213–227. doi: 10.1016/j.diff.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Delcroix GJ, Garbayo E, Sindji L, Thomas O, Vanpouille C, Schiller PC, Montero-Menei CN. Pharmacologically Active Microcarriers enhance the therapeutic potencial of human multipotent mesenchymal stromal cells transplanted in hemiparkinsonian rats. Biomaterials. 2011;32(6):1560–1573. doi: 10.1016/j.biomaterials.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Delcroix GJ, Jacquart M, Lemaire L, Sindji L, Franconi F, Le Jeune JJ, Montero-Menei CN. Mesenchymal and neural stem cells labeled with HEDP-coated SPIO nanoparticles: in vitro characterization and migration potential in rat brain. Brain Res. 2009;1255:18–31. doi: 10.1016/j.brainres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Delcroix GJ, Schiller PC, Benoit JP, Montero-Menei CN. Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials. 2010b;31:2105–2120. doi: 10.1016/j.biomaterials.2009.11.084. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giteau A, Venier-Julienne MC, Marchal S, Courthaudon JL, Sergent M, Montero-Menei C, Verdier JM, Benoit JP. Reversible protein precipitation to ensure stability during encapsulation within PLGA microspheres. Eur J Pharm Biopharm. 2008;70:127–136. doi: 10.1016/j.ejpb.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Jendelova P, Herynek V, Urdzikova L, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- Jomura S, Uy M, Mitchell K, Dallasen R, Bode CJ, Xu Y. Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells. 2007;25:98–106. doi: 10.1634/stemcells.2006-0055. [DOI] [PubMed] [Google Scholar]
- Karoubi G, Ormiston ML, Stewart DJ, Courtman DW. Single-cell hydrogel encapsulation for enhanced survival of human marrow stromal cells. Biomaterials. 2009;30:5445–5455. doi: 10.1016/j.biomaterials.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5(6):485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–106. doi: 10.1002/stem.430. 2010. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G, Bang OY. Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant. 2008;17:1045–1059. doi: 10.3727/096368908786991551. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456:120–123. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, Koc ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- Newman KD, McBurney MW. Poly(D,L lactic-co-glycolic acid) microspheres as biodegradable microcarriers for pluripotent stem cells. Biomaterials. 2004;25:5763–5771. doi: 10.1016/j.biomaterials.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Noh KM, Yokota H, Mashiko T, Castillo PE, Zukin RS, Bennett MV. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc Natl Acad Sci U S A. 2005;102:12230–12235. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando: 1996. [Google Scholar]
- Perez-Pinzon MA, Xu GP, Mumford PL, Dietrich WD, Rosenthal M, Sick TJ. Rapid ischemic preconditioning protects rats from cerebral anoxia/ischemia. Adv Exp Med Biol. 1997;428:155–161. doi: 10.1007/978-1-4615-5399-1_22. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- Rahnemai-Azar ADIG, Gomez LA, Reiner T, Vazquez-Padron R, Perez-Stable C, Roos BA, Pham SM, Schiller PC. Human marrow-isolated adult multilineage inducible (MIAMI) cells protect against peripheral vascular ischemia in a mouse model. Cytotherapy. 2011;2:179–192. doi: 10.3109/14653249.2010.515579. [DOI] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Prado R, Katz LM, Busto R, Sick TJ, Ginsberg MD, Mochly-Rosen D, Perez-Pinzon MA. Protein kinase C delta cleavage initiates an aberrant signal transduction pathway after cardiac arrest and oxygen glucose deprivation. J Cereb Blood Flow Metab. 2005;25:730–741. doi: 10.1038/sj.jcbfm.9600071. [DOI] [PubMed] [Google Scholar]
- Rios C, Garbayo E, Gomez AL, Curtis K, D'Ippolito G, Schiller PC. Stem Cells and their contribution to tissue repair. Stem Cell and Regenerative Medicine. 2010;1:9–22. [Google Scholar]
- Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367–383. doi: 10.1016/S0079-6123(06)61026-1. [DOI] [PubMed] [Google Scholar]
- Tarasenko YI, Yu Y, Jordan PM, Bottenstein J, Wu P. Effect of growth factors on proliferation and phenotypic differentiation of human fetal neural stem cells. J Neurosci Res. 2004;78:625–636. doi: 10.1002/jnr.20316. [DOI] [PubMed] [Google Scholar]
- Tatard VM, Menei P, Benoit JP, Montero-Menei CN. Combining polymeric devices and stem cells for the treatment of neurological disorders: a promising therapeutic approach. Curr Drug Targets. 2005a;6:81–96. doi: 10.2174/1389450053344885. [DOI] [PubMed] [Google Scholar]
- Tatard VM, Sindji L, Branton JG, Aubert-Pouessel A, Colleau J, Benoit JP, Montero-Menei CN. Pharmacologically active microcarriers releasing glial cell line - derived neurotrophic factor: Survival and differentiation of embryonic dopaminergic neurons after grafting in hemiparkinsonian rats. Biomaterials. 2007;28:1978–1988. doi: 10.1016/j.biomaterials.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Tatard VM, Venier-Julienne MC, Benoit JP, Menei P, Montero-Menei CN. In vivo evaluation of pharmacologically active microcarriers releasing nerve growth factor and conveying PC12 cells. Cell Transplant. 2004;13:573–583. doi: 10.3727/000000004783983675. [DOI] [PubMed] [Google Scholar]
- Tatard VM, Venier-Julienne MC, Saulnier P, Prechter E, Benoit JP, Menei P, Montero-Menei CN. Pharmacologically active microcarriers: a tool for cell therapy. Biomaterials. 2005b;26:3727–3737. doi: 10.1016/j.biomaterials.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Xu GP, Dave KR, Vivero R, Schmidt-Kastner R, Sick TJ, Perez-Pinzon MA. Improvement in neuronal survival after ischemic preconditioning in hippocampal slice cultures. Brain Res. 2002;952:153–158. doi: 10.1016/s0006-8993(02)02988-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Zheng W, Honmou O, Miyata K, Harada K, Suzuki J, Liu H, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits of human mesenchymal stem cells derived from bone marrow after global cerebral ischemia. Brain Res. 1310:8–16. doi: 10.1016/j.brainres.2009.11.012. [DOI] [PubMed] [Google Scholar]